Abstract
Introduction
The majority of ovarian cancer (OC) patients receiving standard of care chemotherapy develop chemoresistance within 5 years. The tumor microenvironment (TME) is a dynamic and influential player in disease progression and therapeutic response. However, there is a lack of models that allow us to elucidate the compartmentalized nature of TME in a controllable, yet physiologically relevant manner and its critical role in modulating drug resistance.
Methods
We developed a 3D microvascularized multiniche tumor-on-a-chip formed by five chambers (central cancer chamber, flanked by two lateral stromal chambers and two external circulation chambers) to recapitulate OC-TME compartmentalization and study its influence on drug resistance. Stromal chambers included endothelial cells alone or cocultured with normal fibroblasts or cancer-associated fibroblasts (CAF).
Results
The tumor-on-a-chip recapitulated spatial TME compartmentalization including vessel-like structure, stromal-mediated extracellular matrix (ECM) remodeling, generation of oxygen gradients, and delayed drug diffusion/penetration from the circulation chamber towards the cancer chamber. The cancer chamber mimicked metastasis-like migration and increased drug resistance to carboplatin/paclitaxel treatment in the presence of CAF when compared to normal fibroblasts. CAF-mediated drug resistance was rescued by ECM targeted therapy. Critically, these results demonstrate that cellular crosstalk recreation and spatial organization through compartmentalization are essential to determining the effect of the compartmentalized OC-TME on drug resistance.
Conclusions
Our results present a functionally characterized microvascularized multiniche tumor-on-a-chip able to recapitulate TME compartmentalization influencing drug resistance. This technology holds the potential to guide the design of more effective and targeted therapeutic strategies to overcome chemoresistance in OC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ovarian cancer (OC) is a deadly disease representing the 5th leading cause of cancer-related death among women in developed countries [1]. Due to the lack of specific symptoms in the early stage of the disease, it is mostly diagnosed in the advanced metastatic stage with a 5-year survival rate lower than 15% [1, 2]. Most patients are initially responsive to the standard-of-care taxane/platinum-based therapy, however, more than 80% of patients develop chemotherapy resistance within 5 years leading to elevated mortality and poor prognosis [1,2,3]. In the intricate landscape of OC biology, the tumor microenvironment (TME) has emerged as a dynamic and influential player in disease progression and therapeutic response [4, 5]. The TME encompasses a complex network of cellular and non-cellular components, including fibroblasts, imperfect vascularization, spatial heterogeneity in the distribution of nutrients, oxygen, and signaling molecules, and extracellular matrix (ECM), which collectively create a specialized niche supporting tumor growth. Recent advancements in OC research underscore the critical role of TME in modulating drug resistance, shedding light on the need to understand its compartmentalized nature [6,7,8]. The cellular crosstalk between tumor cells, stromal cancer-associated fibroblasts (CAF) and endothelial cells (EC) in the TME interferes with the cancer cell behavior, progression, and drug response [9, 10]. CAF are the major stromal cellular component surrounding the tumor core that interfere in pathogenesis, progression, invasion, angiogenesis, ECM remodeling, and drug resistance in many cancers [9, 10]. CAF actively participate in ECM remodeling by increased secretion of collagens through transforming growth factor-β (TGF-β) signaling converting the ECM into a stiff physical barrier for drug penetration and generating oxygen gradients that result in hypoxic niches [9, 11,12,13,14]. Interestingly, hypoxia, one of the key hallmarks of OC, is reciprocally implicated in enhanced TGF-β production and CAF activation, increasing the ECM remodeling [15, 16] and also enhancing tumor progression, metastasis, drug resistance, and angiogenesis [12, 17]. The imperfect tumor vasculature generated in the TME influences cancer progression, metastasis, pathogenesis, and it also aggravates hypoxia and causes decreased drug delivery into the tumor, thus having a negative impact in OC patient outcomes [10]. Therefore, a better understanding of the key components of TME compartmentalization and its profound implications for drug resistance in OC will ultimately pave the way for the development of more effective and personalized cancer therapies improving the survival rate in OC patients [18, 19].
Unfortunately, 95% of new cancer chemotherapeutics fail in clinical trials due to a lack of adequate preclinical models to mimic accurately the TME ex vivo [20,21,22]. Animal models remain a key asset in preclinical drug screening assays as they can recapitulate relevant TME components, but they are expensive, time-consuming, have associated ethical problems, and do not always represent the human TME and its key compartments and components [23, 24]. In that regard, recently the U.S. Food and Drug Administration (FDA) passed a law that no longer requires animal testing before human drug trials [25]. The traditionally used 2D cultures are easy to use, high-throughput, and inexpensive, although they are not able to recapitulate the intricacies of the TME, where factors like spatial heterogeneity, nutrient and oxygen gradients, and dynamic cellular interactions play pivotal roles in shaping drug responses [23, 24]. Three-dimensional (3D) cocultures in hydrogels can solve some of these limitations and conserve cellular crosstalk, and gradients but usually cannot recreate the compartmentalization and dynamics of the TME [23, 24, 26]. Moreover, spheroids and organoids are also a widely used model that can partially recapitulate the TME, but there is no fluid flow control or controlled compartmentalization, and the cells inside the spheroids are difficult to image, there is heterogeneity between samples, and not all cell types can naturally form them [23, 24, 26]. Microfluidic systems have emerged as a transformative tool, offering unparalleled insights into the dynamic interplay between tumors and therapeutic agents. Microfluidic systems or tumor-on-a-chip have unique capabilities to study drug resistance by recreating physiological conditions, including fluid shear stress, nutrient and oxygen gradients, and tissue spatial organization and cellular crosstalk, promoting a more accurate representation of the TME [24, 27].
Here, we have developed a multicompartmentalized microvascularized tumor-on-a-chip as a novel preclinical model that recapitulates key components of the dynamic and compartmentalized OC-TME to further evaluate their critical role in modulating OC drug resistance. We have validated our model for generation of oxygen gradients recapitulating the hypoxic OC-TME, formation of vessel-like structures recreating the TME vasculature and mimicking of aberrant ECM remodeling, processes that were enhanced by the presence of CAF in the stromal compartment. Moreover, we have performed drug response studies with standard-of-care treatment and addressed the CAF-induced drug resistance in OC by targeting the TGF-β signaling implicated in ECM remodeling. This enabling technology offers a more biomimetic environment for studying drug resistance offering unparalleled insights into the dynamics and complex compartmentalization of the TME.
Material and Methods
Microdevice Fabrication
Compartmentalized tumor-on-a-chip was designed using KLayout 0.27.5 software with 5 chambers (2 mm × 4 mm) separated by trapezoidal posts with 100 µm gaps. The microdevice was manufactured using the SU-8 lithography technique by pouring polydimethylsiloxane (PDMS, Krayden Dow Sylgard 184 Silicone Elastomer Kit, NC9285739, FicherScientific) on SU8-2075 resin-covered wafer with UV-printed design and baking at 80 °C for 2 hours. The height of the device was 100 µm. The inlets and outlets in the stromal and cancer chambers were cut with 1.5 mm punchers and in the circulation chambers with 3 mm Miltex® Biopsy punchers (Ted Pella, Inc). The PDMS design was bonded to a 24 × 60 mm coverslip (2-541-037, Fisher Scientific) using 115 V plasma cleaner (PDC-001, Harrick Plasma) at 0.5 Torr for 2 min. The devices were sterilized by UV light exposure for 30 min and baked overnight at 80 °C to gain hydrophobicity.
Cell Culture
Ovarian cancer cell lines KURAMOCHI (RRID:CVCL_1345) and SKOV-3 (ATCC HTB-77), representing high-grade serous ovarian carcinoma (HGSC) and ovarian adenocarcinoma respectively, were cultured in specific media for ovarian cancer cells composed by DMEM (MT10013CV, Corning) and Ham’s F-12 (MT10080CV, Corning) media in 1:1 ratio supplemented by 10% (V/V) of fetal bovine serum (FBS, GibCo), 100 U/ml penicillin, and 100 mg/ml streptomycin (Corning CellGro). Human umbilical vein endothelial cells (HUVEC, ATCC CRL-1730) were cultured in F12K media (30-2004, ATCC) supplemented with 0.1 mg/ml heparin (H3393-100KU, Sigma Aldrich) and 30 µg/ml of endothelial cell growth supplement (ECGS, CB-40006, Fisher Scientific) for expansion and in VascuLife® VEGF media (1308000000000, Lifeline Cell Technology) for vessel formation. Primary human adipose-derived stem cells (StemProTM, R7788115, ThermoFisher Scientific) were used as a normal fibroblasts control (NF) and they were cultured in MesenPro RSTM Medium supplemented by MesenPro RSTM Supplement (12746012, Gibco). NOF-151-hTERT (normal stroma immortalized ovarian fibroblast line) was a gift from Dr. Jinsong Liu and was cultured in MCDB131 media and Medium 199 (Gibco) mixed in 1:1 ratio with the addition of 10% FBS, 10 μg/ml epithelial growth factor (EGF), and 1% penicillin/streptomycin. Cancer-associated fibroblasts (CAF) specific for each OC cell line (CAF-KURAMOCHI or CAF-SKOV3) were developed by growing primary human uterine fibroblasts (HUF, ATCC PCS-460-010) in presence of conditioned media from each OC cell line as previously described [28].
Multi-Cultures in Tumor-on-a-Chip
Ovarian cancer cell lines were seeded into the central cancer chamber at a concentration of 4 × 106 cells/ml in a 3D human plasma matrix based on fibrinogen crosslinking as we have previously described [29,30,31]. Briefly, to form the 3D plasma matrix, the cell suspension was mixed with human plasma from healthy donors, calcium chloride as a crosslinker reagent, and tranexamic acid as a stabilizer in 4:4:1:1 ratio, respectively. In the stromal chambers, 20 × 106 cells/ml of EC in coculture with 5 × 106 cells/ml of NF, NOF or CAF were seeded in a 3D human plasma matrix with 2 mg/ml Cultrex collagen I (3440-100-01, R&D Systems) in 1:1 ratio. Alternatively, the monocultures of each stromal cell type were explored in order to study their individual influence in the characterization of the model. In order to preserve the pressure differences inside the device, first, the stromal chambers were filled with cell suspension in the 3D matrix, and after crosslinking at 37 °C for 10 min, the VascuLife® VEGF media was added in both circulation channels to allow vessel formation using 230 µl reservoir connectors (PG-RC-300UL-Q100, PreciGenome). The media was filled with a 2 cm difference (200 µl) between the inlets and the outlets of the circulation chambers to allow hydrostatic pressure and guarantee perfusion based on gravitational difference and interstitial and laminar flow of the fluid inside the microdevice [32]. Finally, the cancer chamber was filled with the OC cell suspension. As a control, a blank 3D matrix with no cells was injected into the stromal chamber. Cell-specific media was added in the inlets and outlets of each chamber to ensure proper media and nutrient gradients across the chip.
Hypoxia Studies
KURAMOCHI cells (3 × 106 cells/ml) were cultured for 7 days in the central chamber with a blank 3D matrix, EC in monoculture, or EC in coculture with NF or CAF in the stromal chamber as described above. KURAMOCHI cells were used to characterize the oxygen levels in the microfluidic device as the most used model cell line for HGSC [33]. A lower number of KURAMOCHI cells than in the drug treatment studies was seeded in the central chamber in order to avoid their migration or growth towards the stromal compartment and study the hypoxic levels in each cell type separately. Image-iTTM Green Hypoxia Reagent (5 μM, EX 488/ EM520 nm, I14833, Invitrogen) was added for 24 hours in the circulation chamber to detect the cells with oxygen levels below 5% and then washed with PBS 1× for 8 h. The images were taken using Nikon A1R Ti2E confocal microscope (EX 488 nm, EM 525 nm) with 10× magnification using large image acquire mode with stitching with 10% overlapping through optimal pathway at 1024 × 1024 pixel resolution.
