Abstract
Conservation efforts have raised awareness about the impact of small-scale fisheries on the distribution of seagrass plants. The patterns of recovery of the seagrass Zostera noltei and of the commercial bivalves Cerastoderma edule, Ruditapes decussatus and Ruditapes philippinarum after shellfish harvesting were studied in a field experiment in a shellfish bed in NW Spain. Sample plots were subjected to a single disturbance in two types of shellfish harvesting treatments in three zones characterized by different harvesting frequency and seagrass density. The photosynthetic efficiency (Fv/Fm), shoot density, leaf length and carbohydrate content of Z. noltei were monitored every three months during one year, and the C and N content of leaves and biomass of plants were measured one year after the disturbance. The abundance of adults, juveniles and recruits and the condition index of adult bivalves were quantified after the experimental harvesting. Shoot density and biomass of Z. noltei remained low during the ten months after the disturbance but recovered to control values after one year. Carbohydrate contents of apical rhizomes were lower in disturbed (treated) plots, whereas no effect was observed on Fv/Fm. Denser and more complex seagrass patches recovered faster. The abundance of adult bivalves below commercial size was lower in the disturbed plots, while the abundance of adults of commercial size, juveniles and recruits did not vary, indicating that abundance and condition were not hampered by harvesting pressure. The findings also suggest that Z. noltei meadows can recover within one year of the impact of shellfish harvesting if the harvesting areas are rotated and dense patches are preserved.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Seagrass meadows are soft-bottom intertidal ecosystems that are usually located at the interface between land and sea. They are formed by angiosperm plants that have evolved to adapt to the marine environment and comprise both large, stable species and small colonizing, opportunistic species (Hemminga and Duarte 2000). Overall, seagrass meadows play an important ecological role, forming the basis of marine food webs and, also providing habitats for diverse faunal communities (Hemminga and Duarte 2000; Carvalho et al. 2006 and references therein). From an anthropocentric perspective, the seagrasses retain organic and inorganic pollutants in the sediment below their rhizosphere, protect the coast against floods or sea level rise, maintain fisheries and, also capture atmospheric carbon (Bas-Ventín et al. 2015). Seagrass ecosystems are therefore characterised as highly valuable, and scientists have focused on protecting and restoring them in recent years (Orth and Heck 2023).
However, the restrictions associated with the protection and conservation of seagrasses can affect other marine activities such as recreation and jobs or food provisioning associated with shellfisheries (Guimarães et al. 2012, Bas-Ventín et al. 2015). Specifically, a global meta-analysis of the interactions between shellfisheries and seagrass revealed shellfishing activities to be among the major threats to seagrass and that the coexistence of shellfisheries and seagrass generate more trade-offs than synergies, specifically because shellfish harvesters physically disturb the meadows with their fishing gear (Dumbauld et al. 2009; Herrera et al. 2022). However, other anthropogenic pressures such as fillings or boats anchoring and mooring, even pose a greater risk for seagrasses conservation (Short and Wyllie-Echevarria 1996, Orth 2006). Although on-bottom bivalve aquaculture (performed directly on the substrate without predation exclusion devices) generally decreases the density of Zostera spp., it also increases growth of these plants; in addition, no effects on the biomass or reproduction of these seagrasses have been reported. However, stronger impacts will lead to longer recovery times (Ferriss et al. 2019).
Ecological disturbance can be defined as an abiotic or biotic process that causes a perturbation in an ecological component or system, relative to a specified reference state. The effects of disturbance and the definition of recovery depend on the reference state (optimal or a pre-existent state), the temporal scale (acute or chronic disturbance) and organizational level (individual to ecosystem) considered (Rykiel 1985). With respect to seagrasses, physical disturbance of the same magnitude (surface, intensity and frequency) applied to a part of a meadow may have less impact on small species, such as Zostera noltei Hornemann, which grow faster than larger species (Duarte 1991). For instance, after shellfishing disturbance in the Ria Formosa (Portugal), the density of Z. noltei shoots recovered to pre-disturbance values after one month (Cabaço et al. 2005). Similarly, in Spain, clam digging in Z. noltei beds resulted in increased shoot density during recovery (4 to 7 months after impact) (Garmendia et al. 2017). Cartographic studies have shown that the spatial cover of Z. noltei patches increased during the growing season in zones chronically disturbed by shellfish harvesting (Román et al. 2022a, Román et al. 2024a). By contrast, the spatial cover increased by 56% over a period of 25 years, corresponding with a ban on shellfish extraction (Román et al. 2020). In Bourgneuf Bay (France), the extent and density of Z. noltei cover increased during 36 years in a meadow disturbed by on-bottom oyster aquaculture structures (Barillé et al. 2010, Zoffoli et al. 2021). After a general decline in Z. noltei cover, the area of some European meadows has increased in recent years (Bertelli et al. 2017; de los Santos et al. 2019), in a change in trend that can be partly explained by the greater resilience of Z. noltei to physical disturbance and by the fact that the recovery of seagrass habitat after disturbance depends on the intensity and frequency of disturbance, as well as on the seagrass species and its life traits (O’Brien et al. 2018, Ferriss et al. 2019).
Bivalve shellfisheries in soft-bottom substrates very often coexist with seagrass meadows (Park et al. 2011; Bas-Ventín et al. 2015) and are subjected to the same stressors. Nonetheless, previous research has shown that the effects of shellfish harvesting on the recruitment and abundance of commercial bivalves, in both vegetated and non-vegetated sediments, are ambiguous. Paradoxically, some studies have indicated that disturbing the sediment enhances the productivity of bivalve stocks. For instance, recruitment of Ruditapes philippinarum (Adams and Reeve, 1850) in areas of Willapa Bay (USA) ploughed in preparation for clam farming was greater in the absence than in the presence of eelgrass beds, apparently due to enhanced fixation of recruits on the bare gravel (Tsai et al. 2010). Moreover, Venerupis corrugata (Gmelin, 1791) was more abundant in Zostera marina Linnaeus meadows in sites where clam harvesting was carried out than in unharvested sites (Barañano et al. 2017). The increase in clam biomass in unvegetated harvested areas can be explained by the enhanced food availability caused by sediment resuspension (Libralato et al. 2002). Nonetheless, the presence of seagrass may be positive for infaunal bivalves, as field experiments have shown that large seagrass patches protect juvenile clams against predation (Irlandi 1997) and environmental stress (Román et al. 2022b). Moreover, recruitment of the commercial clam Mercenaria mercenaria (Linnaeus, 1758) after manual clam harvesting tended to be lower in bare sediment than in seagrass (Peterson et al. 1987). Extrapolating general changes in abundance, condition or recruitment of bivalves to harvesting disturbance is complex, as the changes vary depending on the substrate type (bare or vegetated sediment), the method (mechanical or manual) (Peterson et al. 1987), the size of the disturbed area and the recovery time (Kaiser et al. 2001), and the frequency of disturbance (Brown and Wilson 1997).
The coastline of Galicia (NW Spain), of length 1700 km, encompasses 30.13 km2 of Zostera meadows, of which Z. noltei is the predominant species (25.52 km2 covered by Z. noltei vs. 4.61 km2 covered by Z. marina), although the size of some meadows has gradually decreased in recent decades due to alteration of the coastline and disturbance by shellfishing (Cacabelos et al. 2015). There is evidence that this coastline does not reflect the recently reported reversal in the trend of seagrass loss. Since 1960, the rise in demand for bivalves has caused an increase in commercialisation of clams and has led to the professionalisation of shellfishing activities (creation of regulations, licensing systems and the establishment of fishers’ associations) and to the intensification of harvesting pressure (Frangoudes et al. 2008). In this respect, correlational (Barañano et al. 2017, 2022) and observational (Garcia-Redondo et al. 2019) studies focused on subtidal Z. marina in NW Spain have detected negative effects of clam harvesting from boats on the cover, biomass and density of the plants. However, the causality of the relationship between the impact of shellfishing and the growth and survival of Zostera spp. has not yet been specifically investigated in controlled field experiments in this region. Furthermore, no such studies have been conducted with Z. noltei, the most abundant seagrass in intertidal meadows where shellfishing generally takes place on foot, and which are exposed to manual shellfish harvesting and also to trampling and mechanical tillage.
