Abstract
Using a sediment core covering the last 3,500 years, we analysed photosynthetic pigments’ concentrations in lake sediments and carbon stable isotopic composition of chironomid (Diptera, Chironomidae) remains (δ13CHC). We aimed to reconstruct temporal changes in aquatic primary productivity and carbon resources sustaining chironomid larvae in a high mountain lake (Lake Pyramid Inferior; 5,067 m a.s.l.) located in the Nepalese Himalayas. Both pigments and δ13CHC trends followed a similar fluctuating pattern over time, and we found significant positive relationships between these proxies, suggesting the strong reliance of benthic consumers on the aquatic primary production. Temporal trends matched well with main known climatic phases in the Eastern part of the Himalayan Mountains. Past glacier dynamics and associated in-lake solute concentrations appeared to be the main driver of autochthonous primary productivity, suggesting then the indirect impact of climate change on carbon processing in the benthic food web. During warm periods, the glacier retreat induced a rise in in-lake solute concentrations leading to an increasing primary productivity. Complementary investigations are still needed to strengthen our understanding about the response of past aquatic carbon cycling in CO2-limiting environments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mountain areas play a key role in the regulation of climatic, hydrological and biogeographical processes at a global scale. The Himalayan Mountains are often considered as the “Third Pole” (Qiu, 2008), mainly because they exhibit the largest glacial area outside of the Polar Regions and they are located in the most remote region of the world (Yao et al., 2012a). Glacier dynamics in the Himalayan Mountains induces numerous feedbacks on regional climate, water supply and associated biogeochemical cycles (Yao et al., 2012b). However, numerous studies revealed dramatic decreases in glacial area over the last decades due to climate warming (Salerno et al., 2008; Chudley et al., 2017). Since glacier retreat in the Himalayan Mountains is expected to dramatically increase in a near future due to global warming (Soncini et al., 2016); it has then become necessary to assess climate change impacts on ecosystems in this area.
Lakes are sentinels of climate change, and they are considered as relevant indicators of environmental quality in high mountain landscapes (Adrian et al., 2009; Williamson et al., 2009). Mechanisms by which climate affects lake ecosystems can be summarized by the conceptual energy–mass framework published by Leavitt et al. (2009; Appendix 1). They proposed to distinguish the energy (E effects; atmospheric heat, irradiance, etc.) and mass fluxes (m effects; precipitation patterns, nutrient run-off, etc.) affecting the different compartments of lake functioning. The broader catchment area and the lake itself play then a role of environmental filters to mediate climate change effects (Blenckner, 2005; Appendix 1). In the Nepalese Himalayas, lakes seem to be affected by both E (i.e. water temperature) and m (i.e. nutrients and solute dynamics) fluxes. However, climate-induced glacier dynamics (E effects) appear to play a major role in solute concentrations in lakes (indirect m effects; Salerno et al., 2016) leading to the control of aquatic primary production (Lami et al., 2010), and likely the associated pelagic food web (Nevalainen et al., 2014), but nothing is known about the whole ecosystem response.
The understanding of food web functioning, including all steps of exchange of energy and matter among aquatic consumers (Lindeman, 1942), is an essential knowledge to elucidate the impact of climate change on lakes at ecosystem level. Among the complexity of trophic interactions in lakes, the benthic food web represents an integrative proxy for the understanding of whole lake trophic functioning (Vadeboncoeur et al., 2002). Indeed, lake sediments play a central role in recycling processes of organic matter coming from aquatic ecosystems (i.e. autochthonous primary production) and the associated catchment (i.e. terrestrial organic matter; Meyers & Ishiwatari, 1993). Moreover, the functioning of the benthic food web involves numerous couplings with the surrounding terrestrial environment (Northington et al., 2010) and among in-lake compartments (benthic–pelagic coupling, Wagner et al. 2012; pelagic–benthic coupling, Jónasson, 1972; Johnson & Wiederholm, 1992), making carbon processing in the benthic food web an excellent integrator, in both time and space, of organic matter cycling in lakes. First investigations of the Himalayan lakes were only focused on the characterization of biological communities (Manca et al., 1998). Carbon processing in the benthic food web is then poorly understood in this area. Fortunately, lake sediments and paleolimnological approach can be used to compensate for the lack of hydrobiological surveys in remote areas and to reconstruct the past dynamic of aquatic food webs (Catalan et al., 2013).
