Abstract
Temperate woodland vegetation is initially determined by spatiotemporal and historical factors, mediated by complex biotic interactions. However, catastrophic events such as disease outbreaks (e.g., sweet chestnut blight, ash dieback), infestations of insect pests, and human-accelerated climate change can create canopy gaps due to systematic decline in, or loss of, tree species that was once an important part of the canopy. Resultant cascade effects have the potential to alter the composition of woodland ecosystems quickly and radically, but inherent lag times make primary research into these effects challenging. Here, we explore change in woodland vegetation at 10 sites in response to canopy opening using the Elm Decline, a rapid loss of Ulmus in woodlands across northwestern Europe ~ 5800 years ago that coexisted alongside other stressors such as increasing human activity, as a palaeoecological analogue. For arboreal taxa, community evenness significantly decreased, within-site turnover significantly increased, and richness remained unchanged. Changes in arboreal taxa were highly site-specific but there was a substantial decline in woody climbing taxa, especially Hedera (ivy), across the majority of sites. For shrub taxa, richness significantly increased but evenness and turnover remained consistent. Interestingly, however, there was a significant increase in abundance of shrubs at 70% of sites, including Calluna (heather), Ilex (holly) and Corylus (hazel), suggesting structural change. Surprisingly, there was no change in richness, evenness or turnover for herb taxa, possibly because change was highly variable spatially. However, there was a marked uptick in the disturbance indicator Plantago (plantain). Overall, these findings suggest that woodlands with sustained reduction in, or loss of, a tree species that once formed an important part of the canopy has profound, but often spatially idiosyncratic, impacts on vegetation diversity (richness), composition (evenness), stability (turnover), and on abundance of specific taxa, especially within the shrub layer. Use of this palaeoecological analogue, which was itself complicated by cooccurring changes in human activity, provides a valuable empirical insight into possible cascade effects of similar change in canopy opening in contemporary settings, including Ash Dieback.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Woodland vegetation community composition is initially determined by spatiotemporal factors such as location, connectivity, origin, and age (Peterken 1996; Brudvig and Damschen 2011; Palo et al. 2013; Rackham 2015; Swallow et al. 2020; Uroy et al. 2023). However, because woodlands are complex and dynamic habitats that support multiple co-occurring species, interspecific interactions also modify vegetation composition (Goodenough and Hart 2023; Kermavnar et al. 2023). Such interactions then influence other taxonomic guilds including birds (Hinsley et al. 2009; Hewson et al. 2011), mammals (Holland and Bennett 2007) and invertebrates (Jukes et al. 2001; Tudor et al. 2004). Changes in vegetation assemblage and/or structure can, therefore, have cascade effects throughout the ecosystem (Amar et al. 2006; Gregory et al. 2007; Fox et al. 2015; Homburg et al. 2019; Blumgart et al. 2022; Bowler et al. 2023; Scopes et al. 2023).
Protecting and managing woodlands is important to conserve biodiversity and ecological distinctiveness, as well as to maintain ecosystem services such as carbon sequestration and hydrological regulation (Lorenz and Lal 2010; Bullock et al. 2014; Revell et al. 2021). However, challenges such as climate change, outbreaks of pests or disease, and high levels of herbivory can threaten woodland composition and the ecosystem services they provide (Fuller 2001; Rackham 2008; Gimona et al. 2012; Davies et al. 2017; Freer-Smith and Webber 2017; Green et al. 2021; Yu et al. 2021; Tew et al. 2024). This is especially true when one specific tree species has higher susceptibility to a particular threat than other co-occurring species. Loss of, or severe decline in, any tree species that forms an important part of the canopy can have cascade effects throughout the ecosystem. For example, canopy gaps due to loss of Abies alba (silver fir) can cause understorey homogenization and loss of herb diversity (Nagel et al. 2019). In that case, community-level effects were evident within 30 years and were thus discernible via field studies. However, the lag times usually inherent in ecological systems between measurable cause and quantifiable effect often exceed field study timescales (Goodenough and Webb 2022). Moreover, due to woodland complexity and the presence of local modifiers of vegetation, it is often difficult to predict how canopy opening resulting from loss of a tree species will affect the overall ecosystem (Kermavnar et al. 2023).
Throughout Europe, the fungal pathogen Hymenoscyphus fraxineus is causing severe and widespread Ash Dieback (Mitchell et al. 2014; Needham et al. 2016; Enderle et al. 2019). Indeed, decline in Fraxinus (ash) is already impacting woodlands (Enderle et al. 2019), with predictions that Fraxinus-dominated woodlands will undergo further substantial community change especially where functional diversity is low (Loo 2009; Patacca et al. 2023). Whilst numerous studies have investigated the cause, distribution, and management of Ash Dieback (e.g. Mitchell et al. 2014; Needham et al. 2016; Enderle et al. 2019), and some research has examined ecological correlates of disease magnitude (Erfmeier et al. 2019), studying community-level effects is challenging. To date, because of challenges imposed by lag time in primary ecological studies, such research is largely predictive, being based on likely differential effects of canopy opening on field-layer taxa (Hinsley & Pocock 2014; Mitchell et al. 2014; Needham et al. 2016; Hultberg et al. 2020).
Lack of research on cascade effects of canopy gaps resulting from systematic decline in a specific tree species is not restricted to ash woodland, nor to disease as a cause of change. Other examples include decline in: (1) Acer platanoides (Norway maple) in Massachusetts due to non-native wood-boring pests (Francis and Elmes 2017); (2) Quercus (oak) due to unregulated herbivory in Appalachia (Fajvan and Wood 1996); (3) Fagus due to drought in central Europe (Mette et al. 2013); and (4) Abies and Thuja (cedar) due to elevated summer temperatures in North America (Iverson and Prasad 2001). There is also potential for increasingly extreme weather to create significant canopy gaps in woodland of any composition, especially those with over-mature trees (Forestry Commission 2022). In such instances, lag times mean that understanding community-level effects is necessarily based on qualitative predictions, quantitative modelling, or GIS-based forecasting.
A different approach to overcoming lag time challenges when studying ecological cascade effects is to “learn from the past” (Goodenough and Webb 2022). In palaeoecological research, sub-fossilised pollen is used to analyse vegetation histories, and, ergo, changing community ecology (e.g. Chambers et al. 2007; Barak et al. 2016; Manzano et al. 2020). Richer and Gearey (2018) and Webb and Goodenough (2021) extended this framework to use palaeoecological analogues to predict vegetation responses to contemporary environmental change. Although there are several well-studied tree declines in the palaeoecological record—including Tsuga canadensis (Eastern hemlock) decline in the USA ~ 5000–6000 years ago (Calcote 2003) and Tilia (lime) across the United Kingdom around ~ 2500–3000 years ago (Grant et al. 2011)—the most pronounced tree decline in northwestern Europe was the dramatic loss of Ulmus (Elm) ~ 5800 years ago. This well-studied, abrupt, and often permanent Elm Decline is reported frequently in palaeoecological records in Britain, Ireland and Scandinavia, and occasionally in parts of mainland Europe (Andersen and Rasmussen 1993; Digerfeldt 1997; Gandouin et al. 2009; Jamrichová et al. 2017; Kearney and Gearey 2024; Kaiser et al. 2023). However, although this phenomenon is well studied, research has focused on documenting spatiotemporal trends (e.g. Parker et al. 2002; Giesecke et al. 2017; Kearney and Gearey 2024), with little research exploring community-level ecological consequences.
Here, we explore change in woodland vegetation communities in response to canopy opening, using the Elm Decline as a palaeoecological analogue. Because change in community ecology is inherently site-specific, even when due to the same stimulus, we take a multi-site approach to establish broad trends in response to canopy gaps at ten woodland sites from northwest Europe. We establish temporal trends in richness, evenness and within-site turnover, as well as exploring responses of individual taxa to synthesize general patterns. We hope that a better understanding of woodland community change in response to loss of an important arboreal species will provide useful insights into how woodlands might adapt to current and future tree-loss events, such as those predicted due to diseases, pests, and climatic change.
Methods
Data acquisition and manipulation
Overview
There were many sites that could have been suitable for inclusion in this study, but locating these in an ad-hoc way would inherently have been biased based on incomplete and subjective pre-existing knowledge of authors or sites, whether the original paper specifically mentioned the Elm Decline, and the date of the publication, and whether the paper was available and searchable electronically. Moreover, the existence of a suitable paper did not axiomatically mean that the underpinning data were available for analysis within our research, nor that those data would have a suitable level resolution in dating. Therefore, to ensure that methods were transparent and objective, as well as being effective in the identification of sites that had available data, we followed the precepts of PRISMA (Page et al. 2021) to develop a clear workflow for site selection that would be repeatable.
Identification of potential sites and basic screening
The Neotoma database (Williams et al. 2018) was mined to identify sites across the British Isles—England, Wales, Scotland, Northern Ireland and the Republic of Ireland—with palynological data available in raw format as grain counts. Using this platform, potential sites were identified that met the screening criteria that there was: (1) an associated peer-reviewed published output; (2) at least two radiocarbon dates between 8000 and 4000 calendar Years Before Present (YBP); and (3) ≥ 2% Ulmus in the time-sediment horizon equating to 6500 YBP. The requirement for two radiocarbon dates was predicated on the need for a robust age-depth model to calculate sedimentation rates using IntCal20—the latest calibration curve for terrestrial sites in the Northern Hemisphere (Reimer et al. 2020)—to enable interpolated dates to model time (1000 years) post-dating the Elm decline. The Ulmus criterion was based on 2% being the minimum pre-decline Ulmus percentage observed in a previous study of 25 woodland sites in Ireland affected by the Elm Decline (Kearney and Gearey 2024). Applying the Ulmus threshold at 6500 YBP was based on that same study finding that, although the Elm Decline was not spatially synchronous, it always occurred relatively soon after 6500 YBP. The fourth and final criterion was to ensure that each potential site was likely to be woodland with a relatively closed canopy, such that sites with < 28% arboreal pollen were excluded. This threshold was selected based on minimum values of arboreal pollen found at woodland sites in contemporary studies of pollen deposition being 28–29%, (Bunting 2002; Tinsley and Smith 1974 respectively). Over 100 sites were initially identified. Once each had been screened, 24 sites were carried through for more detailed assessment.
