Abstract
Purpose
Trop2, a cell membrane glycoprotein, is overexpressed in almost all epithelial cancers. This study aimed to explore the mutational characteristics and significance of Trop2 in breast cancer (BC).
Methods
Patients diagnosed with BC (n = 77) were enrolled to investigate expression level and clinical characteristics of Trop2. Database of cBioPortal and Kaplan–Meier Plotter were used to evaluate the effects of Trop2 (TACSTD2) genomic ateration and mRNA expression levels on disease-free survival (DFS) and relapse-free survival (RFS), respectively. Based on next generation sequencing analysis, the Trop2 mutation characteristics of BC patients were deeply depicted. In addition, Trop2 expression, mutation and methylation signature associated with Trop2 mutations were analyzed.
Results
Trop2 mutation and high expression of Trop2 were predictive biomarker for shorter DFS and RFS in BC. The positive rate of Trop2 expression in these 77 BC patients was 96.1% (74/77). Based on the Trop2 expression level, the patients were classified into Trop2 negative group, medium expression group and high expression group. The mutation frequencies of MAP3K1, NOTCH2, PTEN and MAGI2 were significantly higher in Trop2 medium expression group than high expression group. Moreover, we investigated the effect of the Trop2 mutations on other genes, including co-expressed genes, differentially mutated genes, differentially expressed genes, gene methylation and phosphorylation. We found that MED8, DPH2, KDM4A, EBNA1BP2, USP1, IPO13, CGAS, PRKAA2, NCOA7, ASCC3 and ABRACL were differentially expressed, mutated and methylated between Trop2 mutation group and wild group.
Conclusion
MAP3K1, NOTCH2, PTEN and MAGI2 mutations were significantly different between Trop2 medium expression and Trop2 high expression BC patients. The effects of Trop2 mutation on the expression, variation, methylation, and phosphorylation of other genes were comprehensively revealed. High expression level of Trop2 and Trop2 mutation were predictive biomarker for poor prognosis and targeted therapy in BC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Breast cancer (BC) is the main leading form of cancer among women [1, 2]. Triple-negative BC (TNBC), a subtype of BC, stands out for the aggressive nature. Its estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth receptor type 2 (HER2) expression were negative. Patients with TNBC are more prone to treat g distant metastases, anticancer drug resistance and unfavorable survival [3, 4]. Thus, the investigating of innovative therapeutic targets is essential to improve the survival prognosis of patients with TNBC.
Trop2, encoded by Trop2 gene [5], is also known as trophoblast antigen 2, TACSTD2, cell surface glycoprotein Trop2 [6] encoded by. Trop2 is a transmembrane glycoprotein and upregulated expression in all epithelial tumor. When cancer cells start metastasis, Trop2 could be expressed in the cytoplasm. Moreover, Trop2 is related with tumor invasion and proliferation. Thus, Trop2 is a promising target for targeted therapy due to the critical role in cancer progression [7]. Researchers found that Trop2 is overexpressed in the various tumors, such as breast, cervix, colorectal, gastric, certain lung cancers, and uterus [8]. Trop2 expression level was correlated with the prognosis of cancer patients. In prostate cancer and non-small cell lung cancer, Trop2 overexpression portends shorter overall survival (OS) and disease-free survival (DFS) [9, 10]. Trop2 is overexpressed in more than 85% of TNBC [11, 12]. Similarly in BC, membrane-localized Trop2 is an unfavorable prognostic factor in BC patients [13]. Sacituzumab govitecan (IMMU-132), an innovative antibody–drug conjugate (ADC) targeting Trop2, has been proved exhibiting safety and therapeutic activity in a phase I study involving patients with TNBC [14]. Sacituzumab govitecan shows a favourable treatment responses in metastatic TNBC (mTNBC) [15]. A recent phase III ASCENT study revealed that compared with standard-of-care chemotherapy, scituzumab govitecan exhibited superior therapeutic efficacy in treatment of mTNBC patients with high/medium Trop2 expression. The expression level of Trop2 illustrated the response rate and prognosis of BC after scituzumab govitecan treatment. High Trop2 expression demonstrated better treatment efficiency [16]. Thus, Trodelvy (Sacituzumab govitecanhziy) has been approved by the FDA to treat mTNBC patients [11]. However, the response to Trodelvy varies among BC patients, suggesting that additional factors play a role in efficacy of treatment. While significant progress has been made in targeting Trop2 in BC, especially in TNBC, several challenges still be remained. Furthermore, the genetic landscape could potentially influence the prognosis and response to therapy, which has not been extensively explored before.
Thus, we comprehensively investigated the mutation characteristics and prognostic value of Trop2 in BC patients. Herein, by identifying specific genetic alterations and prognostic markers associated with Trop2, this study can provide novel mechanistic insights into how Trop2 contributes to BC progression and resistance to therapy, which could lead to more precise patient stratification and personalized treatment plans. Our study also can help researchers to better understand the efficiency and mechanism of the effect of targeted therapy against Trop2 in BC patients.
