Abstract
The presence of microplastics in freshwater systems can have harmful effects on the food chain. Zooplankton, especially suspension and filter feeders, can ingest microplastics, which can cause adverse effects and transfer them to higher trophic levels. Here, we analyze the presence, abundance, and distribution of microplastics in surface water, zooplankton, and fish in two tropical lakes in central Mexico. We collected water samples in triplicate at three sites in each lake and 120 fish of the genus Chirostoma. From each water sample, 300 rotifers and 150 microcrustaceans were randomly isolated and processed independently. Of the particles found in the water, zooplankton, and fish from both lakes, the fragments were the predominant ones. The total abundance of microplastics in the water column of both lakes varied between 1.2 and 17.0 items L−1. In zooplankton, fragments were found predominantly with up to 0.1 items ind−1, while in fish, up to 4.5 items ind−1 was recorded. Our results confirm the presence of microplastics in different compartments of the food webs of freshwater bodies, water column, zooplankton, and fish. Further work is required on the possible effects of these stressors at the different trophic levels.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Different characteristics of plastic, such as its durability, corrosion resistance, and low cost, generate great social benefits, which is why it has been positioned in practically all aspects of modern life (Andrady & Neal, 2009; Phuong et al., 2016). However, it has also resulted in large quantities of waste that, if improperly managed, means a potential source of contamination of terrestrial, marine, and freshwater environments (Rillig, 2012; Lambert et al., 2013; Eerkes-Medrano et al., 2015). Plastic particles (< 5 mm) are defined as microplastics (MPs) (Thompson et al., 2009), which, depending on their origin, are classified as primary when they are manufactured of microscopic sizes, such as personal care products, and as secondary, formed by the fragmentation of larger plastics by physical or chemical processes (Boucher & Friot, 2017). Due to their small size and low density, microplastics can be easily transported through the air and enter most water bodies (Dris et al., 2016; Rochman, 2018). Microplastics have been widely studied in marine environments (Andrady, 2011; Thompson et al., 2004), where the adverse effects they can cause on marine biota have been revealed (Murray and Cowie, 2011; Cole et al., 2013; Lusher et al., 2013; Nobre et al., 2015). Evidence suggests that microplastic pollution is ubiquitous in terrestrial, marine, estuarine, and freshwater environments (Cole et al., 2011; Forero-López et al., 2021; Rochman, 2018; Xiong et al., 2018). However, the first records in freshwater environments are about 10 years old (Eriksen et al., 2013), so most works have focused on recording microplastic presence, abundance, and distribution in rivers and lakes (reviewed in Zhao et al., 2022).
In freshwater systems, microplastics have been found at average concentrations of 892,777 p/km2 in rivers in Europe (Mani et al., 2015), and in lakes in the USA, concentrations of up to 32 particles/m3 have been recorded (Baldwin et al., 2016), while in China, between 0.013 and 930 items L−1 (Han et al., 2020; Mao et al., 2020) and between 35 and 8925 items/m3 (Jian et al., 2020; Wang et al., 2017) have been recorded in rivers and lakes respectively. The size and presence of microplastics in freshwater systems can have harmful effects on the food chain since various groups of zooplankton, especially suspension and filter feeders, can ingest them directly, affecting them and also transferring them to higher trophic levels (Scherer et al., 2018). However, the literature on freshwater systems is limited and still inconclusive (Nelms et al., 2018). It has been proven that some species of rotifers (Drago et al., 2020; Jeong et al., 2016), cladocerans (De Felice et al., 2019; Rehse et al., 2016), and copepods (Cole et al., 2013) can consume microplastics of different sizes and their consumption can have adverse effects on their demographic variables. Thus, at higher levels of the lake food chain, microplastics can be ingested through two routes: direct ingestion (e.g., zooplanktivorous fish) and indirect ingestion through prey (e.g., piscivorous fish) (Scherer et al., 2018).
Reports on the ingestion of microplastics in freshwater fish represent a low percentage (38%) since most of the work has been carried out on species from marine environments (62%); however, species such as Cyprinus carpio and Oreochromis niloticus have received about a hundred publications (reviewed by Galafassi et al., 2021). Most of the reports on freshwater fish have been carried out mainly in laboratory work (Parker et al., 2021). Field studies (Lin et al., 2020; Park et al., 2020a, 2020b; Garcia et al., 2021) have recorded that the abundance of microplastics in freshwater fish can vary between 0 and 48 particles ind−1. The abundance of microplastics in fish is not necessarily related to the abundance of microplastics in the water column (O'Connor et al., 2020; Wang et al., 2020); it may also be related to the concentration of food or even the type and color of microplastics resembling food particles (Kim et al., 2019). In Mexico, work has been carried out with microplastics in the gastrointestinal tract of a fish of high regional consumption (Oreochromis niloticus), where they have found abundances of up to 24 items fish−1, which may represent a possible trajectory for human exposure (Martinez-Tavera et al., 2021). Also, in the central region of Mexico, there are other species of fish, which, due to their high regional consumption and mode of consumption, may represent a greater risk for human exposure to microplastics, such as pez blanco (Chirostoma).
In the central region of Mexico, there are 18 freshwater fish species from the Atherinopsidae family, particularly from the genus Chirostoma, commonly known as pez blanco or charales. They are species that originated in this region of the country and, given their restricted distribution, are considered endemic (Barbour, 1973; Berlanga-Robles et al., 2002). Species of this genus have been a food source in the region for more than a century; however, overfishing, pollution, and inadequate regulation have decreased populations, placing them at risk of extinction (Berlanga-Robles et al., 2002; Soto-Galera et al., 1998). In recent years, charal fishing has declined due to factors such as overexploitation and environmental degradation (Orbe-Mendoza et al., 2002), a situation that places not only the charales but also the residents of these regions in a vulnerable situation who regularly consume this resource and who are exposed to emerging contaminants such as microplastics. Therefore, in this study, we investigated the abundance, morphology, and distribution of microplastics in water, zooplankton, and fish from two shallow tropical water bodies in Central Mexico. This work will contribute to one of the first references on microplastic contamination in Mexico and could be helpful as a basis for monitoring these contaminants in tropical freshwater systems.
Materials and methods
Study area
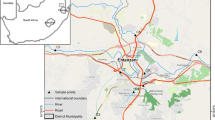
Lakes Cuitzeo and Pátzcuaro are located in the center of the country in the State of Michoacán, Mexico, between 20°05′–19°52′N and 100°50′–101°19′W and 19°32′–19°42′N and 101°32′–101°43′W respectively (Fig. 1). These water bodies are part of the Lerma-Chapala endorheic basin (Bernal-Brooks, 1998; Bradbury, 2000; Torres, 1993). They are shallow tropical lakes of high altitude since Cuitzeo is located at 1820 and Pátzcuaro at 2035 m a.s.l. (Bernal-Brooks, 1998). Lake Cuitzeo has a larger surface area of water bodies, with 420 km2, while Pátzcuaro has a maximum surface area of 116 km2; however, both lakes are considered among the largest in Mexico (Alcocer & Bernal Brooks, 2010). Both water bodies experience rain in summer (May to October) with an average annual precipitation of 906 mm (García Amaro, 2004; Bravo-Inclán et al., 2022). The trophic state of both lakes has varied over time between eutrophic and hypereutrophic, the latter being the prevailing one in aquatic systems (Berry et al., 2011; Lyons et al., 2000). In these lakes, there are five species of the genus Chirostoma, C. estor, C. granduncle, C. patzcuaro, C. attenuatum, and C. humboldtianum, commonly called charales, which have been historically consumed as part of the diet of the populations adjacent (Barbour, 1973; Berlanga-Robles et al., 2002; Lyons et al., 2000; Orbe-Mendoza et al., 2002). Currently, charal populations are subject to multiple stressors, mainly due to the eutrophication of water bodies, the introduction of exotic species, and the municipal and industrial waste dumped into them.