Detailed assessment of screened sites
For the 24 sites identified meeting the four above screening criteria, palynological data were extracted. Because different taxa produce pollen at different rates, there is a disconnect between vegetation assemblage and pollen abundance. Raw pollen counts thus needed to be corrected using taxon-specific Relative Pollen Productivity Estimates (RPPEs) to enable robust analysis of change in vegetation unbiased by differential pollen production (Andersen 1970; Parsons and Prentice 1981; Jackson 1994; Prentice and Webb 1986). To ensure RPPEs were as accurate as possible, the majority of the correction values were taken from Wieczorek and Herzschuh (2020), which collated data from different regions (e.g. Broström et al. 2008; Li et al. 2017). This removed much of the bias in variability inherent in RPPE values usually generated by single-site datasets. For taxa not listed by Wieczorek and Herzschuh (2020), RPPE values were taken from single-site studies (Bradshaw 1981; Bunting and Farrell 2022), or based upon RPPE values from similar taxa (Andersen 1970; Bradshaw 1981; Out and Verhoeven 2014). To apply RPPE corrections at each site, raw pollen data were converted to percentages for each time-sediment horizon, and these values were divided by the relevant taxon-specific RPPE value. RPPE-corrected values were used in analysis of within-turnover and change in individual taxa. Following the protocol of Kearney and Gearey (2024), the presence of a potential Elm Decline—a sustained reduction in Ulmus—at each site was examined by visually inspecting RPPE-corrected Ulmus abundance in each time-sediment horizon. Where no Elm Decline was evident, the site was immediately excluded (n = 14), with the remaining sites (n = 10) being taken through to a final verification step.
Verification
Where there was visual evidence for Elm Decline (n = 10), the period during which Ulmus underwent sustained reduction was identified, together with pre-decline and post-decline periods described below and shown visually in Fig. 1; a minimum of two time-sediment horizons was necessary in each period:
-
“Pre-decline”: the period before sustained Ulmus decline, during which Ulmus abundance was stable at relatively high values or fluctuating within a relatively narrow range of high values, with no overall temporal change; maximum duration 1000 years dependent on sediment deposition and sampling rates.
-
“Within-decline”: the period between the start of sustained Ulmus decline and the lowest value of Ulmus recorded; duration entirely data-driven and thus site dependent.
-
“Post-decline”: the period after sustained Ulmus decline, during which Ulmus was stable at low values or fluctuating within a relatively narrow range of low values with no overall temporal change; maximum duration 1000 years dependent on sediment deposition, sampling rates, and whether Ulmus rebounded.
-
“Rebound”: rebound of Ulmus from sustained low post-decline values; not always present.
Throughout this study, we are using change in the abundance of Ulmus during and after the Elm Decline as a proxy for woodland structural changes and canopy opening, which cannot be quantified directly. The 1000-year maximum window post-decline was selected as being an ecologically relevant timespan during which any vegetative change relating to the canopy changes caused by the Elm Decline, which might be affected by lag times, should be evident. It should be noted that while the process of (and criteria for) designating time periods was standardised, Ulmus abundance was site specific, as was duration and the number of time-sediment horizons. To confirm the presence of the Elm Decline at all potential study sites, a one-way ANOVA with Tukey post-hoc testing was run to ensure Ulmus abundance post-decline was statistically significantly lower than pre-decline. All sites passed the verification step; the final sample size was 10 sites (latitude: 50.622 N to 56.384 N; longitude: − 9.588 W to 0.833 E) that had significant Elm Decline (and as per Fig. 2).
Taxonomic richness
As the taxonomic level to which pollen can be identified based on morphological characteristics varies for different taxa, each site contained a combination of family-level, genus-level and species-level data. This inconsistency was not problematic because analysis of vegetation change was always undertaken at site level, or nested within site, and although taxonomic resolution differed between sites it was temporally consistent within each site. This meant that identified temporal patterns were not an artefact of changing taxonomic resolution over time and rendered taxonomic smoothing unnecessary. Each taxon was classified into one of three categories: (1) arboreal–woody species with the potential to form an overarching canopy; (2) shrub–woody species that typically occur in the scrub stratum within a woodland ecosystem; (3) herb–all non-woody vascular species from the field and herb layers of a woodland ecosystem including forbs, grasses, sedges, and rushes.
In contemporary ecological studies, taxonomic richness is usually a simple count of the number of taxa present. However, in palynological data variability occurs in the number of pollen grains counted by different researchers at different sites, as well as between time-sediment horizons in the same stratified sequence. This can present problems as taxonomic richness is fundamentally, and non-linearly, dependent on the number of grains counted (Birks and Line 1992; Birks 2007). To avoid differences in sample effort biasing analysis, rarefaction was used to create a corrected taxonomic richness metric using PAST v4.12 (Hammer and Harper 2001; Hammer 2022); this approach has been used extensively within palaeoecological research (e.g. Li 2018; Robles-López et al. 2018; Pardoe 2021). Rarefied richness values were calculated for each time-sediment horizon at each site using the grain count for the lowest sample size at that site; this process was undertaken for the overall community and then separately for arboreal, shrub, and herb taxa. All analysis of taxonomic richness thus used (rarefied) pollen counts rather than RPPE-corrected values.
Taxonomic evenness
As noted by Legendre and Legendre (1998) and Peros and Gajewski (2008), taxonomic evenness—the extent to which relative abundance of each taxon is similar (uniform distribution) or dissimilar (skewed distribution)—is an important ecological metric that remains relatively underutilized in palaeoecological research. Here, we calculated Shannon’s Evenness (EH) for each time-sediment horizon. This involved quantifying Shannon’s Diversity Index (H) with rarefaction applied using PAST v4.12. This ensured computed H values were calibrated relative to the lowest sample size at that site, in the same way that taxonomic richness had been corrected. Then, to get rarefied evenness for each time-sediment horizon, we undertook an extra manual step to divide the (rarefied) H value for each horizon by the natural logarithm of the (rarefied) taxonomic richness value for the same horizon to get (rarefied) EH values. This metric was bounded between 0 and 1, with 1 indicating complete evenness (all taxa being uniformly abundant). In all cases, rarefied EH values were calculated for the overall community and then separately for arboreal, shrub, and herb taxa. All analysis of evenness thus used the (rarefied) pollen counts rather than RPPE-corrected values.
Within-site turnover
While pollen data, after rarefaction, were appropriate for calculations of taxonomic richness and taxonomic evenness, consideration of within-site turnover necessitated use of RPPE-corrected values. This ensured analysis was reflective of vegetative change rather than change in the pollen profile. Of the many methods available for quantifying the similarity between two communities, we selected Bray–Curtis (Bray and Curtis 1957). This is highly effective for empirical ecological data as it is based on relative abundance rather than just presence/absence (e.g. Jaccard; Sørensen) and is robust in relation to outliers (Shaw 2003). Bray–Curtis similarity was computed in PAST v4.12 for each time-sediment horizon at each site relative to the previous time-sediment horizon within the stratified sequence. Bray–Curtis distance, which is more intuitive for considering turnover (i.e. dissimilarity), was then manually calculated. As per richness and evenness, turnover was calculated for the overall community and then separately for arboreal taxa, shrub taxa, and herb taxa.
Data analysis
To get an overall understanding of implications of canopy opening due to the effects of the Elm Decline on vegetation communities, we calculated Kruskal–Wallis tests for rarefied taxonomic richness, rarefied taxonomic evenness and within-site turnover, for the overall community and also separately for arboreal, shrub and herb taxa (n = 12 tests). In all cases, the grouping variable was time period (pre-decline, within-decline, and post-decline); rebound was excluded from this analysis as this was not present at all sites. We created graphs to visualise trends, which were supported by Mann–Whitney tests as non-parametric post-hoc analyses to follow Kruskal–Wallis and allow significant pairwise differences to be identified. These analyses were conducted in IBM SPSS v29.
Then, to analyse vegetation change in relation to canopy gaps due to the Elm Decline in more detail, a Generalised Linear Model (GLM) framework was used. Rarefied richness in each time-sediment horizon at each site was entered as the dependent variable and two palynological measures were added as covariate predictors: (1) Ulmus abundance at each time-sediment horizon using RPPE-corrected data; and (2) short-term change in Ulmus for each time-sediment horizon compared to the previous horizon (change direction indicated by negative or positive values; change magnitude indicated by deflection from zero). To allow site-specific differences in effects of the Elm Decline to be quantified, a nested framework was used with each palynological variable nested within Site ID. Four richness models were run to analyze, separately, overall community, arboreal taxa, shrub taxa, and herb taxa. This analytical framework was used also to analyze change in evenness using rarefied EH values and for within-site turnover using Bray–Curtis distance. For all 12 models, GLMs used a gamma distribution with log link function, which consistently returned lower delta Akaike Information Criterion scores than scale models.
