Abstract
Background
Biliary atresia (BA) is one of the main causes of neonatal end-stage liver disease. Without timely diagnosis and treatment, most children with BA will develop irreversible liver fibrosis within the first two months. While current theorized causes of BA include viral infection, immune disorders, and genetic defects, the comprehensive etiology is still largely unknown. Recently, biliatresone attracted much interest for its ability to induce BA in both zebrafish and mice, so we summarized the latest progress of biliatresone research in BA and tried to answer the question of whether it could provide further clues to the etiology of human BA.
Data sources
We conducted a PubMed search for any published articles related to the topic using search terms including “biliary atresia”, “biliatresone”, “GSH”, and “HSP90”. Relevant data were extracted from the original text or supplementary materials of the corresponding articles.
Results
Biliatresone had shown its unique toxicity in multiple species such as zebrafish and mice, and pathogenic factors involved included glutathione (GSH), heat shock protein 90 (HSP90) and the related pathways. In combination with epidemiological evidence and recent studies on the intestinal flora in biliary atresia, a new pathogenic hypothesis that the occurrence of biliary atresia is partly due to biliatresone or its structure-like compounds depositing in human body via vegetables or/and the altered intestinal flora structure can be tentatively established.
Conclusions
Based on the existing evidence, we emphasized that GSH and HSP90 are involved in the development of BA, and the maternal diet, especially higher vegetable intake of Asian women of childbearing age, accompanied by the altered intestinal flora structure, may contribute to the occurrence of biliary atresia and the higher incidence in the Asia group. However, the evidence from large sample epidemiological research is necessary.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biliary atresia (BA), characterized by fibro-inflammation and obstruction of extrahepatic bile ducts (EHBD) that can lead to liver fibrosis [1], remains the most common reason for liver transplantation in infants, and its clinical presentations include high levels of direct or conjugated serum bilirubin, acholic stool, dark urine, and progressive hepatic failure [2]. Typical pathophysiological features of BA livers include EHBD obstruction, portal tract fibrosis, ductular proliferation, and cholestasis with the appearance of bile plugs [3]. BA occurs variably worldwide, affecting all ethnic groups, and has a higher incidence in Asia and the Pacific region [4, 5]. To date, there are still no consistently curative treatment options for BA, and nearly 70% of children still cannot escape the fate of requiring liver transplantation even after undergoing a Kasai portoenterostomy (KPE), a procedure that removes fibrotic EHBD and reestablishes the bile drainage pathway [6]. Therefore, to transfer from effective but symptom-only treatments (such as KPE) to origin-related treatment and/or prevention methods, unraveling the etiology of BA must be a top priority.
Studies conducted on specimen samples (acquired during surgery or tissue biopsy) or experimental animal models, especially the Rhesus rotavirus (RRV)-induced mouse model, which replicates the key pathological features of human BA, such as EHBD damage, portal tract fibrosis and ductular proliferation [7], are trying to answer the question of what causes BA, and some of the factors have been uncovered. Since the theory of viral infection first proposed by Benjamin Landing in 1974, several viruses, including cytomegalovirus (CMV), have been reported to be related to the pathogenesis of BA [8, 9]; however, myxovirus resistance protein 1, the evidence of a preceding viral infection, could be found even in the absence of viral material, thus indicating that the virus infection detected may just be a coincidental prior event, and neither epidemiological investigations nor in vivo virological tests of BA patients confirm a certain virus that causes BA [10, 11]. Immune disorder is another possible cause that has been widely discussed. A variety of immune cells have been shown to be involved in BA [12]. A deficit in the number of circulating regulatory T cells (Tregs) in peripheral blood was reported [13], and the adoptive transfer of Tregs to pups before infection with RRV prevented the obstruction of EHBD [14], but subtle pathogenic mechanisms still need to be investigated. The identification of gene errors linked to BA remains elusive. A genome-wide association study carried out in a large cohort identified glypican-1 (GPC1) and adducin 3 (ADD3) as susceptibility factors of BA [15], and further research confirmed that abnormal localizations of ADD3 and a GPC1-encoded protein exist in BA [16], but a more comprehensive, whole-genome mutation survey in a large cohort is required for further progress. While some pieces of confusion have been identified, the complete immunopathological mechanism of BA is still unclear. Recently, an environmental pathogenic factor, biliatresone, was found to induce BA in zebrafish and mice [17, 18]. Thus, we summarized the latest research progress on biliatresone and its toxicity-related pathogenic mechanisms, with the aim of providing new perspectives for future research on human BA etiology.