Sample collection
The samples were collected in March 2020 in two tropical lakes in Central Mexico, Cuitzeo and Pátzcuaro. Three sampling sites were established in each lake, where triplicate samples of surface water (< 0.30 m) were collected using the bucket sampling method described by Song et al. (2014). For each sample, 30 L of water was filtered with a clean steel bucket through a prewashed stainless steel mesh with a 50-µm mesh size. The residue on the mesh was placed in prewashed glass containers with 100 mL of ultrapure water. The samples were preserved with 5% formalin and 4 °C until laboratory analysis. The fish collection was carried out by local fishermen, who used artisanal methods with traditional techniques that include seines, gillnets, butterfly nets, hooks, and harpoons (Orbe & Acevedo, 1991; Orbe-Mendoza et al., 2002) from whom we acquired the specimens immediately after being caught. Particularly in the collection of the specimens, gillnets were used in the places where the water samples were collected. In each lake, at least 150 fish of the genus Chirostoma were acquired, which were preserved in 10% formalin and then transferred to 70% alcohol and transferred to the laboratory for analysis.
Laboratory analysis
In the laboratory, prior to analysis, 300 rotifers and 150 microcrustaceans (cladocerans and copepods) were randomly isolated from each of the nine water samples from each lake and processed independently. To remove organic matter from water samples and isolated zooplankton, we used the digestion technique with H2O2 in the presence of metal ion (Baldwin et al., 2016; Masura et al., 2015). We use hydrogen peroxide (30%) as an oxidant and an iron sulfate solution as a catalyst. To remove salts and minerals, we use density separation. Subsequently, the samples were vacuum filtered through a 0.22-µm pore size filter (GF/F Whatman). The filters were placed in clean Petri dishes for visual analysis under a microscope. A Nikon SMZ800 stereoscopic microscope (× 10– × 80) was used, where the microplastics were classified, counted, and isolated considering their color, size, and shape according to established categories (Hidalgo-Ruz et al., 2012). Based on their shape, microplastics were classified into three categories: fiber, fragment, and pellet (Rochman et al., 2019; Wang et al., 2017).
Hydrogen peroxide for soft fabrics
The protocol of Li et al. (2016) was used to extract the particles from the gastrointestinal tracts of the fish. A total of 120 individuals from each lake were rinsed with ultrapure water, and biometrics (weight and size) were performed (Lusher et al., 2016), classifying them into four categories according to weight. The specimens were dissected by removing the gastrointestinal tract, from the esophagus to the anus, and ten gastrointestinal tracts were placed in a 500-mL beaker with 200 mL of H2O2 and incubated at 65 °C until the solutions appeared clear without particle evidence. Incubation times were 24 h and, in some cases, 48 h. For each of the four categories, three repetitions were carried out with ten gastrointestinal tracts each, so 120 fish were used (10 specimens × 3 repetitions × 4 categories).
Identification and characterization
From the 60 filters obtained from the water, zooplankton, and fish samples, subsamples of microplastics were obtained that were used for the analysis of the micromorphological characteristics by scanning electron microscopy and for the verification of the polymer composition by Raman spectroscopy (Lusher et al., 2016). For SEM analyses, the microplastics were isolated and placed individually on carbon adhesive tape, coated with gold, and analyzed in a JEOL JSM6360LV microscope (SAMEB of ICMyL UNAM). Microplastics were identified using Raman spectroscopy at the University Laboratory of Spectroscopic Characterization, LUCE ICAT UNAM. Raman spectrum measurements were recorded with a WITec alpha300 RA (WITEc GmbH, Ulm, Germany) instrument using a 300 lines/mm grating and 532-nm laser light excitation, originating from a Nd:YVO4 green laser. The incident laser beam with a power of 44 mW was focused by × 20, × 50, and × 100 objectives (Zeiss, Germany) with 0.4, 0.75, and 0.9 NA, respectively. Punctual Raman spectra were obtained with 0.5-s integration time and ten accumulations. The data processing and analysis were performed with the WITec Project version 5.1 software.
Quality assurance and quality controls
During all stages of the study (sampling and laboratory), measures were implemented to avoid potential contamination of the samples. Any plastic equipment in the sample collection was eliminated, and all field equipment used were carefully prewashed three times with ultrapure water. Cotton laboratory coats and clean nitrile gloves were used during sampling and laboratory work. Water samples at each sampling site were collected in triplicate and were covered, preserved, and stored immediately to avoid exposure to air. White controls were also carried out to verify potential contamination in the field and laboratory, following the same processes used in the samples. In the field, 5 L of ultrapure water was filtered, and after rinsing the 50-µm steel mesh, the water was placed in containers equal to those of the samples and the same analysis process of the environmental samples followed. This procedure allowed us to determine that possible contamination during sampling, transportation, and laboratory procedures was negligible. In addition to the white controls, in the laboratory, all glassware were washed using natural fiber cleaning brushes, rinsed three times with ultrapure water, and immediately covered with aluminum foil. The samples always remained covered during the laboratory processes to avoid airborne contamination. The observation of the filters under a stereoscopic microscope was carried out in clean Petri dishes. Prior to any laboratory analysis, an air purifier with a HEPA filter was used in the workspace.
Statistical analysis
Normality tests were carried out with the Shapiro–Wilk test to compare the abundances of microplastics in water and zooplankton samples, while significant differences between groups were carried out with t-tests. If the normality test failed, a Mann–Whitney test was performed. After checking normality for comparisons between categories of fish, an analysis of variance (one-way ANOVA) was performed, followed by a Tukey multiple comparison. All these analyses were performed using the Prism software GraphPad 9.
Results and discussion
Abundance of microplastics in water, zooplankton, and fish
Microplastics were widely detected in the surface water of the studied areas where average concentrations of 2.6 ± 0.4 items L−1 and 7.7 ± 1.8 items L−1 were recorded in Lakes Cuitzeo and Pátzcuaro respectively (Fig. 2). When comparing the total abundance of microplastics in surface water, significant differences were found (p = 0.01) between Lake Pátzcuaro, which presented values greater than 15 items L−1, and Lake Cuitzeo, where the maximum values were below 5 items L−1 (Fig. 2). The concentrations of microplastics detected in the surface waters of the lakes of Central Mexico were high considering the values obtained in other freshwater systems (Anderson et al., 2017; Fischer et al., 2016), especially from countries like China, which is the largest producer of plastics worldwide and where high concentrations of up to 8925 ± 1591 items/m3 MPs have been recorded (Jian et al., 2020; Wang et al., 2017, 2018). Elevated concentrations of microplastics in freshwater systems have been related to industrial and domestic waste (Browne et al., 2011), so proximity to urban centers impacts the abundance of microplastics (Barrows et al., 2018; Hendrickson et al., 2018; Wang et al., 2017). In the basins of these lakes, there are about 30 municipalities where 1 million people live. These municipalities, together with industrial activities, produce 700 tons of solid waste per day, half of which is plastics and industrial packaging (Delgado et al., 2007, 2008), which can potentially impact aquatic systems.