The study of lake sediments and archived biological remains is a promising approach to better understand the impact of climate change on aquatic ecosystems. Sedimentary pigments of former aquatic primary producers (from both planktonic and benthic populations) are usually well preserved in lake sediments (Leavitt & Hodgson, 2002). Then, sedimentary pigment analysis provides a quantitative reconstruction of the past dynamic of aquatic primary producers, illustrating temporal changes in lake trophic status (Guilizzoni et al., 2011). Moreover, remains of benthic consumers can be found in lake sediments at high concentrations (Walker, 2001). The larvae of Chironomidae (Arthropoda; Diptera; Nematocera) dominate benthic secondary production in high-elevation lakes in the Himalayan Mountains (Manca et al., 1998). In addition, the most sclerotized part of their exoskeleton (the head capsules, HC) shed after each moulting is morphologically and chemically well conserved in lake sediments (Verbruggen et al., 2009). Due to a negligible trophic fractionation (Goedkoop et al., 2006; Frossard et al., 2013) and significant differences in carbon stable isotopic composition (δ13C) of food sources (Rounick & Winterbourn, 1986), δ13C analysis of subfossil chironomid is often used to track carbon sources and energy pathways fuelling chironomid biomass (Wooller et al., 2012; Belle et al., 2014, Frossard et al., 2015). Then, it is possible to study the relationships between phytoplankton dynamics (sedimentary pigment analysis), carbon sources at the base of the benthic food web (δ13C analysis of subfossil chironomid) and past climate dynamics over long time scale.
Using combined analysis of sedimentary pigments and carbon stable isotopic composition of chironomid remains, we aimed to reconstruct the temporal changes in aquatic primary productivity and carbon resources sustaining chironomid larvae in a high mountain lake (Lake Pyramid Inferior; Nepalese Himalayas). We hypothesized that past climate fluctuations influenced the glacier dynamic leading to a control of in-lake solute concentrations and aquatic primary production. We expected to demonstrate the correlation between changes in aquatic primary productivity and δ13CHC values and then better understand the effects of climate change on high-elevation lakes in the Himalayan Mountains.
Methods
Site description
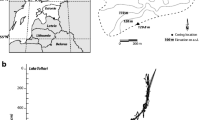
Lake Pyramid Inferior is situated in the Khumbu Valley (Sagarmatha National Park), Nepal (27º96′N, 86º81′E; Fig. 1A). It is a small lake (1.67 ha; Table 1) located above the tree limit in the Nepalese Himalayas (at 5,067 m a.s.l.), with a maximal water depth of 14.8 m (Fig. 1A). A survey conducted in 1998 (Tartari et al., 1998b) estimated that 11% of the entire catchment area was occupied by a glacier (Table 1). Lake Pyramid Inferior exhibits very low nutrient contents (TP = 3 µg.l−1, TN = 204 µgN.l−1; Tartari et al., 1998a) and can be characterized as an ultra-oligotrophic lake (Lami et al., 2010).
A Geographical location of Lake Pyramid Inferior in the Sagarmatha National Park (Khumbu valley; Nepal) and elevation map of its catchment area. Sediment cores were retrieved from the centre of Lake Pyramid Inferior at ca. 14.5 m of water depth. B Pictures of cores collected in 1993 (Pinf-93-01) and 2002 (Pinf-02-03), magnetic susceptibility signals and indication of cross-correlation applied using sedimentological markers. Age–depth models (linear interpolation) of core collected in Lake Pyramid Inferior in 2002 (Pinf-02-03). All sample depths were converted to calibrated year BP (year cal. BP, with 0 cal. BP = AD 1950) according to the age–depth models
Two sediment cores were retrieved from the deepest part of Lake Pyramid Inferior in 1993 (Pinf-93-01; Lami et al., 1998) and 2002 (Pinf-02-03; Lami et al., 2010) using a gravity corer (6 cm in diameter). Magnetic susceptibility was measured on both cores every 1 cm using a Bartington Instruments Magnetic Susceptibility Meter (model MS2) and MS2C Core Logging Sensor. Cross-correlation between cores was performed using a new set of identified lithological boundaries (Fig. 1B). Seven radiocarbon dates (n = 4 for Pinf-93-01 and n = 3 for Pinf-02-03) were obtained from bulk sediment samples by accelerator mass spectrometry performed at the Poznan Radiocarbon Laboratory (Poland) and ANSTO AMS Centre (Australia). The number, name and non-calibrated age of each radiocarbon date are summarized in Table 2. Radiocarbon dates were re-calibrated using the most recent atmospheric calibration dataset (IntCal13; Reimer et al. 2013). Age–depth modelling of the core collected in 2002 (Pinf-02-03) was then obtained by combining all radiocarbon dates (according to the new cross-correlation shown in Fig. 1B), and two different models were produced with linear interpolation using the Clam package for R (Blaauw, 2010). New age–depth models were slightly different than those produced by Lami et al. (2010). Without new radiocarbon dates, it was impossible to select one of these models (Fig. 1B), and the chronology should then be treated cautiously. However, both models shown that 70 cm of sediments from Lake Pyramid Inferior covered the last ca. 3,500 years, corresponding to an average sedimentation rate of about 0.2 mm.year−1 (Fig. 1B). All the following proxies were analysed on the same core collected in 2002 (Pinf-02-03). All sample depths were converted to calibrated year BP (year cal. BP, with 0 cal. BP = AD 1950) according to the two age–depth models (Fig. 1B).