Discovery
Biliatresone originated in livestock. The earliest case of neonatal death with jaundice in livestock was reported by Sven et al. in 1967. The autopsy suggested liver cirrhosis and cholestasis, but the cause was unknown [19]. In 1990, Harper et al. summarized the abnormal deaths in newborn flocks in New South Wales, Australia, in 1964 and 1988. After grazing on unconventional pastures affected by severe drought, the newborn flocks developed jaundice and died shortly after birth. Autopsy showed that the lambs had clay-colored stools in their intestines, with sclerotic liver and narrowed/disappeared EHBD. Further pathological examination revealed EHBD and liver fibrosis, cholestasis, and proliferation of intrahepatic cholangiocytes (IHCs), which were highly similar to human BA, but all ewes appeared normal [20]. The overlap of specific sites and similar pathologic findings suggested that the occurrence of BA in newborn lambs was related to some kind of environmental factor, but no further exploration was conducted by the author. Similar incidents occurred in subsequent years whenever pregnant ewes were exposed to specific pastures during the drought. Park et al. noticed the phenomenon in 2007. BA occurs in newborn lambs after long-term feeding of the Dysphania genus, which only appears in the dry season, suggesting that these plants may contain toxic substances that cause BA. Three crude fractions were then extracted with dichloromethane/methanol or water from the plants and verified in zebrafish larvae [21]. The results showed that the normal fluorescence intensity of the gallbladder of zebrafish exposed to the methanol fraction decreased significantly, indicating the presence of some biliary duct-specific toxins in the methanol fraction. After several rounds of separation and purification, the substance was successfully purified and named biliatresone (approximately 1.84% of the dry weight), which is also the first plant toxin known to selectively destroy the EHBD [22].
Structure, chemical properties and synthesis
Koo et al. identified the molecular and structural formula of biliatresone with high-resolution mass spectrometry and nuclear magnetic resonance analysis in 2015. Biliatresone (molecular formula: C18H16O6) has a 1,2-diary-2-propenone structure, with an α-methylene ketone bridge formed between two phenyl groups; other functional groups include methoxy, hydroxyl and dioxyemethylene functional groups (Supplementary Fig. 1a) [23]. The chemical properties of biliatresone were later verified by Koo et al. with high-performance liquid chromatography and liquid chromatography‒mass spectrometry in 2016. The toxic core of biliatresone is the methylene relative to the α position of 1,2-diaryl-2-propen. The α-methylene, which functions as an electrophilic Michael receptor, undergoes Michael addition reactions with endogenous nucleophiles, including glutathione (GSH) and cysteine (D-NAC and L-NAC). Biliatresone can also react reversibly with established solvent adducts in vivo, including methanol adducts, water adducts, and hemiethylene cholate ketone (C17H16O6). Other functional groups, such as methylene dioxy, dimethoxy, and hydroxyl groups, also play important roles in the reaction [24]. Estrada et al. (USA) and Yang et al. (China) established an in vitro synthetic route of biliatresone in 2017 and 2019, respectively. The artificial product replicated the toxic effect of natural biliatresone and was verified through a zebrafish assay (Supplementary Fig. 1b, c) [25, 26].
Biliatresone animal models
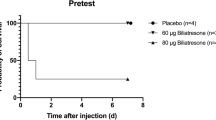
Animal models serve as an important tool for both etiological and damage mechanism studies of BA [27]. Although there is still a lack of direct evidence that biliatresone is the cause of human BA, the discovery of biliatresone does provide the possibility to establish animal models induced by environmental toxins, thus drawing a more complete map of the bile duct injury mechanism in BA. In 2015, Lorent et al. successfully established a biliatresone-induced zebrafish model with natural biliatresone. Experiments were carried out at different concentrations. The results showed that after 24 hours of treatment with biliatresone (0.5 μg/mL), all surviving larvae (93.3%, 14/15) had no significant changes in hepatocytes or intrahepatic bile ducts. Gallbladder showed varying degrees of shrinkage (3/14) or disappearance (11/14), and the effects were dose-dependent and time specific. Treatment on the 5th day post-fertilization (dpf) had more significant effects than intervention on the early stage (6 or 24 hours post-fertilization, hpf). Low-dose intervention (0.0625 μg/mL; 0.125 μg/mL) caused minor gallbladder injury, while the gallbladder underwent morphological changes (0.25 μg/mL) or severe deformation (0.5 μg/mL; 1.0 μg/mL) with increasing dosage, and the elimination of toxin (1.0 μg/mL) did not interrupt the progression of injury [22]. An artificial biliatresone-induced zebrafish model was established by Zhao et al. [28] and Yang et al. [29] in 2020. After treatment with biliatresone at 5 dpf for 24 hours (0.2–0.5 μg/mL) or 48 hours (0.15 μg/mL), the morphology of EHBD was destroyed, and the gallbladder disappeared in 12%–26% of larvae. Yang et al. subsequently constructed an artificial biliatresone-induced mammalian model with newborn BALB/c mice in the same year, and the study was conducted in both newborn and pregnant mice. The results showed that while 48.7% of newborn mice died within seven days after injection (24–48 hours after birth, 80 μg, intraperitoneal), jaundice, slower weight gain and bilirubinuria occurred in 40% of newborn mice within two to four days after injection. Pathological findings included integrity damage to the gallbladder and EHBD epithelium, liver fibrosis with inflammatory infiltration, IHCs hyperplasia in the portal area, and high expression of BA-specific diagnostic index matrix metalloproteinase 7 [29, 30]. All these features replicated the sight of human BA, suggesting it’s a good model for damage mechanism study of human BA (Table 1).