Box and whisker plots showing total microplastic densities in the water column in Lakes Cuitzeo and Pátzcuaro (for each box, the solid horizontal lines from top to bottom indicate maximum value, 75% quartile, median, 25% quartile, and minimum value, respectively; the symbol + inside the box represents the mean value)
Through the concentration analysis, the three forms of microplastics (fiber, fragments, and pellets) found in the study sites were higher. In the fraction corresponding to water, an average of 0.9 ± 0.14 items L−1 fibers was recorded in Lake Cuitzeo, while 1.2 ± 0.17 items L−1 fibers was found in Lake Pátzcuaro (Fig. 3a). Fragments were the most abundant forms in the surface water. They presented significant differences (p = 0.02) between both lakes with averages of 1.5 ± 0.39 and 5.7 ± 1.67 items L−1 fragments in Cuitzeo and Pátzcuaro respectively (Fig. 3b). Finally, in water, the forms with the lowest abundances were pellets with 0.08 ± 0.04 items L−1 in Lake Cuitzeo and 0.5 ± 0.3 items L−1 in Lake Pátzcuaro (Fig. 3c). The three forms of plastics, fibers, fragments, and pellets found in the surface waters of the lakes studied, are some of those that are typically recorded in epicontinental water bodies (Anderson et al., 2017; Jian et al., 2020; Wang et al., 2017, 2018; Xiong et al., 2021). However, our data differ in two main aspects from these works. The first is that in our study, the presence of films was not recorded, and the second is that the shape of the dominant particles was not fibers. Although films are forms that can be found in negligible concentrations (Xiong et al., 2021), they are forms frequently recorded in various continental water bodies (Zhao et al., 2022). On the other hand, fibers were the dominant particles in these works regarding the proportion of shapes, while in our study, the dominant shapes were fragments. In other studies, fragments are one of the most abundant forms in freshwater aquatic systems (reviewed by Koelmans et al., 2019). In Central Mexico, in a study where the presence and abundance of microplastics in sediments of the Atoyac River were analyzed (Shruti et al., 2019), it was found that the two dominant forms were films (25.9%) and fragments (22.2%). Fibers have a greater distribution in freshwater environments, while forms such as fragments and films have been mainly related to areas with anthropogenic influence (Amrutha & Warrier, 2020).
Box and whisker plots showing different microplastic forms: (a) fiber, (b) fragment, and (c) pellet densities in the water column in Lakes Cuitzeo and Pátzcuaro (for each box, the solid horizontal lines from top to bottom indicate maximum value, 75% quartile, median, 25% quartile, and minimum value, respectively; the symbol + inside the box represents the mean value)
Abundance of microplastics in zooplankton
Of the 18 zooplankton samples analyzed in both lakes, the presence of microplastics was found in 94.5%. The abundance of microplastics in zooplankton had higher average values in Lake Cuitzeo in the two forms of microplastics observed in this group. Averages of 0.01 ± 0.003 items ind−1 fibers and 0.11 ± 0.02 items ind−1 fragments were recorded in Lake Cuitzeo, while Lake Pátzcuaro presented 0.005 ± 0.003 items ind−1 fibers and 0.10 ± 0.03 items ind−1 fragments (Fig. 4). No significant differences (p < 0.05) were observed within the zooplankton in the two forms of microplastics recorded in both lakes. Literature on microplastics in zooplankton still needs to be expanded. The initial efforts have been carried out mainly in marine environments and only correlate the concentrations of microplastics with zooplankton abundances (Moore et al., 2001; Lattin et al., 2004; Collignon et al., 2014; Frias et al., 2014). However, the study conducted by Sun et al. (2017) analyzed the ingestion of microplastics by zooplankton in the northern South China Sea. Finding that 100% of the zooplankton samples contained microplastics, so it was possible to verify that groups such as copepods in natural systems can ingest microplastics, as happened in our study where the presence of microplastics was recorded in 94% of the cases. In later works, such as the one carried out in the southern South China Sea (Amin et al., 2020), it was recorded that the consumption of microplastics by cyclopoids and calanoids copepods was between 0.003 and 0.13 particles ind−1, very similar values found in our work (Fig. 4). However, our study was carried out in a freshwater system including other zooplankton groups in addition to copepods such as rotifers and cladocerans.
Box and whisker plots showing different microplastic forms: (a) fiber and (b) fragment densities in zooplankton collected in Lakes Cuitzeo and Pátzcuaro (for each box the solid horizontal lines from top to bottom indicate maximum value, 75% quartile, median, 25% quartile, and minimum value, respectively; the symbol + inside the box represents the mean value)
Work with freshwater zooplankton has mainly been carried out in laboratory tests. It has been documented that zooplankton, particularly rotifers and microcrustaceans (cladocerans and copepods), are capable of consuming microplastics at rates between 2.5–3200 P ind−1 h−1 and 1–28,000 P ind−1 h−1, respectively (reviewed by Scherer et al., 2018). Laboratory studies have not only verified the consumption of microplastics by zooplankton, but they have also documented their adverse effects on the population dynamics of rotifers (Drago et al., 2020; Sun et al., 2019; Xue et al., 2021), cladocerans (De Felice et al., 2019), and copepods (Cole et al., 2013). According to our results, the three main groups of freshwater zooplankton (rotifers, cladocerans, and copepods) are capable of consuming microplastics under natural conditions. However, the effects observed in laboratory tests cannot be comparable with natural populations since higher concentrations of microplastics are used in laboratory tests (Phuong et al., 2016). The consumption of microplastics by zooplankton under natural conditions is a function of the abundance of zooplankton and the number of particles per individual, which is why it becomes inconsistent (Amin et al., 2020). The effect of microplastics on the food web using concentrations close to those recorded in natural water bodies was evaluated by Yıldız et al. (2022), who observed that zooplankton could easily ingest microplastics. However, the degree and incidence of ingestion were lower than those observed in laboratory tests. Likewise, their results show that the effect on the population dynamics of zooplankton due to the consumption of microplastics is less than that observed in laboratory tests.
Abundance of microplastics in fish
The predominant form of microplastics in fish from both lakes was fragments, followed by filaments. No significant differences (p < 0.05) were observed within the fish categories in the two forms of microplastics recorded in this group of organisms. The range of particle abundance in the gastrointestinal tract of fish varied between 0.2 and 4.5 items ind−1 (Fig. 5). Only in the abundance of fragments in the fish from Lake Cuitzeo was a positive correlation between the weight of the organisms and the abundance of particles (Fig. 5b). Our results show that 100% of fish samples in both lakes consumed microplastics, which is consistent with published literature on microplastic consumption by freshwater fish (Campbell et al., 2017; Park et al., 2020a; Peters & Bratton, 2016; Phillips & Bonner, 2015), where variations between 8 and 100% have been recorded in the intake of microplastics. As in work carried out with different species of freshwater fish around the world (Silva-Cavalcanti et al., 2017; Park et al., 2020a; Wang et al., 2020; Garcia et al., 2021), in the fish collected in our survey study in water bodies of Central Mexico, the two predominant forms of microplastics in the gastrointestinal tract were fibers and fragments. The study carried out by Martínez-Tavera et al. (2021) with freshwater fish (Oreochromis niloticus) in a reservoir in central Mexico recorded that the fish predominantly had fibers in their gastrointestinal tract (up to 24 items sample−1), without recording any other form of microplastic.