Sedimentological and sedimentary pigment analysis
Sediment cores were sliced into continuous 5-mm-thick samples (n = 140). Organic matter contents in sediments (OM) were analysed in each sample using loss-on-ignition method following recommendations of Heiri et al. (2001). Results were expressed in terms of percentage of dry weight (% of dry weight). To track the temporal changes in aquatic primary productivity, sedimentary pigments were extracted from about 1 g of fresh sediments for the same 140 samples, overnight under nitrogen, with a solution of acetone and water (90:10), and were then centrifuged at ca. 2,700 g for 10 min. Total carotenoids (TC) and chlorophyll derivatives (CD) were then quantified from the extract via spectrophotometry and expressed in milligrams per gram of organic matter (mg g−1 OM) and in Units per gram of organic matter (U g−1OM), respectively, as described in Guilizzoni et al. (2011). Three outliers were detected in sedimentological data (OM = 46.4%; TC = 15.4 mg g−1 of OM, and CD = 632.1 mg g−1 of OM), and their extreme values were much higher than previously reported from another study conducted on the same lake (Lami et al., 1998). Thus, these samples were removed for further statistical analysis. Results of OM and sedimentary pigment analyses were previously published and discussed by Lami et al. (2010).
Carbon stable isotope analysis of chironomid remains
The rest of sediment samples were pooled to reach a sample thickness of 2 cm (n = 34), and reconstruction of carbon sources sustaining chironomid biomass was performed using carbon stable isotope analysis of chironomid remains archived in lake sediments. We selected Pseudodiamesa nivosa-type morphotype (based on classification provided by Ilyashuk et al., 2010) because this morphotype belongs to the deposit-feeder trophic guild (feeding on phytoplankton deposits, terrestrial detritus and associated bacteria; Pegast, 1947). Thus, it is an excellent indicator of recycling of organic matter in lake sediments. Moreover, Pseudodiamesa nepalensis (belonging to Pseudodiamesa nivosa-type morphotype; Appendix 2) has a widespread occurrence in Nepalese lakes (Manca et al., 1998), and Pseudodiamesa nepalensis has been found at high density in the profundal zone of Lake Pyramid Inferior (Manca et al., 1998). Sediment samples were pretreated using successive washings with NaOH (10%) and HCl (10%) solutions and be sieved through a 100-µm mesh following Schimmelmann & DeNiro (1986) and van Hardenbroek et al. (2009). Following recommendations from Frossard et al. (2013), carbon stable isotope composition of chironomid remains (δ13CHC) belonging to the 4th larval instar of Pseudodiamesa nivosa-type morphotype was then analysed using an isotope ratio mass spectrometer interfaced with an elemental analyser at INRA Nancy (Champenoux; UMR INRA 1137). Approximately 15 entire HC were loaded into tin capsules to reach a sample weight always higher than 20 µg. Results were expressed according to the delta notation with Vienna Pee Dee Belemnite as the standard: δ13C (‰) = ([Rsample/Rstandard]−1) × 1,000, where R = 13C/12C. Replication of sample measurements from internal laboratory standards (with a target weight of 20 µg) produced analytical errors (1σ) of ± 0.2‰ (n = 8).
Data analysis
Zonation in sedimentological data (OM, TC and CD) was determined by constrained hierarchical cluster analysis using a Bray–Curtis distance and CONISS linkage method (Rioja package for R, Juggins, 2017), and significance of zones was assessed using the broken-stick model (Bennett, 1996). For graphical displays, main temporal trends were visually tracked by smoothing the time-series using a LOESS regression (LOESS parameters: span = 0.2; polynomials degree = 2; analogue package for R, Simpson and Oksanen, 2016). Pigment concentrations in lake sediments were expected to have a significant, and potentially non-linear, influence on carbon sources fuelling chironomid biomass. Statistical relationships between δ13CHC values and sedimentary pigment variables (TC and CD) were then examined using a generalized additive model (GAM; fitted using the mgcv package for R; Wood, 2011) approach, with a continuous-time first-order autoregressive process to account for temporal autocorrelation (Simpson & Anderson, 2009). Logarithmic transformations [log(x + 1)] were applied to sedimentary pigment data (i.e. explanatory variables) before statistical analysis in order to approximate a normal distribution. Significance of fitted trends was checked using the standard statistical inference for GAM. All statistical tests and graphical displays were performed using R 3.4.0 statistical software (R Core Team, 2017).