2 Materials and methods
2.1 Patients and tumor samples
A total of 77 BC samples were collected from Guangdong Provincial People's Hospital (GDPH). The expression of Trop2 was detected by immunohistochemistry (IHC) based on SP295 antibody, Abcam. The expression levels of Trop2 were divided into three groups according to previous research by Merve Aslan et al. [17]: score 0 and 1 + were defined as negative, and 2 + and 3 + were defined as positive. In our study, score 0 and 1 + were defined as negative, 2 + is defined as medium expression and 3 + is defined as high expression. This study was approved by the Ethics Committee of the GDPH (No. GDREC2014122H). Informed consent was signed by each patient before study.
2.2 Identification of genomic alterations and tumor mutational burden (TMB)
Microtome sections for pathological examination were prepared from tumor blocks that were formalin-fixed and paraffin-embedded (FFPE). Before sequencing, two experienced pathologists re-reviewed the samples and assessed the FFPE tumor samples for tumor cell percentages. Comprehensive medical records included demographic details such as age and gender, along with clinical data including pathological reports, timing and approach of surgery and survival information. Identification of genomic mutations was facilitated through application of NGS-based YuanSu (OrigiMed, Shanghai, China) gene panel. The specific operation method is the same as our previous article [18]. KEGG pathway enrichment analysis was used for functional pathway analysis. Gene ontology (GO) analysis was performed using the GO database (GO Consortium, geneontology.org). Given the absence of cut-offs criteria for TMB status as defined by American society for clinical pathology (ASCP) have not been defined, we adopted the classification thresholds established in a previous study for other types of tumors. In this study, TMB-Low was defined as ≤ 5 mutations/megabases (mut/Mb) and TMB-High as ≥ 20 mut/Mb of sequenced DNA.
2.3 Public database analysis
From the Kaplan–Meier Plotter database, the mRNA gene chip data of 4929 cases of BC were selected. Thus, the patients were divided into high-Trop2 expression group and low-Trop2 expression group. Screening data from the Breast Invasive Carcinoma project from the public database cBioPortal. Selected data types include: mRNA expression data, CNV variants, DNA mutations, methylation, and protein expression. The effect of Trop2 mutation on RFS and DFS in BC was explored. The multidimensional molecular features and potential functions of Trop2 were deeply investigated.
2.4 Statistical analysis
The online analysis function in cBioPortal was used to analyze the Trop2 mutation characteristics, the correlation between Trop2 mutation and mRNA expression, methylation and mutation count; the correlation between Trop2 mutation and the co-expressed genes, differentially expressed genes, differentially expressed methylated genes and differentially phosphorylation genes. The online tool KOBAS 3.0 (KEGG Orthology Based Annotation System) was used to perform the above gene function annotation (annotation module) and function set enrichment (Enrichment module) [19]. Use the online tool String (https://string-db.org/) to make a protein–protein interaction (PPI) network. Statistical significance was set at P < 0.05. Stata/MP, version 13.0 (StataCorp LP, College Station, TX, USA) and R statistical software (version 4.2.2; R Foundation for Statistical Computing, Vienna, Austria) were exploited to execute the statistical analyses.
3 Results
3.1 Trop2 expression level and coding gene mutation can predict the prognosis of BC
To explore the correction between Trop2 expression and prognosis in BC, we performed survival analysis. Survival analysis from cBioPortal demonstrated that BC patients with Trop2 mutation have shorter DFS (Fig. 1A). Survival analysis from Kaplan–Meier Plotter database indicated that BC patients with high Trop2 expression have short RFS (Fig. 1B).
Prognosis analysis of Trop2 expression and coding gene mutation. A The survival analysis of the cBioportal data showed that breast cancer patients with Trop2 mutations have shorter DFS. B Survival analysis of the data from Kaplan–Meier Plotter database revealed that breast cancer patients with high Trop2 expression have short RFS
3.2 Expression levels of Trop2 in BC patients with different molecular types
77 BC patients with a median age of 47 (26–77) years were collected from the GDPH. According to the results of immunohistochemistry, there were 35 cases of TNBC (45.45%), 31 cases of Hormone Receptor-positive (HR +) HER2-negative (HER2-) BC (40.26%), 7 cases of Hormone Receptor-negative (HR-) HER2-positive (HER2 +) BC (9.09%), and 4 cases of Hormone Receptor-positive (HR +) HER2-positive (HER2 +) BC (5.19%). Using IHC detection and quantitative classification of Trop2 expression in these patients, we found that the proportion of Trop2-positive patients was as high as 96.1% (74/77), of which 57.14% (44/77) were strongly positive (score = 3). The positive rate of Trop2 in TNBC patients was 97.14% (34/35), of which patients with strong positive (score = 3) accounted for 71.43% (25/35). The positive rate of Trop2 in HR + HER2- BC patients was 93.55% (29/31), with strong positive (score = 3) patients accounting for 45.16% (14/31). All HR-HER2 + and HR + HER2 + BC patients were Trop2-positive (Table 1 and Fig. 2).