Galician bivalve shellfisheries are traditionally very important sources of employment and income in coastal areas (Frangoudes et al. 2008). Ruditapes philippinarum, a native of southeastern Asia, was introduced to NW Spain for aquaculture in the 1980s, and it is nowadays seeded in shellfishing beds to preserve stocks (Flassch and Leborgne 1992; Frangoudes et al. 2008). It is currently the main contributor to clam landings (2262.831 tons of R. philippinarum and 260.748 tons of R. decussatus landed in 2022, respectively), because it is more resistant to environmental stress (Domínguez et al., 2020; Domínguez et al. 2021; Román et al. 2022b; Román et al. 2023a; Román et al. 2024b) than the native Ruditapes decussatus (Linnaeus, 1758), thus enhancing the establishment of natural populations in Europe. Despite this, previous research in northern Spain demonstrated that the increasing density of R. philippinarum did not affect the growth or mortality of its native congener R. decussatus (Bidegain and Juanes 2013). Cerastoderma edule (Linnaeus 1758) is a highly productive native species (2299.203 tons landed in 2022), although it is more vulnerable to thermal and saline fluctuations (Domínguez et al. 2020; Domínguez et al. 2021). Although there is a general perception amongst fishers that the presence of seagrass hampers the recruitment and settlement of clams (Herrera et al. 2022), recent studies have demonstrated the positive role of Z. noltei on both clam species under environmental stress. Under an experimental heatwave, Z. noltei dissipated heat more efficiently than bare sediment, which allowed R. philippinarum and R. decussatus to burrow to shallow depths and thus preserve more energy for growth (Román et al. 2023c). Under a slight temperature increase in the field, growth of the native clam was greater when it coexisted with Z. noltei than when in bare sediment (Román et al. 2022b). Moreover, after the application of low salinity treatments and subsequent transplantation to the field, more individuals of the two species survived in patches with Z. noltei than in bare sediment (Román et al. 2024b). Regarding the effects of seagrass on R. philippinarum in its native range, a previous study indicated that the clam condition decreased in the presence of Zostera japonica Ascherson & Graebner (Tsai et al. 2010). Establishi
ng how these species respond to harvesting-related disturbance is important for assessing the sustainability of traditional extractive methods and the potential influence of seagrass presence on the abundance and productivity of stocks.
The following alternative hypotheses were proposed in this study: 1) the morphological (shoot density, leaf length), structural (biomass), physiological (photosynthetic efficiency) and biochemical (C and N contents, starch and sucrose reserves) metrics of Z. noltei and the abundance and condition index of bivalves are capable of recovering to a pre-disturbance state that is statistically indistinguishable from the control state (Rykiel 1985), within one year of shellfish harvesting disturbance; 2) re-establishment of seagrass occurs faster in less disturbed and more complex habitats, i.e. continuous and dense seagrass patches with fine and cohesive sediments (de Boer 2007, Boese et al. 2009, González-Ortiz et al. 2014); and 3) recovery of clam populations is faster in areas where shellfishing activity is more frequent. To test our hypotheses, we performed a year-long field experiment in three zones of a shellfish bed characterized by different harvesting frequency and Z. noltei cover. We chose to conduct the study over a period of one year to include a full seasonal cycle that would allow observation of any recovery (see Román et al. 2018). After the bed was disturbed by shellfish harvesting, by shellfishers or by us (using the same techniques as the shellfishers), we measured different parameters in plants and in recruits, juveniles and adults (commercial and non-commercial sizes) of the three bivalve species. The responses of Z. noltei were assessed by non-destructive measurements every three months throughout the experimental period, before final sampling of bivalves and seagrass was conducted. The experimental set-up enabled us to assess the recovery capacity of Z. noltei meadows and commercial bivalve stocks after manual shellfishing harvesting and to evaluate the long-term sustainability of seagrass meadows coexisting with traditional small-scale fisheries.
Materials and Methods
Description of the Experimental Site
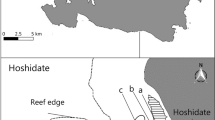
The experiment was conducted in a monospecific Z. noltei meadow in the A Seca shellfishing bed (coordinates 42.43564ºN, 8.68893ºW), which is frequently disturbed by shellfish harvesting on foot, mechanical tillage with tractors, clam seeding and manual reworking of the sediment with rakes for clams harvesting. The study system was therefore not an entirely natural seagrass ecosystem, but a seagrass meadow partly affected by commercial shellfishing activity. The site is an intertidal flat at the outflow of the Mouro creek, in the inner part of the Ria de Pontevedra, a long inlet in NW Spain (Fig. 1a). The tidal cycles in the region are semidiurnal (range, 4 m) and the meteorological regime is oceanic and temperate, with high rainfall (Rodríguez-Guitián and Ramil-Rego 2007).
Experimental scheme. a) Location of the study site in NW Spain, b) Distribution of the zones with different levels of harvesting frequency and seagrass cover (yellow) in the shellfish bed (IH: unvegetated chronically disturbed, LH: vegetated chronically disturbed, NH: vegetated undisturbed), c–e) Detail of the experimental plots (1 m2) in each zone. Disturbance treatments: AD (artificial disturbance), C (control), DS (disturbance by shellfishers). Reference system ETRS89/UTM zone 29 (EPSG: 25829). Ortho-photograph obtained from the photogrammetric flights of the aerial orthophoto Spanish plan (PNOA, 2020).
Three zones were defined according to the harvesting frequency and Z. noltei cover (Fig. 1b), following the same classification used in a previous descriptive study (Román et al. 2023b). The zone with no harvesting (NH) was in the upper intertidal zone, which was the closest to the creek outflow, where no shellfishing has taken place in at least the last five years. The fishing guild has deliberately not harvested clams in the NH zone to preserve areas of undisturbed seagrass in the shellfish bed, and this area was therefore classified as an undisturbed zone. In October 2019, one month before the experiment, in the NH zone where the patches of Z. noltei were continuous, the seagrass density was highest (11,020 ± 1362 shoots m−2), the aboveground biomass (AGB) and below-ground biomass (BGB) were greatest (AGB = 82.24 ± 13.72, BGB = 65.7 ± 7.45 g m−2 respectively), and the sediment size was smallest (280 ± 20 µm) (n = 4).
The zone with low harvesting (LH) frequency was a chronically disturbed vegetated zone in the mid intertidal area, further from the creek, where harvesting is usually performed only once to twice a year (in the present case, the last time was six months before the experiment, in May 2019). In the LH zone, the patches were fragmented, there was a trend toward lower values of seagrass density (6520 ± 1459 shoots m−2, ANOVA: F1,6 = 5.08, p = 0.07) and significantly less biomass than in the NH zone (AGB = 26.06 ± 10.39 g m−2, ANOVA: F1,6 = 11.04, p = 0.02; BGB = 36.03 ± 6.42 g m−2, ANOVA: F1,6 = 9.09, p = 0.03), and an intermediate sediment size (325 ± 14 µm) (n = 4).
The intensely harvested zone (IH), defined as a chronically disturbed, unvegetated zone, occurred in the most seaward location, in the lower intertidal area. The IH zone is a potential seagrass habitat that is harvested monthly, which has caused the complete disappearance of seagrass, as Z. noltei colonizes the substrate in adjacent undisturbed areas at the same tidal range (Fig. 1, see transition area between IH and LH, at the southernmost part of the shellfish bed). Similarly, Zostera spp. meadows reach the lower intertidal and the subtidal fringes in intertidal flats of the region such as the Umia- O Grove intertidal complex (Cacabelos et al. 2015). The particle size of the bare sediment was the largest in the IH zone (536 ± 34 µm) (n = 4) and was significantly larger than in the LH and NH zones (ANOVA post-hoc; IH vs. LH: t-ratio = 6.17, p = 0.004, IH vs. NH = t-ratio = 7.49, p = 0.001) (Román et al. 2023b). The high frequency of harvesting in IH is probably due to a more favourable substrate for harvesting, as the sediment is unvegetated, less cohesive, coarser and more porous than in the LH zone, which reduces the clam harvesting effort and enhances the recruitment and growth of clams (Libralato et al. 2002; Gosling 2015). In the IH and LH zones, the fishing guild usually performs 15 clam “seedings” annually (90–95% with R. philippinarum adults, of size 25–40 mm). The seeding process is performed by dropping clams on the sediment surface and allowing them to burrow into the sediment, without any manual digging or disturbance. During the experimental period, which took place during the COVID-19 pandemic, only two seedings were performed in the IH, on 17 October and 6 November 2020, but not in the experimental plots or nearby (personal communication, technical assistant at the Pontevedra fishing guild).