Finally, to understand the responses of specific taxa to the structural changes in the woodlands caused by the Elm Decline, we undertook SIMPER analysis for each site in Past v4.12. This used Bray–Curtis scores on RPPE-corrected data to allow identification of the most influential taxa driving the dissimilarity. As this analysis was pairwise, we undertook comparisons firstly for post-decline versus pre-decline and secondly for rebound versus post-decline. Together, this allowed consideration of which taxa differed after Ulmus had decreased post-decline relative to initial conditions and recovery.
Results
Evidence for Elm decline
There was conclusive evidence for the Elm Decline at our study sites. On average there was a 79% decrease in Ulmus abundance in ecological communities post-decline relative to pre-decline at the same site (minimum = 67% decline; maximum = 94% decline). This decline was statistically significant at all sites (one-way ANOVAs P < 0.05 in all cases; tests not shown). Follow-up Tukey post-hoc pairwise comparisons showed that Ulmus abundance was significantly lower after the Elm Decline relative to before the Elm Decline at all sites (Fig. 3a–j), with eight out of ten sites also showing at least one additional significant pairwise difference (Fig. 3a, b, d, f–j). This provided good evidence for structural changes in the woodland, including canopy opening.
Mean Ulmus abundance (RPPE-corrected values) before the Elm Decline (blue), within the decline (yellow), after the decline(green) and, where relevant, during rebound (grey). Sites listed: a Clara Bog; b Cornaher Lough; c Cranes Moor; d Gallanech Beg; e Hockham Mere; f Lough Aisling; g Lough Doo; h Cockoo; i Saham Mere; and j Winney’s Down. One-way ANOVAs with Tukey post-hoc testing show statistical differences between communities. Asterisks indicates a significant difference * < 0.05; ** < 0.01; *** < 0.001
Although Ulmus decline was observed at all sites, there were notable differences in pre-decline values from RPPE-corrected Ulmus abundance being as high as ~ 18% (e.g. Clara Bog and Gallanech Beg; Fig. 3a, d) to as low as ~ 2% (Lough Aisling and Winney’s Down; Fig. 3f, j). Notwithstanding these initial differences, Ulmus abundance dropped to zero or very close to zero for at least one time-sediment horizon post-decline at all sites, although it was notable that Ulmus was not lost completely for > 1 horizon at any site. When averaging across all sites, Ulmus abundance using RPPE-corrected values were: pre-decline = 7.71%, within-decline = 4.33% and post-decline = 2.13%. When figures were recalculated just for sites that had a recovery phase (Fig. 3h–j) values were: pre-decline = 5.76%, within-decline = 1.99%; post-decline = 1.09%; rebound = 2.02%. This compared with sites where Ulmus did not rebound (Fig. 3a–g) thus: pre-decline = 8.33%, within-decline = 5.33%; post-decline = 2.35%. This suggested that sites with a higher initial Ulmus abundance declined to low Ulmus levels (~ 2%) post-decline that then remained fairly consistent, whereas sites with lower pre-decline Ulmus abundance originally decreased even further but subsequently rebounded to a similar Ulmus abundance (~ 2%).
Effects of Elm decline on vegetation community metrics
Across all sites and all time-sediment horizons, rarefied taxonomic richness calculated using pollen counts was lowest for shrub taxa (mean = 3.06 taxa), intermediate for arboreal taxa (mean = 6.98), and highest for herb taxa (mean = 7.71). Rarefied evenness calculated on pollen counts differed substantially between taxa, being highly uneven within the shrub sub-community (mean EH = 0.30), while the arboreal and herb communities were considerably more even (mean EH = 0.73 and 0.78, respectively). Within-site turnover calculated using Bray–Curtis dissimilarity on RPPE-corrected values was, on average, lowest for arboreal taxa (mean = 0.18), intermediate for shrub taxa (mean = 0.33) and highest for herb taxa (mean = 0.44).
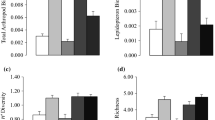
When testing for any impact of the canopy opening associated with the Elm Decline on richness, evenness and within-site turnover using Kruskal–Wallis and Mann–Whitney tests, several patterns emerged. For each taxonomic group, richness values were approximately equal pre-decline and within-decline (Fig. 4a–d). Visually, richness in the overall community and the arboreal sub-community decreased by ~ 50% post-decline in comparison to pre-decline levels (Fig. 4a, b). The opposing trend was found for shrub and herb taxa, where richness increased by ~ 100% post-decline relative to pre-decline (Fig. 4c, d). High levels of within- and between-site variation meant that the only statistically significant pattern was for shrub richness (Table 1; Fig. 4c). For evenness, a significant pattern was found for the arboreal sub-community due to a significant decrease in evenness post-decline relative to pre-decline levels (Table 1; Fig. 4f). This significant pattern contrasted with fairly uniform evenness temporally for the overall community (Fig. 4e) and for shrub and herb taxa (Fig. 4g–h). For turnover, significant patterns were found for arboreal taxa due to turnover being lower pre-decline and higher post-decline (Table 1; Fig. 4j). There was little change for other taxonomic groupings (Fig. 4i, k − l).
Mean values across all sites pre-decline, within-decline and post-decline for: a − d rarefied taxonomic richness (number of taxa); e − h rarefied taxonomic evenness (0 − 1 index); and i − l within-site turnover (0 − 1 index) calculated on RPPE-corrected data. Asterisks indicate a significant difference via Mann–Whitney pairwise tests * < 0.05; ** < 0.01; *** < 0.001
There were notable differences between community metrics during rebound (closing or partial closing of canopy) relative to other periods of the Elm Decline, as detailed in Table 2. Most significant findings related to within-site turnover, which was significantly higher during rebound compared to pre-decline for arboreal taxa (mean = 0.29 vs 0.21) and shrub taxa (mean = 0.42 vs 0.25). A significant counter pattern was observed for herb taxa, with turnover being lower during rebound relative to pre-decline (mean = 0.41 vs 0.70). Turnover was also significantly higher during rebound than post-decline for shrub taxa (mean 0.42 vs 0.33). The only other significant pairwise comparison was a significant increase in evenness for arboreal taxa during rebound relative to pre-decline (mean = 0.78 vs 0.72).
Site-specific trends
All nested GLMs undertaken to better understand the influence of (RPPE-corrected) Ulmus abundance and short-term change in Ulmus abundance on community richness, evenness and within-site turnover were significant, with the single exception of turnover for shrub taxa (Table 3). Ulmus abundance was a significant term in each of the 11 significant models, while short-term Ulmus change (i.e. relative difference in Ulmus compared to the previous time-sediment horizon) was significant in only five models. In the five models where both Ulmus predictors were significant, the Ulmus abundance was notably stronger as a predictor. This suggests that the overall amount of Ulmus, which was driven by long-term change, was the main driver in community metrics, rather than short-term changes between time-sediment horizons.
One of the major strengths of the nested analytical framework was the finding that the same analysis resulted in markedly different patterns at different sites (Table 3). To understand this complex picture, we investigated the relative number of sites where change in Ulmus abundance and/or short-term Ulmus change was positively or negatively associated with the dependent variable, as well as the mean positive and mean negative gradients as a measure of effect size. Together, this information captured both the trend prevalence and trend strength. In most models, the ratio between whether a significant factor was positively or negatively related to the dependent variable was approximately even (no more unbalanced than 60% of sites in one direction versus 40% of sites in the opposing direction) and effect sizes were also approximately equal., However, there were some clear trends: (1) evenness of arboreal taxa was significantly higher when Ulmus was more abundant at nine out of ten sites, and the average effect size was considerably larger than found at the single site with the opposing trend; (2) within-site turnover in the overall community was significantly lower when Ulmus was more abundant at seven of ten sites; (3) within-site turnover in arboreal taxa was significantly lower when Ulmus was more abundant and significantly higher when recent Ulmus change was negative (i.e. when Ulmus was declining over the short-term) at the majority of sites, with larger effect sizes than for opposing trends. For richness of shrubs, it was also noted that although there was little difference in the ratio of sites with positive and negative trends, at the six sites where higher Ulmus abundance was associated with greater richness there was a much larger effect size than found at the four sites where lower Ulmus abundance was associated with greater richness.
SIMPER analysis (Table 4) showed substantial dissimilarity in post-decline communities compared to pre-decline (mean dissimilarity = 44.32; minimum = 30.47; maximum = 57.01). Unsurprisingly, the taxa driving dissimilarity were largely site-specific, but there were some general trends. Abundance of shrub taxa increased at seven of the ten sites (Calluna at four sites; Ilex at three sites; and Corylus at two sites). Woody climbing taxa (Hedera and Lonicera) changed at eight of ten sites although in the case of Hedera these changes were conflicting with decreases at five sites contrasting with increases at two sites. Plantago, a species indicative of disturbance, increased in abundance at seven of the ten sites. SIMPER analysis comparing communities immediately post-decline with the Ulmus rebound period revealed that Hedera increased (after having decreased throughout the Elm Decline), while Plantago abundance continued to increase at all three sites with Ulmus rebound.