Results
Organic matter content values (OM) in lake sediments ranged from 2.9 to 30.1% (Fig. 2A), whereas total carotenoids (TC) and chlorophyll derivatives (CD) ranged from 0 to 5.4 mg g−1 of OM, and from 0.5 to 245.8 U g−1 of OM, respectively (Fig. 2A). Dendrogram based on sedimentological data (Bray–Curtis distance and CONISS linkage method) showed 5 significant zones defined by their major temporal patterns (Fig. 2A). The succession of zones along the sediment core seemed to correspond to a regular temporal pattern (Fig. 2A). “Black” zones (1, 3 and 5) referred to the periods with the high OM contents, and TC and CD values (Fig. 2A). On the opposite side, the lowest OM contents and TC and CD values were observed in “white” zones (2 and 4; Fig. 2A).
A Temporal dynamics of organic matter content (OM; %), sedimentary pigments (total carotenoids (TC) and chlorophyll derivatives (CD) expressed in mg g−1 OM and U g−1 OM, respectively). The dendrogram was constructed by hierarchical clustering analysis (Bray–Curtis distance, CONISS linkage method). The shaded areas in stratigraphic diagram indicate significant zones according to the broken-stick model. The sample ages were also reported on the y-axis according to the two possible age–depth models (Fig. 1B). B Temporal trend in carbon isotopic composition of Pseudodiamesa nivosa type (δ13CHC; ‰)
Subfossil chironomid δ13C values (δ13CHC) were relatively high and ranged from − 16.9 to − 8.1‰ (Fig. 2B). The temporal trend of δ13CHC values seemed also to follow a regular pattern (Fig. 2B), matching very well with those observed in sedimentological data (Fig. 2A). The highest δ13CHC values (i.e. less 13C-depleted) were reported during the highest sedimentary pigment concentrations (Fig. 2A). A GAM approach was applied to δ13CHC values to understand how aquatic primary production (TC and CD) dynamics affected carbon sources sustaining chironomid biomass. After logarithmic transformation of explanatory variables, model residues followed a normal distribution and the use of the Gaussian family was then relevant. Both GAMs showed strong significant positive effects of TC (F = 14.8; edf = 2.42) and CD concentrations (F = 7.5; edf = 2.44) on δ13CHC values, explaining 57 and 34% of δ13CHC variability, respectively (Fig. 3B and Table 3).
A Conceptual overview showing the impact of climate change on the energy–mass framework in Lake Pyramid Inferior. Energy (E) refers to irradiance and atmospheric heat, while mass (m) transfers correspond to nutrients and solute dynamics. The dashed arrow represents a minor process, whereas the full arrows correspond to main processes that affected the Lake Pyramid Inferior dynamic. In the case of Lake Pyramid Inferior, a warmer climate induces a decrease in the glacial area in the catchment, leading to increase in solute concentrations in lake. B Fitted smooth function between explanatory variables (TC and CD) and δ13CHC values from generalized additive models (GAMs), with a continuous-time first-order autoregressive process to account for temporal autocorrelation. Grey surface marks the 95% uncertainty interval of the fitted function. On the x-axis, black ticks show the distribution of the observed values for variables. Numbers in brackets on the y-axis are the effective degrees of freedom (edf) of the smooth function
Discussion
Climate change, E–m flux and benthic secondary production
Reconstructions of past climate changes in the Himalayan Mountains are scarce, but they revealed marked alternations of warm and cold periods over the last 3,500 years (Röthlisberger & Geyh, 1985; Owen et al., 2009; Holmes et al., 2009; Henderson & Holmes, 2009; Yang et al., 2009). Since current glacier dynamics and associated in-lake solute concentrations are strongly regulated by climate variability (Salerno et al., 2008; Salerno et al., 2016; Chudley et al., 2017), past warming periods should also correspond to periods of glacial retreats. Clustering analysis of the sedimentary pigment stratigraphy of Lake Pyramid Inferior revealed a strong concordance between primary producer’s dynamics and main known climatic phases in the Eastern part of the Himalayan Mountains (Lami et al., 2010). During warm phases (“dark” zones in Fig. 2A), autochthonous primary productivity was higher due to increase in in-lake solute concentrations, whereas in contrast the lowest aquatic primary productivity was reported during cold events (“white” zones in Fig. 2A). According to the conceptual E–m flux framework (Leavitt et al., 2009), phytoplankton community seemed then to be indirectly controlled by climate change through temporal changes in in-lake solute concentrations (m-fluxes; Fig. 3A; Lami et al., 2010). Effects of climate change were then mediated by the surrounding catchment and the glacier dynamics, playing the role of environmental filters.