Collagen Idetection
KURAMOCHI cells (3 × 106 cells/ml) were cultured for 7 days in the presence of a blank scaffold, and monoculture of EC, NF, or CAF in the stromal chamber. One set of microfluidic devices was treated with DMSO as a control group and the other set with halofuginone 10 nM (S8144, SelleckChem), an inhibitor of TGF-β signaling pathway, to study its influence on collagen secretion in the different cell types. Similarly, as in the hypoxia studies, the KURAMOCHI cell line was used as the most relevant model for HGSC, and a lower concentration of OC cells compared to drug studies was used to guarantee the spatial separation of the cells and collagen I detection in the specific cell type focusing mainly on stromal cells as the main secretors of the ECM. Microchips were washed with PBS 1×, fixed with 1% paraformaldehyde, washed again with PBS 1×, and blocked with 4% (w/V) BSA in VascuLife® VEGF media. Finally, the tumor-on-a-chip was incubated with AF488-anti-collagen I antibody (1:1000, ab275996, Abcam) and DAPI 1 µg/ml (62248, ThermoScientificTM) in VascuLife® VEGF media supplemented with 0.1% (w/V) BSA (A2508, Sigma-Aldrich) for 24 h, next washed with PBS 1x and imaged on Nikon A1R Ti2E confocal microscope (EX 488 nm, EM 525 nm). Close-up images were taken in the different compartments of the device. Then, the 3D scaffolds inside the microfluidic device were digested with 20 mg/ml collagenase I (GibcoTM, USA) for 3 h, and imaging flow cytometry was performed on Cytek® Amnis® ImageStream®X MkII Imaging Flow Cytometer (Cytek Biosciences) using the INSPIRE software at 40× magnification at low speed. Images were acquired with a 488 nm laser power of 35 mW, a 785 nm laser at 2 mW for side scatter and in brightfield. A similar approach was taken to study the differences in collagen fiber orientation in the different stromal scenarios for both control and halofuginone-treated groups in the microfluidic device by second harmonic generation microscopy (SHG) using FLUOVIEW FVMPE-RS Multi Photon Laser Scanning Microscope (Olympus). A tunable laser (InSight, SpectraPhysics) was used at 840 nm and z-stacks of 50 μm with a step size of 1 μm in 2 different positions of the stromal chamber in the microfluidic device were acquired using 25x water-immersion objective. Scan size was set to 800 × 800 px with line averaging of 2.
Epithelial-Mesenchymal Transition Studies
For epithelial-mesenchymal transition (EMT) studies, KURAMOCHI cells were cocultured with EC, NF, or CAF in a 1:1 ratio in 3D scaffold in hypoxia for 7 days to determine the expression of EMT markers fibronectin and N-cadherin in the cancer cells. Also, a monoculture of each cell type (KURAMOCHI, EC, NF, CAF) was used to determine the fibronectin expression in the different cell types. The 3D scaffolds were digested with collagenase and, when needed, the cells were fixed and permeabilized with Cyto-FastTM Fix/Perm Buffer Set (426803, Biolegend) following the manufacturer’s instructions. Then, they were stained with APC-anti-CD325 (N-cadherin) antibody (350808, Biolegend) and with anti-cellular fibronectin antibody (SAB42000784, Sigma-Aldrich) and secondary CFTM-488A-anti-mouse antibody (SAB4600042, Sigma-Aldrich) and analyzed with Cytek® Amnis® ImageStream®X MkII Imaging Flow Cytometer or BD LSRFortessa Flow Cytometer. The data was analyzed using FlowJo v10.0 (BD Biosciences).
TGF-β Secretion Detection
KURAMOCHI cells, EC, NF and CAF were cultured in monocultures in 3D hypoxic scaffolds for 7 days in the presence of the DMSO (control) or 10 nM halofuginone and then the scaffolds were digested, and the media was used to detect the active TGF-β1 secreted by the cells using the LEGEND MAXTM Free Active TGF- β1 ELISA kit (437707, BioLegend) following the manufacturer’s instructions. The absorbance was measured at 450 nm on Biotek Cytation 3 Microplate Reader.
Drug Penetration and Uptake
For drug penetration assays, the tumor-on-a-chip was filled with a blank 3D matrix and incubated for 30 min at 37 °C for crosslinking. For drug uptake assays the microchips were filled with SKOV-3 cells (3 × 106 cells/ml) in the cancer chamber surrounded by a blank 3D scaffold, EC, or EC in coculture with NF or with CAF in the stromal chamber as described above. SKOV-3 cells were used in this assay for imaging purposes because of their low clustering potential. The cells were grown for 5 days to allow ECM remodeling and tumor-stroma interactions. For both drug penetration and drug uptake assays, doxorubicin 100 µM (100280, MedKoo Biosciences), a naturally fluorescent drug, was added in both circulation chambers using the reservoir connectors. The images were taken at different time points (0; 0.25; 0.5; 0.75, 1; 1.25; 1.5; 1.75; 2, 2.5; 3; 4; 5; 6; 7; 8; 24 hours) for drug penetration and at 24 h for drug uptake using Nikon Eclipse Ni-E fluorescent microscope (EX 559.5 nm/EM 645 nm) at 10× magnification. The velocity of the fluid diffusion (μm/s) through the microfluidic device was measured experimentally by determining the difference of the distance (μm) traveled by the fluid in each time frame divided by the time (s).
Migration Studies
OC cell lines (KURAMOCHI and SKOV-3) were stained with surface membrane marker InvitrogenTM DiD (D307, Invitrogen) and seeded at 4 × 106 cells/ml concentration in the cancer chamber surrounded by blank 3D scaffold, endothelial cells stained with BV605-anti-CD31 antibody (303122, Biolegend) in monoculture or coculture with either NF or CAF detected with FITC-anti-CD90 antibody (328108, Biolegend) in the stromal chamber. VascuLife® VEGF media was changed daily to allow vessel formation. The microfluidic devices were imaged on days 3, 7, and 14 using fluorescent confocal microscope Nikon A1R-Ti2E at 10× magnification with 1024 × 1024 pixel image resolution and in large image acquiring mode with 10% overlapping through the optimal pathway.
Drug Treatment
KURAMOCHI or SKOV-3 cells (4 × 106 cells/ml) were seeded in the cancer chamber in the 3D human plasma model in the presence of a blank 3D scaffold, or EC (20 × 106 cells/ml) in coculture with NF or CAF (5 × 106 cells/ml) in the stromal chamber in collagen I:human plasma (1:1) 3D model for 3 days to allow cellular crosstalk and ECM remodeling. Also, the monocultures of EC, NF, or CAF were used in the stromal chamber in order to elucidate the influence of each cell type in drug response. Alternatively, a mixture of 3D human plasma matrix and Cultrex collagen I was also used in the central chamber to grow KURAMOCHI cells to study the influence of the type of 3D matrix in drug resistance. Moreover, the KURAMOCHI cell line was seeded in monoculture or coculture with EC or triculture with EC and NF or EC and CAF in the central chamber in the 3D human plasma matrix and blank plasma:collagenI matrix (no cells) in the stromal chamber to study the role of the compartmentalization in drug response. Then, the standard-of-care chemotherapeutic drugs paclitaxel (12.5 nM, S1150, Selleckchem) and carboplatin (25 µM, 100130, MedKoo) were added into both circulation chambers for 4 days. For ECM remodeling targeting studies, the cells were pre-incubated with halofuginone 10 nM also injected into both circulation chambers for 72 h, and then paclitaxel/carboplatin/halofuginone combo was added for 4 days. A microfluidic device treated with DMSO was used as a control for cell viability. The day before analysis, the cells were stained with Nuclear ID® Blue/Red cell viability kit (ENZ-53005-C100, Enzo Life Sciences) and FITC-anti-MUC-1 antibody to identify OC cells and images were taken in Nikon Eclipse Ni-E fluorescent microscope at 10× magnification in DAPI (EX 383-408 nm/EM 435-485 nm), GFP (EX450-490 nm/EM500-550) and TexRed channels (EX 540-580 nm/EM 593-668 nm).
Image Data Analysis
Fluorescent images acquired on Nikon Eclipse Ni-E or confocal microscope Nikon A1R-Ti2E, unless otherwise stated, were processed, and analyzed with NIS-Elements AR Analysis 5.21.03 Software. Binary thresholding was used to detect the cells of interest. For the vessel’s characterization, area restriction in a range of 50% higher than the calculated mean value of all objects and the largest object area value (3000 µm2–50,000 µm2) was applied to detect vessel-like structures of EC in TexRed channel (BV605-anti-CD31 antibody). Circularity (0-1), elongation, and area (μm2) parameters were determined by automatic measurement of selected objects and the inner diameter of the vessels (μm) was manually determined with the distance measurement tool. In the collagen I secretion studies, the unspecific green background from the 3D scaffold was subtracted in the fluorescent images of AF488-anti-collagen I, and the mean fluorescence intensity (MFI) of AF488 inside individual cells was measured with automatic measurement. The images taken on ImageStream®X MkII Imaging Flow Cytometer were analyzed using IDEAS v6.2 analysis software. Each sample was processed by first using the area of the brightfield and the brightfield aspect ratio to identify single round cells discarding debris and speed beads. Then, the brightfield gradient RMS value was used to gate in-focus events for further analysis. Finally, events with no Mean Pixel AF488 intensity were excluded from the analysis discarding debris (Supplementary Fig. 1a). The area of each cell was calculated using a brightfield-based object mask and the AF488-colagen I intensity was calculated from the pixel intensity within the cell mask (Supplementary Fig. 1b). All images show the median AF488-collagen I intensity for each sample and were window-leveled based on the median intensity of the CAF images. Finally, the MFI of AF488-collagen I was analyzed in individual cells in FlowJo_v10.8.1_CL software and representative histograms were generated for each condition. The same analysis was performed with the cells stained with CF488- anticellular-anti-fibronectin antibody. For the SHG image analysis, six iterations of constrained iterative deconvolution were uniformly applied to the images in CellSens analysis software (Olympus), and the orientation of the collagen fibers was determined in ImageJ (FIJI) using the OrientationJ plugin for Hue, Saturation and Brightness (HSB) color-survey maps and OrientationJ distribution measurement. In the oxygen gradients studies, the tumor-on-a-chip was divided into 9 equal vertical portions from left to right and the mean fluorescent intensity of the Image-iTTM green hypoxia reagent was measured inside individual cells through binary thresholding and automatic detection of MFI. In the migration studies, binary thresholding was used to detect the KURAMOCHI cells, and the number and area of the clusters were measured in the different conditions using automated measurement and applying specific restrictions of area in a range of 50% higher than the calculated mean value of all objects in cancer chamber and the largest object area value (1300 µm2–90,000 µm2) (Supplementary Fig. 2). To characterize the metastasis-like migration of SKOV-3 cells towards the stromal chamber, the migration distance was measured manually using the distance measurement tool and MFI of DiD-marked OC cells (Cy5 channel) was determined by automatic measurement feature in rectangular region of interest (ROI) selected in the stromal chamber of the device to detect the number of cells that migrated. For drug penetration studies, the MFI of doxorubicin was quantified in stromal and cancer chambers separately using rectangular ROI selection and for drug uptake images, the red background of doxorubicin penetration into the 3D matrix was subtracted to detect intracellular drug fluorescence. To measure the velocity of the doxorubicin diffusion, the distance of penetration of the drug in the microfluidic device was measured manually using the distance measurement tool in NIS-Elements software. The representative pictures in the drug treatment studies were processed adjusting the blue staining for live cells into green color for better visualization and the number of live (green) and dead (red) cells was determined by manual counting in ImageJ software using the cell counter plugin. The counterstain with FITC-MUC-1 for KURAMOCHI cells was processed and changed to white color for better visualization with the live/dead staining.
Statistical Analysis
Experiments were performed in triplicates and repeated at least three times. All graphs were generated and analyzed using GraphPad Prism 9 software as Mean ± SD, and statistical significance was analyzed using t-test, One-way or Two-way ANOVA, and p-value lower than 0.05 was considered significant. The data that did not follow a normal (Gaussian) distribution were plotted as a violin plot with median, and statistical significance was determined with the Mann-Whitney nonparametric test considering p-value lower than 0.05 significant.