Discussion
Baseline trends
All sites within this study had substantial, sustained and significant Elm Decline; indeed, this was a prerequisite for inclusion in this research, which uses the Elm Decline as a proxy for changing canopy openness. Relative decline in Ulmus varied in magnitude between sites (67–94%) and there was also notable spatial variation in actual Ulmus abundance both pre- and post-decline. The pre-decline abundance of Ulmus varied from ~ 2 to 18% between our sites across the British Isles. This roughly accords with ~ 2–22% across the Irish sites studied by Kearney and Gearey (2024), despite our data being RPPE-corrected rather than raw pollen percentages. Post-decline, Kearney and Gearey’s sites ranged from “minimal values” to ~ 5%, with 1–2% being typical; this again agrees with our findings despite differences in data. These comparisons are not confounded by site overlap as only one site (Lough Aisling) was common to both studies.
Partial rebound was identified at three study sites (30%): Ulmus abundance showed a marked uptick relative to abundance immediately post-decline but remained substantially and significantly lower than pre-decline levels. Similar partial recovery has been seen in other cases where an important tree species has been lost, including Fraxinus woodland affected by Ash Dieback (Heinze et al. 2017). This could be driven by natural (and likely heritable) disease resistance, which allows some persistence of the species (Coker et al. 2019), followed by a negative correlation between disease susceptibility and reproductive success facilitating partial recovery as trees with natural immunity have higher reproductive fitness (Semizer-Cuming et al. 2019).
Reduction in a prevalent tree species in woodland will axiomatically change community composition (Needham et al. 2016). Primary research in the USA by Fajvan and Wood (1996) found that loss of mature Quercus resulted in increases in subdominant Acer species, while high mortality in Ulmus due to contemporary Dutch Elm Disease in Sweden led to increases in competitively inferior Quercus and Fagus (Brunet et al. 2014), which, quite literally, filled the gap. Both these situations accord with the competitor release hypothesis (Crawley 1997). As expected, therefore, there were significant effects of Elm Decline on richness, evenness and within-site turnover in the palaeoecological analogue examined here. Most importantly, the post-decline period was associated with greater unevenness in arboreal taxa and higher rates of arboreal turnover when compared to pre-decline; there was also a substantial and significant increase in shrub richness post-decline relative to pre-decline.
Change in arboreal taxa
Evidence for higher within-site turnover in arboreal taxa both during and post-decline compared to pre-decline is unsurprising. This is driven not only by a decrease in Ulmus but also by increases in other arboreal taxa that were, filling the gaps. As such, this metric is essentially recording the magnitude of the ecological response to biotic change at community level, in a similar way to that which has been observed for climate change, both in primary ecological research (Hillebrand et al. 2010; Ulrich et al. 2014; Gibson‐Reinemer et al. 2015; James et al. 2017) and when using palaeoecological analogues (Webb and Goodenough 2021). However, change in the arboreal sub-community to become less even after decline in a key tree species at first seems counter-intuitive, since this might be expected to rebalance the community and promote greater evenness (Yang et al. 2018). However, this is likely due to canopy gaps at most sites being filled by whatever other arboreal species is/are next most abundant in that community rather than being randomly selected from those present, especially if sub-dominant species are pioneer trees within secondary succession (Fajvan and Wood 1996) or if there is one highly-dominant species where decline in Ulmus would likely make the other taxon more dominant as the probability of that species filling the gap would be high. Together, these processes could act to make communities more strongly skewed than previously (Mitchell et al. 2016). This situation has been observed in models of lowland woodland in the UK in relation to simulated Ash Dieback (Needham et al. 2016), and observed in primary studies on loss of dominant trees in the USA and mainland Europe (Fajvan and Wood 1996; Brunet et al. 2014). It was also notable in our data that arboreal richness did not increase, suggesting that gaps were filled by taxa already present in the community, which also supports this explanation. Our findings thus indicate the potential of the palaeoecological record for researching this phenomenon over timespans that exceed those typically possible (and fundable) in primary ecological research, without the assumptions inherent in predictive modelling.
Investigating the effects of Ulmus abundance (driven by long-term Ulmus decline) and short-term change in Ulmus on arboreal taxa in more detail produced some consistent patterns across the majority of sites: (1) evenness was significantly higher when Ulmus was more abundant; and (2) turnover was significantly lower when Ulmus was more abundant and significantly higher when recent Ulmus change was negative. These patterns reinforce the overarching conclusions above and highlight the generality of these findings across most sites. They also suggest that turnover was driven both by long- and short-term change in Ulmus, although the former was more important. When considering specific taxa, change in the abundance of Quercus, Taxus, and Tilia was recorded at different sites. Change in Quercus and Taxus was noted in some of the Irish sites studied by Kearney and Gearey (2024), although that research only documented arboreal taxa that increased contemporaneously with, or immediately after, the Elm Decline rather than change in either direction. This methodological difference, and smaller study area, might explain why there was a more consistent palaeoecological signal in response to canopy changes associated with the Elm Decline in Ireland than the strongly site-specific patterns across the British Isles presented here. Similar idiosyncratic site-specific differences have been found previously when considering the effects of the same broadscale changes, for example, patterns of climate variability 6000–9000 years ago (Seddon et al. 2015) and in response to the abrupt climate perturbation 8200 years ago (Webb and Goodenough 2021). This also agrees with Williams et al. (2011) who found that even when there was widespread directional vegetation change, this was mediated by local biotic and abiotic processes and by stochastic processes such as disturbance events.
A decrease in the woody climbing taxon Hedera was noted at 50% of sites post-decline when compared with abundance of this same species pre-decline, with recovery subsequently noted at two of the three sites showing Ulmus rebound. This change is unlikely to be linked directly to loss of Ulmus as a host species as Hedera has limited host preference, however, as the majority of Hedera biomass is on older trees (Castagneri et al. 2013), loss of mature (Ulmus) and replacement with younger trees (any species) could have an effect. Alternatively, or in combination, it is possible that loss of Hedera post-decline, and subsequent rebound, links to the creation and refilling of canopy gaps. The effect of canopy openness has been noted in modelling of Hedera between different woodlands (van Couwenberghe et al. 2011), and is likely driven by Hedera preferring deep or intermediate shade (Ellenberg 1991). Hedera is also known to decrease at more exposed sites, especially if that increases vulnerability to colder conditions (Metcalfe 2005; Birks 2011).
Change in shrub taxa
The increase in shrub richness and an increase in abundance of important shrub taxa (Calluna, Ilex and Corylus) at seven of 10 sites with documented Elm Decline in our dataset is interesting since it hints at a likely structural change in woodland habitat in strata other than the canopy. This might result from these species, many of which are woody pioneers within secondary successional processes, increasing in response to greater sunlight and physical space caused by Ulmus loss. This was not expected, especially given research by Nagel et al., (2019) in coniferous woodland when the loss of Abies from the canopy led to lower shrub richness. However, it does reflect the descriptive palaeoecological findings of Kearney and Gearey (2024) ~ 40% sites were noted to have an increase in shrub species, primarily Corylus—it is recognised that, on occasion, Corylus can be tall and single-stemmed; classification as a shrub is based likely place in woodland strata at the majority of sites.
If a similar increase in the shrub layer abundance and richness in deciduous woodlands to that seen during the Elm Decline occurs in future scenarios involving canopy gaps as the result of systematic decline in, or loss of, a specific tree species due to non-native pests (Fajvan and Wood 1996; Francis and Elmes 2017), disease (Enderle et al. 2019), and climate change (Iverson & Prasad 2001; Mette et al. 2013), this is likely to alter the composition of faunal guilds in complex ways. For example, an increase in understory would likely change the avian guild, which is strongly determined by habitat (Amar et al. 2006), benefiting some specialist taxa, such as Luscinia megarhynchos (nightingale) and Poecile montanus (willow tit) (Hewson et al. 2007; Dyda et al. 2009) but decreasing suitability for aerial insectivores such as Ficedula hypolucea (pied flycatcher) (Vanhinsbergh et al. 2003). As all three of these species are currently declining rapidly (Amar et al. 2006; Gregory et al. 2007), structural changes in woodlands related to loss of specific tree species would have differential impacts on fauna. This is a level of complexity that has not been fully considered in work, for example, on the impacts of Ash Dieback, which has focused more on changes to Fraxinus-associated species and deadwood abundance.
Change in herb taxa
The systematic decline in, or loss of, a specific tree species within woodland will, at least temporarily, create canopy gaps and thus increase sunlight reaching the forest floor. Because this would be to the advantage of light-loving taxa and the disadvantage shade-loving taxa, it was expected that the past Elm Decline—considered in this research as a palaeoecological analogue for tree loss in contemporary settings—would increase turnover in the herb layer. This has been found in primary ecological research (Aszalós et al. 2023) and in predictive work into the likely effects of contemporary Ash Dieback on woodland flora (Needham et al. 2016). It was also expected that such turnover might have altered richness and evenness metrics, although it is noted that turnover can occur in the absence of change in richness and evenness if taxa changes remain balanced (Santini et al. 2017; Webb and Goodenough 2021). Somewhat surprisingly, therefore, we found no significant differences in herb taxa metrics in our study. This might be because the effect of the Elm Decline on herbs, even using relative high-level community metrics of richness, evenness and turnover, was highly site-specific. This is suggested by the split in the number of sites with positive and negative relationships in the influence of Ulmus abundance and Ulmus change on herb richness, evenness and turnover. Alternatively, the influence of Elm Decline on annual herbs could have been so rapid and short-lived that it is not well reflected in the palaeoecological record. It is also possible that taxonomic resolution played a role if turnover involved species in the same genus (or, in some cases, genera in the same family), which might not be detected given the level to which some taxa can be identified using pollen (Moore et al. 1991; Birks 1993, 2007).