Damage mechanism
EHBD atresia is an important characteristic of BA. Lorent et al. further demonstrated the specific targeting of biliatresone to EHBD and gallbladder in 2015. Exposure to natural biliatresone (0.5 g/mL) caused loss of epithelial monolayer integrity in extrahepatic cholangiocytes (EHCs) and gallbladder epithelial cells, while hepatocytes showed only slight tubulin changes even with high doses (5 g/mL) of stimulation [22]. Inflammatory infiltration is another important feature of BA. Although inflammatory cell aggregation was observed in the portal vein region of the mouse model, the adaptive immune system of zebrafish was not established until 4–6 weeks after birth [31]. Lorent et al. showed that clearance of macrophages in zebrafish did not affect disease progression, indicating that differences exist in injury mechanisms among models, and the immune system plays a limited role in it [22, 29]. The above evidence suggests the following characteristics of biliatresone: specifically targeting EHBD and gallbladder, and perhaps not primarily dependent on the immune system.
Toxicity to extrahepatic cholangiocytes depends on glutathione consumption
GSH plays a vital role in cellular protective antioxidant responses and has been shown to be involved in various cholestatic diseases, including BA [32,33,34,35]. Zhao et al. conducted RNA sequencing of biliatresone-treated zebrafish livers in 2016 and found that multiple genes related to GSH synthesis were upregulated after treatment, including gclc, gclm and nrf2. Mass spectrometry results showed that biliatresone caused a dose-dependent decrease in the level of GSH. The opposing fates of EHCs compared with hepatocytes during stimulation depend in large part on their differences in basal redox states. The GSH storage of EHCs was significantly lower than that of hepatocytes, and the reduction of GSH expression by BSO (a specific inhibitor of de novo glutathione synthesis) sensitized the EHCs to a low dose of biliatresone, while exogenous supplementation with NAC (precursor of GSH) alleviated the damage [36]. In 2020, Zhao et al. further revealed that in addition to differences in basal reserves, different parts of the liver had different ways to maintain GSH storage. Mutations in the glutathione reductase gene sensitize EHCs to low-dose biliatresone, while the same changes do not affect IHCs, which suggests that EHCs’s maintenance of GSH is more dependent on the conversion of GSSG (oxidized GSH) to GSH. IHCs may have a better synthetic ability to tolerate the toxin [28].
GSH-RhoU-Hey2-SOX17 pathway
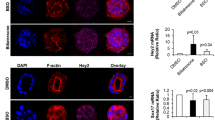
SOX17 is a master regulator of EHBD development in various vertebrate species, and reduced SOX17 expression induces hypoplastic gallbladders at late stages of organogenesis [37]. In 2016, Waisbourd-Zinman et al. found that biliatresone intervention resulted in decreased expression of GSH and SOX17 in mouse EHCs organoid [38], and the reduction in SOX17 expression by siRNA mimics the effect of biliatresone without affecting GSH levels [39]. In 2020, Sophia et al. applied gene chip technology and further found that the intracellular expression of RhoU/Wrch1 and Hey2 changed after treatment. RhoU/Wrch1 is a known regulator of cytoskeleton-related genes and actin organization, and the upregulation of RhoU disrupts epithelial cell polarization, which is necessary for tight junction assembly in MDCK cells [40]. Hey2 is a Notch signaling protein. The Notch signaling pathway is important for bile duct development regulation and plays an important role in various liver diseases [41]. The overexpression of RhoU/Wrch1 simulates the effect of biliatresone and simultaneously leads to an upregulation of Hey2 and downregulation of SOX17 without affecting the level of GSH, while overexpression of Hey2 can only decrease the expression of SOX17 without changing the expression of RhoU. This evidence jointly indicates that decreased GSH expression initiates a pathway leading to increased RhoU/Wrch1 expression, which in turn leads to a sequential increase in Hey2 expression and a decrease in SOX17 expression [42].