Based on the findings of a previous observational study (Román et al. 2023b), bivalve abundance increased in the different zones with shellfishing pressure and decreased with the complexity of seagrass patches. The NH zone was characterized by lower abundances of the commercial species C. edule, R. philippinarum and R. deccusatus (59 ± 18 ind. m−2), whereas the abundances of species were greatest in the IH zone (70 ± 62 ind. m−2). The most abundant species in the shellfish bed was R. philippinarum (96 ± 17 ind. m−2), with recruits (antero-posterior length < 10 mm, 64 ± 18 ind. m−2) and adults of commercial size and larger, i.e. ≥ 35 mm, (51 ± 20 ind. m−2) being most abundant in the IH zone. By contrast, the least abundant species was R. deccusatus, with recruits only in the NH zone (32 ± 43 ind. m−2) and only 9 ± 10 adults m−2 of commercial size in the IH zone (n = 4) (Román et al. 2023b).
Experimental Set-up
The experiment was established on 25 November 2019, during a period when the shellfishers were not disturbing the seagrass. It was developed in close collaboration with the Pontevedra fishing guild, which is responsible for managing the shellfish bed. A total of 32 experimental plots (1 m2) were marked with PVC stakes. Of these plots, 8 were located in the NH zone, 12 in the LH zone and 12 in the IH zone. In each zone, each treatment was applied to 4 plots. The treatments consisted of three different types of shellfishing-related disturbance: disturbance by shellfishers (DS), artificial disturbance (AD) and control (C) (no disturbance). Each type of disturbance was applied once at the beginning of the experiment. The DS treatment was applied in the LH and IH zones but not in NH zone, where no harvesting was conducted. The DS treatment consisted of allowing the shellfishers to harvest the bivalves as usual, by uprooting the seagrass and/or sediment and digging the sediment with a rake, then collecting specimens of commercial sizes (C. edule ≥ 25 mm, R. decussatus ≥ 40 mm, R. philippinarum ≥ 35 mm). The smaller individuals were left on the surface and allowed to burrow into the sediment by themselves; the sediment was not manipulated and was left to be redistributed by the flowing tides. The shellfishers were not told that an experiment was being carried out, to prevent any influence on their way of working. The spatial cover in the DS treatment was therefore not exactly constrained by plot size and was generally larger. After the shellfishers had disturbed the substrate, we searched for disturbed zones of size as close as possible to 1 m2 and marked each with four PVC stakes. The AD treatment was applied in NH, LH and IH zones by researchers, extracting commercial size bivalves as well as Z. noltei, if any, by emulating harvesting by shellfishers and applying a similar effort in all zones. The C treatment in NH, LH and IH zones consisted of leaving the plots undisturbed and was considered a pre-disturbance state (see detail of zones and treatments in Fig. S1). The experimental area within each zone was delimited with PVC stakes attached to buoys, and fishers were warned by the fishing guild to avoid disturbing the plots. All plots were separated by a distance of at least 2 m, and they were visited and visually inspected every three months for any signs of perturbance (Fig. 1c-e).
In Situ Measurements and Final Sampling
Non-destructive measurements of Z. noltei were performed in March (i.e. four months after the disturbance) and then in June, September and November 2020. The quantum efficiency of photosystem II photochemistry, an indicator of photosynthetic efficiency (Fv/Fm), was measured in 6 shoots per plot. The total number of shoots was counted, and the length of 30 leaves was measured in three small (10 × 10 cm) quadrats placed randomly within each plot. The average shoot density was calculated to estimate the values for each quadrat. Samples consisting of apical shoots and rhizomes, including the first four internodes after the apex, were collected at the interface of the plots and the undisturbed meadow to avoid disturbing the seagrass inside plots during the recovery process. The samples were frozen for further analysis of carbohydrate (starch and sucrose content) on apical rhizomes. We expected to observe an effect on the carbohydrate reserves at the interface of the disturbed plots due to the mobilization of carbon storage resources to sustain growth and lateral expansion. In each of the four non-destructive samplings, pore water was extracted at the interface of the plots and the undisturbed meadows at 3 and 8 cm with Rhizon™ samplers (0.15 μm pore size filters: Rhizosphere research products) in control plots throughout the experiment and only once (at the end of the experiment) in all plots. Samples for sulphide measurements were preserved with a solution of 50 mM zinc acetate (25% of sample volume) (Fossing and Jørgensen 1989), whereas samples used for nitrate, ammonium and phosphate determination were immediately frozen on dry ice in the field. The concentrations (μmol L−1) of nitrate, ammonium and phosphate in the sediment pore water were determined by continuous segmented flow analysis (nitrate: ISO 13395:1997, ammonium: ISO 11732:2005a, phosphate: ISO 6878:2005b). The sulphide concentrations were determined by colorimetry (ISO 10530:1992) in the CACTI facilities at the Universidade de Vigo. All analyses of inorganic nutrients were accredited by Entidad Nacional de Acreditación (ENAC; signatory of all Multilateral Recognition Agreements established in international organizations of accreditation bodies, European co-operation for Accreditation-EA).
The final sampling was performed one year after the start of the experiment, on 16 November 2020. The non-invasive measurements were performed as before and the pore water was extracted in all treatments. Quadrats of 0.5 × 0.5 m were then placed in centre of each 1 m2 plot, and adult and juvenile bivalves were collected from these smaller quadrats (by sieving at 2 mm). Quadrats of 0.25 × 0.25 m were used to sample Z. noltei (for further measurement of biomass and C:N ratio) and juvenile and recruit bivalves (sieving at 1 mm). All samples (plants, bivalves and pore water) were stored at -20ºC.
The sediment temperature was recorded every 30 min, at depths of 3, 8 and 13 cm, with Envloggers (Electric blue) attached to methacrylate sticks (n = 2) placed in each zone between 1 December 2019 and the end of the experiment. These depths were selected because of their influence on seagrass rhizosphere (3 cm) and on R. philippinarum (3 cm) and R. decussatus (8 and 13 cm) burrowing depths (Domínguez et al. 2020 and references therein).
Water temperatures were obtained from the MeteoGalicia MOHID Water Modelling System (Text S1). The degree days (DD), which represent the amount of heating experienced by bivalves and Z. noltei above their optimum temperatures, were calculated as the sum of positive differences between mean hourly temperatures and the threshold of 20 ºC, divided by 24 h per day. The optimal sediment temperature at 3 and 8 cm for the performance of adult R. philippinarum and R. decussatus, respectively is 20 ºC (Domínguez et al. 2021), and the optimal water temperature for the growth of Z. marina is also 20 ºC (Kim et al. 2020 and references therein). The number of DD for water were normalized by the different immersion times in each zone (Text S1).
Sample Processing
The C and N contents were determined in 100 samples of Z. noltei leaves collected in the final sampling. From each plot with Z. noltei (20), 5 replicates consisting of 15 shoots (equivalent to 20 mg dw) were dried (60 ºC, 48 h) and ground to a fine powder for determination of the C and N contents in a Fisons Carlo Erba EA1108 elemental microanalyzer, in the analytical facilities at the Universidade de Vigo (CACTI-UVIGO).
The apical rhizomes collected in the four non-destructive samplings were dried (60 ºC, 48 h) and ground for analysis of starch and sucrose contents by resorcinol and anthrone assays, respectively, both standardized to sucrose (Olivé et al. 2007). The final AGB and BGB of Z. noltei were quantified as the dry weight (dw) after heating at 60 ºC for 48 h.
Bivalve recruits collected in the final sampling were counted in a binocular microscope, and their size, (antero-posterior length) was measured with Leica Application Suite V4 image analysis software. Adult, commercial sized and juvenile bivalves were counted and measured. Adult and commercial sized bivalves were dried at 60ºC for 48 h, to determine their dry weight. The condition index (CI) was calculated using Eq. (1) (Walne and Mann 1975).
Data Treatment
The effect of the fixed factors Disturbance (C, DS, AD) and Time (March, June, September and November) on Fv/Fm, leaf length, shoot density, starch and sucrose in apical rhizomes of Z. noltei was tested using linear mixed-effects models, with the lme function in the nlme package (Pinheiro et al. 2022) and with Plot as a random factor. In March, Z. noltei was not growing in the AD plots in the LH zones, and therefore there are no observations for any seagrass response variable, except shoot density. As the design was not orthogonal, models for March in LH zones were therefore run separately from those for the other months. The effect of the disturbance treatments at the end of the experiment on the AGB and BGB was tested with linear models, whereas the effect on C and N contents in leaves was tested with linear mixed-effects models, including the random factor Plot.