Results
Multiniche Microvascularized Tumor-on-a-Chip Recapitulates the Compartmentalized OC-TME
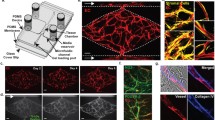
To develop a new physiologically relevant model to mimic the spatial organization and the biological compartments of the OC-TME where the cancer cells grow surrounded by cancer-associated fibroblasts, imperfect vasculature, and the gradients of nutrients, oxygen and drugs (Fig. 1a left), we have designed a novel microvascularized multicompartmentalized PDMS tumor-on-a-chip. Specifically, this device consisted of a central chamber to seed OC cell lines, flanked by two stromal chambers on both sides to grow EC in monoculture or coculture with NF or CAF to study tumor-stroma crosstalk and the influence of TME compartmentalization in drug resistance in OC. Two outer circulation channels on each side were included to perfuse media or drugs into the device. Besides the recapitulation of the specific tumor compartments, the dual symmetric design was also used in order to maintain the pressure difference at the interface with the proximal compartments during the filling process to avoid the leakage of the fluid [34]. The dimensions of each chamber were 2 mm × 4 mm × 0.1 mm. All compartments were separated by trapezoidal pillars with 100 µm gaps to maintain the compartmentalization, and allow cell-cell interactions, migration, and media and drug flow (Fig. 1a right). The tumor-on-a-chip was functionally characterized for the recreation of key physical components of the complex OC-TME. First, the design was measured for the formation of vascularization in the stromal chambers surrounding the cancer chamber. Vessel-like structures were formed in the three experimental groups including EC, either in monoculture or coculture with NF or CAF. No significant differences were observed in the circularity and area of the vessels between the groups, although the presence of CAF significantly increased the inner diameter of the vessels from 60 µm to 80 µm and the elongation from 1.9 to 2.1 when compared to EC monoculture (Fig. 1b). Second, the tumor-on-a-chip was evaluated for the recapitulation of ECM remodeling associated with tumor growth and driven by stromal cells, which is a key contributor to reduction of therapeutic efficacy. Collagen I secretion, as the most common and abundant ECM protein in the OC-TME, was assessed by immunofluorescence staining both by confocal imaging and imaging flow cytometry providing high throughput single cell measurements coupled with single cell imaging. While confocal images revealed no collagen I expression in blank, OC and EC cells, monoculture NF and CAF had positive collagen staining and CAF had significantly higher expression measured by MFI than the NF (Fig. 1c). Confocal imaging of whole chips had significant matrix background when stained for collagen, therefore, cells were isolated from the device and analyzed by imaging flow cytometry. Similarly, collagen I expression was significantly higher in CAF compared to OC, EC, and NF (Fig. 1d). Moreover, we evaluated the tumor-on-a-chip for the recapitulation of key spatial tissue gradients, particularly for oxygen gradients allowing to recreate the hypoxic OC-TME. The four experimental groups were stained with green hypoxia ImageiTTM reagent that fluoresces when cells are exposed to less than 5% oxygen. A hypoxic niche core in the central cancer chamber was validated in all 4 conditions, but only the incorporation of CAF in the stromal chamber allowed for a spatial tissue oxygen gradient from the circulation chambers towards the cancer chamber (Fig. 1e). Finally, we have evaluated the epithelial-mesenchymal transition (EMT) potential of the different cell types in monoculture by measuring fibronectin with imaging flow cytometry, where the stromal cells (EC, NF, and CAF) expressed significantly higher fibronectin compared to OC cells (Fig. 2a). Then, OC cells were cocultured with EC, NF or CAF and fibronectin and N-cadherin expression was measured in the OC cells by flow cytometry. The OC cells presented significantly higher expression of both EMT markers (Fig. 2b, c) when cocultured with stromal cells. These findings validated our model as suitable for recreating the compartmentalized OC-TME and the influence of CAF in vessel-like structure formation, ECM remodeling, and spatial oxygen gradients and their potential to induce EMT in the OC cells.
Multiniche microvascularized tumor-on-a-chip recapitulates the compartmentalized OC-TME. a Schematic representation of the tumor microenvironment (left) and tumor-on-a-chip device and close-up view of the different chambers and cell types seeded inside (right) and real image of microfluidic device filled with dyes next to a nickel representing the size of the platform (bottom). b Representative images of the stromal chamber with vessel-like structures formation of endothelial cells (EC) stained with BV605-anti-CD31 (red) in monoculture or coculture with normal fibroblasts (NF) or cancer-associated fibroblasts (CAF) stained with FITC-anti-CD90 (green) and the quantification of the circularity, area, inner diameter, and elongation of the vessels. Median value is marked in red. Scale Bar = 100 µm. *p < 0.05, **p < 0.01, Mann-Whitney nonparametric test compared to EC. c Representative images of the stromal chamber by immunofluorescent staining with AF488-anti-collagen I in blank gel or mono-culture cells grown in the microfluidic device for 7 days and quantification of the mean fluorescent intensity (MFI) of AF488. Scale Bar = 100 µm. Mean ± SD, ****p < 0.0001, t-test. d Imaging flow cytometry analysis of cells isolated from microfluidic device and analyzed for collagen I, including representative images, representative histogram of each condition and violin plot for quantification of MFI of AF488 inside individual cells. Median value is marked in red. ****p < 0.0001, Mann-Whitney nonparametric test. Scale Bar = 10 µm. e Representative images of OC cells grown in presence of blank scaffold, EC, EC + NF or EC + CAF in the stromal chambers for 7 days and stained with hypoxic Image-iTTM reagent (green) and quantification of MFI of the device divided in 9 sections from left to right. Scale Bar = 1 mm. Mean ± SD, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, Two-Way ANOVA compared to Blank. Figure a created with Biorender.com
Hypoxic stroma induces epithelial-mesenchymal transition in OC cells. a KURAMOCHI cells (OC), EC, NF and CAF were grown in monoculture in hypoxic 3D scaffolds for 7 days and the expression of cellular fibronectin in each cell type was detected with imaging flow cytometry with CF488-anti-fibronectin antibody. Representative images are shown on the left and violin plots of the quantification of MFI of CF488 inside individual cells on the right. Median value is marked in red. Scale bar = 10 µm, ****p < 0.0001, Mann-Whitney nonparametric test. Moreover, the OC cells were grown in monoculture or cocultured with EC, NF or CAF in the hypoxic 3D scaffold for 7 days and then stained with b CF488-anti-fibronectin or c APC-anti-N-cadherin antibodies and OC were analyzed by flow cytometry. Representative histograms are shown on the left and the quantification of MFI of each antibody on the right. Mean ± SD, **p < 0.01, ****p < 0.0001, Two-Way ANOVA compared to OC
Tumor-on-a-Chip Allows for Cluster Formation and Metastasis-Like Migration While Recapitulating Tumor-Stroma Interactions
To study cancer cell behavior and cellular crosstalk, tumor-on-a-chip devices were monitored over 14 days in culture. On one hand, KURAMOCHI, a non-metastatic high-grade serous OC cell line that forms clusters in 3D culture [35] was evaluated and found that over time KURAMOCHI formed more and bigger clusters and established cell interactions with the stromal compartment (Fig. 3a). Number of clusters and cluster size were significantly higher in OC cells when no stroma was present (blank) on days 3 and 7. In the presence of stromal cells on day 3, the number and area of clusters surrounded by CAF were higher than in EC and NF. While day 7 showed a significantly elevated formation of clusters in the presence of CAF and EC compared to NF, day 14 had a similar number of clusters in all conditions even though EC, NF and CAF had significantly higher areas compared to blank (Fig. 3b). On the other hand, SKOV-3, a clear cell carcinoma very aggressive and metastasis-like cell line [33, 36, 37], was also monitored and identified that formed tumor-stromal interactions and initiated metastasis-like migration towards stromal chambers on days 7 and 14 (Fig. 3c). The distance of migration of the OC cells was measured and while there were no significant differences on day 7, migration was significantly reduced on day 14 in the conditions that contain stromal fibroblasts (NF and CAF) compared to blank. When no stroma was included, the SKOV-3 cells migrated significantly longer distances than when stroma cells were present (335 ± 33 µm on day 7 and 663 ± 54 µm on day 14), followed by SKOV-3 cultured with EC (280 ± 24 µm on day 7 and 349 ± 26 µm on day 14). When fibroblasts were seeded in the stromal chambers, even though the differences were not statistically significant, CAF presented a trend of higher migration of SKOV-3 cells compared to NF (216 ± 21 µm on day 7 and 273 ± 33 µm on day 14 compared to 188 ± 23 µm on day 7 and 258 ± 32 µm on day 14 in NF) (Fig. 3d). Also, the number of SKOV-3 cells that migrated towards stromal chambers determined by MFI measurement of DiD-stained cells in the stromal compartment was significantly higher on day 14 when no stroma was present, and CAF induced significantly more metastasis-like migration than NF.
Tumor-on-a-chip allows for cluster formation and metastasis-like migration while recapitulating tumor-stroma interactions. a KURAMOCHI cells (DiD, purple) were grown in cancer chamber with blank scaffold, EC (BV605-anti-CD31, red), EC + NF (FITC-anti-CD90, green) or EC + CAF (FITC-anti-CD90, green) in stromal chambers and images were taken on days 3, 7 and 14. Scale bar = 1 mm. Close-up magnification images show the interactions between cancer and stromal chambers (yellow rectangles) and yellow arrows show the cluster formation on day 14. Scale Bar = 0.5 mm. b Quantification of number of clusters on the left, Mean ± SD, Two-Way ANOVA compared to blank, and cluster area for KURAMOCHI cultures (low-metastatic potential) on the right, Mann-Whitney nonparametric test, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 c SKOV-3 cells (DiD, purple) were grown in cancer chamber with blank scaffold, EC (BV605-anti-CD31, red), EC + NF (FITC-anti-CD90, green) or EC + CAF (FITC-anti-CD90, green) in stromal chambers and images were taken on days 3, 7 and 14. Scale bar = 1 mm. Close-up magnification images show the interactions between cancer and stromal chambers (yellow rectangles). Scale bar = 0.5 mm. White discontinued lines denote distance of migration towards stromal chamber. d Quantification of distance of migration and number of migrated cells (MFI of DiD) for SKOV-3 cultures (high metastatic potential). *p < 0.05,***p < 0.001, ****p < 0.0001, Two-Way ANOVA
Microfluidic Device Mimics Drug Penetration Gradients in OC-TME
To analyze the fluid flow inside of our tumor-on-a-chip, a blank 3D scaffold (without cells) was injected into cancer and stromal chambers, and once polymerized, doxorubicin was injected in both circulation channels and fluorescent time-lapse microscopy was performed (Fig.4a). A laminar flow of doxorubicin (red) can be seen on the representative images in Fig. 4b over time, reaching full penetration of the whole central chamber at 24 h. The velocity of the diffusion of the drug inside the microfluidic device was determined experimentally (Fig. 4c (left), and a significant decrease was observed 15–30 min post-injection reaching a slow fluid flow during the following hours. The doxorubicin penetration was quantified by measuring its MFI at different time points (Fig. 4c right). As expected, doxorubicin penetrated faster in the stromal chambers at around 2–4 h, but there was a delay in drug penetration into the central chamber (7 h), recapitulating tumor drug penetration. Importantly, both stromal and cancer chambers reached plateau full drug penetration at 24 h (Fig. 4c). Having this in mind, SKOV-3 cells were seeded in the central chamber in the presence of blank scaffold, EC, or EC in coculture with NF or CAF and grown for 5 days to allow TME spatial compartmentalization including vessel formation, ECM remodeling and oxygen gradients, and then doxorubicin was injected in both circulation channels and fluorescent images were taken at 24 h (Fig. 4d). As observed in Fig. 4e and in the supplementary video, complete drug penetration and uptake in cells were reached at 24 h, a time point that was established as a minimal time for drug screening incubations.