The lack of overall patterns in herb richness, evenness and within-site turnover should not be considered synonymous with there being no effect on herb taxa. Indeed, considering taxa-level changes demonstrates that findings are very site-specific, but there is a general trend in the disturbance-loving Plantago lanceolata, which increased at 70% of our study sites and 76% of the sites documented by Kearney and Gearey (2024). It has previously been postulated (e.g., Whitehouse et al. 2014) that the Elm Decline was linked to the contemporaneous onset of Neolithic agriculture rather than disease, with Plantago increases at this time being evidence of this. However, Kearney and Gearey (2024) suggested that while the two events might share similar timing, this is correlation rather than causality. For the purposes of our research, the causality of the Elm Decline and subsequent interplay between human activity and natural disturbances in woodland is largely irrelevant in a study of the biotic impacts, especially as contemporary changes in woodland, for example due to Ash Dieback, will be similarly complex due to multiple co-occurring natural and anthropogenic stressors.
Conclusions and relevance to current woodland challenges
In this paper, we have built on the extensive literature on the abrupt, widespread, and often permanent, Elm Decline that occurred throughout much of northwestern Europe ~ 5800 years ago leading to an opening of the tree canopy. Whereas most research to date has focused on spatiotemporal synchrony and causal mechanisms, or has considered the effects of Elm Decline within single-site reconstructions, our aim was to quantify cascade effects of Ulmus canopy loss throughout the ecological community. The raison d'etre for the research was thus not about past Elm Decline per se, but rather use of this as a palaeoecological analogue through which to consider possible consequences of current and future declines in specific tree species on the wider vegetation community (and thus ecosystem services).
It is important to highlight that because of the rigorous site selection criteria used here, the number of sites that could be included in this study was lower than would be ideal in terms of ensuring general applicability of findings to similar contexts in other parts of the world and should, as with any research on relatively few sites, be considered as indicative rather than definitive. However, the fact that the phytogeography of Ulmus is unchanged throughout the last 9500 years (Parker et al. 2002) means that using community responses to change Ulmus abundance in the past (at a time of natural and human change) as a proxy for contemporary change (also at a time of natural and human change) is well founded. Moreover, this approach allows consideration of change over much longer time periods than would be possible (or fundable) in primary ecological work and avoids the assumptions and uncertainty implicit in predictive modelling.
The key findings and caveats are outlined below:
-
1.
There were comparatively few generalized findings, even in the relatively high-level community metrics of richness, evenness and within-site turnover. Trends in the abundance of specific taxa contemporaneously with, or immediately after, the Elm Decline were especially spatially idiosyncratic and might change in other locations dependent upon spatiotemporal variables and local conditions.
-
2.
Many, but not all, of the cascade effects in the vegetation community could be linked back to change in ecosystem structure, especially canopy gaps and resultant impacts on understory taxa.
-
3.
Decline in, or loss of, a tree species decreased arboreal evenness rather than increased it, likely because recruitment of arboreal taxa to “fill the gap” was driven by relative abundance of dominant or subdominant taxa.
-
4.
There were substantial and significant decreases in woody climbing taxa, which are not often a focus in primary ecological surveys (Castagneri et al. 2013).
-
5.
Shrub richness and density increased. Mirroring of this finding in the future would have substantial implications on non-vegetative species including invertebrates, birds and mammals, thereby further extending the cascade effects it has been possible to document here. However, high browser density in contemporary ecosystems might reduce scrub invasion (and arboreal regeneration) (Dolman 2010).
-
6.
Vegetation responses in contemporary situations would be complicated by human influence, including silviculture, management and conservation. Indirect effects, such as an increase in non-native species, could also have an effect especially where these species have invasive tendencies or are pioneer species (Needham et al. 2016).
References
Amar A, Fitzpatrick P, Smith KW, Lindsell JA (2006) What’s happening to our Woodland Birds? BTO Research report, 169
Andersen ST (1970) The relative pollen productivity and pollen representation of North European trees, and correction factors for tree pollen spectra. Determined by surface pollen analyses from forests. Raekke 96:1–99. https://doi.org/10.34194/raekke2.v96.6887
Andersen ST, Rasmussen KL (1993) Radiocarbon wiggle-dating of elm declines in northwest Denmark and their significance. Veg Hist Archaeobot 2(3):125–135. https://doi.org/10.1007/BF00198583
Aszalós R, Kovács B, Tinya F, Németh C, Horváth CV, Ódor P (2023) Canopy gaps are less susceptible to disturbance-related and invasive herbs than clear-cuts: temporal changes in the understorey after experimental silvicultural treatments. For Ecol Manag 549:121438. https://doi.org/10.1016/j.foreco.2023.121438
Barak RS, Hipp AL, Cavender-Bares J, Pearse WD, Hotchkiss SC, Lynch EA, Callaway JC, Calcote R, Larkin DJ (2016) Taking the long view: integrating recorded, archeological, paleoecological, and evolutionary data into ecological restoration. Int J Plant Sci 177(1):90–102. https://doi.org/10.1086/683394
Bennett KD (1983) Devensian late-glacial and flandrian vegetational history at hockham mere, Norfolk, England. New Phytol 95(3):457–487. https://doi.org/10.1111/j.1469-8137.1983.tb03512.x
Bennett KD (1988) Holocene pollen stratigraphy of central East Anglia, England, and comparison of pollen zones across the British Isles. New Phytol 109(2):237–253. https://doi.org/10.1111/j.1469-8137.1988.tb03712.x
Birks HJB (1993) Quaternary palaeoecology and vegetation science—current contributions and possible future developments. Rev Palaeobot Palynol 79(1–2):153–177. https://doi.org/10.1016/0034-6667(93)90045-V
Birks HJB (2007) Estimating the amount of compositional change in late-quaternary pollen-stratigraphical data. Veg Hist Archaeobot 16(2):197–202. https://doi.org/10.1007/s00334-006-0079-1
Birks HJB (2011) Strengths and weaknesses of quantitative climate reconstructions based on late-quaternary biological proxies. Open Ecol J 3(1):68–110. https://doi.org/10.2174/1874213001003020068
Birks HJB, Line JM (1992) The use of rarefaction analysis for estimating palynological richness from quaternary pollen-analytical data. Holocene 2(1):1–10. https://doi.org/10.1177/095968369200200101
Blumgart D, Botham MS, Menéndez R, Bell JR (2022) Moth declines are most severe in broadleaf woodlands despite a net gain in habitat availability. Insect Conserv Divers 15(5):496–509. https://doi.org/10.1111/icad.12578
Bowler DE, Cunningham CA, Beale CM, Emberson L, Hill JK, Hunt M, Maskell L, Outhwaite CL, White PCL, Pocock MJO (2023) Idiosyncratic trends of woodland invertebrate biodiversity in Britain over 45 years. Insect Conserv Divers 16(6):776–789. https://doi.org/10.1111/icad.12685
Bradshaw RHW (1981) Modern pollen-representation factors for woods in south-east England. J Ecol 69(1):45. https://doi.org/10.2307/2259815
Bray JR, Curtis JT (1957) An ordination of the upland forest communities of southern Wisconsin. Ecol Monogr 27(4):325–349. https://doi.org/10.2307/1942268
Broström A, Nielsen AB, Gaillard MJ, Hjelle K, Mazier F, Binney H, Bunting J, Fyfe R, Meltsov V, Poska A, Räsänen S, Soepboer W, von Stedingk H, Suutari H, Sugita S (2008) Pollen productivity estimates of key European plant taxa for quantitative reconstruction of past vegetation: a review. Veg Hist Archaeobot 17(5):461–478. https://doi.org/10.1007/s00334-008-0148-8
Browne PR (1986) Pollen stratigraphy in the Nephin Begs Co. Mayo. Unpublished PhD thesis. Trinity College, Dublin
Brudvig LA, Damschen EI (2011) Land-use history, historical connectivity, and land management interact to determine longleaf pine woodland understory richness and composition. Ecography 34(2):257–266. https://doi.org/10.1111/j.1600-0587.2010.06381.x