Microplastic densities: (a) fiber and (b) fragment from the gastrointestinal tract of Chirostoma fish collected in Lakes Cuitzeo and Pátzcuaro. Categories were established according to the fish weight: Lake Cuitzeo C1 0.5–0.7 g, C2 0.8–1.1 g, C3 1.2–1.4 g, and C4 1.5–2.1 g. Lake Pátzcuaro C1 2.4–2.8 g, C2 2.9–3.2 g, C3 3.3–3.6 g, and C4 3.7–4.3 g
The abundances recorded in Chirostoma fish in our study were lower than those recorded in the digestive tract of freshwater fish from South Korea, where average concentrations of 22.0 ± 14.6 particles fish−1 were recorded (Park et al., 2020a). However, our data fit more closely with those obtained in freshwater fish from Colombia (Garcia et al., 2021), Brazil (Silva-Cavalcanti et al., 2017), and the USA (Peters & Bratton, 2016) where averages of 2.1 ± 1.26 individual items−1, 3.6 items fish−1, and 1.65 items fish−1, respectively, were observed. Although the concentration of microplastics in both water bodies was high, this is not always reflected in fish’s high ingestion of microplastics. The ingestion of microplastics by fish is not necessarily related to the availability or abundance of microplastics in the water column; it is also related to the feeding habits of fish (McNeish et al., 2018). For example, fish capable of exploiting a wider variety of resources could ingest microplastics from the water column, mistaking them for prey or ingest them through contaminated prey (Scherer et al., 2018). In this case, the importance of the shape and color of microplastics becomes especially relevant since it has been shown that some species of fish preferentially consume some shapes and colors of microplastics, which may be related to the similarity to their natural prey regardless of its concentration in the water column (Okamoto et al., 2022; Ory et al., 2017; Scherer et al., 2018). In the case of the charal (Chirostoma) studied in our work, they are considered primarily zooplanktivorous and with the possibility of consuming benthic invertebrates (Berlanga-Robles et al., 2002; Orbe-Mendoza et al., 2002) so they would mainly be consuming microplastics from contaminated prey or by mistaking their prey for some microplastics. Finally, in different works, it has been suggested that the consumption of microplastics by fish is a route of transfer to humans (Erkes-Medrano et al., 2015; Akoueson et al., 2020; Garcia et al., 2021), in the case of Chirostoma fish perhaps more pronounced, because these fish are widely consumed in the center of the country and the entire organism is usually consumed (Orbe-Mendoza et al., 2002).
Surface morphology, physical appearance, and elemental composition of microplastics
The surface of the particles analyzed by SEM showed various surface textures, among which grooves, fractures, mechanical pits, and scales stand out among others (Fig. 6). The color distribution of the microplastics in both bodies of water (Fig. 7) was black, white, blue, red, brown, and green; additionally, other colors such as purple, pink, transparent, yellow, and green were found in low percentages (< 1%). In the three fractions analyzed, water, zooplankton, and fish, the color with the highest percentage was black (> 50%), followed by white (< 28%) and blue (< 22%). In this work, 20 particles were analyzed with Raman spectroscopy to determine their chemical composition. The analysis showed that the main polymers found were polypropylene (PP), polyamide (nylon), and polyethylene terephthalate (PET). The surface textures of the particles analyzed in SEM, regardless of their shape, fibers, or fragments, clearly showed a degradation phenomenon. Coincidentally, in studies where secondary microplastics have been analyzed in freshwater bodies of water, the same signs of degradation have been observed in the particles such as fractures and mechanical pits, grooves, and scales (Jian et al., 2020; Garcia et al., 2021; Martinez-Tavera et al., 2021). Various factors are responsible for the erosion and degradation of plastics in the environment, including mechanical factors such as waves, oxidative weathering due to exposure to UV rays, and biological degradation (Zbyszewski & Corcoran, 2011, Zbyszewski et al., 2014). Analyzing the ultrastructure of microplastics not only can contribute to the historical reconstruction of the particles (Erkes-Medrano et al., 2015) but also gives us an idea about the effects it can have on its interactions with the biota since degradation signals can be related to more significant physical damage or increased toxicity in the organisms that consume them (Teuten et al., 2007; Wardrop et al., 2016). Coinciding with the results of our work, the color distribution of microplastics in various works around the world shows that colored plastics are dominant in water samples and ingested by organisms (De Sá et al., 2018; Eriksen et al., 2013; Wang et al., 2017, 2018).
Additionally, black color was the dominant color in the particles (> 50%) in our study, while in other works, the blue particles were the dominant ones (Güven et al., 2017; Peters & Bratton, 2016; Shruti et al., 2019; Lusher et al., 2020; Wu et al., 2018). In Mexico, Martinez-Tavera et al. (2021) recorded a dominance of black particles in fish collected in a reservoir, highlighting that this color of particles mainly comes from agricultural processes. Finally, the main polymers recorded in our work, polypropylene (PP), polyamide (nylon), and polyethylene terephthalate (PET), are the main polymers found in bodies of water, zooplankton, and freshwater fish (reviewed by De Sá et al., 2018; Koelmans et al., 2019; Parker et al., 2021; Zhao et al., 2022) and that have been related to urbanization and various human activities such as fishing in the case of polyamide (Barrows et al., 2018; Hossain et al., 2019; Peters & Braton, 2016; Shruti et al., 2019).
Conclusions
This research is the first to provide data on the distribution of microplastics in three different strata of tropical water bodies in Mexico. The results show the presence of microplastics in the water column, zooplankton, and fish from both water bodies in the country’s center. The chemical composition of the microplastics was mainly polypropylene (PP), polyamide (nylon), and polyethylene terephthalate (PET). The dominance of the fragments in the three strata of the waterbody does not allow us to distinguish whether the presence of microplastics in the digestive tract of fish of the genus Chirostoma is carried out indirectly through the consumption of their prey (zooplankton) or directly from the water column, so future work is required to clarify the route of entry of these contaminants into fish.
Data availability
No datasets were generated or analysed during the current study.