The protein quality control mechanism
The heat shock protein 90 (HSP90) chaperone mechanism is a key regulator of protease homeostasis during eukaryotic cell physiology and stress conditions and is involved in many cellular processes, including protein folding and DNA repair [43, 44]. Rajagopalan et al. performed exome sequencing in 101 North American BA patients in 2020 [45]. The results suggested decreased HSP90 expression in BA and mutations in STIP1 (a chaperone of HSP90) and REV1 (a key role in DNA repair pathways) genes [45], which was consistent with the study of Dong et al. [46]. Zhao et al. reported evidence of the involvement of the protein quality control mechanism in biliatresone injury in 2020. The RNA sequencing results showed that heat shock response-related gene expression was upregulated after treatment with biliatresone and that the inhibition of HSP90 expression by the HSP90 inhibitor 17-N-allylamino-17-demethoxy-geldmycin made EHCs sensitive to a low dose of biliatresone. In addition, activation of cyclic guanosine monophosphate (cGMP) signaling rescued the damage caused by HSP90 decline, but NAC was unable to reverse the sensitization induced by HSP90 inhibition. This evidence suggests that the cGMP signaling pathway is independent of the GSH pathway and acts synergistically with NAC to reduce biliatresone-mediated damage by enhancing protein quality control [28]. A detailed map of the relevant mechanisms is shown in (Fig. 1).
Environmental pathogenic factors of human biliary atresia
Biliatresone is a kind of 1,2-diarylacetone isoflavone that is rarely found in the environment. Currently, there is no evidence indicating that pregnant women could be directly exposed to biliatresone; however, Elliger et al. found in 1994 that beet roots inoculated with rhizocorrhiza produced an isoflavone with a similar biliatresone-like structure but without the necessary disease-causing core α-methylene [47, 48]. In 2002, Hur et al. screened the bacteria involved in phytoestrogen transformation in the human intestinal tract by 16S ribosomal RNA gene sequencing and found that clostridia existing in the human intestinal tract could split the C ring in the soybean isoflavone structure in vitro, resulting in the same methylate structure as the pathogenic structure of biliatresone [49]. These findings suggest that the human gut microflora has the ability to alter the structure of isoflavone in plants and that nontoxic precursors in food are likely to be converted to toxic substances through the gut microflora. The intestinal flora has been widely reported to be associated with liver functions in various liver diseases [50, 51]. Recently, the role of intestinal flora in BA has received attention. A disturbed gut microbiota structure was identified in the BA group [52] and correlated with the occurrence of postoperative cholangitis and jaundice clearance [53, 54]. Wang et al. conducted 16S ribosomal RNA gene sequencing on the intestinal flora of 16 BA patients after surgery in 2020, and the results showed that the intestinal flora diversity of BA patients decreased and the overall structure changed, among which the proportion of Clostridium decreased significantly [55]. Jee et al. found in 2022 that modification of intestinal microbiota composition by butyrate intake in newborn mice significantly improved EHBD injury induced by RRV, and this effect was associated with increased fecal Clostridium [56]. However, it is unknown whether this change in intestinal flora structure facilitates the transformation of nontoxic precursors in food.