The surrounding catchment of Lake Pyramid Inferior is mainly constituted by bare rocks (ca. 90%; Tartari et al., 1998b), and inputs of terrestrial organic matter to lakes can then assume to be negligible. In this context, the availability of food sources at the base of aquatic food webs was only controlled by the intensity of aquatic primary productivity, and it was likely the limiting factor of benthic secondary production (as suggested by Morgan et al., 1980), inducing a bottom-up control on benthic consumers. Indeed, benthic secondary production of lakes can be strongly reliant on phytoplankton dynamics in a phenomenon also described as the pelagic–benthic coupling (Goedkoop & Johnson, 1996). In this study, we demonstrated the positive relationship between photosynthetic pigments’ concentrations in sediments and carbon sources incorporated in chironomid biomass (illustrated by δ13C values of Pseudodiamesa nivosa type; Fig. 3B). The target morphotype used in this study belonged to Pseudodiamesa nivosa-type morphotype which is known to feed on phytoplankton deposits and associated bacteria (and terrestrial detritus if any; Pegast, 1947). The deposit-feeder trophic guild is then particularly able to feed on phytoplankton deposits on lake sediments especially during phytoplankton blooms (e.g. Johnson, 1987). Warm periods and associated rise in aquatic primary production (Fig. 2A; Lami et al., 2010) induced changes in food source availabilities at the base of aquatic food webs (i.e. phytoplankton), impacting trophic interactions in the benthic food web. Finally, the present study also confirmed the strong sensitivity of carbon processing in the benthic food web to climate change. Indeed, previous studies revealed that temperature was the principal driver of the food availability at the base of the benthic food web in small lakes (van Hardenbroek et al., 2013; Belle et al., 2017a, c).
High elevation, partial pressure and isotopic fractionation
The range of δ13CHC values reported in this study (ranging from − 16.9 to − 8.1‰, Fig. 2B) was considerably higher than that previously reported from different types of lakes (from − 49.2 to − 22‰; -van Hardenbroek et al., 2013; Frossard et al., 2015; Belle et al., 2016; Belle et al., 2017c). Accurate data interpretation needs a reliable estimation of isotopic baselines (Vander Zanden and Rasmussen, 1999; Post, 2002), and too few stable isotopic studies have been conducted in the Nepalese Himalayas. Whereas δ13C values of pelagic primary production usually range from − 36 to − 25‰ (Vuorio et al., 2006; Wang et al., 2013), the range of δ13CHC values could match well with that of the δ13C values of phytoplankton frequently observed in CO2-limiting environment (Street-Perrott et al., 1997; Reuss et al., 2013). Lake Pyramid Inferior is located at 5,067 m a.s.l., and atmospheric pressure is consequently about the half of the value reported at the sea level. Based on calculations combining pH and alkalinity values measured in water column of Lake Pyramid Inferior, Tartari et al. (1998a) assumed that dissolved inorganic carbon (DIC) concentrations in the lake may be in equilibrium with ambient atmosphere. The DIC concentrations are then very low and might be a limiting factor for the development of phytoplankton (Hein, 1997). In this CO2-limiting context, CO2 invasion from atmospheric origin can be observed during rising phytoplankton productivity (Schindler & Fee, 1974), which could explain the observed high δ13C values. In addition, a lower fractionation by phytoplankton during periods of high aquatic primary productivity (“dark zones”, Fig. 2A) could lead to increase in phytoplankton δ13C values observed during productive periods. Indeed, it is well known that a substrate competition (for the light isotope, 12C) can occur when the aquatic primary productivity is high and DIC concentrations are very low (Smyntek et al., 2012), leading to the decrease in the trophic fractionation. Similar observations were previously suggested from sediment records in temperate lakes (Belle et al., 2017b; Schilder et al., 2017).
Future challenges for (paleo)-limnological studies in the Nepalese Himalayas
Further investigations are still needed to better understand the carbon cycle in high-elevation lakes (i.e. from biogeochemical cycles to aquatic food webs), and in particular fractionation by phytoplankton and aquatic primary producers in CO2-limiting environment (Street-Perrott et al., 1997). The use of compound-specific stable isotopes of phytoplankton markers (e.g. Ninnes et al., 2017) could help us better estimate the temporal changes in isotopic baselines for chironomid larvae. In addition, complementary studies combining stable isotope and chironomid abundance investigations should be conducted to assess the impact on benthic secondary productivity (similarly to Frossard et al., 2015). The reconstruction of carbon processing in aquatic food webs using carbon stable isotopic analysis of biological remains seems to be promising in Nepalese Himalayas lakes and could be coupled with those of cladoceran remains (Rantala et al., 2015), to better assess the complexity of the response of lake carbon cycling to past climate change.
References
Adrian, R., et al., 2009. Lakes as sentinels of climate change. Limnology and Oceanography 54: 2283–2297.
Belle, S., et al., 2014. Temporal changes in the contribution of methane-oxidizing bacteria to the biomass of chironomid larvae determined using stable carbon isotopes and ancient DNA. Journal of Paleolimnology 52: 215–228.
Belle, S., et al., 2016a. Increase in benthic trophic reliance on methane in 14 French lakes during the Anthropocene. Freshwater Biology 61: 1105–1118.