References
Akoueson, F., Sheldon, L. M., Danopoulos, E., Morris, S., Hotten, J., Chapman, E., Li, J., & Rotchell, J. M. (2020). A preliminary analysis of microplastics in edible versus non-edible tissues from seafood samples. Environmental Pollution, 263, 114452. https://doi.org/10.1016/j.envpol.2020.114452
Alcocer, J., & Bernal-Brooks, F. W. (2010). Limnology in Mexico. Hydrobiologia, 644, 1–54. https://doi.org/10.1007/s10750-010-0211-1
Amin, R. M., Sohaimi, E. S., Anuar, S. T., & Bachok, Z. (2020). Microplastic ingestion by zooplankton in Terengganu coastal waters, southern South China Sea. Marine Pollution Bulletin, 150, 110616. https://doi.org/10.1016/j.marpolbul.2019.110616
Amrutha, K., & Warrier, A. K. (2020). The first report on the source-to-sink characterization of microplastic pollution from a riverine environment in tropical India. Science of the Total Environment, 739, 140377. https://doi.org/10.1016/j.scitotenv.2020.140377
Anderson, P. J., Warrack, S., Langen, V., Challis, J. K., Hanson, M. L., & Rennie, M. D. (2017). Microplastic contamination in Lake Winnipeg, Canada. Environmental Pollution, 225, 223–231. https://doi.org/10.1016/j.envpol.2017.02.072
Andrady, A. L. (2011). Microplastics in the marine environment. Marine Pollution Bulletin, 62(8), 1596–1605. https://doi.org/10.1016/j.marpolbul.2011.05.030
Andrady, A. L., & Neal, M. A. (2009). Applications and societal benefits of plastics. Philosophical Transactions of the Royal Society b: Biological Sciences, 364(1526), 1977–1984. https://doi.org/10.1098/rstb.2008.0304
Baldwin, A. K., Corsi, S. R., & Mason, S. A. (2016). Plastic debris in 29 Great Lakes tributaries: Relations to watershed attributes and hydrology. Environmental Science & Technology, 50(19), 10377–10385. https://doi.org/10.1021/acs.est.6b02917
Barbour, C. D. (1973). A biogeographical history of Chirostoma (Pisces: Atherinidae): A species flock from the Mexican Plateau. Copeia, 533–556. https://doi.org/10.2307/1443118
Barrows, A. P., Christiansen, K. S., Bode, E. T., & Hoellein, T. J. (2018). A watershed-scale, citizen science approach to quantifying microplastic concentration in a mixed land-use river. Water Research, 147, 382–392. https://doi.org/10.1016/j.watres.2018.10.013
Berlanga-Robles, C. A., Madrid-Vera, J., & Ruiz-Luna, A. (2002). Fish abundance and trophic structure from the commercial catch in Lake Patzcuaro. Mexico. Hydrobiologia, 467(1), 117–122. https://doi.org/10.1023/A:1014965504486
Bernal-Brooks, F. (1998). The lakes of Michoacán (Mexico): A brief history and alternative point of view. Freshwater Forum, 10, 20–34.
Berry, J. P., Lee, E., Walton, K., Wilson, A. E., & Bernal-Brooks, F. (2011). Bioaccumulation of microcystins by fish associated with a persistent cyanobacterial bloom in Lago de Patzcuaro (Michoacan, Mexico). Environmental Toxicology and Chemistry, 30(7), 1621–1628. https://doi.org/10.1002/etc.548
Boucher, J., & Friot, D. (2017). Primary microplastics in the oceans: A global evaluation of sources (Vol. 10). Gland, Switzerland: Iucn.
Bradbury, J. P. (2000). Limnologic history of Lago de Patzcuaro, Michoacan, Mexico for the past 48,000 years: Impacts of climate and man. Palaeogeography, Palaeoclimatology, Palaeoecology, 163(1–2), 69–95. https://doi.org/10.1016/S0031-0182(00)00146-2
Bravo-Inclán, L., Sánchez-Chávez, J. J., Tomasini-Ortiz, A. C., González-Villela, R., Mijangos-Carro, M., & Bernal-Brooks, F. (2022). Long term studies in Lake Pátzcuaro, eutrophication and recovery efforts during one decade. In Kajitvichyanukul, P., D’Arcy, B. (eds) Land use and water quality: The impacts of diffuse pollution. IWAP, London, https://doi.org/10.2166/9781789061123_045
Browne, M. A., Crump, P., Niven, S. J., Teuten, E., Tonkin, A., Galloway, T., & Thompson, R. (2011). Accumulation of microplastic on shorelines worldwide: Sources and sinks. Environmental Science & Technology, 45(21), 9175–9179. https://doi.org/10.1021/es201811s
Campbell, S. H., Williamson, P. R., & Hall, B. D. (2017). Microplastics in the gastrointestinal tracts of fish and the water from an urban prairie creek. Facets, 2(1), 395–409. https://doi.org/10.1139/facets-2017-0008
Cole, M., Lindeque, P., Fileman, E., Halsband, C., Goodhead, R., Moger, J., & Galloway, T. S. (2013). Microplastic ingestion by zooplankton. Environmental Science & Technology, 47(12), 6646–6655. https://doi.org/10.1021/es400663f
Cole, M., Lindeque, P., Halsband, C., & Galloway, T. S. (2011). Microplastics as contaminants in the marine environment: A review. Marine Pollution Bulletin, 62(12), 2588–2597. https://doi.org/10.1016/j.marpolbul.2011.09.025
Collignon, A., Hecq, J. H., Galgani, F., Collard, F., & Goffart, A. (2014). Annual variation in neustonic micro-and meso-plastic particles and zooplankton in the Bay of Calvi (Mediterranean–Corsica). Marine Pollution Bulletin, 79(1–2), 293–298. https://doi.org/10.1016/j.marpolbul.2013.11.023
De Felice, B., Sabatini, V., Antenucci, S., Gattoni, G., Santo, N., Bacchetta, R., Ortenzi, M. A., & Parolini, M. (2019). Polystyrene microplastics ingestion induced behavioral effects to the cladoceran Daphnia magna. Chemosphere, 231, 423–431. https://doi.org/10.1016/j.chemosphere.2019.05.115
De Sá, L. C., Oliveira, M., Ribeiro, F., Rocha, T. L., & Futter, M. N. (2018). Studies of the effects of microplastics on aquatic organisms: What do we know and where should we focus our efforts in the future? Science of the Total Environment, 645, 1029–1039. https://doi.org/10.1016/j.scitotenv.2018.07.207
Delgado, O. B., Mendoza, M., Granados, E. L., & Geneletti, D. (2008). Analysis of land suitability for the siting of inter-municipal landfills in the Cuitzeo Lake Basin. Mexico. Waste Management, 28(7), 1137–1146. https://doi.org/10.1016/j.wasman.2007.07.002
Delgado, O. B., Ojeda-Benítez, S., & Márquez-Benavides, L. (2007). Comparative analysis of hazardous household waste in two Mexican regions. Waste Management, 27(6), 792–801. https://doi.org/10.1016/j.wasman.2006.03.022
Drago, C., Pawlak, J., & Weithoff, G. (2020). Biogenic aggregation of small microplastics alters their ingestion by a common freshwater micro-invertebrate. Frontiers in Environmental Science, 8, 574274. https://doi.org/10.3389/fenvs.2020.574274
Dris, R., Gasperi, J., Saad, M., Mirande, C., & Tassin, B. (2016). Synthetic fibers in atmospheric fallout: A source of microplastics in the environment? Marine Pollution Bulletin, 104(1–2), 290–293. https://doi.org/10.3389/fenvs.2020.574274
Eerkes-Medrano, D., Thompson, R. C., & Aldridge, D. C. (2015). Microplastics in freshwater systems: A review of the emerging threats, identification of knowledge gaps and prioritisation of research needs. Water Research, 75, 63–82. https://doi.org/10.1016/j.watres.2015.02.012
Eriksen, M., Mason, S., Wilson, S., Box, C., Zellers, A., Edwards, W., Farley, H., & Amato, S. (2013). Microplastic pollution in the surface waters of the Laurentian Great Lakes. Marine Pollution Bulletin, 77(1–2), 177–182. https://doi.org/10.1016/j.marpolbul.2013.10.007
Fischer, E. K., Paglialonga, L., Czech, E., & Tamminga, M. (2016). Microplastic pollution in lakes and lake shoreline sediments a case study on Lake Bolsena and Lake Chiusi (Central Italy). Environmental Pollution, 213, 648–657. https://doi.org/10.1016/j.envpol.2016.03.012
Forero-López, A. D., Rimondino, G. N., Truchet, D. M., Colombo, C. V., Buzzi, N. S., Malanca, F. E., Spetter, C. V., & Fernández-Severini, M. D. (2021). Occurrence, distribution, and characterization of suspended microplastics in a highly impacted estuarine wetland in Argentina. Science of the Total Environment, 785, 147141. https://doi.org/10.1016/j.scitotenv.2021.147141
Frias, J. P., Otero, V., & Sobral, P. (2014). Evidence of microplastics in samples of zooplankton from Portuguese coastal waters. Marine Environmental Research, 95, 89–95. https://doi.org/10.1016/j.marenvres.2014.01.001
Galafassi, S., Campanale, C., Massarelli, C., Uricchio, V. F., & Volta, P. (2021). Do freshwater fish eat microplastics? A review with a focus on effects on fish health and predictive traits of MPs ingestion. Water, 13(16), 2214. https://doi.org/10.3390/w13162214
García Amaro, E. (2004). Modificaciones al sistema de clasificación climática de Köppen. Universidad Nacional Autónoma de México, Instituto de Geografía.