BA occurs worldwide; the epidemiological survey showed that the worldwide distribution of BA was ethnically influenced. The incidence of BA is higher in Asia and the Pacific, among which the prevalence rate in the Chinese population (1:5000) [57] is approximately four times higher than that in North America (1:20,000) [58]. Regarding diet structure, a global diet analysis published in Lancet in 2019 showed that while the daily vegetable intake in both East Asia and the Pacific is above the global average, that in America is less than half the level [59]. A more detailed diet report summarized by Pomerleau et al. in 2005 showed that the daily fruit and vegetable consumption of Chinese women (15–44 years old) (325.5 g/day) was approximately 1.3 times higher than that of North American women (15–44 years old) (247.5 g/day) [60]. In addition, a study to identify potential risk factors for isolated BA conducted by The et al. in 2007 indicated that low maternal intake of some specific nutrients, including vitamin E, copper, phosphorus, and beta tocopherol, was associated with the occurrence of isolated BA [61]. Although the evidence is still limited, these results, including the discovery of biliatresone, remind us that diet structure could be one of the possible triggers of BA. Combined with epidemiological data, we try to establish such a hypothesis that food diet serves as a potential environmental factor of BA. The structural analogs in some specific foods are enriched in the intestinal tract of the Asian population and then transformed into toxins similar to biliatresone through the altered intestinal flora in BA. These toxins eventually affect the normal biliary system development of the fetus or newborn through the placenta or breast milk and ultimately lead to the occurrence of BA alone or accompanied by other environmental factors, such as CMV infection, which was verified to exist in 48%–50% of Chinese BA patients and is much higher than that of the European group (approximately 10%) [62]. In recent years, BA has been hypothesized to have begun as early as in utero, and syndromic BA is presumed to be an embryonic defect due to its unique visceral anomalies, such as polysplenia or situs inversus, although the actual genes involved are inconclusive [63]. Approximately 22.2% of fetuses diagnosed with hilar hepatic cysts develop cystic BA after birth, but it is not usually accompanied by organ abnormalities other than liver and gall [64, 65]. As for isolated BA, the evidence shows that it may develop in utero from the increased blood direct bilirubin levels that can be detected in the first hour after birth [1, 66]. These studies provide some extra support for our hypothesis, but larger cohort studies are needed for evidence based on epidemiology.
Conclusions
Biliatresone is a kind of toxin that specifically targets EHCs and the gallbladder. GSH serves as a key molecule responsible for the toxic effects. Differences in the storage, synthesis and regeneration of GSH in various parts of the liver are the basis of injury, and the HSP90 pathway is also involved, but the mechanism has not been confirmed in mammalian models other than zebrafish. The discovery of biliatresone suggests that exposure to structure-related compounds from food or other environmental sources may be an important trigger event for BA in humans, and it is speculated that diet, especially the high vegetable intake in Asian populations, may be one of the reasons for the high incidence accompanied by the altered intestinal flora structure.
Future studies should focus on (1) the enrichment of biliatresone and structural analogs in the human environment, their relationship with the spatial distribution characteristics of BA cases and whether the corresponding structural analogs have the same toxicological effects in vivo; (2) the role of intestinal flora in BA development, especially the difference in intestinal flora structure between Asia and Western BA groups; and (3) the important roles of redox homeostasis and protein quality control mechanisms in BA in animal models other than zebrafish.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Harpavat S, Garcia-Prats JA, Anaya C, Brandt ML, Lupo PJ, Finegold MJ, et al. Diagnostic yield of newborn screening for biliary atresia using direct or conjugated bilirubin measurements. JAMA. 2020;323:1141–50.
Feldman AG, Mack CL. Biliary atresia: cellular dynamics and immune dysregulation. Semin Pediatr Surg. 2012;21:192–200.
Lendahl U, Lui VCH, Chung PHY, Tam PKH. Biliary Atresia–emerging diagnostic and therapy opportunities. EBioMedicine. 2021;74:103689.
Chardot C, Carton M, Spire-Bendelac N, Le Pommelet C, Golmard JL, Auvert B. Epidemiology of biliary atresia in France: a national study 1986–96. J Hepatol. 1999;31:1006–13.
Hsiao CH, Chang MH, Chen HL, Lee HC, Wu TC, Lin CC, et al. Universal screening for biliary atresia using an infant stool color card in Taiwan. Hepatology. 2008;47:1233–40.
Burns J, Davenport M. Adjuvant treatments for biliary atresia. Transl Pediatr. 2020;9:253–65.
Yang L, Mizuochi T, Shivakumar P, Mourya R, Luo Z, Gutta S, et al. Regulation of epithelial injury and bile duct obstruction by NLRP3, IL-1R1 in experimental biliary atresia. J Hepatol. 2018;69:1136–44.
Hays DM, Woolley MM, Snyder WH, Reed GB, Gwinn JL, Landing BH. Diagnosis of biliary atresia: relative accuracy of percutaneous liver biopsy, open liver biopsy, and operative cholangiography. J Pediatr. 1967;71:598–607.
Letter AG. Cytomegalovirus and biliary atresia. Lancet. 1973;2:1206.
Tyler KL, Sokol RJ, Oberhaus SM, Le M, Karrer FM, Narkewicz MR, et al. Detection of reovirus RNA in hepatobiliary tissues from patients with extrahepatic biliary atresia and choledochal cysts. Hepatology. 1998;27:1475–82.