The effect of fixed factors Disturbance and Zone on the abundances of commercial adults, adults below commercial size (size > 25 mm), juveniles (10 mm < size < 25 mm) and recruits (size < 10 mm) of the three species of bivalves were tested using generalized linear models (GLMs) with Poisson distributions of errors. The effects of the fixed factors on the CI of bivalves were only tested for the adult specimens of R. philippinarum (due the low abundances of C. edule and R. decussatus) by using linear-mixed effect models, with Plot as a random factor. The bivalve responses were modelled separately per species, as specific responses were expected. The highest bivalve densities in the plots (~ 400 ind m−2) were within the optimal range of densities in shellfish beds in Spain (300–800 ind. m–2; Navajas et al. 2003). This ensured that no density-dependent interactions among species would occur (Royo et al. 2002; Melià and Gatto 2005). The DS treatment was not applied in the NH zones, and therefore, to ensure orthogonality, the responses of Z. noltei and of bivalves in NH were modelled separately from those in the LH and IH zones.
Prior to the statistical tests, outliers of all variables that were 3 times the inter-quartile range above or below the third or first quartiles were removed (modified from Crawley 2013), to increase the confidence of the statistical tests (never more than 5% of data removed from any variable, see Table S1). The normality of the residuals and homogeneity of variances were checked by examining qq-plots and plots of standardized residuals against fitted values, respectively. When the residuals of the models were not homogeneously distributed, the heteroscedastic structure of the data in the model was specified using the VarIdent variance structure. Effects of fixed factors were considered statistically significant when the p-values were below 0.05. Analysis of variance tables for the fitted models were applied using type 3 sum of squares to identify significant terms. Post hoc pairwise multiple comparisons tests were run with the significant terms by calculating the least-squares means for the significant factors or their combinations with the emmeans function from the homonymous package (Lenth 2022). To reduce the comparison-wise error rates, the post-hoc p-values were adjusted with Tukey correction (Underwood 1997). All data are reported as means ± SE. All statistical tests were conducted using R software version 4.2.2. (R core Team 2022).
Results
Description of Temperatures and Nutrients in the Experimental Site
The mean sediment temperatures were maximal in the NH zone and minimal in the IH zone at all three depths (Fig. 2, Table S2). Within each zone, daily 90th percentiles decreased with sediment depth, being maximal at 3 cm (NH = 19.39 ± 4.6 ºC, LH = 19.43 ± 4.6 ºC, IH = 17.88 ± 3.5 ºC) and minimal at 13 cm (NH = 18.13 ± 3.9 ºC, LH = 17.89 ± 3.8 ºC, IH = 17.26 ± 3.0 ºC). The number of DD with sediment temperatures above 20 ºC decreased from NH to LH and to IH. Within each zone, the number of DD decreased with sediment depth (NH: 3 cm = 234, 8 cm = 188, 13 cm = 167; LH: 3 cm = 211, 8 cm = 169, 13 cm = 137; IH: 3 cm = 104, 8 cm = 81, 13 cm = 72). The temperature spikes recorded in the sediment between 26 and 28 May occurred during low spring tide at noon, coinciding with a period of hot weather (www.meteogalicia.gal). The number of DD in water, normalized by immersion times, was lower in IH (0.16) than in NH (0.19) and LH (0.19) (Table S2). The nutrient concentrations in pore water were very similar at the different sampling depths and did not differ significantly between treatments at the end of the experiment (Fig. S2).
Zostera noltei Metrics
In March, four months after the treatments, Z. noltei recolonized the DS plots, but not the AD plots, in which seagrass was not observed until June (Fig. 3a, LH zone). In the NH zone, leaf length and number of shoots in the 10 × 10 cm quadrats were smaller in AD than in C in March, June and September, but not in November, when both variables recovered to values statistically indistinguishable from the control values (leaf length: AD = 10.9 ± 1.2 cm, C = 10.8 ± 0.8 cm; shoots: AD = 72.8 ± 12.2, C = 96.8 ± 9.7). In the LH zone, leaf length and number of shoots were larger in the DS (leaf length: 13.8 ± 0.3 cm; shoots = 96.5 ± 6.5) than in C (leaf length: 6.6 ± 0.7 cm; shoots = 66.3 ± 10.4) and in AD (leaf length: 6.6 ± 0.3 cm; shoots = 40.0 ± 7.4) plots in November (Fig. 3a-b, Tables 1 and S3).
Zostera noltei. Responses of a) number of shoots in quadrats of 10 × 10 cm, b) length, c) Fv/Fm, d) starch and e) sucrose contents to disturbance treatments in zones with different harvesting frequency during the experiment. Zone levels: NH = no harvesting and LH = low harvesting. Levels of disturbance: C = control (no disturbance), DS = disturbance by shellfishers, AD = Artificial disturbance. Asterisks indicate significant differences indicated by pairwise post-hoc tests between levels of treatments. (***: p < 0.001, **: p < 0.01, *: p < 0.05)
In both zones, Fv/Fm increased from March to June, then decreased in September; the values were higher at the end than at the beginning of the experiment (6% and 14% higher in LH and NH, respectively). In the NH zone, Fv/Fm was not different in the disturbance treatments. In the LH zone, the Fv/Fm was lower in AD than in C and DS treatments only in June. At the end of the experiment, no differences in Fv/Fm were observed between the treatments (Fig. 3c, Table 1).
The treatments (DS and AD) generally led to a decrease in the starch content in apical rhizomes. In the LH zone, it was higher in the C than in DS plots in June, September and November (November: C = 19.9 ± 4.9 mg g−1, AD = 9.6 ± 1.5 mg g−1, IS = 9.9 ± 1.9 mg g−1), and in the NH zone, the content was higher in C than in AD plots in June and November (November: C = 23.5 ± 5.7 mg g−1, AI = 8.6 ± 0.7 mg g−1). The starch content increased in summer and then decreased in autumn, particularly in the NH zones, and was also lower in LH than in NH zones in June and September, during the growing season. The sucrose content of the apical rhizomes was greater in the C than in the DS and AD treatments in both zones in March, but increased over time in disturbed plots, so that by November, concentrations were not different between treatments (Fig. 3d-e, Tables 1 and S3).
At the end of the experiment, the AGB and BGB of Z. noltei did not differ between treatment plots at NH. The AGB was only significantly higher in the DS plots (0.29 ± 0.02 g) than in the C (0.13 ± 0.01 g) and AD (0.16 ± 0.03 g) plots in the LH zones. At the same time, in the LH zones the C % in leaves in DS plots (42.4 ± 0.2 g) was significantly smaller than in the other treatments (C = 44.2 ± 0.2 g, AD = 43.6 ± 0.4 g). The N % did not vary significantly between treatments in both zones (Fig. 4, Table 2).
Zostera noltei. a) Above- and b) below-ground biomass in 0.25 × 0.25 cm quadrats (n = 4), c) carbon and d) nitrogen contents in leaves (n = 20). Zone level: NH = no harvesting and LH = low harvesting. Levels of disturbance: C = control (no disturbance), DS = disturbance by shellfishers, AD = artificial disturbance. Letters above the bars indicate the differences between treatments indicated by post-hoc tests
Bivalve Abundance and Condition Index
The most abundant bivalve species was R. philippinarum in the IH zone, particularly the commercial sized individuals (24.0 ± 6.2 ind.) and recruits (39.0 ± 6.6 ind.). Specimens of R. decussatus of commercial size were also more abundant in the IH zone (4.0 ± 0.9 ind.). Moreover, the C. edule juveniles were the most abundant, in contrast to the scarce adult sizes of this species (Fig. 5, Table 3).
Abundance of a) commercial size bivalves (C. edule ≥ 25 mm, R. decussatus ≥ 40 mm, R. philippinarum ≥ 35 mm), b) adult bivalves (size > 25 mm), c) juvenile bivalves (10 mm < size < 25 mm) and d) recruits (size < 10 mm) in 50 × 50 cm (adults and juveniles) and of 25 × 25 cm (recruits) sampling quadrats, collected at the end of the experiment (November 2020). Zone levels: NH = no harvesting, LH = low harvesting, and IH = intense harvesting. Levels of disturbance: C = control (no disturbance), DS = disturbance by shellfishers, AD = artificial disturbance. Dashed lines indicate that data were analysed in each zone separately to ensure orthogonality. Letters indicate the differences between treatments indicated by post-hoc tests
The shellfishing disturbance treatments did not have a clear effect on the bivalve abundance and the strongest effects were observed in adults. The R. decussatus adults were more abundant in C than in the DS and AD plots, in both the LH (C = 2.0 ± 1.1 ind., DS = 1.0 ± 0.4 ind., AD = 0.5 ± 0.3 ind.) and IH (C = 3.0 ± 0.6 ind., DS = 0.5 ± 0.3 ind., AD = 1.0 ± 0.3 ind.) zones. In the NH zones, R. decussatus also tended to be more abundant in C (3.0 ± 0.5 ind.) than in AD (1.0 ± 0.3 ind.), although the difference was not significant. The R. philippinarum adults were less abundant in DS than in C and AD plots in the LH (C = 8.0 ± 2.2 ind., AD = 4.0 ± 1.6 ind., DS = 3.0 ± 0.9 ind.) and IH zones (C = 5.0 ± 0.4 ind., AD = 4.0 ± 2.1 ind., DS = 3.0 ± 0.5 ind.) (Fig. 5b, Table 3).