Microfluidic device mimics drug penetration gradients in OC-TME. a Schematic representation of method used for doxorubicin penetration assay, where doxorubicin (100 µM) was injected in both circulation channels and images were taken in time-lapse fluorescent microscope up to 24 h. b Representative images at 5 min, 30 min, 2, 4, 7 and 24 h of doxorubicin penetration. Scale Bar = 1 mm. c Quantification of the fluid velocity (μm/s) inside the microfluidic device over the time (h) (left) and MFI of doxorubicin penetration inside the cancer and stromal chambers at each time point (right). d Schematic representation of method used for doxorubicin uptake, cancer cells were cultured surrounded by different stromal cells (EC, NF, or CAF) or blank scaffold as a control for 5 days and then doxorubicin (100 µM) was injected in both circulation channels and images were taken at 24 h. e Representative images of doxorubicin uptake by cells at 24 h. Scale Bar = 1 mm. Figure a and d created with Biorender.com
Multiniche Microvascular Tumor-on-a-Chip Recapitulates CAF-Induced Drug Resistance in OC-TME
The microfluidic model was evaluated for drug screening and to study the influence of TME compartmentalization in drug resistance in OC. OC cell lines were grown in the absence of stroma (blank 3D scaffold) or presence of the different stromal cells (EC, EC in coculture with NF or CAF) for 3 days to allow spatial TME compartmentalization (vascularization, oxygen gradients and ECM remodeling), then, the devices were treated with a combination of standard-of-care chemotherapeutic drugs paclitaxel and carboplatin (PC) (Fig. 5a). Representative close-up images of the central chamber seeded with KURAMOCHI or SKOV-3 cells are shown in Fig. 5b and d, respectively. There were no significant dead cells observed in either of the stromal cells as seen in Supplementary Fig. 3 and the counterstain with MUC-1 antibody (white) showed that the cytotoxic effect is exclusive on the cancer cells located in the central chamber (Supplementary Fig. 4a). The number of live (green) and dead (red) cells was determined, and drug cytotoxicity for KURAMOCHI (Fig. 5c) and for SKOV-3 cells (Fig. 5e) was expressed as a percentage of dead cells normalized by the total number of cells in each condition. In both cell lines, in the absence of stroma (blank) or presence of normal stroma (EC or EC + NF), there was a significant cytotoxic effect of the paclitaxel/carboplatin combination, but in the presence of CAF, there was no killing effect observed compared to DMSO treated condition. Alternatively, the KURAMOCHI cells were cocultured with NF or CAF in the stromal chamber in the presence or absence of EC (Supplementary Fig. 4b) to confirm the specific influence of the CAF in the drug resistance, and no significant differences in the cytotoxic effect of PC between the conditions were observed (Supplementary Fig. 4c). Moreover, to further explore the effect of the composition of the 3D matrix and ECM used in the central chamber in drug response, the 3D human plasma scaffold in the central chamber was mixed with collagen I. The presence of external collagen I in the central chamber caused drug resistance in the presence of CAF (Supplementary Fig. 5a-c), similar to when a plasma matrix was used in the central chamber (Fig. 5b). Additionally, there were no significant differences when EC were cocultured with the stroma. These last two studies together suggest that the chemoresistance effect is driven by the stromal cells and not the EC included in the stromal compartment. Finally, the KURAMOCHI cells were grown in monoculture or in coculture with EC, EC + NF, or EC + CAF inside the cancer chamber with a blank 3D scaffold in the stromal compartments to examine the influence of the compartmentalization in the drug response (Supplementary Fig. 5d–f). Without compartmentalization, CAF were not able to induce the same degree of drug resistance as with compartmentalization. Still the coculture with CAF protected OC cells more than NFs, where there was significantly more killing compared to DMSO control. Moreover, NOF cells were explored as an alternative for NF because of their ovarian origin in the drug treatment studies, where KURAMOCHI cells were seeded in the presence of EC in coculture with NF, NOF or CAF (Supplementary Fig. 6a, b) and there was a similar drug response in the OC cells cocultured with EC + NOF as in EC + NF, having a significantly higher cytotoxic effect compared to the DMSO treated condition and confirming the drug resistance in the presence of CAF. These findings have validated our model to recapitulate the influence of stromal cells in drug response in OC cancer, specifically CAF-induced drug resistance.
Multiniche microvascular tumor-on-a-chip recapitulates CAF-induced drug resistance in OC-TME. a Schematic representation of the methods used in this experiment where OC cell lines were cultured in presence of different stromal components (blank 3D scaffold, EC, EC + NF and EC + CAF) in stromal chamber for 3 days to allow compartmentalization and then treated with paclitaxel and carboplatin (PC) for 4 days. DMSO-treated chips were used as a control. Nuclear ID live (green)/dead (red) reagent was used to determine cell viability. b Representative images of merged green(live)/red(dead) and red channel (dead cells) for KURAMOCHI in cancer chamber. Scale Bar = 500 µm. c Quantification of percentage of cell death normalized by the total number of cells per condition. Mean ± SD, *p < 0.05, ***p < 0.001, ****p < 0.0001, Two-Way ANOVA. d Representative images of merged green(live)/red(dead) and red channel (dead cells) for SKOV3 in cancer chamber. Scale Bar = 500 µm. e Quantification of percentage of cell death normalized by the total number of cells in each condition. Mean ± SD, *p < 0.05, t test to corresponding DMSO control. Figure a created with Biorender.com
CAF-Mediated Drug Resistance can be Rescued by an Anti-TGF-β Targeting Agent
We used halofuginone as a targeting agent inhibiting TGF-β signaling to overcome the drug resistance in OC caused by CAF. First, we have evaluated the potential of HALO to inhibit collagen secretion by blocking the TGF-β pathway. KURAMOCHI cells were seeded in the central chamber surrounded by a blank scaffold, EC, NF, or CAF in two sets of microdevices. One set of the microfluidic devices was grown untreated to allow spatial TME compartmentalization and the other was pre-incubated with halofuginone in order to avoid TGF-β-mediated ECM remodeling by CAF. Halofuginone-treated CAF presented significantly decreased levels of collagen secretion in the fluorescent confocal microscopy and imaging flow cytometry (Fig. 6a, b). Second, SHG microscopy revealed densely packed and aligned fibers in the CAF with a clear peak of distribution of orientation compared to the rest of the stromal cells with more disorganized collagen fibers. Preincubation with halofuginone generated a disorganized orientation of the collagen fibers in the CAF similar to the normal stroma orientation (Fig. 6c–e). To further confirm the antagonist effect of halofuginone on the TGF-β pathway, free active TGF-β1 was measured in the media and revealed a significantly higher secretion of active TGF-β1 by KURAMOCHI cells and CAF than in EC and NF, which was significantly inhibited by the preincubation with HALO (Fig. 6f). Same studies were also performed in the NOF cell line and confirmed similar collagen secretion patterns and response to HALO as the NF (Supplementary Fig. 6c-e). Finally, KURAMOCHI cells were seeded in the central chamber surrounded by a blank scaffold, EC, EC in coculture with NF or CAF in two sets of microdevices in presence or absence of HALO treatment for 3 days. After 3 days, paclitaxel/carboplatin combination was added to the first set and paclitaxel/carboplatin/halofuginone to the second set of microfluidic devices for another 4 days. Then, cells were stained with viability reagent as previously described (Fig. 7a). Representative images of the central chamber of both sets, paclitaxel/carboplatin-treated (left) and paclitaxel/carboplatin/halofuginone-treated (right) are shown in Fig. 7b and revealed increased death in the coculture CAF condition with halofuginone when compared to standard of care alone treatment. No significant dead cells were observed in the stromal chambers as seen in the representative images of whole microfluidic devices in Supplementary Fig. 7. The cytotoxic effect was expressed as a percentage of dead cells normalized by the total number of cells per condition and as seen in Fig. 7c where halofuginone did not have any effect on the cancer cells grown in the presence of normal stroma, however, it caused significantly moderated improvement in the drug response in the CAF-mediated drug resistance to standard-of-care drugs.
CAF-mediated collagen secretion is inhibited by an anti-TGF-β targeting agent. KURAMOCHI, EC, NF and CAF were grown in monoculture in the microfluidic device for 7 days in the presence or absence of halofuginone (HALO) as a targeting agent for TGF-β a Representative images of the stromal chamber by immunofluorescent staining with AF488-anti-collagen I (left) and quantification of the mean fluorescent intensity (MFI) of AF488 (right). Scale Bar = 100 µm. Mean ± SD, *p < 0.05, **p < 0.01, ****p < 0.0001, Two-Way ANOVA. b Representative images of imaging flow cytometry of the cells stained with AF488-anti-collagen I (left) and violin plots with quantification of the mean fluorescent intensity (MFI) of AF488 (right) in the individual cells. Scale Bar = 10 µm. Median value is marked in red. ****p < 0.0001, Mann-Whitney test compared to DMSO. c Representative images of collagen fibers in SHG microscopy. Scale bar = 20 µm. d Hue, Saturation and Brihgtness (HSB) color orientation maps generated with OrientationJ plugin in ImageJ software to visualize the orientation of the collagen fibers. Scale bar = 20 µm. e Representative orientation histograms in degrees generated with OrientationJ plugin in ImageJ software. f Quantification of the secretion of the free active TGF-β1 (pg/ml) by ELISA, *p < 0.05,***p < 0.001, ****p < 0.0001, Two-Way ANOVA
CAF-mediated drug resistance can be rescued by an anti- TGF-β targeting agent. a Schematic representation of methods used in this approach where the chips were pre-treated with halofuginone (HALO) as a targeting agent for TGF-β then treated with paclitaxel and carboplatin (PC) for 4 days. b Representative close-up images of KURAMOCHI grown in central chamber with blank scaffold, endothelial cells (EC), EC in co-culture with NF or CAF in stromal chamber treated with PC or PC with halofuginone (PC + H) (as pre-treatment). Live/Dead Nuclear ID dye was used to detect live cells (green) and dead cells (red). Scale Bar = 500 µm. c Quantification of percentage of cell death normalized by the total number of cells in each condition. Mean ± SD, *p < 0.05, t test to corresponding PC condition. Figure a created with Biorender.com
Discussion
In this study, we have developed a versatile multi-compartmentalized microvascularized PDMS tumor-on-a-chip, where spatial TME compartmentalization was recapitulated including vascularization, ECM remodeling, oxygen gradients, as well as drug penetration, and uptake kinetics into tumor. Importantly, the device allowed us to investigate tumor-stroma cellular crosstalk, compare normal or cancer-associated fibroblast’s role in modulating drug response, and further investigate targeted therapies that can overcome this induced drug resistance. This study demonstrates that CAF are key contributors in the OC-TME compartmentalization and influence drug response, which is in accordance with ours and other reports [28, 38,39,40]. This device grants drug screening under a faithful recapitulation of the OC-TME, which would provide strong evidence for allowing new opportunities to improve chemotherapy effectiveness and mitigating chemoresistance in ovarian cancer.
Ovarian tumors are heterogeneous where the cancer-stroma interplay is important in tumor progression and therapeutic response and it is vital to mimic the cellular and acellular components of the TME, and its spatial organization in preclinical models in order to find new therapies and improve the outcomes of OC patients [41]. In terms of key cellular compartment recapitulation for mimicking the OC-TME compartmentalization, this study has used two distinct and valuable OC cell lines. We used the KURAMOCHI cell line, as a model for HGSC which is the most common and deadliest subtype of OC [1, 35], and the SKOV-3 cell line as a model for clear cell ovarian adenocarcinoma, as the most used cell line in OC preclinical research [33, 36, 37]. To model the stromal component of the OC-TME, we used endothelial cells, or their coculture with normal fibroblasts or cancer-associated fibroblasts. Specifically, we used human umbilical vein endothelial cells (HUVEC) which are widely used in vascularization microfluidic studies because of their easy growth in 3D scaffolds and formation of vessel-like structures [42,43,44,45,46,47,48,49,50,51], even though they do not represent exactly the behavior of the native EC in OC tumors, which could be included in future studies [52]. Moreover, human adipose-derived stem cells (ADSC) were used as a normal fibroblast model because of their high expansion ability and reproducibility suitable for high-throughput research, although they are not directly representative of normal tissue origin including fallopian tube or ovary [53]. Currently, the OC-stroma interaction studies use a variety of normal fibroblasts from different non-ovarian origins such are NIH3T3 fibroblasts from mouse embryos [28], lung fibroblasts [54], or dermal fibroblasts [55]. Therefore, we selected ADSC as it is demonstrated that human mesenchymal stem cells are functionally resting normal fibroblasts having similar phenotypes and characteristics and are widely used in bioengineering studies [56, 57]. Also, a normal stroma immortalized ovarian fibroblast line (NOF-151-hTERT) was used as an alternative for ADSC, constituting a more physiologically relevant model due to their ovarian origin [58]. The effect of NOF in drug resistance was similar to the ADSC, validating the use of ADSC as normal fibroblasts. Moreover, it is known that in the TME cancer cells recruit stromal cells into their vicinity through tumor-stromal interactions and signaling molecules and activate them into cancer-like phenotype [38]. In this study, we have used cancer-associated fibroblasts, as activated fibroblasts, derived from primary human uterine fibroblasts by their exposure to conditioned media from specific OC cell line (KURAMOCHI or SKOV-3) that have been previously functionally characterized in terms of CAF-like phenotype, higher proliferation rate, contractility, ECM remodeling, tumor progression, and drug resistance [28]. CAF are the most important cell type in the OC-TME constituting a heterogeneous population of cells because they can be derived from normal fibroblasts, bone marrow mesenchymal stem cells, adipocytes, endothelial cells, or epithelial cells and they can be activated through different signaling pathways, exosomes, or cytokines such as TGF-β and also through ECM remodeling, although most of these CAF subtypes have not been fully characterized yet. Therefore, using a well-characterized cell source is important to avoid the heterogeneity from primary CAF cultures that also have low reproducibility, low growth rate, and finite life span, thus they are not suitable for high throughput studies.