Shivakumar P, Campbell KM, Sabla GE, Miethke A, Tiao G, McNeal MM, et al. Obstruction of extrahepatic bile ducts by lymphocytes is regulated by IFN-gamma in experimental biliary atresia. J Clin Invest. 2004;114:322–9.
Wang J, Xu Y, Chen Z, Liang J, Lin Z, Liang H, et al. Liver immune profiling reveals pathogenesis and therapeutics for biliary atresia. Cell. 2020;183:1867–83.e26.
Wen J, Zhou Y, Wang J, Chen J, Yan W, Wu J, et al. Retraction note: interactions between Th1 cells and Tregs affect regulation of hepatic fibrosis in biliary atresia through the IFN-gamma/STAT1 pathway. Cell Death Differ. 2020;27:2295.
Tucker RM, Feldman AG, Fenner EK, Mack CL. Regulatory T cells inhibit Th1 cell-mediated bile duct injury in murine biliary atresia. J Hepatol. 2013;59:790–6.
Bai MR, Niu WB, Zhou Y, Gong YM, Lu YJ, Yu XX, et al. Association of common variation in ADD3 and GPC1 with biliary atresia susceptibility. Aging (Albany NY). 2020;12:7163–82.
Smith K. Biliary tract: GPC1 genetic risk further links Hedgehog signalling with pathogenesis of biliary atresia. Nat Rev Gastroenterol Hepatol. 2013;10:127.
Davenport M. Biliary atresia: from Australia to the zebrafish. J Pediatr Surg. 2016;51:200–5.
Patman G. Biliary tract: newly identified biliatresone causes biliary atresia. Nat Rev Gastroenterol Hepatol. 2015;12:369.
Joest E. Handbook of special pathological anatomy of domestic animals. 3rd ed. Paul Parey; 1949.
Harper P, Plant JW, Unger DB. Congenital biliary atresia and jaundice in lambs and calves. Aust Vet J. 1990;67:18–22.
Lemaigre FP. Development of the intrahepatic and extrahepatic biliary tract: a framework for understanding congenital diseases. Ann Rev Pathol. 2020;15:1–22.
Lorent K, Gong W, Koo KA, Waisbourd-Zinman O, Karjoo S, Zhao X, et al. Identification of a plant isoflavonoid that causes biliary atresia. Sci Transl Med. 2015;7:286.
Koo KA, Lorent K, Gong W, Windsor P, Whittaker SJ, Pack M, et al. Biliatresone, a reactive natural toxin from Dysphania glomulifera and D. littoralis: discovery of the toxic moiety 1,2-diaryl-2-propenone. Chem Res Toxicol. 2015;28:1519–21.
Koo KA, Waisbourd-Zinman O, Wells RG, Pack M, Porter JR. Reactivity of biliatresone, a natural biliary toxin, with glutathione, histamine, and amino acids. Chem Res Toxicol. 2016;29:142–9.
Estrada MA, Zhao X, Lorent K, Kriegermeier A, Nagao SA, Berritt S, et al. Synthesis and structure-activity relationship study of biliatresone, a plant isoflavonoid that causes biliary atresia. ACS Med Chem Lett. 2017;9:61–4.
Yang Y, Dong R, Jia L, Qiang L, Shan Z. Synthesis study of biliatresone, a plant isoflavonoid that causes biliary atresia in zebrafish. Chin J Exp Surg. 2019;36:3 (in Chinese).
Pal N, Joy PS, Sergi CM. Biliary atresia animal models: is the needle in a haystack? Int J Mol Sci. 2022;23:7838.
Zhao X, Lorent K, Escobar-Zarate D, Rajagopalan R, Loomes KM, Gillespie K, et al. Impaired redox and protein homeostasis as risk factors and therapeutic targets in toxin-induced biliary atresia. Gastroenterology. 2020;159:1068–84.e2.
Yang Y, Wang J, Zhan Y, Chen G, Shen Z, Zheng S, et al. The synthetic toxin biliatresone causes biliary atresia in mice. Lab Invest. 2020;100:1425–35.
Thomas H. Biliary tract: MMP7–a diagnostic biomarker for biliary atresia. Nat Rev Gastroenterol Hepatol. 2018;15:68.
Iwanami N, Hess I, Schorpp M, Boehm T. Studying the adaptive immune system in zebrafish by transplantation of hematopoietic precursor cells. Methods Cell Biol. 2017;138:151–61.