Belle, S., et al., 2016b. 20th century human pressures drive reductions in deep-water oxygen leading to losses of benthic methane-based food webs. Quaternary Science Reviews 137: 209–220.
Belle, S., et al., 2017a. Chironomid paleo diet as an indicator of past carbon cycle in boreal lakes: Lake Kylmänlampi (Kainuu province; Eastern Finland) as a case study. Hydrobiologia 785: 149–158.
Belle, S., et al., 2017b. Impact of eutrophication on the carbon stable-isotopic baseline of benthic invertebrates in two deep soft-water lakes. Freshwater Biology 62: 1105–1115.
Belle, S., et al., 2017c. 14,000 years of climate-induced changes in chironomid paleo-diet in a small boreal lake (Lake Tollari, Estonia). Climatic Change 145: 205–219.
Bennett, K. D., 1996. Determination of the number of zones in a biostratigraphical sequence. New Phytologist 132: 155–170.
Blaauw, M., 2010. Methods and code for “classical” age-modelling of radiocarbon sequences. Quaternary Geochronology 5: 512–518.
Blenckner, T., 2005. A conceptual model of climate-related effects on lake ecosystems. Hydrobiologia 533: 1–14.
Catalan, J., S. Pla-Rabés, A. P. Wolfe, J. P. Smol, K. M. Rühland, N. J. Anderson, J. Kopáček, E. Stuchlík, R. Schmidt, K. A. Koinig, L. Camarero, R. J. Flower, O. Heiri, C. Kamenik, A. Korhola, P. R. Leavitt, R. Psenner & I. Renberg, 2013. Global change revealed by palaeolimnological records from remote lakes: a review. Journal of Paleolimnology 49: 513–535.
Chudley, T. R., et al., 2017. Glacier characteristics and retreat between 1991 and 2014 in the Ladakh Range, Jammu and Kashmir. Remote Sensing Letters 8: 518–527.
Frossard, V., et al., 2013. A study of the δ13C offset between chironomid larvae and their exuvial head capsules: implications for palaeoecology. Journal of Paleolimnology 50: 379–386.
Frossard, V., et al., 2015. Changes in carbon sources fueling benthic secondary production over depth and time: coupling Chironomidae stable carbon isotopes to larval abundance. Oecologia 178: 603–614.
Goedkoop, W. & R. K. Johnson, 1996. Pelagic-benthic coupling: profundal benthic community response to spring diatom deposition in mesotrophic Lake Erken. Limnology and Oceanography 41: 636–647.
Goedkoop, W., et al., 2006. Trophic fractionation of carbon and nitrogen stable isotopes in Chironomus riparius reared on food of aquatic and terrestrial origin. Freshwater Biology 51: 878–886.
Guilizzoni, P., et al., 2011. Use of sedimentary pigments to infer past phosphorus concentration in lakes. Journal of Paleolimnology 45: 433–445.
Hein, M., 1997. Inorganic carbon limitation of photosynthesis in lake phytoplankton. Freshwater Biology 37: 545–552.
Heiri, O., et al., 2001. Loss on ignition as a method for estimating organic and carbonate content in sediments: reproducibility and comparability of results. Journal of Paleolimnology 25: 101–110.
Henderson, A. C. G. & J. A. Holmes, 2009. Palaeolimnological evidence for environmental change over the past millennium from Lake Qinghai sediments: a review and future research prospective. Quaternary International 194: 134–147.
Holmes, J. A., et al., 2009. Climate change over the past 2000 years in Western China. Quaternary International 194: 91–107.
Ilyashuk, B. P., et al., 2010. Midges of the genus Pseudodiamesa Goetghebuer (Diptera, Chironomidae): current knowledge and palaeoecological perspective. Journal of Paleolimnology 44: 667–676.
Johnson, R. K., 1987. Seasonal variation in diet of Chironomus plumosus (L.) and C. anthracinus Zett. (Diptera: Chironomidae) in mesotrophic Lake Erken. Freshwater Biology 17: 525–532.
Johnson, R. K. & T. Wiederholm, 1992. Pelagic-benthic coupling-The importance of diatom interannual variability for population oscillations of Monoporeia affinis. Limnology Oceanography 37: 1596–1607.
Jónasson, P. M., 1972. Ecology and production of profundal benthos in relation to phytoplankton in Lake Esrom. Oikos 14: 1–148.
Juggins, S., 2017. Rioja: Analysis of Quaternary Science Data.
Lami, A., et al., 2010. Chemical and biological response of two small lakes in the Khumbu Valley, Himalayas (Nepal) to short-term variability and climatic change as detected by long-term monitoring and paleolimnological methods. Hydrobiologia 648: 189–205.
Lami, A., et al., 1998. Palaeolimnological evidence of environmental changes in some high altitude Himalayan lakes (Nepal). Memorie dell’Istituto italiano di idrobiologia 57: 130–131.