The abundance of R. philippinarum and C. edule juveniles and recruits and of R. decussatus recruits did not decrease because of the shellfishing treatments. By contrast, C. edule juveniles were more abundant in DS than in C and AD plots, in both the LH and IH zones, and in the NH zone, juveniles were more abundant in the AD plots than in the other plots. The only negative effect of shellfishing disturbance was a lower abundance of R. decussatus juveniles in AD (1.0 ± 0.6 ind.) than in C (5.0 ± 0.6 ind.) plots in the NH zones (Fig. 5c-d, Table 3).
The CI of commercial sized individuals and adults of R. philippinarum was not affected by shellfishing disturbance, but it was significantly greater in the IH (commercial size = 65.9 ± 0.8 ‰; adults = 57.5 ± 2.3 ‰) than in LH (commercial size = 42.9 ± 2.8 ‰, adults = 47.2 ± 1.4 ‰) zones. Despite the lower abundance of R. decussatus, the CI of this species was higher than that of the other species, especially in the IH zones (Fig. 6, Table S4).
Mean (± SE) condition index of a) bivalves of commercial size and b) adult bivalves. Numbers within the bars indicate the abundance per group. Zone levels: NH = No harvesting, LH = low harvesting and IH = intense harvesting. Levels of disturbance: C = control, DS = disturbance by shellfishers, AD = artificial disturbance. Letters indicate the differences between treatments indicated by post-hoc tests of linear mixed-effects models
Discussion
In the present study, we observed that Z. noltei metrics in treated (disturbed) plots were statistically indistinguishable from those in control plots in both NH and LH zones one year after a single shellfishing-related disturbance. The recovery of Z. noltei was faster in the NH zone, characterised by more complex seagrass patches, which were continuous and dense with finer, cohesive and more stable sediments.
The abundance and condition of bivalves also recovered in vegetated (NH, LH) and unvegetated (IH) zones, although adults tended to be less abundant in the treated plots. Bivalves were more abundant, and their condition values were higher in the chronically disturbed IH zone, subjected to more intense shellfishing pressure than in LH, and characterized by coarser, more dynamic and unvegetated sediments.
Re-establishment of Zostera noltei
The study findings showed that Z. noltei metrics in the study area re-established rapidly after a single shellfish harvesting disturbance in both vegetated zones (NH and LH), and within each area after disturbance (AD and DS in LH and AD in NH). The experimental treatments led to the disappearance of visible above-ground biomass, and the shoot density and leaf length were negatively affected ten months after the disturbance. However, one year after, the disturbed plots did not show distinguishable differences relative to control plots. Indeed, the leaf length, shoot density and AGB values were higher in plots disturbed by shellfishers than in the control plots in the LH zone, demonstrating the capacity of Z. noltei to recover after occasional disturbance. Recovery of Z. noltei shoot density in the treated plots was faster in the NH zones, which were characterized by higher density of shoots and apical meristems to initiate re-growth on the disturbed surfaces. The more continuous canopy with higher shoot density in NH than in LH zone suggested that greater structural complexity may enhance the recovery process through rhizome elongation from the edges of the disturbed plots (Boese et al. 2009). Similar recovery patterns in Zostera spp. affected by manual clam harvesting disturbance were observed in the shoot density and biomass of Z. japonica in Korea (Park et al. 2011), in the biomass of Z. marina in Yakina Bay (USA) (Boese et al. 2002), and in the shoot density of Zostera capensis Setchell, 1933 in Mozambique (Amone-Mabuto et al. 2023). Even shorter periods were observed for Z. marina in NW Spain, as shoot density and biomass had recovered two months after subtidal shellfishing disturbance involving manual raking from boats (Barañano et al. 2017).
The lower starch and sucrose contents of apical rhizomes of Z. noltei in the treated plots can probably be attributed to the depletion of carbon reserves as they were assimilated into new tissue growth, especially during the growing season (Hemminga and Duarte 2000). Respiration of the below-ground biomass, together with the absence of C fixation and O2 provisioning by photosynthetic leaves, could exacerbate the depletion of carbohydrate reserves (Hemminga 1998). In June and September, during the periods of highest light availability, the increased photosynthetic rates probably enhanced carbohydrate storage (Vermaat and Verhagen 1996), especially in the control plots, even though the lower Fv/Fm values recorded during those months suggested a period of stronger thermal stress for the plants.
Recovery of Z. noltei plants was observed in AD and DS treatments, although there may be some differences in the magnitude of disturbance. For example, the harvesting intensity applied by the shellfishers is an important variable in this context as it may be greater in plots with more clams, leading to some variability in the strength of disturbance between the DS plots. Another difference is that the affected surfaces in DS plots were not limited to a size of 1 m2 size and were in general larger than in the AD plots. The greater extension of the disturbed areas may have slowed down the recovery of the seagrass. Nonetheless, this was not observed in the present study because seagrass metrics in DS plots recovered control values, and the values were even higher than in AD plots.
Although sexual reproduction may have partly contributed to the recovery in the plots disturbed by shellfishers, the findings suggest that the initial rapid revegetation of the disturbed plots occurred via asexual reproduction through new shoot formation from the adjacent undisturbed seagrass. In fact, plants with reproductive shoots, i.e. bearing visible seeds, only represented about 1% and 2% in June and September, respectively, whereas in the other zones and treatment plots, no reproductive shoots were observed.
We observed that Z. noltei showed a high degree of resilience when nutrient levels in sediment pore water and temperature did not exceed stressful thresholds for the seagrass. In vegetated zones (NH and LH), ammonium and phosphate concentrations were typical of seagrasses occurring at the same latitudes (Hemminga et al. 2000; Carvalho et al. 2006) and were lower than eutrophication levels (Douglas et al. 2016), indicating good water quality and/or good light penetration. Moreover, there was no thermal stress during immersion, as the mean and 90th percentiles of water temperatures did not exceed the optimal threshold of 20 ºC for seagrasses, and the number of DD above 20 ºC was negligible. The potential thermal stress during low tide could not be evaluated as the air temperature during emersion was not recorded. Nonetheless, the greater number of DD in the sediment in the NH zone suggested a potentially stronger thermal stress than in LH, which did not prevent the recovery of seagrass, as evidenced by our results. Therefore, in this study the absence of further physical disturbance, together with the favourable environmental context, promoted the recovery of Z. noltei. The maximum Fv/Fm values observed in all plots in November suggested improvement in the photosynthetic performance of plants at the end of the growing season. This can be explained by the absence of thermal stress coincident with the presence of more above-ground biomass before the winter decline, as observed in a previous seasonal study of Z. noltei in the same region (Román et al., 2018).
Bivalve Abundance and Condition Index
The treatments did not affect the abundance of bivalves of commercial size. Similarly, clam digging and raking did not affect the abundance of the clams Macoma balthica (Linnaeus, 1758) and Cryptomya californica (Conrad, 1837) in Yakina Bay (USA) (Boese et al. 2002). Given that our experimental plots were not horizontally isolated, the lateral migration of juvenile and adult bivalves towards the experimental plots during the recovery period may have homogenised the densities (McLusky et al. 1983). Our findings suggest that the abundance and sustainability of commercial bivalve stocks would not be threatened a year after a single disturbance.
By contrast, the non-commercial size adults of R. decussatus and R. philippinarum were less abundant in the treated plots one year after the disturbance. Ruditapes philippinarum, the most abundant species in the shellfish bed, may reach more than 25 mm in size before an age of one year (Moura et al. 2018). Therefore, most of the adults collected at the end of the experiment were recruits or larvae at the time of disturbance and during the recovery period. Previous research has shown that juvenile clams exhibit greater settlement rejection rates, burrow slower and remain longer at the surface in disturbed sediments, which enhances their displacement by currents (Woodin et al. 1995). Therefore, a plausible (partial) explanation for the observed negative effects of harvesting disturbance on adult abundances is the greater vulnerability of juveniles and recruits to currents and/or predation after rejecting the disturbed substrate. We can rule out the effect of the two seedings events carried out during 2020 on clam abundance in the experimental plots because they took place outside the experimental areas.
The absence of any effects of shellfish harvesting on bivalve recruitment is consistent with the findings of previous experimental field research (Peterson et al. 1987). The abundance of recruits followed the same trends and seemed to be influenced in the long term by the abundance of commercial size bivalves. Indeed, in a 44-year-long study in the Wadden Sea, low recruitment of M. balthica was linked to low abundances of mature individuals (Beukema et al. 2017).