Garcia, A. G., Suárez, D. C., Li, J., & Rotchell, J. M. (2021). A comparison of microplastic contamination in freshwater fish from natural and farmed sources. Environmental Science and Pollution Research, 28(12), 14488–14497. https://doi.org/10.1007/s11356-020-11605-2
Güven, O., Gökdağ, K., Jovanović, B., & Kıdeyş, A. E. (2017). Microplastic litter composition of the Turkish territorial waters of the Mediterranean Sea, and its occurrence in the gastrointestinal tract of fish. Environmental Pollution, 223, 286–294. https://doi.org/10.1016/j.envpol.2017.01.025
Han, M., Niu, X., Tang, M., Zhang, B. T., Wang, G., Yue, W., Kong, X., & Zhu, J. (2020). Distribution of microplastics in surface water of the lower Yellow River near estuary. Science of the Total Environment, 707, 135601. https://doi.org/10.1016/j.scitotenv.2019.135601
Hendrickson, E., Minor, E. C., & Schreiner, K. (2018). Microplastic abundance and composition in western Lake Superior as determined via microscopy, Pyr-GC/MS, and FTIR. Environmental Science & Technology, 52(4), 1787–1796. https://doi.org/10.1021/acs.est.7b05829
Hidalgo-Ruz, V., Gutow, L., Thompson, R. C., & Thiel, M. (2012). Microplastics in the marine environment: A review of the methods used for identification and quantification. Environmental Science & Technology, 46(6), 3060–3075. https://doi.org/10.1021/es2031505
Hossain, M. S., Sobhan, F., Uddin, M. N., Sharifuzzaman, S. M., Chowdhury, S. R., Sarker, S., & Chowdhury, M. S. N. (2019). Microplastics in fishes from the Northern Bay of Bengal. Science of the Total Environment, 690, 821–830. https://doi.org/10.1016/j.scitotenv.2019.07.065
Jeong, C. B., Won, E. J., Kang, H. M., Lee, M. C., Hwang, D. S., Hwang, U. K., Zhou, B., Souissi, S., Lee, S. J., & Lee, J. S. (2016). Microplastic size-dependent toxicity, oxidative stress induction, and p-JNK and p-p38 activation in the monogonont rotifer (Brachionus koreanus). Environmental Science & Technology, 50(16), 8849–8857. https://doi.org/10.1021/acs.est.6b01441
Jian, M., Zhang, Y., Yang, W., Zhou, L., Liu, S., & Xu, E. G. (2020). Occurrence and distribution of microplastics in China’s largest freshwater lake system. Chemosphere, 261, 128186. https://doi.org/10.1016/j.chemosphere.2020.128186
Kim, S. W., Chae, Y., Kim, D., & An, Y. J. (2019). Zebrafish can recognize microplastics as inedible materials: Quantitative evidence of ingestion behavior. Science of the Total Environment, 649, 156–162. https://doi.org/10.1016/j.scitotenv.2018.08.310
Koelmans, A. A., Nor, N. H. M., Hermsen, E., Kooi, M., Mintenig, S. M., & De France, J. (2019). Microplastics in freshwaters and drinking water: Critical review and assessment of data quality. Water Research, 155, 410–422. https://doi.org/10.1016/j.watres.2019.02.054
Lambert, S., Sinclair, C., & Boxall, A. (2013). Occurrence, degradation, and effect of polymer-based materials in the environment. Reviews of Environmental Contamination and Toxicology, 227, 1–53. https://doi.org/10.1007/978-3-319-01327-5_1
Li, J., Qu, X., Su, L., Zhang, W., Yang, D., Kolandhasamy, P., Li, D., & Shi, H. (2016). Microplastics in mussels along the coastal waters of China. Environmental Pollution, 214, 177–184. https://doi.org/10.1016/j.envpol.2016.04.012
Lin, L., Ma, L. S., Li, H. X., Pan, Y. F., Liu, S., Zhang, L., Peng, J. P., Fok, L., Xu, X. R., & He, W. H. (2020). Low level of microplastic contamination in wild fish from an urban estuary. Marine Pollution Bulletin, 160, 111650. https://doi.org/10.1016/j.marpolbul.2020.111650
Lusher, A. L., Mchugh, M., & Thompson, R. C. (2013). Occurrence of microplastics in the gastrointestinal tract of pelagic and demersal fish from the English Channel. Marine Pollution Bulletin, 67(1–2), 94–99. https://doi.org/10.1016/j.marpolbul.2012.11.028
Lusher, A. L., O’Donnell, C., Officer, R., & O’Connor, I. (2016). Microplastic interactions with North Atlantic mesopelagic fish. ICES Journal of Marine Science, 73(4), 1214–1225. https://doi.org/10.1093/icesjms/fsv241
Lusher, A. L., Welden, N. A., Sobral, P., & Cole, M. (2020). Sampling, isolating and identifying microplastics ingested by fish and invertebrates. In Analysis of nanoplastics and microplastics in food CRC Press., 9(9), 1346–1360. https://doi.org/10.1039/C6AY02415G
Lyons, J., Gutiérrez-Hernández, A., Díaz-Pardo, E., Soto-Galera, E., Medina-Nava, M., & Pineda-López, R. (2000). Development of a preliminary index of biotic integrity (IBI) based on fish assemblages to assess ecosystem condition in the lakes of central Mexico. Hydrobiologia, 418, 57–72. https://doi.org/10.1023/A:1003888032756
Mani, T., Hauk, A., Walter, U., & Burkhardt-Holm, P. (2015). Microplastics profile along the Rhine River. Scientific Reports, 5(1), 17988. https://doi.org/10.1038/srep17988
Mao, Y., Li, H., Gu, W., Yang, G., Liu, Y., & He, Q. (2020). Distribution and characteristics of microplastics in the Yulin River, China: Role of environmental and spatial factors. Environmental Pollution, 265, 115033. https://doi.org/10.1016/j.envpol.2020.115033
Martinez-Tavera, E., Duarte-Moro, A. M., Sujitha, S. B., Rodriguez-Espinosa, P. F., Rosano-Ortega, G., & Expósito, N. (2021). Microplastics and metal burdens in freshwater Tilapia (Oreochromis niloticus) of a metropolitan reservoir in Central Mexico: Potential threats for human health. Chemosphere, 266, 128968. https://doi.org/10.1016/j.chemosphere.2020.128968
Masura, J., Baker, J. E., Foster, G. D., Arthur, C., & Herring, C. (2015). Laboratory methods for the analysis of microplastics in the marine environment: Recommendations for quantifying synthetic particles in waters and sediments. https://doi.org/10.25607/OBP-604
McNeish, R. E., Kim, L. H., Barrett, H. A., Mason, S. A., Kelly, J. J., & Hoellein, T. J. (2018). Microplastic in riverine fish is connected to species traits. Scientific Reports, 8(1), 11639. https://doi.org/10.1038/s41598-018-29980-9
Moore, C. J., Moore, S. L., Leecaster, M. K., & Weisberg, S. B. (2001). A comparison of plastic and plankton in the North Pacific central gyre. Marine Pollution Bulletin, 42(12), 1297–1300. https://doi.org/10.1016/S0025-326X(01)00114-X
Murray, F., & Cowie, P. R. (2011). Plastic contamination in the decapod crustacean Nephrops norvegicus (Linnaeus, 1758). Marine Pollution Bulletin, 62(6), 1207–1217. https://doi.org/10.1016/j.marpolbul.2011.03.032