Brunet J, Bukina Y, Hedwall PO, Holmström E, von Oheimb G (2014) Pathogen induced disturbance and succession in temperate forests: evidence from a 100-year data set in southern Sweden. Basic Appl Ecol 15(2):114–121. https://doi.org/10.1016/j.baae.2014.02.002
Bullock C, Hawe J, Little D (2014) Realising the ecosystem-service value of native woodland in Ireland. New Zealand J for Sci 44(Suppl 1):S4. https://doi.org/10.1186/1179-5395-44-s1-s4
Bunting MJ (2002) Detecting woodland remnants in cultural landscapes: modern pollen deposition around small woodlands in northwest Scotland. Holocene 12(3):291–301. https://doi.org/10.1191/0959683602hl545rp
Bunting MJ, Farrell M (2022) Do local habitat conditions affect estimates of relative pollen productivity and source area in heathlands? Front Ecol Evol 10:787345. https://doi.org/10.3389/fevo.2022.787345
Calcote R (2003) Mid-Holocene climate and the hemlock decline: the range limit of Tsuga canadensis in the western Great Lakes region, USA. Holocene 13(2):215–224. https://doi.org/10.1191/0959683603hl608rp
Castagneri D, Garbarino M, Nola P (2013) Host preference and growth patterns of ivy (Hedera helix L.) in a temperate alluvial forest. Plant Ecol 214(1):1–9. https://doi.org/10.1007/s11258-012-0130-5
Chambers FM, Mauquoy D, Gent A, Pearson F, Daniell JRG, Jones PS (2007) Palaeoecology of degraded blanket mire in South Wales: data to inform conservation management. Biol Conserv 137(2):197–209. https://doi.org/10.1016/j.biocon.2007.02.002
Coker TLR, Rozsypálek J, Edwards A, Harwood TP, Butfoy L, Buggs RJA (2019) Estimating mortality rates of European ash (Fraxinus excelsior) under the ash dieback (Hymenoscyphus fraxineus) epidemic. Plants People Planet 1(1):48–58. https://doi.org/10.1002/ppp3.11
Connolly A (1999) The Palaeoecology of Clara Bog. Unpublished PhD thesis. Trinity College, Dublin
Crawley MJ (1997) Plant ecology. Blackwell Scientific
Davies S, Patenaude G, Snowdon P (2017) A new approach to assessing the risk to woodland from pest and diseases. Forestry (Lond) 90(3):319–331. https://doi.org/10.1093/forestry/cpx001
Digerfeldt G (1997) Reconstruction of Holocene lake-level changes in Lake Kalvsjön, southern Sweden, with a contribution to the local palaeohydrology at the Elm Decline. Veg Hist Archaeobot 6(1):9–14. https://doi.org/10.1007/BF01145881
Dolman P, Fuller R, Gill R, Hooton D, Tabor R (2010) Escalating; ecological impacts: of Deer in lowland woodland. Br Wildl 21(4):242–254
Dyda J, Symes N, Lamacraft D (2009) Woodland management for birds: a guide to managing woodland for priority birds in Wales. The RSPB and Forestry Commission, Wales
Ellenberg H (1991) Zeigerwerte von pflanzen in Mitteleuropa. Scripta Geobotanica 18:1–248
Enderle R, Stenlid J, Vasaitis R (2019) An overview of ash (Fraxinus spp.) and the ash dieback disease in Europe. CABI Rev. https://doi.org/10.1079/pavsnnr201914025
Erfmeier A, Haldan KL, Beckmann LM, Behrens M, Rotert J, Schrautzer J (2019) Ash dieback and its impact in near-natural forest remnants—a plant community-based inventory. Front Plant Sci 10:658. https://doi.org/10.3389/fpls.2019.00658
Fajvan MA, Wood JM (1996) Stand structure and development after Gypsy moth defoliation in the Appalachian Plateau. For Ecol Manag 89(1–3):79–88. https://doi.org/10.1016/S0378-1127(96)03865-0
Forestry Commission (2022) Forestry Commission Chair calls for change in approach to tree planting as last year’s winter storm damage revealed-GOV.UK. Available at: https://www.gov.uk/government/news/forestry-commission-chair-calls-for-change-in-approach-to-tree-planting-as-last-years-winter-storm-damage-revealed#:~:text=Chair%20of%20the%20Forestry%20Commission%2C%20Sir%20William%20Worsley%20said%3A&text=The%20woodlands%20of%20the%20future,age%20profiles%20across%20the%20country. Accessed 10 June 2024
Fox R, Brereton TM, Asher J, August TA, Botham MS, Bourn NAD, Cruickshanks KL, Bulman CR, Ellis S, Harrower CA, Middlebrook I, Noble DG, Powney GD, Randle Z, Warren MS, Roy DB (2015) The state of the UK’s Butterflies. Wareham
Francis A, Elmes M (2017) Assessing the impact of asian longhorned beetle in Worcester, MA: thermal effects, community responses, and future vulnerability. Clark University
Freer-Smith PH, Webber JF (2017) Tree pests and diseases: the threat to biodiversity and the delivery of ecosystem services. Biodivers Conserv 26(13):3167–3181. https://doi.org/10.1007/s10531-015-1019-0
Fuller RJ, Gill RMA (2001) Ecological impacts of increasing numbers of Deer in British woodland. Forestry (Lond) 74(3):193–199. https://doi.org/10.1093/forestry/74.3.193
Fyfe RM, Woodbridge J (2012) Differences in time and space in vegetation patterning: analysis of pollen data from Dartmoor, UK. Landsc Ecol 27(5):745–760. https://doi.org/10.1007/s10980-012-9726-3
Gandouin E, Ponel P, Andrieu-Ponel V, Guiter F, de Beaulieu JL, Djamali M, Franquet E, Van Vliet-Lanoë B, Alvitre M, Meurisse M, Brocandel M, Brulhet J (2009) 10, 000 years of vegetation history of the Aa palaeoestuary, St-Omer Basin, northern France. Rev Palaeobot Palynol 156(3–4):307–318. https://doi.org/10.1016/j.revpalbo.2009.03.008
García-Nieto AP, García-Llorente M, Iniesta-Arandia I, Martín-López B (2013) Mapping forest ecosystem services: from providing units to beneficiaries. Ecosyst Serv 4:126–138. https://doi.org/10.1016/j.ecoser.2013.03.003
Gibson-Reinemer DK, Sheldon KS, Rahel FJ (2015) Climate change creates rapid species turnover in montane communities. Ecol Evol 5(12):2340–2347. https://doi.org/10.1002/ece3.1518
Giesecke T, Brewer S, Finsinger W, Leydet M, Bradshaw RHW (2017) Patterns and dynamics of European vegetation change over the last 15,000 years. J Biogeogr 44:1441–1456
Gimona A, Poggio L, Brown I, Castellazzi M (2012) Woodland networks in a changing climate: threats from land use change. Biol Conserv 149(1):93–102. https://doi.org/10.1016/j.biocon.2012.01.060
Goodenough AE, Hart AG (2023) Applied ecology: monitoring. Manag Conserv Monit. https://doi.org/10.1093/hesc/9780198723288.001.0001
Goodenough AE, Webb JC (2022) Learning from the past: opportunities for advancing ecological research and practice using palaeoecological data. Oecologia 199(2):275–287. https://doi.org/10.1007/s00442-022-05190-z
Grant MJ, Hughes PDM, Barber KE (2009) Early to mid-Holocene vegetation-fire interactions and responses to climatic change at Cranes Moor, New Forest. In: Briant RM, Bates MR, Hosfield RT, Wenban-Smith FF (eds) The quaternary of the Solent Basin and West Sussex: a field guide. Quaternary Research Association, London, pp 198–214
Grant MJ, Waller MP, Groves JA (2011) The Tilia decline: vegetation change in lowland Britain during the mid and late Holocene. Quat Sci Rev 30(3–4):394–408. https://doi.org/10.1016/j.quascirev.2010.11.022
Grant MJ, Hughes PDM, Barber KE (2014) Climatic influence upon early to mid-Holocene fire regimes within temperate woodlands: a multi-proxy reconstruction from the New Forest, southern England. J Quatern Sci 29(2):175–188. https://doi.org/10.1002/jqs.2692
Green S, Cooke DEL, Dunn M, Barwell L, Purse B, Chapman DS, Valatin G, Schlenzig A, Barbrook J, Pettitt T, Price C, Pérez-Sierra A, Frederickson-Matika D, Pritchard L, Thorpe P, Cock PJA, Randall E, Keillor B, Marzano M (2021) PHYTO-THREATS: addressing threats to UK forests and woodlands from Phytophthora; identifying risks of spread in trade and methods for mitigation. Forests 12(12):1617. https://doi.org/10.3390/f12121617
Gregory RD, Vorisek P, Van Strien A, Gmelig Meyling AW, Jiguet F, Fornasari L, Reif J, Chylarecki P, Burfield IJ (2007) Population trends of widespread woodland birds in Europe. Ibis 149(s2):78–97. https://doi.org/10.1111/j.1474-919x.2007.00698.x
Hammer Ø, Harper DAT (2001) Past: paleontological statistics software package for education and data analysis. Palaeontol Electron 4:1
Hammer Ø (2022) PAST: PAleontological STatistics. Version 4.12. Reference manual. Available at: https://www.nhm.uio.no/english/research/resources/past/downloads/past4manual.,pdf. Accessed 10 June 2024
Heinze B, Tiefenbacher H, Litschauer R, Kirisits T (2017) Ash dieback in Austria—history, current situation and outlook. In: Vasaitis R, Enderle R (eds) Dieback of European Ash (Fraxinus spp.): consequences and guidelines for sustainable management. SLU Service/Repro, Uppsala, pp 33–52
Hewson CM, Amar A, Lindsell JA, Thewlis RM, Butler S, Smith K, Fuller RJ (2007) Recent changes in bird populations in British broadleaved woodland. Ibis 149(s2):14–28. https://doi.org/10.1111/j.1474-919x.2007.00745.x