Cao P, Sun J, Sullivan MA, Huang X, Wang H, Zhang Y, et al. Angelica sinensis polysaccharide protects against acetaminophen-induced acute liver injury and cell death by suppressing oxidative stress and hepatic apoptosis in vivo and in vitro. Int J Biol Macromol. 2018;111:1133–9.
Ali FEM, Bakr AG, Abo-Youssef AM, Azouz AA, Hemeida RAM. Targeting Keap-1/Nrf-2 pathway and cytoglobin as a potential protective mechanism of diosmin and pentoxifylline against cholestatic liver cirrhosis. Life Sci. 2018;207:50–60.
Luo Z, Shivakumar P, Mourya R, Gutta S, Bezerra JA. Gene expression signatures associated with survival times of pediatric patients with biliary atresia identify potential therapeutic agents. Gastroenterology. 2019;157:1138–52.e14.
Wang J, Xu J, Xia M, Yang Y, Shen Z, Chen G, et al. Correlation between hepatic oxidative damage and clinical severity and mitochondrial gene sequencing results in biliary atresia. Hepatol Res. 2019;49:695–704.
Zhao X, Lorent K, Wilkins BJ, Marchione DM, Gillespie K, Waisbourd-Zinman O, et al. Glutathione antioxidant pathway activity and reserve determine toxicity and specificity of the biliary toxin biliatresone in zebrafish. Hepatology. 2016;64:894–907.
Merino-Azpitarte M, Lozano E, Perugorria MJ, Esparza-Baquer A, Erice O, Santos-Laso A, et al. SOX17 regulates cholangiocyte differentiation and acts as a tumor suppressor in cholangiocarcinoma. J Hepatol. 2017;67:72–83.
Bock C, Boutros M, Camp JG, Clarke L, Clevers H, Knoblich JA, et al. The organoid cell atlas. Nat Biotechnol. 2021;39:13–7.
Waisbourd-Zinman O, Koh H, Tsai S, Lavrut PM, Dang C, Zhao X, et al. The toxin biliatresone causes mouse extrahepatic cholangiocyte damage and fibrosis through decreased glutathione and SOX17. Hepatology. 2016;64:880–93.
Krneta-Stankic V, Corkins ME, Paulucci-Holthauzen A, Kloc M, Gladden AB, Miller RK. The Wnt/PCP formin Daam1 drives cell-cell adhesion during nephron development. Cell Rep. 2021;36:109340.
Wang DP, Tang XZ, Liang QK, Zeng XJ, Yang JB, Xu J. MicroRNA-599 promotes apoptosis and represses proliferation and epithelial-mesenchymal transition of papillary thyroid carcinoma cells via downregulation of Hey2-depentent Notch signaling pathway. J Cell Physiol. 2020;235:2492–505.
Fried S, Gilboa D, Har-Zahav A, Lavrut PM, Du Y, Karjoo S, et al. Extrahepatic cholangiocyte obstruction is mediated by decreased glutathione, Wnt and Notch signaling pathways in a toxic model of biliary atresia. Sci Rep. 2020;10:7599.
Schopf FH, Biebl MM, Buchner J. The HSP90 chaperone machinery. Nat Rev Mol Cell Biol. 2017;18:345–60.
Moran Luengo T, Mayer MP, Rudiger SGD. The Hsp70-Hsp90 chaperone cascade in protein folding. Trends Cell Biol. 2019;29:164–77.
Rajagopalan R, Tsai EA, Grochowski CM, Kelly SM, Loomes KM, Spinner NB, et al. Exome sequencing in individuals with isolated biliary atresia. Sci Rep. 2020;10:2709.
Dong R, Deng P, Huang Y, Shen C, Xue P, Zheng S. Identification of HSP90 as potential biomarker of biliary atresia using two-dimensional electrophoresis and mass spectrometry. PLoS One. 2013;8:e68602.
Elliger CA, Halloin JM. Phenolics induced in beta vulgaris by Rhizoctonia solani infection. Phytochemistry. 1994;37:691–3.
Geigert J, Stermitz FR, Johnson G, Maag DD, Johnson DK. Two phytoalexins from sugarbeet (Beta vulgaris) leaves. Tetrahedron. 1973;29:2703–6.
Hur HG, Beger RD, Heinze TM, Lay JO Jr, Freeman JP, Dore J, et al. Isolation of an anaerobic intestinal bacterium capable of cleaving the C-ring of the isoflavonoid daidzein. Arch Microbiol. 2002;178:8–12.