Once CAF in the TME are activated, they interact with the cancer cells, other stromal cells, and ECM through different signaling molecules, cytokines, chemokines, and exosomes, and participate in tumor progression, invasion, metastasis, angiogenesis, ECM remodeling and drug resistance [26, 39, 59, 60]. Even though the OC drug resistance mechanisms remain still unclear, it is known that CAF may play an important role as they are the key component of the OC stroma implicated in ECM remodeling [9]. Activated CAF secrete ECM proteins such are collagens mediated by TGF-β signaling, converting the ECM into a stiff obstacle for drug penetration and generating gradients of oxygen that result in a hypoxic tumor core [38, 39]. Hypoxia also enhances drug resistance by inducing different changes in gene and protein expression affecting many cellular and physiological functions, resulting in poor prognosis of OC patients [6, 12, 39, 60]. CAF-induced ECM remodeling can compress the vessels in the TME reducing drug delivery inside the tumor and CAF also secrete hypoxia-induced angiogenesis regulator (HIAR) and vascular endothelial growth factor (VEGF-A) promoting aberrant and exacerbated angiogenesis [60]. Furthermore, CAF enhance vascular permeability and leakage that impede drug penetration into the tumor by different factors such are VEGF, lipoma preferred partner (LPP), and platelet-derived growth factor receptor (PDGFR) [60]. CAF also interfere with drug resistance by inducing and maintaining the stemness of the tumor, promoting increased efflux of chemotherapeutic drugs, and by secreting pro-inflammatory cytokine IL-6 that has been correlated with resistance to paclitaxel [8, 60, 61]. CAF are also implicated in the induction of epithelial-mesenchymal transition (EMT) increasing the invasiveness of the cancer cells and cisplatin resistance [62]. Moreover, CAF can reprogram metabolically the cancer cells to enhance their survival, and they secrete long non-coding RNA ANRIL that suppresses the expression of drug transporters to promote drug efflux in OC and induce cisplatin resistance [60, 62, 63]. Stromal cells from ascites have been reported to confer drug resistance to OC cells, as the cancer cells acquire the functional p-glycoproteins for drug efflux through trogocytosis of the stromal cell’s membrane [64]. Current therapeutic strategies to overcome drug resistance induced by CAF consist in targeting (I) the cells of origin of CAF, (II) CAF markers (FAP), (III) CAF activation signaling pathways, (IV) or CAF-directed therapeutic delivery [60]. Also, the treatment with chemotherapeutics in OC can stimulate TGF-β secretion and induce more drug resistance [65], therefore targeting TGF-β signaling could improve the OC patient outcomes. For example, halofuginone, an anticoccidial drug, is a TGF-β signaling inhibitor that had antitumor effects and reduced collagen synthesis in animal models of several solid cancers (lung, melanoma, breast, pancreas, etc.) [66], and had synergetic effect with chemotherapeutic drugs overcoming chemotherapeutic resistance in lung, prostate, and colorectal cancers, and it is currently explored in several clinical trials in bladder cancer, AIDS-related Kaposi sarcoma, and in advanced solid tumors [67]. Geyer et al. used halofuginone to target stroma in a microfluidic device modeling the TME in pancreatic ductal adenocarcinoma increasing immune infiltration that was impaired by pancreatic stromal cells [68]. Halofuginone plays an antitumor role by inducing tumor apoptosis and autophagy, inhibiting cancer cell proliferation and metastasis, and cell cycle arrest through different signaling pathways such as TGF-β, caspase inhibition, and collagen synthesis inhibition [67].
In terms of acellular components of the TME and its spatial organization, we have validated our model to recreate the spatial arrangement and characteristics of the OC-TME and its influence on drug resistance. In this study, we have demonstrated that the incorporation of cancer-associated fibroblasts can modify cancer cell behavior, progression, invasion, and drug response. First, the endothelial cells grown in our model in the presence of VEGF were able to generate vessel-like structures mimicking the imperfect leaky vasculature in the TME [23, 24, 59]. In the presence of CAF, even though the area of the vessels has not been affected, the inner diameter and elongation of the vessels were increased confirming that the CAF enhance the leaky vascularization and angiogenesis in OC-TME [10, 69]. Also, we confirmed that CAF are key contributors to ECM remodeling by higher collagen I secretion compared to normal stroma (NF and EC) and therefore converting the ECM in a physical obstacle with higher stiffness that impairs the penetration of drugs and oxygen into the tumor. We were able to recreate hypoxic tumor core characteristic for OC to mimic the role of hypoxia in drug resistance [70] and we validated that the presence of CAF in the OC stroma generated spatial gradients of oxygen from circulation chambers towards the cancer chamber compared to other stromal cells sole recreation of core hypoxic niches in the central chamber. Moreover, it is known that CAF can induce epithelial-mesenchymal transition (EMT) [62] and secrete metabolites to fuel cancer cell growth under hypoxic and undernourished conditions and therefore increase tumor progression and invasion [39]. In our studies, we observed a higher expression of two EMT markers, fibronectin [71,72,73] and N-cadherin [74] in the cancer cells in the presence of the CAF confirming their ability to induce epithelial-mesenchymal transition implicated in the drug resistance and cancer invasiveness [62]. Also, we compared KURAMOCHI cells known for their low migratory potential and generation of clusters, and the SKOV-3 cell line with high metastatic potential [35]. As expected, a higher formation of the number of clusters in KURAMOCHI and higher migration in SKOV-3 were observed in the absence of stroma as there is no competition with stromal cells and lower gradients of nutrients, therefore SKOV-3 cells can freely migrate towards the blank scaffold in the stromal compartment. It should be noted that is an experimental culture control condition, but not physiologically relevant. In the presence of stroma, CAF induced a higher formation of clusters than NF confirming their pro-tumorigenic potential [39]. In the migration studies of SKOV-3 cells, EC induced higher migration than when both types of fibroblasts were present, and of both stromal cocultures, migration in CAF was higher than NF. These effects are expected as it is known that both CAF and especially endothelial cells influence cancer invasiveness and metastasis in OC [9, 10, 23, 75]. In these studies, we observed that the cancer cells could interact with the stromal cell through the gaps between the pillars that separates the different compartments and recreate the tumor-stroma interactions, and also that the NF cultured for 14 days had low viability meanwhile CAF could still proliferate after 14 days in the tumor-on-a-chip thus the rest of the studies were performed for a maximum of 7 days in order to guarantee the viability of all cell types. Moreover, our microfluidic device allowed us to generate drug gradients as there was a delay of drug penetration in the cancer chamber compared to the stromal chamber, recapitulating the drug gradients in the TME. Even though there was a fast significant decrease of the fluid velocity across the device caused by the resistance of the 3D matrix [76], the laminar flow of the liquid injected in the circulation channel was still assured, confirming the ability to study drug pharmacokinetics in our microdevice. In future experiments, pressure-driven devices, such are syringe pumps [77] could be used to establish and control more physiologically relevant continuous flow rate and shear stress that can influence cancer cell stemness, invasiveness, and drug resistance [78, 79]. Furthermore, we confirmed that all cell types seeded in our microfluidic device could uptake the drug after 24 h of incubation indicating our device is a suitable platform for drug screening that could be used in preclinical studies in a time-optimal manner. Moreover, we have validated that our model could mimic the tumor-stroma interactions and the influence of stroma in drug response to standard-of-care therapy (combination of paclitaxel and carboplatin) in OC [80]. Of note, while drug response studies were performed with paclitaxel and carboplatin, cell uptake assay included doxorubicin due to its fluorescent properties. Several publications have benefited similarly of the doxorubicin fluorescent profile for penetration characterization [76, 81,82,83]. The therapeutic response was studied after 96 h of incubation with standard-of-care treatment to ensure their successful cell uptake and cytotoxic effect despite the fact they could have different diffusion patterns, molecular weights or mechanism of action than doxorubicin. As expected, normal stroma did not significantly influence the drug response in OC meanwhile the presence of CAF in the stromal compartment had a negative effect and caused drug resistance when compared to normal fibroblasts validating that the tumor-on-a-chip mimics the drug resistance mechanisms produced by CAF. The presence or the absence of the endothelial cells in the coculture with CAF did not change the therapeutic response in the OC cells indicating that CAF were the cell type responsible for the drug resistance. We used different 3D matrices in the different compartments due to the specific characteristics of the cells. A fibrinogen-based human plasma 3D model was used in the central chamber as the cancer cells proliferate well in this matrix and high-throughput drug screenings can be easily implemented [30]. The stromal chamber used a hybrid collagen:fibrinogen hydrogel to promote better proliferation in HUVEC [84] and to include extra collagen and stiffness to the stromal compartment. Using different matrices for the specific cell type is a common practice in others’ research to provide a specific environment for each cell type [47, 85, 86]. Importantly, we investigated the effect of the composition of the ECM and the 3D matrix in the central chamber on tumor therapeutic response [87]. We found that both a fibrinogen-based hydrogel, as well as a hybrid fibrinogen:collagen hydrogel in the central chamber caused similar drug resistance. Furthermore, we validated that the compartmentalization of our model is crucial for modeling CAF-driven drug resistance as cocultures of the cancer cells with EC and CAF in the central chamber could not recapitulate the same level of chemoresistance. Finally, our studies suggest that targeting TGF-β signaling with halofuginone can moderately rescue CAF-driven drug resistance to standard-of-care treatment. Targeting TGF-β in CAF cocultures decreased collagen I secretion and altered the high alignment of the collagen fibers, characteristics linked with higher tumor progression, drug resistance, and worse outcomes in the cancer patients [88,89,90]. The majority of the normal ovarian stroma comprises collagen type I with nonspecific fiber orientation/alignment, other minor matrix proteins, and stromal cells (e.g., fibroblasts and myofibroblasts) [91]. For example, the collagen in breast cancer is characterized by perpendicularly oriented fibers to the tumor boundary, whereas HGSC tumors display newly synthesized, densely packed, highly aligned wavy fibers [92]. Critically, these findings suggest that tumor-stroma interactions with CAF and endothelial cells and the biophysical properties of the OC-TME including hypoxia, ECM remodeling, and drug penetration are key contributors to OC chemoresistance. Moreover, we demonstrated that a precise recreation of the cellular crosstalk in the TME, as well as spatial organization through compartmentalization, are fundamental to determining the effect of these physical and biological mechanisms on drug resistance.