Hewson CM, Austin GE, Gough SJ, Fuller RJ (2011) Species-specific responses of woodland birds to stand-level habitat characteristics: the dual importance of forest structure and floristics. For Ecol Manag 261(7):1224–1240. https://doi.org/10.1016/j.foreco.2011.01.001
Hillebrand H, Soininen J, Snoeijs P (2010) Warming leads to higher species turnover in a coastal ecosystem. Glob Change Biol 16(4):1181–1193. https://doi.org/10.1111/j.1365-2486.2009.02045.x
Hinsley SA, Hill RA, Fuller RJ, Bellamy PE, Rothery P (2009) Bird species distributions across woodland canopy structure gradients. Commun Ecol 10(1):99–110. https://doi.org/10.1556/ComEc.10.2009.1.12
Hinsley SA, Pocock MJO (2014) Ash dieback: long-term monitoring of impacts on biodiversity. JNCC Report
Holland GJ, Bennett AF (2007) Occurrence of small mammals in a fragmented landscape: the role of vegetation heterogeneity. Wildl Res 34(5):387. https://doi.org/10.1071/wr07061
Homburg K, Drees C, Boutaud E, Nolte D, Schuett W, Zumstein P, von Ruschkowski E, Assmann T (2019) Where have all the beetles gone? Long-term study reveals carabid species decline in a nature reserve in Northern Germany. Insect Conserv Diversity 12(4):268–277. https://doi.org/10.1111/icad.12348
Hultberg T, Sandström J, Felton A, Öhman K, Rönnberg J, Witzell J, Cleary M (2020) Ash dieback risks an extinction cascade. Biol Conserv 244:108516. https://doi.org/10.1016/j.biocon.2020.108516
Iverson LR, Prasad AM (2001) Potential changes in tree species richness and forest community types following climate change. Ecosystems 4(3):186–199. https://doi.org/10.1007/s10021-001-0003-6
Jackson ST (1994) Pollen and spores in quaternary lake sediments as sensors of vegetation composition: theoretical models and empirical evidence. In: Sedimentation of organic particles. Cambridge University Press, pp 253–286. https://doi.org/10.1017/cbo9780511524875.015
James CS, Reside AE, VanDerWal J, Pearson RG, Burrows D, Capon SJ, Harwood TD, Hodgson L, Waltham NJ (2017) Sink or swim? Potential for high faunal turnover in Australian rivers under climate change. J Biogeogr 44(3):489–501. https://doi.org/10.1111/jbi.12926
Jamrichová E, Hédl R, Kolář J, Tóth P, Bobek P, Hajnalová M, Procházka J, Kadlec J, Szabó P (2017) Human impact on open temperate woodlands during the middle Holocene in Central Europe. Rev Palaeobot Palynol 245:55–68. https://doi.org/10.1016/j.revpalbo.2017.06.002
Jukes MR, Peace AJ, Ferris R (2001) Carabid beetle communities associated with coniferous plantations in Britain: the influence of site, ground vegetation and stand structure. For Ecol Manag 148(1–3):271–286. https://doi.org/10.1016/S0378-1127(00)00530-2
Kaiser K, Theuerkauf M, Hieke F (2023) Holocene forest and land-use history of the Erzgebirge, central Europe: a review of palynological data. E&G Quat Sci J 72(2):127–161. https://doi.org/10.5194/egqsj-72-127-2023
Kearney K, Gearey BR (2024) The elm decline is dead! long live declines in elm: revisiting the chronology of the elm decline in Ireland and its association with the Mesolithic/neolithic transition. Environ Archaeol 29(1):6–19. https://doi.org/10.1080/14614103.2020.1721694
Kermavnar J, Kutnar L, Pintar AM (2023) Ecological factors affecting the recent Picea abies decline in Slovenia: the importance of bedrock type and forest naturalness. Iforest 16(2):105–115. https://doi.org/10.3832/ifor4168-016
Kirby K, Peterken GF (1996) Natural woodland: ecology and conservation in northern temperate regions. J Ecol 84(5):790. https://doi.org/10.2307/2261344
Legendre P, Legendre L (1998) Numerical ecology. Elsevier, Amsterdam
Li Q (2018) Spatial variability and long-term change in pollen diversity in Nam Co catchment (central Tibetan Plateau): implications for alpine vegetation restoration from a paleoecological perspective. Sci China Earth Sci 61(3):270–284. https://doi.org/10.1007/s11430-017-9133-0
Li FR, Gaillard MJ, Sugita S, Mazier F, Xu QH, Zhou ZZ, Zhang YY, Li YC, Laffly D (2017) Relative pollen productivity estimates for major plant taxa of cultural landscapes in central Eastern China. Veg Hist Archaeobot 26(6):587–605. https://doi.org/10.1007/s00334-017-0636-9
Loo JA (2009) Ecological impacts of non-indigenous invasive fungi as forest pathogens. In: Langor DW, Sweeney J (eds) Ecological impacts of non-native invertebrates and fungi on terrestrial ecosystems. Springer, Netherlands, pp 81–96. https://doi.org/10.1007/978-1-4020-9680-8_6
Lorenz K, Lal R (2010) Carbon sequestration in forest ecosystems. Springer, Netherlands. https://doi.org/10.1007/978-90-481-3266-9
Macklin MG, Bonsall C, Davies FM, Robinson MR (2000) Human-environment interactions during the Holocene: new data and interpretations from the Oban area, Argyll, Scotland. Holocene 10(1):109–121. https://doi.org/10.1191/095968300671508292
Manzano S, Julier ACM, Dirk CJ, Razafimanantsoa AHI, Samuels I, Petersen H, Gell P, Hoffman MT, Gillson L (2020) Using the past to manage the future: the role of palaeoecological and long-term data in ecological restoration. Restor Ecol 28:1335–1342. https://doi.org/10.1111/rec.13285
Metcalfe DJ (2005) Hedera helix L. J Ecol 93(3):632–648. https://doi.org/10.1111/j.1365-2745.2005.01021.x
Mette T, Dolos K, Meinardus C, Bräuning A, Reineking B, Blaschke M, Pretzsch H, Beierkuhnlein C, Gohlke A, Wellstein C (2013) Climatic turning point for beech and oak under climate change in Central Europe. Ecosphere 4(12):1–19. https://doi.org/10.1890/es13-00115.1
Mitchell RJ, Beaton JK, Bellamy PE, Broome A, Chetcuti J, Eaton S, Ellis CJ, Gimona A, Harmer R, Hester AJ, Hewison RL, Hodgetts NG, Iason GR, Kerr G, Littlewood NA, Newey S, Potts JM, Pozsgai G, Ray D, Sim DA, Stockan JA, Taylor AFS, Woodward S (2014) Ash dieback in the UK: a review of the ecological and conservation implications and potential management options. Biol Conserv 175:95–109. https://doi.org/10.1016/j.biocon.2014.04.019
Mitchell RJ, Hewison RL, Hester AJ, Broome A, Kirby KJ (2016) Potential impacts of the loss of Fraxinus excelsior (Oleaceae) due to ash dieback on woodland vegetation in Great Britain. New J Bot 6(1):2–15. https://doi.org/10.1080/20423489.2016.1171454
Moore P, Webb J, Collinson M (1991) Pollen analysis. Blackwell Scientific, Oxford
Nagel TA, Iacopetti G, Javornik J, Rozman A, De Frenne P, Selvi F, Verheyen K (2019) Cascading effects of canopy mortality drive long-term changes in understorey diversity in temperate old-growth forests of Europe. J Veg Sci 30(5):905–916. https://doi.org/10.1111/jvs.12767
Needham J, Merow C, Butt N, Malhi Y, Marthews TR, Morecroft M, McMahon SM (2016) Forest community response to invasive pathogens: the case of ash dieback in a British woodland. J Ecol 104(2):315–330. https://doi.org/10.1111/1365-2745.12545
Ninan KN, Inoue M (2013) Valuing forest ecosystem services: what we know and what we don’t. Ecol Econ 93:137–149. https://doi.org/10.1016/j.ecolecon.2013.05.005
O’Connell M, Mitchell FJG, Readman PW, Doherty TJ, Murray DA (1987) Palaeoecological investigations towards the reconstruction of the post-glacial environment at Lough Doo, County Mayo, Ireland. J Quat Sci 2(2):149–164. https://doi.org/10.1002/jqs.3390020208
Out WA, Verhoeven K (2014) Late Mesolithic and early Neolithic human impact at Dutch wetland sites: the case study of Hardinxveld-Giessendam De Bruin. Veg Hist Archaeobot 23(1):41–56. https://doi.org/10.1007/s00334-013-0396-0
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li TJ, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg 88:105906. https://doi.org/10.1016/j.ijsu.2021.105906
Palo A, Ivask M, Liira J (2013) Biodiversity composition reflects the history of ancient semi-natural woodland and forest habitats—compilation of an indicator complex for restoration practice. Ecol Indic 34:336–344. https://doi.org/10.1016/j.ecolind.2013.05.020
Pardoe HS (2021) Identifying floristic diversity from the pollen record in open environments; considerations and limitations. Palaeogeogr Palaeoclimatol Palaeoecol 578:110560. https://doi.org/10.1016/j.palaeo.2021.110560
Parker AG, Goudie AS, Anderson DE, Robinson MA, Bonsall C (2002) A review of the mid-Holocene elm decline in the British Isles. Prog Phys Geogr Earth Environ 26(1):1–45. https://doi.org/10.1191/0309133302pp323ra
Parsons RW, Prentice IC (1981) Statistical approaches to R-values and the pollen—vegetation relationship. Rev Palaeobot Palynol 32(2–3):127–152. https://doi.org/10.1016/0034-6667(81)90001-4