Tripathi A, Debelius J, Brenner DA, Karin M, Loomba R, Schnabl B, et al. The gut-liver axis and the intersection with the microbiome. Nat Rev Gastroenterol Hepatol. 2018;15:397–411.
Albillos A, de Gottardi A, Rescigno M. The gut-liver axis in liver disease: pathophysiological basis for therapy. J Hepatol. 2020;72:558–77.
Song W, Sun LY, Zhu ZJ, Wei L, Qu W, Zeng ZG, et al. Association of gut microbiota and metabolites with disease progression in children with biliary atresia. Front Immunol. 2021;12:698900.
Yang T, Yang S, Zhao J, Wang P, Li S, Jin Y, et al. Comprehensive analysis of gut microbiota and fecal bile acid profiles in children with biliary atresia. Front Cell Infect Microbiol. 2022;12:914247.
van Wessel D, Nomden M, Bruggink J, de Kleine R, Kurilshikov A, Verkade H, et al. Gut microbiota composition of biliary atresia patients before Kasai portoenterostomy associates with long-term outcome. J Pediatr Gastroenterol Nutr. 2021;73:485–90.
Wang J, Qian T, Jiang J, Yang Y, Shen Z, Huang Y, et al. Gut microbial profile in biliary atresia: a case-control study. J Gastroenterol Hepatol. 2020;35:334–42.
Jee JJ, Yang L, Shivakumar P, Xu PP, Mourya R, Thanekar U, et al. Maternal regulation of biliary disease in neonates via gut microbial metabolites. Nat Commun. 2022;13:18.
Chung PHY, Zheng S, Tam PKH. Biliary atresia: east versus west. Semin Pediatr Surg. 2020;29:150950.
Hopkins PC, Yazigi N, Nylund CM. Incidence of biliary atresia and timing of hepatoportoenterostomy in the United States. J Pediatr. 2017;187:253–7.
GBD 2017 Diet Collaborators. Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2019;393:1958–72.
Lock K, Pomerleau J, Causer L, Altmann DR, McKee M. The global burden of disease attributable to low consumption of fruit and vegetables: implications for the global strategy on diet. Bull World Health Organ. 2005;83:100–8.
The NS, Honein MA, Caton AR, Moore CA, Siega-Riz AM, Druschel CM, et al. Risk factors for isolated biliary atresia, national birth defects prevention study, 1997–2002. Am J Med Genet A. 2007;143A:2274–84.
Zhao D, Gong X, Li Y, Sun X, Chen Y, Deng Z, et al. Effects of cytomegalovirus infection on the differential diagnosis between biliary atresia and intrahepatic cholestasis in a Chinese large cohort study. Ann Hepatol. 2021;23:100286.
Davenport M, Muntean A, Hadzic N. Biliary atresia: clinical phenotypes and aetiological heterogeneity. J Clin Med. 2021;10:5675.
Shen O, Sela HY, Nagar H, Rabinowitz R, Jacobovich E, Chen D, et al. Prenatal diagnosis of biliary atresia: a case series. Early Hum Dev. 2017;111:16–9.
Chen L, He F, Zeng K, Wang B, Li J, Zhao D, et al. Differentiation of cystic biliary atresia and choledochal cysts using prenatal ultrasonography. Ultrasonography. 2022;41:140–9.
Harpavat S, Finegold MJ, Karpen SJ. Patients with biliary atresia have elevated direct/conjugated bilirubin levels shortly after birth. Pediatrics. 2011;128:e1428–33.
Funding
This study received financial support from Shanghai Key Clinical Specialty (No. shslczdzk05703), National Natural Science Foundation of China (No. 81974059), International Joint Laboratory Project of Haiju, National Children’s Medical Center (No. EK1125180104), Shenkang Three-year Action Plan of Precision Diagnosis and Treatment Project for Difficult Diseases (No. SHDC2020CR2009A), National Natural Science Foundation of China (No. 82001595).
Author information
Authors and Affiliations
Contributions
ZJJ contributed to conceptualization, writing of original draft, reviewing and editing. ZS, DR and YYF contributed to conceptualization, reviewing and editing. All the authors approved the final manuscript as submitted.
Corresponding author
Ethics declarations
Ethical approval
Not needed.
Conflict of interest
No financial or non-financial benefits have been received or will be received from any party related directly or indirectly to the subject of this article. The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhu, JJ., Yang, YF., Dong, R. et al. Biliatresone: progress in biliary atresia study. World J Pediatr 19, 417–424 (2023). https://doi.org/10.1007/s12519-022-00619-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12519-022-00619-0