Currently, many studies have been conducted to assess the influence of the stroma in drug resistance in OC using different 3D preclinical models including spheroids, tumoroids, and 3D coculture models [59]. However, they do not recreate the compartmentalization, and dynamic properties of the TME [23, 26]. Therefore, microfluidic devices could bridge the gap between the preclinical in vitro and in vivo models as they are easy to use, high throughput, cost effective, and biocompatible, and allow to recapitulate compartmentalized TME, cell-cell interaction, gradients, cellular characteristics, migration, and fluid flow control [19, 27, 93, 94]. Several groups had developed microfluidic devices to assess the OC poor prognosis and study tumor-stroma interactions and migration of OC cells [95], biomarkers for early diagnosis [96], and shear stress [97]. For example, Ibrahim et al. developed a microchip to study the role of different stromal cell types such are mesothelial cells, endothelial cells, and adipocytes to model metastatic OC into peritoneum, but they did not model the primary OC tumors [52]. Dadgar et al. used OC patient-derived xenograft (PDX) tumors to generate spheroids in a multichambered microfluidic device demonstrating that the cell viability and epithelial markers in the cells grown in the microfluidic device were higher than in Matrigel 3D cultures but the generation of PDX models can be complicated, time-consuming and expensive and also it doesn’t meet the 3R (replacement, reduction, and refinement) for animal research [18, 98]. Alkmin et al. studied drug response to carboplatin in spheroids of different OC cell lines in channel-based microfluidic design with different collagen fiber morphology but they did not consider any stromal cell component [91]. Even though a similar approach was used to study tumor-stroma interactions in the microfluidic models for other cancer types such are breast cancer [85, 99, 100], pancreatic cancer [68, 101], glioma [47], colorectal [102], etc. Shirure et al. created a similar multi-compartmentalized model to recreate a hypoxic tumor microenvironment that induced higher tumor growth and migration of breast cancer cells, however, they generated hypoxia by injecting sodium sulfide [48], meanwhile, our design was bioengineered to recreate the hypoxic TME without the need for external agents. Also, microvascular networks were similarly created by other groups using the HUVEC cells in coculture with fibroblasts. Sewell-Loftin et al. used a similar model to study vascularization in the presence of normal fibroblasts or CAF and confirmed enhanced angiogenesis in the presence of CAF, but they did not study cancer behavior in this approach [103]. Angelidakis et al. generated microvascular networks in a simpler microfluidic device that consisted of one central chamber and two lateral media channels for studying the metastatic potential of breast cancer cells [50] and a similar design was used to incorporate spheroids from patient tissues and study monocyte transport into the tumor and immunotherapy efficacy by Nguyen et al. [51]. Moreover, Barcus et al. used a multi-compartmentalized microfluidic device similar to ours, to study the invasion and migration of mice breast cancer organoids and the influence of CAF using only one half of the device [49]. To our knowledge, our model is the first microfluidic model for ovarian cancer that takes into account the spatial distribution of the key cell types present in its heterogeneous and complex TME, their interactions, and ECM remodeling and also recapitulates the hypoxic TME, allowing to study their influence in the drug resistance and targeting therapies that could improve the OC patient outcomes that have not significantly changed in the last 50 years [1].
Despite the fascinating findings in our study, there are some limitations that we would like to acknowledge. We used cell lines to model the OC-TME, the incorporation of primary cells would be more physiologically relevant and would recapitulate better individual drug response in OC patients allowing personalized therapeutic efficacy prediction [19]. We acknowledge that the hydrostatic pressure difference for laminar flow generation inside the microfluidic device lacks dynamic control and there is a pressure drop as the media flows through the channel. Alternatives to control fluid shear stress and fluid flow should be investigated [77]. Additional CAF- or ECM-targeting drugs or combination of drugs [80] should be further explored, as well as validation of TME compartmentalization with additional cell types present in the OC-TME such as adipocytes, mesothelial cells [52], and immune cells [104]. Investigating tumor-immune interactions and immunotherapies could improve outcomes for OC patients, considering that high grade serous carcinoma was one of the first cancer types were the presence of tumor infiltrated lymphocytes was correlated with higher survival rates and CAF act as a barrier for immune infiltration and induce tumor inflammatory processes [28, 39]. As there is an urgent need for developing preclinical models for studying immune infiltration, immune-tumor interactions, and immunotherapy [104], our versatile model would be a suitable platform to perform these studies in the future as we were able to recreate the physical and chemical barrier by modeling hypoxia and CAF-induced ECM remodeling involved in the generation of an immunosuppressive OC-TME. Also, our model would allow automatization as it is compatible with standard glass slides for automatic confocal microscopy or automatic injection by liquid handler or syringe pumps. Moreover, additional studies could be performed to explore the influence of a higher height of the device (for example 200 μm) in the OC-TME recreation.
Conclusions
In conclusion, our results present a functionally characterized microvascularized multiniche tumor-on-a-chip able to recapitulate key spatial OC-TME compartmentalization including vascularization, ECM remodeling, oxygen gradients, and drug penetration kinetics, which directly influence drug resistance. Using this device, we have confirmed CAF’s role in modulating drug response and implemented targeted therapeutic approaches to overcome this chemoresistance. Our results are expected to have an important positive impact because they will provide strong evidence for providing new opportunities for improving chemotherapy effectiveness and mitigating chemoresistance in ovarian cancer.
Data availibility
The original contributions presented in the study are included in the article material, and further inquiries can be directed to the corresponding author.
References
Lisio, M. A., L. Fu, A. Goyeneche, Z. H. Gao, and C. Telleria. High-grade serous ovarian cancer: basic sciences, clinical and therapeutic standpoints. Int. J. Mol. Sci. 2019. https://doi.org/10.3390/ijms20040952.
Morand, S., M. Devanaboyina, H. Staats, L. Stanbery, and J. Nemunaitis. Ovarian cancer immunotherapy and personalized medicine. Int. J. Mol. Sci. 2021. https://doi.org/10.3390/ijms22126532.
Ortiz, M., E. Wabel, K. Mitchell, and S. Horibata. Mechanisms of chemotherapy resistance in ovarian cancer. Cancer Drug. Resist. 5(2):304–316, 2022. https://doi.org/10.20517/cdr.2021.147.
Gertych, A., A. E. Walts, K. Cheng, M. Liu, J. John, J. Lester, et al. Dynamic changes in the extracellular matrix in primary, metastatic, and recurrent ovarian cancers. Cells. 2022. https://doi.org/10.3390/cells11233769.
Mendoza-Martinez, A. K., D. Loessner, A. Mata, and H. S. Azevedo. Modeling the tumor microenvironment of ovarian cancer: the application of self-assembling biomaterials. Cancers (Basel). 2021. https://doi.org/10.3390/cancers13225745.
Alatise, K. L., S. Gardner, and A. Alexander-Bryant. Mechanisms of drug resistance in ovarian cancer and associated gene targets. Cancers (Basel). 2022. https://doi.org/10.3390/cancers14246246.
Guo, C., C. Song, J. Zhang, Y. Gao, Y. Qi, Z. Zhao, et al. Revisiting chemoresistance in ovarian cancer: mechanism, biomarkers, and precision medicine. Genes Dis. 9(3):668–681, 2022. https://doi.org/10.1016/j.gendis.2020.11.017.
Kim, S., Y. Han, S. I. Kim, H. S. Kim, S. J. Kim, and Y. S. Song. Tumor evolution and chemoresistance in ovarian cancer. NPJ Precis. Oncol. 2:20, 2018. https://doi.org/10.1038/s41698-018-0063-0.
Zhang, M., Z. Chen, Y. Wang, H. Zhao, and Y. Du. The role of cancer-associated fibroblasts in ovarian cancer. Cancers (Basel). 2022. https://doi.org/10.3390/cancers14112637.
Leung, C. S., T. L. Yeung, K. P. Yip, K. K. Wong, S. Y. Ho, L. S. Mangala, et al. Cancer-associated fibroblasts regulate endothelial adhesion protein LPP to promote ovarian cancer chemoresistance. J. Clin. Invest. 128(2):589–606, 2018. https://doi.org/10.1172/JCI95200.
Zhang, Y., H. Gan, F. Zhao, X. Ma, X. Xie, R. Huang, et al. CPEB4-promoted paclitaxel resistance in ovarian cancer in vitro relies on translational regulation of CSAG2. Front. Pharmacol. 11:600994, 2020. https://doi.org/10.3389/fphar.2020.600994.
Shih, H. J., H. F. Chang, C. L. Chen, and P. L. Torng. Differential expression of hypoxia-inducible factors related to the invasiveness of epithelial ovarian cancer. Sci. Rep. 11(1):22925, 2021. https://doi.org/10.1038/s41598-021-02400-1.
Fang, Y., M. Chen, G. Li, Y. Yang, P. He, J. Chen, et al. Cancer-associated fibroblast-like fibroblasts in vocal fold leukoplakia suppress CD8(+)T cell functions by inducing IL-6 autocrine loop and interacting with Th17 cells. Cancer Lett. 546:215839, 2022. https://doi.org/10.1016/j.canlet.2022.215839.
Huang, J., L. Zhang, D. Wan, L. Zhou, S. Zheng, S. Lin, et al. Extracellular matrix and its therapeutic potential for cancer treatment. Signal Transduct. Target. Ther. 6(1):153, 2021. https://doi.org/10.1038/s41392-021-00544-0.
Mallikarjuna, P., Y. Zhou, and M. Landstrom. The synergistic cooperation between TGF-beta and hypoxia in cancer and fibrosis. Biomolecules. 2022. https://doi.org/10.3390/biom12050635.
Huang, Y., Z. Chen, T. Lu, G. Bi, M. Li, J. Liang, et al. HIF-1alpha switches the functionality of TGF-beta signaling via changing the partners of smads to drive glucose metabolic reprogramming in non-small cell lung cancer. J. Exp. Clin. Cancer Res. 40(1):398, 2021. https://doi.org/10.1186/s13046-021-02188-y.
Selvendiran, K. Hypoxia induces chemoresistance in ovarian cancer cells by activation of signaltransducer and activator of transcription 3. Int. J. Cancer. 2009. https://doi.org/10.1002/ijc.24601.
Dadgar, N., A. M. Gonzalez-Suarez, P. Fattahi, X. Hou, J. S. Weroha, A. Gaspar-Maia, et al. A microfluidic platform for cultivating ovarian cancer spheroids and testing their responses to chemotherapies. Microsyst. Nanoeng. 6:93, 2020. https://doi.org/10.1038/s41378-020-00201-6.
Colombo, E., and M. G. Cattaneo. Multicellular 3D models to study tumour-stroma interactions. Int. J. Mol. Sci. 2021. https://doi.org/10.3390/ijms22041633.
Caballero, D., B. Kundu, C. M. Abreu, S. Amorim, D. C. Fernandes, R. A. Pires, et al. Forecast cancer: the importance of biomimetic 3D in vitro models in cancer drug testing/discovery and therapy. In Vitro Models. 1(2):119–123, 2022. https://doi.org/10.1007/s44164-022-00014-z.
Wong, C. H., K. W. Siah, and A. W. Lo. Estimation of clinical trial success rates and related parameters. Biostatistics. 20(2):273–286, 2019. https://doi.org/10.1093/biostatistics/kxx069.
Seyhan, A. A. Lost in translation: the valley of death across preclinical and clinical divide—identification of problems and overcoming obstacles. Transl. Med. Commun. 2019. https://doi.org/10.1186/s41231-019-0050-7.
Li, W., Z. Zhou, X. Zhou, B. L. Khoo, R. Gunawan, Y. R. Chin, et al. 3D biomimetic models to reconstitute tumor microenvironment in vitro: spheroids, organoids, and tumor-on-a-chip. Adv. Healthc. Mater. 12(18):e2202609, 2023. https://doi.org/10.1002/adhm.202202609.
Cauli, E., M. A. Polidoro, S. Marzorati, C. Bernardi, M. Rasponi, and A. Lleo. Cancer-on-chip: a 3D model for the study of the tumor microenvironment. J. Biol. Eng. 17(1):53, 2023. https://doi.org/10.1186/s13036-023-00372-6.
Wadman, M. FDA no longer has to require animal testing for new drugs. Science. 379(6628):127–128, 2023. https://doi.org/10.1126/science.adg6276.
Rodrigues, J., M. A. Heinrich, L. M. Teixeira, and J. Prakash. 3D in vitro model (r)evolution: unveiling tumor-stroma interactions. Trends Cancer. 7(3):249–264, 2021. https://doi.org/10.1016/j.trecan.2020.10.009.
Mehta, P., Z. Rahman, P. Ten Dijke, and P. E. Boukany. Microfluidics meets 3D cancer cell migration. Trends Cancer. 8(8):683–697, 2022. https://doi.org/10.1016/j.trecan.2022.03.006.
Axemaker, H., S. Plesselova, K. Calar, M. Jorgensen, J. Wollman, and P. de la Puente. Normal uterine fibroblast are reprogramed into ovarian cancer-associated fibroblasts by ovarian tumor-derived conditioned media. bioRxiv. 2023. https://doi.org/10.1101/2023.09.29.560158.
Bhattacharya, S., K. Calar, C. Evans, M. Petrasko, and P. de la Puente. Bioengineering the oxygen-deprived tumor microenvironment within a three-dimensional platform for studying tumor-immune interactions. Front. Bioeng. Biotechnol. 8:1040, 2020. https://doi.org/10.3389/fbioe.2020.01040.