The abundance of bivalves and the condition of the most abundant species, R. philippinarum, was highest in the zone without Z. noltei (IH), which is consistent with the reported increase in the condition index of R. philippinarum after removal of Z. japonica (Tsai et al. 2010). In the IH zone, the continuous physical disturbance caused by harvesting and the absence of seagrass could increase the particle size and porosity, sediment oxygenation and irrigation and enhance the availability of food (Dumbauld et al. 2009). This result is consistent with the “Tapes paradox”, in which the productivity of commercial bivalves is enhanced by the continuous reworking and resuspension of the substrate caused by the exploitation (Libralato et al. 2002). Nonetheless, the preference of bivalves for sites with fewer episodes of tidal exposure during emersion cannot be ruled out as another potential explanation for the largest abundances and high CI values observed in the IH zone.
Conclusions
The study findings showed that a single shellfish harvesting event had short-term effects on the morphological, structural and biochemical metrics of Z. noltei in undisturbed (NH) and chronically disturbed (LH) vegetated areas. However, after one year, these metrics were statistically indistinguishable from control values. The ability of Z. noltei to recover was probably enhanced by the absence of further disturbance from clam harvesting and by favourable levels of temperature and nutrients that did not impair Z. noltei growth and reproduction. Nonetheless, some of the ecological functions and services provided by the seagrass in the undisturbed state were probably lost during the experimental period. By contrast, shellfish harvesting did not appear to affect commercial size or juvenile bivalves or recruits, although the abundance of adults decreased, which could compromise the abundance of commercial stocks. The findings suggest that the sustainability of the seagrass and commercial bivalve stocks would not be threatened by the harvesting frequency applied in this experiment, which was lower than harvesting performed by shellfishers (from twice a year to monthly). Therefore, rotation of the space where extractive pressure is applied, i.e. maintaining undisturbed areas with no harvesting activity in both vegetated and bare sediment areas for at least one year, may favour the re-establishment of bivalves and seagrass and enhance the long-term stability of the system. Moreover, the preservation of dense, undisturbed patches of seagrass could favour the coexistence of bivalve harvesting and seagrass in shellfish beds.
Data Availability
Data will be made available on request.
References
Amone-Mabuto, M., J. Hollander, B. Lugendo, J.B. Adams, and S. Bandeira. 2023. A field experiment exploring disturbance-and-recovery, and restoration methodology of Zostera capensis to support its role as a coastal protector. Nordic Journal of Botany. https://doi.org/10.1111/njb.03632.
Barañano, C., E. Fernández, G. Méndez, and J.S. Troncoso. 2017. Resilience of Zostera marina habitats and response of the macroinvertebrate community to physical disturbance caused by clam harvesting. Marine Biology Research 1000: 1–12. https://doi.org/10.1080/17451000.2017.1307989.
Barañano, C., E. Fernández, P. Morán, P. Urbieta, and G. Méndez. 2022. Population dynamics of a fragmented subtidal Zostera marina population affected by shell fishing. Estuarine, Coastal and Shelf Science 269: 107818. https://doi.org/10.1016/j.ecss.2022.107818.
Barillé, L., M. Robin, N. Harin, A. Bargain, and P. Launeau. 2010. Increase in seagrass distribution at Bourgneuf Bay (France) detected by spatial remote sensing. Aquatic Botany 92: 185–194. https://doi.org/10.1016/j.aquabot.2009.11.006.
Bas-Ventín, L., J. de Souza Troncoso, and S. Villasante. 2015. Towards adaptive management of the natural capital: Disentangling trade-offs among marine activities and seagrass meadows. Marine Pollution Bulletin 101: 29–38. https://doi.org/10.1016/j.marpolbul.2015.11.031.
Beukema, J.J., R. Dekker, and J. Drent. 2017. Dynamics of a Limecola (Macoma) balthica population in a tidal flat area in the western Wadden Sea: Effects of declining survival and recruitment. Helgoland Marine Research 71: 18. https://doi.org/10.1186/s10152-017-0498-7.
Bertelli, C.M., M.T. Robinson, A.F. Mendzil, L.R. Pratt, and R.K.F. Unsworth. 2017. Finding some seagrass optimism in Wales, the case of Zostera noltii. Marine Pollution Bulletin 134: 216–222. https://doi.org/10.1016/j.marpolbul.2017.08.018.
Bidegain, G., and J.A. Juanes. 2013. Does expansion of the introduced Manila clam Ruditapes philippinarum cause competitive displacement of the European native clam Ruditapes decussatus? Journal of Experimental Marine Biology and Ecology 445: 44–52. https://doi.org/10.1016/j.jembe.2013.04.005.
Boese, B.L. 2002. Effects of recreational clam harvesting on eelgrass (Zostera marina) and associated infaunal invertebrates: In situ manipulative experiments. Aquatic Botany 73: 63–74. https://doi.org/10.1016/S0304-3770(02)00004-9.
Boese, B.L., J.E. Kaldy, P.J. Clinton, P.M. Eldridge, and C.L. Folger. 2009. Recolonization of intertidal Zostera marina L. (eelgrass) following experimental shoot removal. Journal of Experimental Marine Biology and Ecology 374: 69–77. https://doi.org/10.1016/j.jembe.2009.04.011.
Brown, B., and W.H. Wilson. 1997. The role of commercial digging of mudflats as an agent for change of infaunal intertidal populations. Journal of Experimental Marine Biology and Ecology 218: 49–61. https://doi.org/10.1016/S0022-0981(97)00067-1.
Cabaço, S., A. Alexandre, and R. Santos. 2005. Population-level effects of clam harvesting on the seagrass Zostera noltii. Marine Ecology Progress Series 298: 123–129. https://doi.org/10.3354/meps298123.
Cacabelos, E., P. Quintas, J.S. Troncoso, J. Sánchez, J. Amigo, I. Romero, V. García, J. Cremades, and I. Bárbara. 2015. Galicia. In Atlas de las praderas marinas de España, ed. J.M. Ruiz, J.E. Guillén, A. Ramos Segura, and M.M. Otero, 489–530. Spain: IEO/IEL/UICN, Murcia-Alicante-Málaga. https://www.ieo.es/documents/10192/26809/Atlas-praderas-marinas-de-Espa%C3%B1a-244-1.pdf/ee4e0dd6-e30c-443e-a6dd-14cc445068ad.
Carvalho, S., A. Moura, and M. Sprung. 2006. Ecological implications of removing seagrass beds (Zostera noltii) for bivalve aquaculture in southern Portugal. Cahiers de Biologie Marine 47: 321–329. https://doi.org/10.21411/cbm.a.b8e380e1.
Crawley, M.J. 2013. Classical tests. In The R Book, ed. M.J. Crawley, 344–387. United Kingdom: Wiley.
de Boer, W.F. 2007. Seagrass-sediment interactions, positive feedbacks and critical thresholds for occurrence: A review. Hydrobiologia 591: 5–24. https://doi.org/10.1007/s10750-007-0780-9.
de los Santos, C. B., D. Krause-Jensen, T. Alcoverro, N. Marbà, C. M. Duarte, M. M. van Katwijk, M. Pérez, et al. 2019. Recent trend reversal for declining European seagrass meadows. Nature Communications 10: 1–8. https://doi.org/10.1038/s41467-019-11340-4.
Domínguez, R., E. Vázquez, S. A. Woodin, D. S. Wethey, L. G. Peteiro, G. Macho, and C. Olabarria. 2020. Sublethal responses of four commercially important bivalves to low salinity. Ecological Indicators 111. Elsevier: 106031. https://doi.org/10.1016/j.ecolind.2019.106031
Domínguez, R., C. Olabarria, S.A. Woodin, D.S. Wethey, L.G. Peteiro, G. Macho, and E. Vázquez. 2021. Contrasting responsiveness of four ecologically and economically important bivalves to simulated heat waves. Marine Environmental Research 164: 105229. https://doi.org/10.1016/j.marenvres.2020.105229.
Douglas, E.J., C.A. Pilditch, L.V. Hines, C. Kraan, and S.F. Thrush. 2016. In situ soft sediment nutrient enrichment: A unified approach to eutrophication field experiments. Marine Pollution Bulletin 111: 287–294. https://doi.org/10.1016/j.marpolbul.2016.06.096.