Nelms, S. E., Galloway, T. S., Godley, B. J., Jarvis, D. S., & Lindeque, P. K. (2018). Investigating microplastic trophic transfer in marine top predators. Environmental Pollution, 238, 999–1007. https://doi.org/10.1016/j.envpol.2018.02.016
Nobre, C. R., Santana, M. F. M., Maluf, A., Cortez, F. S., Cesar, A., Pereira, C. D. S., & Turra, A. (2015). Assessment of microplastic toxicity to embryonic development of the sea urchin Lytechinus variegatus (Echinodermata: Echinoidea). Marine Pollution Bulletin, 92(1–2), 99–104. https://doi.org/10.1016/j.marpolbul.2014.12.050
O’Connor, J. D., Mahon, A. M., Ramsperger, A. F., Trotter, B., Redondo-Hasselerharm, P. E., Koelmans, A. A., Lally, H. T., & Murphy, S. (2020). Microplastics in freshwater biota: A critical review of isolation, characterization, and assessment methods. Global Challenges, 4(6), 1800118. https://doi.org/10.1002/gch2.201800118
Okamoto, K., Nomura, M., Horie, Y., & Okamura, H. (2022). Color preferences and gastrointestinal-tract retention times of microplastics by freshwater and marine fishes. Environmental Pollution, 304, 119253. https://doi.org/10.1016/j.envpol.2022.119253
Orbe, A., & Acevedo, J. (1991). Análisis de la selectividad de las artes de pesca y el esfuerzo pesquero en el lago de Pátzcuaro. México: Michoacán. Instituto Nacional de la Pesca. Secretaría de Pesca. Informe.
Orbe-Mendoza, A. A., Acevedo-García, J., & Lyons, J. (2002). Lake Pátzcuaro fishery management plan. Reviews in Fish Biology and Fisheries, 12, 207–217. https://doi.org/10.1023/A:1025087705940
Ory, N. C., Sobral, P., Ferreira, J. L., & Thiel, M. (2017). Amberstripe scad Decapterus muroadsi (Carangidae) fish ingest blue microplastics resembling their copepod prey along the coast of Rapa Nui (Easter Island) in the South Pacific subtropical gyre. Science of the Total Environment, 586, 430–437. https://doi.org/10.1016/j.scitotenv.2017.01.175
Park, T. J., Lee, S. H., Lee, M. S., Lee, J. K., Lee, S. H., & Zoh, K. D. (2020a). Occurrence of microplastics in the Han River and riverine fish in South Korea. Science of the Total Environment, 708, 134535. https://doi.org/10.1016/j.scitotenv.2019.134535
Park, T. J., Lee, S. H., Lee, M. S., Lee, J. K., Park, J. H., & Zoh, K. D. (2020b). Distributions of microplastics in surface water, fish, and sediment in the vicinity of a sewage treatment plant. Water, 12(12), 3333. https://doi.org/10.3390/w12123333
Parker, B., Andreou, D., Green, I. D., & Britton, J. R. (2021). Microplastics in freshwater fishes: Occurrence, impacts and future perspectives. Fish and Fisheries, 22(3), 467–488. https://doi.org/10.1111/faf.12528
Peters, C. A., & Bratton, S. P. (2016). Urbanization is a major influence on microplastic ingestion by sunfish in the Brazos River Basin, Central Texas, USA. Environmental Pollution, 210, 380–387. https://doi.org/10.1016/j.envpol.2016.01.018
Phillips, M. B., & Bonner, T. H. (2015). Occurrence and amount of microplastic ingested by fishes in watersheds of the Gulf of Mexico. Marine Pollution Bulletin, 100(1), 264–269. https://doi.org/10.1016/j.marpolbul.2015.08.041
Phuong, N. N., Zalouk-Vergnoux, A., Poirier, L., Kamari, A., Châtel, A., Mouneyrac, C., & Lagarde, F. (2016). Is there any consistency between the microplastics found in the field and those used in laboratory experiments? Environmental Pollution, 211, 111–123. https://doi.org/10.1016/j.envpol.2015.12.035
Rehse, S., Kloas, W., & Zarfl, C. (2016). Short-term exposure with high concentrations of pristine microplastic particles leads to immobilisation of Daphnia magna. Chemosphere, 153, 91–99. https://doi.org/10.1016/j.chemosphere.2016.02.133
Rillig, M. C. (2012). Microplastic in terrestrial ecosystems and the soil? Environmental Science and Technology, 46(12), 6453–6454. https://doi.org/10.1021/es302011r
Rochman, C. M. (2018). Microplastics research—from sink to source. Science, 360(6384), 28–29. https://doi.org/10.1126/science.aar7734
Rochman, C. M., Brookson, C., Bikker, J., Djuric, N., Earn, A., Bucci, K., & Hung, C. (2019). Rethinking microplastics as a diverse contaminant suite. Environmental Toxicology and Chemistry, 38(4), 703–711. https://doi.org/10.1002/etc.4371
Scherer, C., Weber, A., Lambert, S., & Wagner, M. (2018). Interactions of microplastics with freshwater biota (pp. 153–180). Springer International Publishing. https://doi.org/10.1007/978-3-319-61615-5_8
Shruti, V. C., Jonathan, M. P., Rodriguez-Espinosa, P. F., & Rodríguez-González, F. (2019). Microplastics in freshwater sediments of Atoyac River Basin, Puebla City, Mexico. Science of the Total EnviroNment, 654, 154–163. https://doi.org/10.1016/j.scitotenv.2018.11.054
Silva-Cavalcanti, J. S., Silva, J. D. B., de França, E. J., de Araújo, M. C. B., & Gusmão, F. (2017). Microplastics ingestion by a common tropical freshwater fishing resource. Environmental Pollution, 221, 218–226. https://doi.org/10.1016/j.envpol.2016.11.068
Song, Y. K., Hong, S. H., Jang, M., Kang, J. H., Kwon, O. Y., Han, G. M., & Shim, W. J. (2014). Large accumulation of micro-sized synthetic polymer particles in the sea surface microlayer. Environmental Science & Technology, 48(16), 9014–9021. https://doi.org/10.1021/es501757s
Soto-Galera, E., Díaz-Pardo, E., López-López, E., & Lyons, J. (1998). Fish as indicators of environmental quality in the Río Lerma Basin. México. Aquatic Ecosystem Health & Management, 1(3–4), 267–276. https://doi.org/10.1080/14634989808656923
Sun, X., Li, Q., Zhu, M., Liang, J., Zheng, S., & Zhao, Y. (2017). Ingestion of microplastics by natural zooplankton groups in the northern South China Sea. Marine Pollution Bulletin, 115(1–2), 217–224. https://doi.org/10.1016/j.marpolbul.2016.12.004
Sun, Y., Xu, W., Gu, Q., Chen, Y., Zhou, Q., Zhang, L., Gu, L., Huang, Y., Lyu, K., & Yang, Z. (2019). Small-sized microplastics negatively affect rotifers: Changes in the key life-history traits and rotifer–Phaeocystis population dynamics. Environmental Science & Technology, 53(15), 9241–9251. https://doi.org/10.1021/acs.est.9b02893