Patacca M, Lindner M, Lucas-Borja ME, Cordonnier T, Fidej G, Gardiner B, Hauf Y, Jasinevičius G, Labonne S, Linkevičius E, Mahnken M, Milanovic S, Nabuurs G, Nagel TA, Nikinmaa L, Panyatov M, Bercak R, Seidl R, Ostrogović Sever MZ, Socha J, Thom D, Vuletic D, Zudin S, Schelhaas M (2023) Significant increase in natural disturbance impacts on European forests since 1950. Glob Change Biol 29:1359–1376. https://doi.org/10.1111/gcb.16531
Peros MC, Gajewski K (2008) Testing the reliability of pollen-based diversity estimates. J Paleolimnol 40(1):357–368. https://doi.org/10.1007/s10933-007-9166-2
Prentice IC, Webb T III (1986) Pollen percentages, tree abundances and the Fagerlind effect. J Quat Sci 1(1):35–43. https://doi.org/10.1002/jqs.3390010105
Rackham O (2008) Ancient woodlands: modern threats. New Phytol 180(3):571–586. https://doi.org/10.1111/j.1469-8137.2008.02579.x
Rackham O (2015) Woodlands. William Collins, London
Reimer PJ, Austin WEN, Bard E, Bayliss A, Blackwell PG, Bronk Ramsey C, Butzin M, Cheng H, Edwards RL, Friedrich M, Grootes PM, Guilderson TP, Hajdas I, Heaton TJ, Hogg AG, Hughen KA, Kromer B, Manning SW, Muscheler R, Palmer JG, Pearson C, van der Plicht J, Reimer RW, Richards DA, Scott EM, Southon JR, Turney CSM, Wacker L, Adolphi F, Büntgen U, Capano M, Fahrni SM, Fogtmann-Schulz A, Friedrich R, Köhler P, Kudsk S, Miyake F, Olsen J, Reinig F, Sakamoto M, Sookdeo A, Talamo S (2020) The IntCal20 Northern Hemisphere radiocarbon age calibration curve (0–55 cal kBP). Radiocarbon 62(4):725–757. https://doi.org/10.1017/rdc.2020.41
Revell N, Lashford C, Blackett M, Rubinato M (2021) Modelling the hydrological effects of woodland planting on infiltration and peak discharge using HEC-HMS. Water 13(21):3039. https://doi.org/10.3390/w13213039
Richer S, Gearey B (2018) From Rackham to REVEALS: reflections on palaeoecological approaches to woodland and trees. Environ Archaeol 23(3):286–297. https://doi.org/10.1080/14614103.2017.1283765
Robles-López S, Fernández Martín-Consuegra A, Pérez-Díaz S, Alba-Sánchez F, Broothaerts N, Abel-Schaad D, López-Sáez JA (2018) The dialectic between deciduous and coniferous forests in central Iberia: a palaeoenvironmental perspective during the late Holocene in the Gredos range. Quat Int 470:148–165. https://doi.org/10.1016/j.quaint.2017.05.012
Santini L, Belmaker J, Costello MJ, Pereira HM, Rossberg AG, Schipper AM, Ceaușu S, Dornelas M, Hilbers JP, Hortal J, Huijbregts MAJ, Navarro LM, Schiffers KH, Visconti P, Rondinini C (2017) Assessing the suitability of diversity metrics to detect biodiversity change. Biol Conserv 213:341–350. https://doi.org/10.1016/j.biocon.2016.08.024
Scopes ER, Goodwin CED, Al-Fulaij N, White I, Langton S, Walsh K, Broome A, McDonald RA (2023) Shifting baselines for species in chronic decline and assessment of conservation status. Are hazel dormice Muscardinus avellanarius endangered? Ecol Solut Evid 4(1):e12206. https://doi.org/10.1002/2688-8319.12206
Seddon AW, Macias-Fauria M, Willis KJ (2015) Climate and abrupt vegetation change in Northern Europe since the last deglaciation. Holocene 25(1):25–36. https://doi.org/10.1177/0959683614556383
Semizer-Cuming D, Finkeldey R, Nielsen LR, Kjær ED (2019) Negative correlation between ash dieback susceptibility and reproductive success: good news for European ash forests. Ann for Sci 76(1):16. https://doi.org/10.1007/s13595-019-0799-x
Shaw PJA (2003) Multivariate statistics for the environmental sciences. Hodder Arnold, London
Swallow KA, Wood MJ, Goodenough AE (2020) Relative contribution of ancient woodland indicator and non-indicator species to herb layer distinctiveness in ancient semi-natural, ancient replanted, and recent woodland. Appl Veg Sci 23(4):471–481. https://doi.org/10.1111/avsc.12501
Tew ER, Ambrose-Oji B, Beatty M, Büntgen U, Butterworth H, Clover G, Cook D, Dauksta D, Day W, Deakin J, Field A, Gardiner B, Harrop P, Healey JR, Heaton R, Hemery G, Hill L, Hughes O, Khaira-Creswell PK, Kirby K, Leitch A, MacKay J, McIlhiney R, Murphy B, Newton L, Norris D, Nugee R, Parker J, Petrokofsky G, Prosser A, Quine C, Randhawa G, Reid C, Richardson M, Ridley-Ellis DJ, Riley R, Roberts JE, Schaible R, Simpson LE, Spake R, Tubby I, Urquhart J, Wallace-Stephens F, Wilson JD, Sutherland WJ (2024) A horizon scan of issues affecting UK forest management within 50 years. Forestry (Lond) 97(3):349–362. https://doi.org/10.1093/forestry/cpad047
Tinsley HM, Smith RT (1974) Surface pollen studies across a woodland/heath transition and their application to the interpretation of pollen diagrams. New Phytol 73(3):547–565. https://doi.org/10.1111/j.1469-8137.1974.tb02132.x
Tudor O, Dennis RLH, Greatorex-Davies JN, Sparks TH (2004) Flower preferences of woodland butterflies in the UK: nectaring specialists are species of conservation concern. Biol Conserv 119(3):397–403. https://doi.org/10.1016/j.biocon.2004.01.002
Ulrich W, Soliveres S, Maestre FT, Gotelli NJ, Quero JL, Delgado-Baquerizo M, Bowker MA, Eldridge DJ, Ochoa V, Gozalo B, Valencia E, Berdugo M, Escolar C, García-Gómez M, Escudero A, Prina A, Alfonso G, Arredondo T, Bran D, Cabrera O, Cea AP, Chaieb M, Contreras J, Derak M, Espinosa CI, Florentino A, Gaitán J, Muro VG, Ghiloufi W, Gómez-González S, Gutiérrez JR, Hernández RM, Huber-Sannwald E, Jankju M, Mau RL, Hughes FM, Miriti M, Monerris J, Muchane M, Naseri K, Pucheta E, Ramírez-Collantes DA, Raveh E, Romão RL, Torres-Díaz C, Val J, Veiga JP, Wang DL, Yuan X, Zaady E (2014) Climate and soil attributes determine plant species turnover in global drylands. J Biogeogr 41(12):2307–2319. https://doi.org/10.1111/jbi.12377
Uroy L, Ernoult A, Alignier A, Mony C (2023) Unveiling the ghosts of landscapes past: changes in landscape connectivity over the last decades are still shaping current woodland plant assemblages. J Ecol 111(5):1063–1078. https://doi.org/10.1111/1365-2745.14079
Van Couwenberghe R, Collet C, Lacombe E, Gégout JC (2011) Abundance response of western European forest species along canopy openness and soil pH gradients. For Ecol Manag 262(8):1483–1490. https://doi.org/10.1016/j.foreco.2011.06.049
Vanhinsbergh D, Fuller RJ, Noble D (2003) A review of possible causes of recent changes in populations of Woodland birds in Britain. BTO Research Report No. 245
Webb JC, Goodenough AE (2021) Vegetation community changes in European woodlands amid a changing climate: a palaeoecological modelling perspective. Commun Ecol 22(3):319–330. https://doi.org/10.1007/s42974-021-00057-4
Whitehouse NJ, Schulting RJ, McClatchie M, Barratt P, McLaughlin TR, Bogaard A, Colledge S, Marchant R, Gaffrey J, Bunting MJ (2014) Neolithic agriculture on the European western frontier: the boom and bust of early farming in Ireland. J Archaeol Sci 51:181–205. https://doi.org/10.1016/j.jas.2013.08.009
Wieczorek M, Herzschuh U (2020) Compilation of relative pollen productivity (RPP) estimates and taxonomically harmonised RPP datasets for single continents and Northern Hemisphere extratropics. Earth Syst Sci Data 12(4):3515–3528. https://doi.org/10.5194/essd-12-3515-2020
Williams JW, Blois JL, Shuman BN (2011) Extrinsic and intrinsic forcing of abrupt ecological change: case studies from the late quaternary. J Ecol 99(3):664–677. https://doi.org/10.1111/j.1365-2745.2011.01810.x
Williams JW, Grimm EC, Blois JL, Charles DF, Davis EB, Goring SJ, Graham RW, Smith AJ, Anderson M, Arroyo-Cabrales J, Ashworth AC, Betancourt JL, Bills BW, Booth RK, Buckland PI, Curry BB, Giesecke T, Jackson ST, Latorre C, Nichols J, Purdum T, Roth RE, Stryker M, Takahara H (2018) The Neotoma Paleoecology Database, a multiproxy, international, community-curated data resource. Quat Res 89(1):156–177. https://doi.org/10.1017/qua.2017.105
Yang XX, Jiang WY, Yang SL, Kong ZC, Luo YL (2018) Changes in plant richness and evenness since Marine Isotope Stage 2 on the Chinese Loess Plateau. Palaeogeogr Palaeoclimatol Palaeoecol 509:137–143. https://doi.org/10.1016/j.palaeo.2017.02.021
Yu JJ, Berry P, Guillod BP, Hickler T (2021) Climate change impacts on the future of forests in Great Britain. Front Environ Sci 9:640530. https://doi.org/10.3389/fenvs.2021.640530
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Corresponding editor: Lei Yu
The online version is available at http://www.springerlink.com.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Webb, J., Goodenough, A.E. Applying palaeoecological analogues to contemporary challenges: community-level effects of canopy gaps caused by systematic decline of a prevalent tree species. J. For. Res. 35, 132 (2024). https://doi.org/10.1007/s11676-024-01781-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11676-024-01781-z