Duarte, C. M. 1991. Allometric scaling of seagrass form and productivity. Marine Ecology Progress Series 77: 289–300. https://www.int-res.com/articles/meps/77/m077p289.pdf
Dumbauld, B.R., J.L. Ruesink, and S.S. Rumrill. 2009. The ecological role of bivalve shellfish aquaculture in the estuarine environment: A review with application to oyster and clam culture in West Coast (USA) estuaries. Aquaculture 290: 196–223. https://doi.org/10.1016/j.aquaculture.2009.02.033.
Ferriss, B.E., L.L. Conway-Cranos, B.L. Sanderson, and L. Hoberecht. 2019. Bivalve aquaculture and eelgrass: A global meta-analysis. Aquaculture 498: 254–262. https://doi.org/10.1016/J.AQUACULTURE.2018.08.046.
Flassch, J.P., and Y. Leborgne. 1992. Introduction in Europe, from 1972 to 1980, of the Japanese Manila clam (Tapes philippinarum) and the effects on aquaculture production and natural settlement. ICES Marine Science Symposium 194, 92–96. https://archimer.ifremer.fr/doc/00037/14871/12197.pdf
Fossing, H., and B.B. Jørgensen. 1989. Measurement of bacterial sulfate reduction in sediments: Evaluation of a single-step chromium reduction method. Biogeochemistry 8: 205–222. https://doi.org/10.1007/BF00002889.
Frangoudes, K., B. Marugán-Pintos, and J.J. Pascual-Fernández. 2008. From open access to co-governance and conservation: The case of women shellfish collectors in Galicia (Spain). Marine Policy 32: 223–232. https://doi.org/10.1016/j.marpol.2007.09.007.
García-Redondo, V., I. Bárbara, and P. Díaz-Tapia. 2019. Zostera marina meadows in the northwestern Spain: Distribution, characteristics and anthropogenic pressures. Biodiversity and Conservation 28: 1743–1757. https://doi.org/10.1007/s10531-019-01753-4.
Garmendia, J.M., M. Valle, Á. Borja, G. Chust, D.-J. Lee, J. Germán Rodríguez, and J. Franco. 2017. Effect of trampling and digging from shellfishing on Zostera noltei (Zosteraceae) intertidal seagrass beds. Scientia Marina 81: 121–128. https://doi.org/10.3989/scimar.04482.17A.
González-Ortiz, V., L.G. Egea, R. Jimeńez-Ramos, F. Moreno-Mariń, J.L. Pérez-Lloréns, T.J. Bouma, and F.G. Brun. 2014. Interactions between seagrass complexity, hydrodynamic flow and biomixing alter food availability for associated filter-feeding organisms. PLoS ONE 9 (8): e104949. https://doi.org/10.1371/journal.pone.0104949.
Gosling, E. 2015. How bivalves feed. In Marine bivalve molluscs, 2nd ed., ed. E. Gosling, 99–156. United Kingdom: Wiley Blackwell. https://doi.org/10.1002/9781119045212.
Guimarães, M.H.M.E., A.H. Cunha, R.L. Nzinga, and J.F. Marques. 2012. The distribution of seagrass (Zostera noltii) in the Ria Formosa lagoon system and the implications of clam farming on its conservation. Journal for Nature Conservation 20: 30–40. https://doi.org/10.1016/j.jnc.2011.07.005.
Hemminga, M.A. 1998. The root/rhizome system of seagrasses: An asset and a burden. Journal of Sea Research 39: 183–196. https://doi.org/10.1016/S1385-1101(98)00004-5.
Hemminga, M.A., and C.M. Duarte. 2000. Seagrass Ecology. Cambridge University Press, Cambridge, United Kingdom. https://doi.org/10.1017/CBO9780511525551.
Herrera, M., A. Tubío, P. Pita, E. Vázquez, C. Olabarria, C.M. Duarte, and S. Villasante. 2022. Trade-offs and synergies between seagrass ecosystems and fishing activities: A global literature review. Frontiers in Marine Science 9: 781713. https://doi.org/10.3389/fmars.2022.781713.
Irlandi, E.A. 1997. Seagrass patch size and survivorship of an infaunal bivalve. Oikos 78: 511–518. https://doi.org/10.2307/3545612.
ISO (1997). UNE-EN ISO 13395:1997. Water quality. Determination of nitrite nitrogen and nitrate nitrogen and the sum of both by flow analysis (CFA and FIA) and spectrometric detection. (ISO 13395:1996). https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma?c=N0014189
ISO (2005a). UNE-EN ISO 11732:2005. Water quality - Determination of ammonium nitrogen - Method by flow analysis (CFA and FIA) and spectrometric detection (ISO 11732:2005). https://www.une.org/encuentra-tunorma/busca-tu-norma/norma?c=norma-une-en-iso-11732-2005-n0035122
ISO (2005b). Water quality - Determination of phosphorus - Ammonium molybdate spectrometric method (ISO 6878:2004). https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma?c=N0032755
ISO (1992). Water quality — Determination of dissolved sulfide — Photometric method using methylene blue (ISO 10530:1992). https://tienda.aenor.com/norma-iso-10530-1992-018599
Kaiser, M.J., G. Broad, and S.J. Hall. 2001. Disturbance of intertidal soft-sediment benthic communities by cockle hand raking. Journal of Sea Research 45: 119–130. https://doi.org/10.1016/S1385-1101(01)00052-1.
Kim, M., L.Z. Qin, S.H. Kim, H.J. Song, Y.K. Kim, and K.S. Lee. 2020. Influence of water temperature anomalies on the growth of Zostera marina Plants held under high and low irradiance levels. Estuaries and Coasts 43: 463–476. https://doi.org/10.1007/s12237-019-00578-2.
Lenth, R. 2022. Emmeans: Estimated marginal means, aka least-squares means. R Package Version. https://CRAN.R-project.org/package=emmeans.
Libralato, S., R. Pastres, F. Pranovi, S. Raicevich, A. Granzotto, O. Giovanardi, and P. Torricelli. 2002. Comparison between the energy flow networks of two habitats in the Venice Lagoon. Marine Ecology 23: 228–236. https://doi.org/10.1111/j.1439-0485.2002.tb00022.x.
McLusky, D., F. Anderson, and S.W. Wolfe-Murphy. 1983. Distribution and population recovery of Arenicola marina and other benthic fauna after bait digging. Marine Ecology Progress Series 11: 173–179. https://doi.org/10.3354/meps011173.
Melià, P., and M.A. Gatto. 2005. Stochastic bioeconomic model for the management of clam farming. Ecological Modelling 184: 163–174. https://doi.org/10.1016/j.ecolmodel.2004.11.011.
Moura, P., P. Vasconcelos, F. Pereira, P. Chainho, J.L. Costa, and M.B. Gaspar. 2018. Reproductive cycle of the Manila clam (Ruditapes philippinarum): An intensively harvested invasive species in the Tagus Estuary (Portugal). Journal of the Marine Biological Association of the United Kingdom 98: 1645–1657. https://doi.org/10.1017/S0025315417001382.
Navajas R., J.L., González, and A. Royo. 2003. Revisión del cultivo de la almeja japonesa (Ruditapes philippinarum, Adams & Reeve, 1850) en España. IX Congreso Nacional de Acuicultura, Cádiz.
O’Brien, K.R., M. Waycott, P. Maxwell, G.A. Kendrick, J.W. Udy, A.J.P. Ferguson, K. Kilminster, P. Scanes, L.J. McKenzie, K. McMahon, M.P. Adams, J. Samper-Villareal, C. Collier, M. Lyons, P.J. Mumby, L. Radke, M.J.A. Christianen, and W.C. Dennison. 2018. Seagrass ecosystem trajectory depends on the relative timescales of resistance, recovery and disturbance. Marine Pollution Bulletin 134: 166–176. https://doi.org/10.1016/j.marpolbul.2017.09.006.
Olivé, I., F.G. Brun, J.J. Vergara, and J.L. Pérez-Lloréns. 2007. Effects of light and biomass partitioning on growth, photosynthesis and carbohydrate content of the seagrass Zostera noltii Hornem. Journal of Experimental Marine Biology and Ecology 345: 90–100. https://doi.org/10.1016/j.jembe.2007.02.008.
Orth, R.J., and K.L. Heck. 2023. The dynamics of seagrass ecosystems: History, past accomplishments, and future prospects. Estuaries and Coasts 46: 1653–1676. https://doi.org/10.1007/s12237-023-01252-4.
Orth, R.J., T.J.B. Carruthers, W.C. Dennison, C.M. Duarte, J.W. Fourqurean, and Heck jr., K.L., Hugues, A.R Kendrick, G.A., Kenworthy, W.J., Olyarnik, S., Short, F. T., Waycott, M., Williams, S.L. 2006. A global crisis for seagrass ecosystems. Bioscience 56 (12): 987–996. https://doi.org/10.1641/0006-3568(2006)56[987:AGCFSE]2.0.CO;2.