Teuten, E. L., Rowland, S. J., Galloway, T. S., & Thompson, R. C. (2007). Potential for plastics to transport hydrophobic contaminants. Environmental Science & Technology, 41(22), 7759–7764. https://doi.org/10.1021/es071737s
Thompson, R. C., Moore, C. J., Vom Saal, F. S., & Swan, S. H. (2009). Plastics, the environment and human health: Current consensus and future trends. Philosophical Transactions of the Royal Society b: Biological Sciences, 364(1526), 2153–2166. https://doi.org/10.1098/rstb.2009.0053
Thompson, R. C., Olsen, Y., Mitchell, R. P., Davis, A., Rowland, S. J., John, A. W. G., McGonigle, D., & Russell, A. E. (2004). Lost at sea: Where is all the plastic? Science, 304(5672), 838–838. https://doi.org/10.1126/science.1094559
Torres, A. C. (1993). Lake Patzcuaro, Mexico: Watershed and water quality deterioration in a tropical high-altitude Latin American lake. Lake and Reservoir Management, 8(1), 37–47. https://doi.org/10.1080/07438149309354457
Wang, S., Zhang, C., Pan, Z., Sun, D., Zhou, A., Xie, S., ... & Zou, J. (2020). Microplastics in wild freshwater fish of different feeding habits from Beijiang and Pearl River Delta regions, south China. Chemosphere, 258, 127345. https://doi.org/10.1016/j.chemosphere.2020.127345
Wang, W., Ndungu, A. W., Li, Z., & Wang, J. (2017). Microplastics pollution in inland freshwaters of China: A case study in urban surface waters of Wuhan, China. Science of the Total Environment, 575, 1369–1374. https://doi.org/10.1016/j.scitotenv.2016.09.213
Wang, W., Yuan, W., Chen, Y., & Wang, J. (2018). Microplastics in surface waters of Dongting Lake and Hong Lake, China. Science of the Total Environment, 633, 539–545. https://doi.org/10.1016/j.scitotenv.2018.03.211
Wardrop, P., Shimeta, J., Nugegoda, D., Morrison, P. D., Miranda, A., Tang, M., & Clarke, B. O. (2016). Chemical pollutants sorbed to ingested microbeads from personal care products accumulate in fish. Environmental Science & Technology, 50(7), 4037–4044. https://doi.org/10.1021/acs.est.5b06280
Wu C, Zhang K, Xiong X (2018) Microplastic pollution in inland waters focusing on Asia. In M. Wagner, S. Lambert (Eds.) Freshwater microplastics. The handbook of environmental chemistry, (vol 58, pp. 85–99). Springer https://doi.org/10.1007/978-3-319-61615-5_8
Xiong, X., Liu, Q., Chen, X., Wang, R., Duan, M., & Wu, C. (2021). Occurrence of microplastic in the water of different types of aquaculture ponds in an important lakeside freshwater aquaculture area of China. Chemosphere, 282, 131126. https://doi.org/10.1016/j.chemosphere.2021.131126
Xiong, X., Zhang, K., Chen, X., Shi, H., Luo, Z., & Wu, C. (2018). Sources and distribution of microplastics in China’s largest inland lake–Qinghai Lake. Environmental Pollution, 235, 899–906. https://doi.org/10.1016/j.envpol.2017.12.081
Xue, Y. H., Sun, Z. X., Feng, L. S., Jin, T., Xing, J. C., & Wen, X. L. (2021). Algal density affects the influences of polyethylene microplastics on the freshwater rotifer Brachionus calyciflorus. Chemosphere, 270, 128613. https://doi.org/10.1016/j.chemosphere.2020.128613
Yıldız, D., Yalçın, G., Jovanović, B., Boukal, D. S., Vebrová, L., Riha, D., Stanković, J., Savić-Zdraković, D., Metin, M., Akyürek, Y. N., Balkanlı, D., Filiz, N., Milošević, D., Feuchtmayr, H., Richardson, J. A., & Beklioğlu, M. (2022). Effects of a microplastic mixture differ across trophic levels and taxa in a freshwater food web: In situ mesocosm experiment. Science of the Total Environment, 836, 155407. https://doi.org/10.1016/j.scitotenv.2022.155407
Zbyszewski, M., & Corcoran, P. L. (2011). Distribution and degradation of fresh water plastic particles along the beaches of Lake Huron, Canada. Wat Air and Soil Poll, 220, 365–372. https://doi.org/10.1007/s11270-011-0760-6
Zbyszewski, M., Corcoran, P. L., & Hockin, A. (2014). Comparison of the distribution and degradation of plastic debris along shorelines of the Great Lakes. North America. J Great Lakes Res, 40(2), 288–299. https://doi.org/10.1016/S0380-1330(01)70665-X
Zhao M, Cao Y, Chen T, Li H, Tong Y, Fan W, Xie Y, Tao Y, Zhou J (2022) Characteristics and source-pathway of microplastics in freshwater system of China: A review. Chemosphere 134192. https://doi.org/10.1016/j.chemosphere.2022.134192
Acknowledgements
This work was supported by UNAM-PAPIIT IN230120. RIFM has received scholarship (53890) from PAPIIT IN230120. We thank Dr. Selene Rubí Islas Sánchez from the Laboratorio Universitario de Caracterización Espectroscópica, LUCE_ICAT_UNAM for the characterization of the samples using the Raman scattering spectroscopy technique. We thank M. in C. Gómez-Lizárraga for the SEM analyzes in the SAMEB of the ICMyL-UNAM.
Funding
This work was supported by Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica of the Universidad Nacional Autónoma de México (project number IN230120). RIFM has received scholarship (53890) from PAPIIT IN230120.
Author information
Authors and Affiliations
Contributions
Jiménez-Contreras contributed with the conceptualization and experimental design of the study, with the sample collection, material preparation and laboratory analysis, with the data interpretation and analysis, with funding acquisition, and with the writing of the manuscript. Fernández-Medina contributed with the experimental design of the study, with the collection, processing, and analysis of the samples, with data analysis, and writing of the manuscript. Fernández-Araiza contributed with the conceptualization and experimental design of the study, with funding acquisition, and review and editing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Declarations
All the authors have read and understood, and have complied as applicable with the statement on ethical responsibilities of authors as found in the Instructions for Authors.
Ethics approval
The authors declare that local fishers provided all biological specimens.
Consent to participate
Not applicable.
Consent to publish
All authors agree to publish.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jiménez-Contreras, J., Fernández-Medina, R.I. & Fernández-Araiza, M.A. Microplastics pollution in tropical lakes: water, zooplankton, and fish in Central Mexico. Environ Monit Assess 196, 813 (2024). https://doi.org/10.1007/s10661-024-12978-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-024-12978-4