Leavitt, P. R. & D. A. Hodgson, 2002. Sedimentary Pigments. In Last, W. M. & J. P. Smol (eds), Tracking Environmental Change Using Lake Sediments. Developments in Paleoenvironmental Research. Springer, Dordrecht: 295–325.
Leavitt, P. R., et al., 2009. Paleolimnological evidence of the effects on lakes of energy and mass transfer from climate and humans. Limnology Oceanography 54: 2330–2348.
Lindeman, R. L., 1942. The trophic-dynamic aspect of ecology. Ecology 23: 157–176.
Manca, M., et al., 1998. Report on a collection of aquatic organisms from high mountain lakes in the Khumbu Valley (Nepalese Himalayas). Memorie dell’Istituto italiano di Idrobiologia 57: 77–98.
Meyers, P. A. & R. Ishiwatari, 1993. Lacustrine organic geochemistry – an overview of indicators of organic matter sources and diagenesis in lake sediments. Organic Geochemistry 20: 867–900.
Morgan, N. C., et al., 1980. Secondary production. In LeCren, E. D. & R. H. Lowe-McConnel (eds), The functioning of freshwater ecosystems. Cambridge University Press, Cambridge: 247–340.
Nevalainen, L., et al., 2014. Fossil cladoceran record from Lake Piramide Inferiore (5067 m asl) in the Nepalese Himalayas: biogeographical and paleoecological implications. Journal of Limnology 73: 358–368.
Ninnes, S., et al., 2017. Investigating molecular changes in organic matter composition in two Holocene lake-sediment records from central Sweden using pyrolysis-GC/MS. Journal of Geophysical Research 122: 1423–1438.
Northington, R. M., et al., 2010. Benthic secondary production in eight oligotrophic arctic Alaskan lakes. Journal of the North American Benthological Society 29: 465–479.
Owen, L. A., et al., 2009. Quaternary glaciation of Mount Everest. Quaternary Science Reviews 28: 1412–1433.
Pegast, F., 1947. Systematik und Verbreitung der um die Gattung Diamesa gruppierten Chironomiden. Archiv für Hydrobiologie 45: 435–596.
Post, D. M., 2002. Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83: 703–718.
Qiu, J., 2008. China: The third pole. Nature News 454: 393–396.
R Core Team. 2017. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org.
Rantala, M. V., et al., 2015. Climate controls on the Holocene development of a subarctic lake in northern Fennoscandia. Quaternary Science Reviews 126: 175–185.
Reimer, P. J., E. Bard, A. Bayliss, J. W. Beck, P. G. Blackwell, C. B. Ramsey, C. E. Buck, H. Cheng, R. L. Edwards, M. Friedrich, P. M. Grootes, T. P. Guilderson, H. Haflidason, I. Hajdas, C. Hatté, T. J. Heaton, D. L. Hoffmann, A. G. Hogg, K. A. Hughen, K. F. Kaiser, B. Kromer, S. W. Manning, M. Niu, R. W. Reimer, D. A. Richards, E. M. Scott, J. R. Southon, R. A. Staff, C. S. M. Turney, & J. van der Plicht, 2013. IntCal13 and Marine13 Radiocarbon Age Calibration Curves 0–50,000 Years cal BP. Radiocarbon 55: 1869–1887.
Reuss, N. S., et al., 2013. Stable isotopes reveal that chironomids occupy several trophic levels within West Greenland lakes: implications for food web studies. Limnology Oceanography 58: 1023–1034.
Röthlisberger, R. & M. A. Geyh, 1985. Glacier variations in Himalayas and Karakorum. Zeitschrift für Gletscherkunde und Glazialgeologie 21: 237–249.
Rounick, J. S. & M. J. Winterbourn, 1986. Stable carbon isotopes and carbon flow in ecosystems. BioScience 36: 171–177.
Salerno, F., et al., 2008. Glacier surface-area changes in Sagarmatha national park, Nepal, in the second half of the 20th century, by comparison of historical maps. Journal of Glaciology 54: 738–752.
Salerno, F., et al., 2016. Glacier melting increases the solute concentrations of Himalayan Glacial Lakes. Environmental Science Technology 50: 9150–9160.
Schilder, J., et al., 2017. Trophic state changes can affect the importance of methane-derived carbon in aquatic food webs. Proceedings of the Royal Society B 284: 20170278.
Schimmelmann, A. & M. J. DeNiro, 1986. Stable isotopic studies on chitin. III. The D/H and 18O/16O ratios in arthropod chitin. Geochimica et Cosmochimica Acta 50: 1485–1496.
Schindler, D. W. & E. J. Fee, 1974. Experimental lakes area: whole-lake experiments in eutrophication. Journal of the Fisheries Research Board of Canada 31: 937–953.
Simpson, G. L. & N. J. Anderson, 2009. Deciphering the effect of climate change and separating the influence of confounding factors in sediment core records using additive models. Limnology Oceanography 54: 2529–2541.