Park, S.R., Y.K. Kim, J.H. Kim, C.K. Kang, and K.S. Lee. 2011. Rapid recovery of the intertidal seagrass Zostera japonica following intense Manila clam (Ruditapes philippinarum) harvesting activity in Korea. Journal of Experimental Marine Biology and Ecology 407: 275–283. https://doi.org/10.1016/j.jembe.2011.06.023.
Peterson, C.H., H.C. Summerson, and S.R. Fegley. 1987. Ecological consequences of mechanical harvesting of clams. Fishery Bulletin 85: 281–298.
Pinheiro, J., D. Bates, S. DebRoy, D. Sarkar, S. Heisterkamp, B. Van Willingen, and J. Ranke. 2022. Nlme: linear and nonlinear mixed effects models. Version. https://CRAN.R-project.org/package=nlme.
PNOA Plan Nacional de ortofotografía aérea (2020). Available online: https://centrodedescargas.cnig.es/CentroDescargas/busquedaSerie.do?codSerie=02211. Accessed on 1 Oct 2023.
R Core Team (2022) R: a language and environment for sta- tistical computing. R Foundation for Statistical Computing, Vienna. https://www.r-project.org/
Rodríguez-Guitián, M., and P. Ramil-Rego. 2007. Clasificaciones climáticas aplicadas a Galicia: revisión desde una perspectiva biogeográfica. Recursos Rurais 3: 31–53. https://doi.org/10.15304/rr.id5318.
Román, M., S. Rendal, E. Fernández, and G. Méndez. 2018. Seasonal variability of the carbon and nitrogen isotopic signature in a Zostera noltei meadow at the NW Iberian Peninsula. Wetlands 38: 739–753. https://doi.org/10.1007/s13157-018-1019-4.
Román, M., E. Fernández, J. Zamborain-Mason, L. Martínez, and G. Méndez. 2020. Decadal changes in the spatial coverage of Zostera noltei in two seagrass meadows (Ría de Vigo; NW Spain). Regional Studies in Marine Science 36: 101264. https://doi.org/10.1016/j.rsma.2020.101264.
Román, M., C.B. de los Santos, S. Román, R. Santos, J.S. Troncoso, E. Vázquez, and C. Olabarria. 2022a. Loss of surficial sedimentary carbon stocks in seagrass meadows subjected to intensive clam harvesting. Marine Environmental Research. https://doi.org/10.1016/j.marenvres.2022.105570.
Román, S., E. Vázquez, M. Román, R.M. Viejo, S.A. Woodin, D.S. Wethey, J.S. Troncoso, and C. Olabarria. 2022b. Effects of warming on biological interactions between clams and the seagrass Zostera noltei: A case study using open top chambers. Estuarine, Coastal and Shelf Science 276: 108027. https://doi.org/10.1016/j.ecss.2022.108027.
Román, S., E. Vázquez, M. Román, R.M. Viejo, N. Weidberg, J.S. Troncoso, S.A. Woodin, D.S. Wethey, and C. Olabarria. 2023a. The stress response of the seagrass Zostera noltei and three commercial clam species to low salinity associated with heavy rainfall. ICES Journal of Marine Science 0: 1–17. https://doi.org/10.1093/icesjms/fsad203.
Román, S., C. Olabarria, N. Weidberg, M. Román, and E. Vázquez. 2023b. Population structure and habitat assessment for two commercial clam species exploited in small-scale fisheries. Reviews in Fish Biology and Fisheries 33: 1483–1504. https://doi.org/10.1007/s11160-023-09791-6.
Román, M., F. Gilbert, R.M. Viejo, S. Román, J.S. Troncoso, V. Elsa, and C. Olabarria. 2023c. Are clam-seagrass interactions affected by heatwaves during emersion? Marine Environmental Research 186: 105906. https://doi.org/10.1016/j.marenvres.2023.105906.
Román, S., C. Olabarria, M. Román, and E. Vázquez. 2024a. Recovery of fishery-stressed seagrass meadows is driven by improvements in wastewater management. Marine Pollution Bulletin 201: 116282. https://doi.org/10.1016/j.marpolbul.2024.116282.
Román, M., E. Vázquez, R.M. Viejo, S.A. Woodin, D.S. Wethey, S. Román, N. Weidberg, J.S. Troncoso, M.M. Mendez, and C. Olabarria. 2024b. Context-dependent resilience of intertidal seagrass and venerid clams after hyposalinity stress. Marine Ecology Progress Series 729: 117–133. https://doi.org/10.3354/meps14516.
Royo, A., D. Quintero, M. Hurtado Burgos, and M. Hurtado Cancelo. 2002. Cultivo de almeja japonesa Ruditapes philippinarum (Adams and Reeve, 1850) a altas densidades de siembra y en zona intermareal. Boletín del Instituto Español de Oceanografía 18: 349–56. https://digital.csic.es/bitstream/10261/313233/3/205-206-1-PB.pdf.
Rykiel, E.J. 1985. Towards a definition of ecological disturbance. Australian Journal of Ecology 10: 361–365. https://doi.org/10.1111/j.1442-9993.1985.tb00897.x.
Short, F.T., and S. Wyllie-Echeverria. 1996. Natural and human-induced disturbance of seagrasses. Environmental Conservation 23 (1): 17–27. https://doi.org/10.1017/S0376892900038212.
Tsai, C., S. Yang, A.C. Trimble, and J.L. Ruesink. 2010. Interactions between two introduced species: Zostera japonica (dwarf eelgrass) facilitates itself and reduces condition of Ruditapes philippinarum (Manila clam) on intertidal flats. Marine Biology 157: 1929–1936. https://doi.org/10.1007/s00227-010-1462-0.
Underwood, A.J. 1997. Factorial experiments. In Experiments in Ecology, ed. A.J. Underwood, 298–358. Their Logical Design and Interpretation Using Analyses of Variance: Cambridge University Press, Cambridge, UK.
Vermaat, J.E., and F.C.A. Verhagen. 1996. Seasonal variation in the intertidal seagrass Zostera noltii Hornem.: Coupling demographic and physiological patterns. Aquatic Botany 52: 259–281. https://doi.org/10.1016/0304-3770(95)00510-2.
Walne, P.R., and R. Mann. 1975. Growth and biochemical composition of Ostrea edulis and Crassostrea gigas. In Proceedings of the 9th European Marine Biology Symposium, Oban, 2–8 October 1974, ed. H. Barnes, 587–607. Aberdeen: Aberdeen University Press.
Woodin, S.A., S.M. Lindsay, and D.S. Wethey. 1995. Process-specific recruitment cues in marine sedimentary systems. Biological Bulletin 189: 49–58. https://doi.org/10.2307/1542201.
Zoffoli, M.L., P. Gernez, L. Godet, S. Peters, S. Oiry, and L. Barillé. 2021. Decadal increase in the ecological status of a North-Atlantic intertidal seagrass meadow observed with multi-mission satellite time-series. Ecological Indicators 130: 108033. https://doi.org/10.1016/j.ecolind.2021.108033.
Acknowledgements
This research was funded by the Spanish Ministerio de Ciencia e Innovación / Agencia Estatal de Investigación (https://doi.org/10.13039/501100011033) and the Spanish Ministerio para la Transición Ecológica y Reto Demográfico for the ZEUS (grant RTI2018-095583-B-I00) and for the RECOBI (grant TED2021-129524B-I00) projects, respectively, and by the Autonomous government Xunta de Galicia-FEDER (project ED431C 2021/42): “ERDF A way of making Europe”. Marta Román received funding through a Postdoctoral fellowship from the Xunta de Galicia (ED481B-2023-006). Salvador Román received funding through a PhD fellowship from the Xunta de Galicia (ED481A-2020/199). The authors thank Esther Pérez, the fishing guild of Pontevedra and the technical staff of CACTI for technical support, and two anonymous reviewers for their constructive comments that improved the quality of the manuscript. Funding for open access charge: Universidade de Vigo/ CRUE-CISUG
Funding
Xunta de Galicia,ED481B-2023-006,Marta Román,ED481A-2020/199,Salvador Román,ED431C 2021/42,Celia Olabarria,Ministerio de Ciencia e Innovación,RTI2018-095583-B-I00,Celia Olabarria,TED2021-129524B-I00,Celia Olabarria.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Just Cebrian
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Román, M., Vázquez, E., Simón, A. et al. Recovery of the Seagrass Zostera noltei and Commercial Bivalve Populations After Shellfish Harvesting. Estuaries and Coasts (2024). https://doi.org/10.1007/s12237-024-01402-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12237-024-01402-2