Calar, K., S. Plesselova, S. Bhattacharya, M. Jorgensen, and P. de la Puente. Human plasma-derived 3D cultures model breast cancer treatment responses and predict clinically effective drug treatment concentrations. Cancers (Basel). 2020. https://doi.org/10.3390/cancers12071722.
Bhattacharya, S., K. Calar, and P. de la Puente. Mimicking tumor hypoxia and tumor-immune interactions employing three-dimensional in vitro models. J. Exp. Clin. Cancer Res. 39(1):75, 2020. https://doi.org/10.1186/s13046-020-01583-1.
Komeya, M., K. Hayashi, H. Nakamura, H. Yamanaka, H. Sanjo, K. Kojima, et al. Pumpless microfluidic system driven by hydrostatic pressure induces and maintains mouse spermatogenesis in vitro. Sci. Rep. 7(1):15459, 2017. https://doi.org/10.1038/s41598-017-15799-3.
Elias, K. M., M. M. Emori, E. Papp, E. MacDuffie, G. E. Konecny, V. E. Velculescu, et al. Beyond genomics: critical evaluation of cell line utility for ovarian cancer research. Gynecol. Oncol. 139(1):97–103, 2015. https://doi.org/10.1016/j.ygyno.2015.08.017.
Huang, C. P., J. Lu, H. Seon, A. P. Lee, L. A. Flanagan, H. Y. Kim, et al. Engineering microscale cellular niches for three-dimensional multicellular co-cultures. Lab. Chip. 9(12):1740–1748, 2009. https://doi.org/10.1039/b818401a.
Haley, J., S. Tomar, N. Pulliam, S. Xiong, S. M. Perkins, A. R. Karpf, et al. Functional characterization of a panel of high-grade serous ovarian cancer cell lines as representative experimental models of the disease. Oncotarget. 7(22):32810–32820, 2016. https://doi.org/10.18632/oncotarget.9053.
Kerslake, R., B. Belay, S. Panfilov, M. Hall, I. Kyrou, H. S. Randeva, et al. Transcriptional landscape of 3D vs 2D ovarian cancer cell models. Cancers (Basel). 2023. https://doi.org/10.3390/cancers15133350.
Domcke, S., R. Sinha, D. A. Levine, C. Sander, and N. Schultz. Evaluating cell lines as tumour models by comparison of genomic profiles. Nat. Commun. 4:2126, 2013. https://doi.org/10.1038/ncomms3126.
Zhang, Q., J. Ding, Y. Wang, L. He, and F. Xue. Tumor microenvironment manipulates chemoresistance in ovarian cancer (review). Oncol. Rep. 2022. https://doi.org/10.3892/or.2022.8313.
Feng, B., J. Wu, B. Shen, F. Jiang, and J. Feng. Cancer-associated fibroblasts and resistance to anticancer therapies: status, mechanisms, and countermeasures. Cancer Cell. Int. 22(1):166, 2022. https://doi.org/10.1186/s12935-022-02599-7.
Huang, C. Y., C. L. Chung, T. H. Hu, J. J. Chen, P. F. Liu, and C. L. Chen. Recent progress in TGF-beta inhibitors for cancer therapy. Biomed. Pharmacother. 134:111046, 2021. https://doi.org/10.1016/j.biopha.2020.111046.
Yang, Y., Y. Yang, J. Yang, X. Zhao, and X. Wei. Tumor microenvironment in ovarian cancer: function and therapeutic strategy. Front. Cell Dev. Biol. 8:758, 2020. https://doi.org/10.3389/fcell.2020.00758.
Jeon, et al. Human 3D vascularized organotypic microfluidic assays to study breast cancer cell extravasation. Proc. Natl. Acad. Sci. USA. 112(7):E818, 2015. https://doi.org/10.1073/pnas.1501426112.
Quintard, C., G. Jonsson, C. Laporte, C. Bissardon, A. Pitaval, N. Werschler, et al. An automated microfluidic platform integrating functional vascularized organoids-on-chip. bioRxiv. 2021. https://doi.org/10.1101/2021.12.29.474327.
Kim, S., W. Kim, S. Lim, and J. S. Jeon. Vasculature-on-a-chip for in vitro disease models. Bioengineering (Basel). 2017. https://doi.org/10.3390/bioengineering4010008.
Silvestri, V. L., E. Henriet, R. M. Linville, A. D. Wong, P. C. Searson, and A. J. Ewald. A tissue-engineered 3D microvessel model reveals the dynamics of mosaic vessel formation in breast cancer. Cancer Res. 80(19):4288–4301, 2020. https://doi.org/10.1158/0008-5472.CAN-19-1564.
Nguyen, D. H., S. C. Stapleton, M. T. Yang, S. S. Cha, C. K. Choi, P. A. Galie, et al. Biomimetic model to reconstitute angiogenic sprouting morphogenesis in vitro. Proc. Natl. Acad. Sci. USA. 110(17):6712–6717, 2013. https://doi.org/10.1073/pnas.1221526110.
Adjei-Sowah, E. A., S. A. O’Connor, J. Veldhuizen, C. Lo Cascio, C. Plaisier, S. Mehta, et al. Investigating the interactions of glioma stem cells in the perivascular niche at single-cell resolution using a microfluidic tumor microenvironment model. Adv. Sci. (Weinh). 9(21):e2201436, 2022. https://doi.org/10.1002/advs.202201436.
Shirure, V. S., S. F. Lam, B. Shergill, Y. E. Chu, N. R. Ng, and S. C. George. Quantitative design strategies for fine control of oxygen in microfluidic systems. Lab. Chip. 20(16):3036–3050, 2020. https://doi.org/10.1039/d0lc00350f.
Barcus, C. E., P. Y. Hwang, V. Morikis, A. Brenot, P. Pence, M. Clarke, et al. Tyrosine kinase-independent actions of DDR2 in tumor cells and cancer-associated fibroblasts influence tumor invasion, migration and metastasis. J. Cell Sci. 2021. https://doi.org/10.1242/jcs.258431.
Angelidakis, E., S. Chen, S. Zhang, Z. Wan, R. D. Kamm, and S. E. Shelton. Impact of fibrinogen, fibrin thrombi, and thrombin on cancer cell extravasation using in vitro microvascular networks. Adv. Healthc. Mater. 12(19):e2202984, 2023. https://doi.org/10.1002/adhm.202202984.
Nguyen, H. T., N. Gurvich, M. R. Gillrie, G. Offeddu, M. Humayun, E. L. Kan, et al. Patient-specific vascularized tumor model: blocking TAM recruitment with multispecific antibodies targeting CCR2 and CSF-1R. bioRxiv. 2023. https://doi.org/10.1101/2023.11.28.568627.
Ibrahim, L. I., C. Hajal, G. S. Offeddu, M. R. Gillrie, and R. D. Kamm. Omentum-on-a-chip: a multicellular, vascularized microfluidic model of the human peritoneum for the study of ovarian cancer metastases. Biomaterials. 288:121728, 2022. https://doi.org/10.1016/j.biomaterials.2022.121728.
Shih, I. M., Y. Wang, and T. L. Wang. The origin of ovarian cancer species and precancerous landscape. Am. J. Pathol. 191(1):26–39, 2021. https://doi.org/10.1016/j.ajpath.2020.09.006.
Rangasudhagar Radhakrishnan, J. H. H., M. Jayaraman, J. Liu, K. M. Moxley, Ciro Isidoro, A. K. Sood, Y. S. Song, and D. N. Dhanasekaran. Ovarian cancer cell-derived lysophosphatidic acid induces glycolytic shift and cancer-associated fibroblast-phenotype in normal and peritumoral fibroblasts. Cancer Lett. 442:464–474, 2019. https://doi.org/10.1016/j.canlet.2018.11.023.
Mohammad Hadi, L., K. Stamati, E. Yaghini, A. J. MacRobert, and M. Loizidou. Treatment of 3D in vitro tumoroids of ovarian cancer using photochemical internalisation as a drug delivery method. Biomedicines. 2023. https://doi.org/10.3390/biomedicines11020572.
Zhou, Z. Q., Y. Chen, M. Chai, R. Tao, Y. H. Lei, Y. Q. Jia, et al. Adipose extracellular matrix promotes skin wound healing by inducing the differentiation of adipose-derived stem cells into fibroblasts. Int. J. Mol. Med. 43(2):890–900, 2019. https://doi.org/10.3892/ijmm.2018.4006.
Denu, R. A., S. Nemcek, D. D. Bloom, A. D. Goodrich, J. Kim, D. F. Mosher, et al. Fibroblasts and mesenchymal stromal/stem cells are phenotypically indistinguishable. Acta Haematol. 136(2):85–97, 2016. https://doi.org/10.1159/000445096.
Yeung, T. L., C. S. Leung, K. K. Wong, G. Samimi, M. S. Thompson, J. Liu, et al. TGF-beta modulates ovarian cancer invasion by upregulating CAF-derived versican in the tumor microenvironment. Cancer Res. 73(16):5016–5028, 2013. https://doi.org/10.1158/0008-5472.CAN-13-0023.
Franchi-Mendes, T., R. Eduardo, G. Domenici, and C. Brito. 3D cancer models: depicting cellular crosstalk within the tumour microenvironment. Cancers (Basel). 2021. https://doi.org/10.3390/cancers13184610.
Zhao, Z., T. Li, L. Sun, Y. Yuan, and Y. Zhu. Potential mechanisms of cancer-associated fibroblasts in therapeutic resistance. Biomed. Pharmacother. 166:115425, 2023. https://doi.org/10.1016/j.biopha.2023.115425.
Niu, N., J. Yao, R. C. Bast, A. K. Sood, and J. Liu. IL-6 promotes drug resistance through formation of polyploid giant cancer cells and stromal fibroblast reprogramming. Oncogenesis. 10(9):65, 2021. https://doi.org/10.1038/s41389-021-00349-4.
Ashrafizadeh, M., A. Zarrabi, K. Hushmandi, M. Kalantari, R. Mohammadinejad, T. Javaheri, et al. Association of the epithelial-mesenchymal transition (EMT) with cisplatin resistance. Int. J. Mol. Sci. 2020. https://doi.org/10.3390/ijms21114002.
Guo, H., C. Ha, H. Dong, Z. Yang, Y. Ma, and Y. Ding. Cancer-associated fibroblast-derived exosomal microRNA-98-5p promotes cisplatin resistance in ovarian cancer by targeting CDKN1A. Cancer Cell. Int. 19:347, 2019. https://doi.org/10.1186/s12935-019-1051-3.
Rafii, A., P. Mirshahi, M. Poupot, A. M. Faussat, A. Simon, E. Ducros, et al. Oncologic trogocytosis of an original stromal cells induces chemoresistance of ovarian tumours. PLoS One. 3(12):e3894, 2008. https://doi.org/10.1371/journal.pone.0003894.
Zhu, H., X. Gu, L. Xia, Y. Zhou, H. Bouamar, J. Yang, et al. A novel TGFbeta trap blocks chemotherapeutics-induced TGFbeta1 signaling and enhances their anticancer activity in gynecologic cancers. Clin. Cancer Res. 24(12):2780–2793, 2018. https://doi.org/10.1158/1078-0432.CCR-17-3112.
Huang, Y., J. Yuan, E. Righi, W. S. Kamoun, M. Ancukiewicz, J. Nezivar, et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc. Natl. Acad. Sci. USA. 109(43):17561–17566, 2012. https://doi.org/10.1073/pnas.1215397109.
Mi, L., Y. Zhang, A. Su, M. Tang, Z. Xing, T. He, et al. Halofuginone for cancer treatment: a systematic review of efficacy and molecular mechanisms. J. Funct. Foods. 2022. https://doi.org/10.1016/j.jff.2022.105237.
Geyer, M., L. M. Gaul, S. L. D’Agosto, V. Corbo, and K. Queiroz. The tumor stroma influences immune cell distribution and recruitment in a PDAC-on-a-chip model. Front. Immunol. 14:1155085, 2023. https://doi.org/10.3389/fimmu.2023.1155085.
Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer. 16(9):582–598, 2016. https://doi.org/10.1038/nrc.2016.73.
Klemba, A., L. Bodnar, H. Was, K. K. Brodaczewska, G. Wcislo, C. A. Szczylik, et al. Hypoxia-mediated decrease of ovarian cancer cells reaction to treatment: significance for chemo- and immunotherapies. Int. J. Mol. Sci. 2020. https://doi.org/10.3390/ijms21249492.