Simpson, G. L. & J. Oksanen, 2016. Analogue: analogue and weighted averaging methods for palaeoecology.
Smyntek, P. M., et al., 2012. Dissolved carbon dioxide concentration controls baseline stable carbon isotope signatures of a lake food web. Limnology Oceanography 57: 1292–1302.
Soncini, A., et al., 2016. Future hydrological regimes and glacier cover in the Everest region: The case study of the upper Dudh Koshi basin. Science of the Total Environment 565: 1084–1101.
Street-Perrott, F. A., et al., 1997. Impact of lower atmospheric carbon dioxide on tropical mountain ecosystems. Science 278: 1422–1426.
Tartari, G. A., et al., 1998a. Lake cadastre of Khumbu Himal Region: geographical-geological-limnological data base. Memorie dell’Istituto italiano di Idrobiologia 7: 151–235.
Tartari, G. A., et al., 1998b. Water chemistry of high altitude lakes in the Khumbu and Imja Kola valleys (Nepalese Himalayas). Memorie dell’Istituto italiano di Idrobiologia 57: 51–76.
Vadeboncoeur, Y., et al., 2002. Putting the lake back together: reintegrating Benthic pathways into lake food web models. BioScience 52: 44–54.
Vander Zanden, M. J. V. & J. B. Rasmussen, 1999. Primary Consumer δ 13C and δ 15N and the Trophic Position of Aquatic Consumers. Ecology 80: 1395–1404.
van Hardenbroek, M., et al., 2009. Fossil chironomid δ13C as a proxy for past methanogenic contribution to benthic food webs in lakes? Journal of Paleolimnology 43: 235–245.
van Hardenbroek, M., et al., 2013. Evidence for past variations in methane availability in a Siberian thermokarst lake based on δ13C of chitinous invertebrate remains. -. Quaternary Science Reviews 66: 74–84.
Verbruggen, F., et al., 2009. Effects of chemical pretreatments on δ18O measurements, chemical composition, and morphology of chironomid head capsules. Journal of Paleolimnology 43: 857–872.
Vuorio, K., M. Meili & J. Sarvala, 2006. Taxon-specific variation in the stable isotopic signatures (δ13C and δ15N) of lake phytoplankton. Freshwater Biology 51: 807–822.
Wagner, A., S. Volkmann & P. M. A. Dettinger-Klemm, 2012. Benthic–pelagic coupling in lake ecosystems: the key role of chironomid pupae as prey of pelagic fish. Ecosphere 3: art14.
Walker, I. R., 2001. Midges: Chironomidae and Related Diptera. In Smol, J. P. et al. (eds), Tracking environmental change using lake sediments. Developments in Paleoenvironmental Research. Springer, Netherlands: 43–66.
Wang, B., et al., 2013. Mechanisms controlling the carbon stable isotope composition of phytoplankton in karst reservoirs. Journal of Limnology 72: 11.
Williamson, C. E., et al., 2009. Sentinels of change. Science 323: 887–888.
Wood, S. N., 2011. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. Journal of the Royal Statistical Society: Series B 73: 3–36.
Wooller, M. J., et al., 2012. Reconstruction of past methane availability in an Arctic Alaska wetland indicates climate influenced methane release during the past ~ 12,000 years. Journal of Paleolimnology 48: 27–42.
Yang, B., et al., 2009. Temperature changes on the Tibetan Plateau during the past 600 years inferred from ice cores and tree rings. Global and Planetary Change 69: 71–78.
Yao, T., et al., 2012a. Third pole environment (TPE). Environmental Development 3: 52–64.
Yao, T., et al., 2012b. Different glacier status with atmospheric circulations in Tibetan Plateau and surroundings. Nature Climate Change 2: 663–667.
Acknowledgements
This study was part of the framework of the Ev-K2-CNR ‘Scientific and Technological Research in Himalayas and Karakorum’ Project with financial support from the Ev-K2-CNR Committee and in collaboration with the Nepal Academy of Science and Technology (NAST). Financial support was provided by institutional research grants from the Italian National Research Council (CNR) and the Italian Ministry of Foreign Affairs. We are grateful to Christian Hossann (INRA Nancy, Champenoux) for assistance in stable isotope analysis of carbon. The PTEF facility is supported by the French National Research Agency through the Laboratory of Excellence ARBRE (ANR-11-LABX-0002-01). The authors gratefully acknowledge the constructive comments of two anonymous reviewers on this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Jasmine Saros
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Belle, S., Musazzi, S. & Lami, A. Glacier dynamics influenced carbon flows through lake food webs: evidence from a chironomid δ13C-based reconstruction in the Nepalese Himalayas. Hydrobiologia 809, 285–295 (2018). https://doi.org/10.1007/s10750-017-3477-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-017-3477-8