Jun, B. H., T. Guo, S. Libring, M. K. Chanda, J. S. Paez, A. Shinde, et al. Fibronectin-expressing mesenchymal tumor cells promote breast cancer metastasis. Cancers (Basel). 2020. https://doi.org/10.3390/cancers12092553.
Lin, T. C., C. H. Yang, L. H. Cheng, W. T. Chang, Y. R. Lin, and H. C. Cheng. Fibronectin in cancer: friend or foe. Cells. 2019. https://doi.org/10.3390/cells9010027.
Petrini, I., S. Barachini, V. Carnicelli, S. Galimberti, L. Modeo, R. Boni, et al. ED-B fibronectin expression is a marker of epithelial-mesenchymal transition in translational oncology. Oncotarget. 8(3):4914–4921, 2017. https://doi.org/10.18632/oncotarget.13615.
Loh, C. Y., J. Y. Chai, T. F. Tang, W. F. Wong, G. Sethi, M. K. Shanmugam, et al. The E-CADHERIN and N-Cadherin switch in epithelial-to-mesenchymal transition: signaling, therapeutic implications, and challenges. Cells. 2019. https://doi.org/10.3390/cells8101118.
Fang, Z., Q. Meng, J. Xu, W. Wang, B. Zhang, J. Liu, et al. Signaling pathways in cancer-associated fibroblasts: recent advances and future perspectives. Cancer Commun. (Lond). 43(1):3–41, 2023. https://doi.org/10.1002/cac2.12392.
Ayuso, J. M., M. Virumbrales-Munoz, A. Lacueva, P. M. Lanuza, E. Checa-Chavarria, P. Botella, et al. Development and characterization of a microfluidic model of the tumour microenvironment. Sci. Rep. 6:36086, 2016. https://doi.org/10.1038/srep36086.
Iakovlev, A. P., A. S. Erofeev, and P. V. Gorelkin. Novel pumping methods for microfluidic devices: a comprehensive review. Biosensors (Basel). 2022. https://doi.org/10.3390/bios12110956.
Nath, S., M. Pigula, A. P. Khan, W. Hanna, M. K. Ruhi, F. M. Dehkordy, et al. Flow-induced shear stress confers resistance to carboplatin in an adherent three-dimensional model for ovarian cancer: a role for EGFR-targeted photoimmunotherapy informed by physical stress. J. Clin. Med. 2020. https://doi.org/10.3390/jcm9040924.
Ip, C. K., S. S. Li, M. Y. Tang, S. K. Sy, Y. Ren, H. C. Shum, et al. Stemness and chemoresistance in epithelial ovarian carcinoma cells under shear stress. Sci. Rep. 6:26788, 2016. https://doi.org/10.1038/srep26788.
Cortez, A. J., P. Tudrej, K. A. Kujawa, and K. M. Lisowska. Advances in ovarian cancer therapy. Cancer Chemother. Pharmacol. 81(1):17–38, 2018. https://doi.org/10.1007/s00280-017-3501-8.
Grist, S. M., S. S. Nasseri, L. Laplatine, J. C. Schmok, D. Yao, J. Hua, et al. Long-term monitoring in a microfluidic system to study tumour spheroid response to chronic and cycling hypoxia. Sci. Rep. 9(1):17782, 2019. https://doi.org/10.1038/s41598-019-54001-8.
Degerstedt, O., P. O’Callaghan, A. L. Clavero, J. Grasjo, O. Eriksson, E. Sjogren, et al. Quantitative imaging of doxorubicin diffusion and cellular uptake in biomimetic gels with human liver tumor cells. Drug Deliv. Transl. Res. 14(4):970–983, 2024. https://doi.org/10.1007/s13346-023-01445-1.
Moon, H. S., C. E. Yoo, S. Kim, J. E. Lee, and W. Y. Park. Application of an open-chamber multi-channel microfluidic device to test chemotherapy drugs. Sci. Rep. 10(1):20343, 2020. https://doi.org/10.1038/s41598-020-77324-3.
Sgarioto, M., P. Vigneron, J. Patterson, F. Malherbe, M. D. Nagel, and C. Egles. Collagen type I together with fibronectin provide a better support for endothelialization. Comptes Rendus Biol. 335(8):520–528, 2012. https://doi.org/10.1016/j.crvi.2012.07.003.
Truong, D. D., A. Kratz, J. G. Park, E. S. Barrientos, H. Saini, T. Nguyen, et al. A human organotypic microfluidic tumor model permits investigation of the interplay between patient-derived fibroblasts and breast cancer cells. Cancer Res. 79(12):3139–3151, 2019. https://doi.org/10.1158/0008-5472.CAN-18-2293.
Agrawal, A., S. Shahreza, Y. Javanmardi, N. Szita, and E. Moeendarbary. The tumour microenvironment modulates cancer cell intravasation. Organs-on-a-Chip. 2022. https://doi.org/10.1016/j.ooc.2022.100024.
Langhans, S. A. Three-dimensional in vitro cell culture models in drug discovery and drug repositioning. Front. Pharmacol. 9:6, 2018. https://doi.org/10.3389/fphar.2018.00006.
Nissen, N. I., A. Z. Johansen, I. Chen, J. S. Johansen, R. S. Pedersen, C. P. Hansen, et al. Collagen biomarkers quantify fibroblast activity in vitro and predict survival in patients with pancreatic ductal adenocarcinoma. Cancers (Basel). 2022. https://doi.org/10.3390/cancers14030819.
Belhabib, I., S. Zaghdoudi, C. Lac, C. Bousquet, and C. Jean. Extracellular matrices and cancer-associated fibroblasts: targets for cancer diagnosis and therapy? Cancers (Basel). 2021. https://doi.org/10.3390/cancers13143466.
Nadiarnykh, O., R. B. LaComb, M. A. Brewer, and P. J. Campagnola. Alterations of the extracellular matrix in ovarian cancer studied by second harmonic generation imaging microscopy. BMC Cancer. 10:94, 2010. https://doi.org/10.1186/1471-2407-10-94.
Alkmin, S., R. Brodziski, H. Simon, D. Hinton, R. H. Goldsmith, M. Patankar, et al. Role of collagen fiber morphology on ovarian cancer cell migration using image-based models of the extracellular matrix. Cancers (Basel). 2020. https://doi.org/10.3390/cancers12061390.
Rentchler, E. C., K. L. Gant, R. Drapkin, M. Patankar, and P. J. Campagnola. Imaging collagen alterations in STICs and high grade ovarian cancers in the fallopian tubes by second harmonic generation microscopy. Cancers (Basel). 2019. https://doi.org/10.3390/cancers11111805.
Byrne, M. B., M. T. Leslie, H. R. Gaskins, and P. J. A. Kenis. Methods to study the tumor microenvironment under controlled oxygen conditions. Trends Biotechnol. 32(11):556–563, 2014. https://doi.org/10.1016/j.tibtech.2014.09.006.
Virumbrales-Muñoz, M., and J. M. Ayuso. From microfluidics to microphysiological systems: Past, present, and future. Organs-on-a-Chip. 2022. https://doi.org/10.1016/j.ooc.2022.100015.
Lopez, E., S. Kamboj, C. Chen, Z. Wang, S. Kellouche, J. Leroy-Dudal, et al. In vitro models of ovarian cancer: bridging the gap between pathophysiology and mechanistic models. Biomolecules. 2023. https://doi.org/10.3390/biom13010103.
Dabbagh Moghaddam, F., D. Dadgar, Y. Esmaeili, S. Babolmorad, E. Ilkhani, M. Rafiee, et al. Microfluidic platforms in diagnostic of ovarian cancer. Environ. Res. 237(Pt 2):117084, 2023. https://doi.org/10.1016/j.envres.2023.117084.
Rizvi, I., U. A. Gurkan, S. Tasoglu, N. Alagic, J. P. Celli, L. B. Mensah, et al. Flow induces epithelial-mesenchymal transition, cellular heterogeneity and biomarker modulation in 3D ovarian cancer nodules. Proc. Natl. Acad. Sci. USA. 110(22):E1974–E1983, 2013. https://doi.org/10.1073/pnas.1216989110.
Pape, J., M. Emberton, and U. Cheema. 3D cancer models: the need for a complex stroma, compartmentalization and stiffness. Front. Bioeng. Biotechnol. 2021. https://doi.org/10.3389/fbioe.2021.660502.
Rogers, M., T. Sobolik, D. K. Schaffer, P. C. Samson, A. C. Johnson, P. Owens, et al. Engineered microfluidic bioreactor for examining the three-dimensional breast tumor microenvironment. Biomicrofluidics. 12(3):034102, 2018. https://doi.org/10.1063/1.5016433.
Truong, D., J. Puleo, A. Llave, G. Mouneimne, R. D. Kamm, and M. Nikkhah. Breast cancer cell invasion into a three dimensional tumor-stroma microenvironment. Sci. Rep. 6:34094, 2016. https://doi.org/10.1038/srep34094.
Lee, J. H., S. K. Kim, I. A. Khawar, S. Y. Jeong, S. Chung, and H. J. Kuh. Microfluidic co-culture of pancreatic tumor spheroids with stellate cells as a novel 3D model for investigation of stroma-mediated cell motility and drug resistance. J. Exp. Clin. Cancer Res. 37(1):4, 2018. https://doi.org/10.1186/s13046-017-0654-6.
Seaman, K., Y. Sun, and L. You. Recent advances in cancer-on-a-chip tissue models to dissect the tumour microenvironment. Med-X. 2023. https://doi.org/10.1007/s44258-023-00011-1.
Sewell-Loftin, M. K., J. B. Katz, S. C. George, and G. D. Longmore. Micro-strains in the extracellular matrix induce angiogenesis. Lab Chip. 20(15):2776–2787, 2020. https://doi.org/10.1039/d0lc00145g.
Lee, J. Y., and O. Chaudhuri. Modeling the tumor immune microenvironment for drug discovery using 3D culture. APL Bioeng. 5(1):010903, 2021. https://doi.org/10.1063/5.0030693.
Acknowledgements
This project used Sanford Research Histology and Imaging Core and the Flow Cytometry Core Facilities that are supported in part by a Center for Cancer Research CoBRE grant from the National Institutes of Health (5P20GM103548). We want to thank Jared Wollman and Kelly Graber from the Sanford Research Flow Cytometry and Histology and Imaging Cores, respectively for training and help in experimental set up. Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award number R21CA259158 (P.P), American Cancer Society Research Scholar RSG-23-1149466-01-CDP (P.P), and Pilot and Feasibility Enabling Technologies Award from Sanford Research and Sanford Health Foundation (P.P). E.S was funded by SPUR summer program under award R25HD097633. Work at South Dakota State University was supported by the National Institute of General Medical Sciences of the National Institutes of Health COBRE under award number P20GM135008 and National Science Foundation/EPSCoR Grant No. 0091948. We also acknowledge the South Dakota State University Functional Genomics Core Facility (RRID: SCR_023786). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding institutions.
Funding
This work was funded by National Cancer Institute, R21CA259158, Pilar de la Puente, Sanford Health Foundation, National Institute of Health, 5P20GM103548, Pilar de la Puente, American Cancer Society, RSG-23-1149466-01-CDP, Pilar de la Puente.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Pilar de la Puente and Kristin Calar have a patent for the 3D culture method described in this manuscript, US Patent Application #2022/0228124. Pilar de la Puente is the co-founder of Cellatrix LLC; however, there has been no contribution of the aforementioned entity to the current study. Simona Plesselova, Hailey Axemaker, Emma Sahly, Amrita Bhagia, Jessica Faragher, and Darci Fink state no conflicts of interest.
Ethical Approval
This research did not involve animal experiments. The studies involving human participants were reviewed and approved by Sanford Health Institutional Review Board. The patients/participants provided their written informed consent to participate in this study.
Additional information
Associate Editor Michael R. King oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (MP4 2853 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Plesselova, S., Calar, K., Axemaker, H. et al. Multicompartmentalized Microvascularized Tumor-on-a-Chip to Study Tumor-Stroma Interactions and Drug Resistance in Ovarian Cancer. Cel. Mol. Bioeng. (2024). https://doi.org/10.1007/s12195-024-00817-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12195-024-00817-y