3.3 Clinical characteristics and differentially mutated genes of patients with different Trop2 expression levels
Among the 77 patients, 52 of them received NGS, and correlation analysis between the clinical characteristics and the Trop2 expression was performed (Table 2).
Score 0 and 1 + were defined as negative, 2 + was defined as medium, and 3 + was defined as high. We divided our patients into negative-Trop2 group, medium-Trop2 group and high-Trop2 group. By analyzing these 52 NGS testing, the mutation landscape of top 50 mutated genes was showed in Fig. 3, and the top 4 mutated genes were TP53 (76.9%), PIK3CA (34.6%), ERBB2 (26.9%) and KMT2D (19.2%) (Fig. 3).
A total of 361 variations from 147 genes, substitution/indel was the main type of variations, followed by gene amplification. Genes such as ERBB2, CDK12, MYC, CCND1, FGF3, FGF19 and FGF4 have higher frequency of gene amplification.
In order to identify the molecular differences of samples with different Trop2 expression levels, we compared the gene variations of samples with different Trop2 expression levels. Due to the small sample size of the negative group (n = 3), we only compared the medium-Trop2 group and the high-Trop2 group in this study. The variation characteristics of the top30 genes in high-Trop2 group and medium-Trop2 group were shown in Fig. 4.
The result revealed that the high group is mainly dominated by substitution/indel and gene amplification variants, and fusion/rearrangement, truncation and gene homozygous deletion were rare. The medium-Trop2 group had more fusion/rearrangement variants, and 10 of the 30 (33.3%) genes had fusion/rearrangement variants, including ERBB2, MAP3K1, NOTCH2, PTEN, ADGRA, AMER1, CFTR, COL1A1, FBXW7 and KDM5B. We further analyzed the differentially mutated genes of the two groups. MAP3K1, NOTCH2, PTEN and MAGI2 mutations only happened in the medium group, with significant differences. The mutation frequency of TP53 in high group was higher than that in medium group (83.87% (26/31) vs 61.11% (11/18)), but due to the small sample size with 52 samples, there was no statistical difference (P = 0.094).
3.4 Mutation characteristics of Trop2 in BC
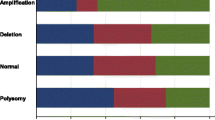
Above, we found Trop2 expression level might relate with other genes but limited by our small samples. Furthermore, we used data from cBioPortal databases to explore Trop2 mutation signature. Trop2 mutations were found in 22 (2.0%) of 1,094 patients, including 20 amplifications, 1 deep deletion, and 1 single nucleotide polymorphism (SNP, c. V253G, p. V253G, missense) (Fig. 5A–C). The characteristics of all Trop2 CNV (including amplification and deletion) of the 21 patients were shown in Fig. 5D. Gene amplifications were clustered in 1p32, 8q24.13 regions, and gene deletion was clustered in 11q22 and 11q24 regions. Trop2 methylation and mRNA expression levels were moderately negatively correlated (Spearman −0.47(p < 0.001), Pearson −0.56(p < 0.001)) (Fig. 5E), and Trop2 mutation and mutation count were moderately positively correlated (Spearman 0.47(p < 0.001) Pearson: 0.35(p < 0.001)) (Fig. 5F).
Mutation characteristics of Trop2. A Mutation profile of Trop2 in 1094 patients. B Lollipop map of Trop2 mutations. C Trop2 variant sites we annotated using GISTIC 2.0. D Trop2 CNV characteristics in 21 cases. E Correlation analysis of Trop2 methylation and mRNA expression levels. F Correlation analysis of Trop2 variation and mutation count
3.5 Effects of Trop2 mutations on other genes
To further explore the function of the Trop2, we continued to analyze Trop2 mutations data of 1094 patients. We first investigated the effect of the gene mutations on other genes, including co-expressed genes, differentially mutated genes, differentially expressed genes, gene methylation and phosphorylation. The mRNA expression of EFNA1, LGALS3, SLPI, and PDZK1IP1 was significantly positively correlated with Trop2 mutation (Fig. 6A–D). Comparing the Trop2 mutant group with the wild group, 612 differentially expressed genes, 952 differentially mutated genes, 450 differentially methylated genes and 459 differentially phosphorylated genes were screened, respectively. Drawing the Venn diagram of the above differentially expressed genes, differentially mutated genes and differentially methylated genes, we observed that these three groups share 11 genes, namely MED8, DPH2, KDM4A, EBNA1BP2, USP1, IPO13, CGAS, PRKAA2, NCOA7, ASCC3 and ABRACL. Excluding the above 11 genes, there are 81 shared genes between differentially expressed genes and differentially mutated genes, 28 shared genes between differentially expressed genes and differentially methylated genes, and 32 shared genes between differentially mutated genes and differentially methylated genes (Fig. 6E).
3.6 Functional exploration of Trop2 mutations
KOBAS 3.0 was used to enrich 11 genes that were differentially expressed, mutated and methylated, 81 genes that were simultaneously differ differentially expressed/differentially mutated, 28 genes that were simultaneously differentially expressed/differentially methylated, and 32 genes that were simultaneously differentially mutated/differentially methylated. 11 genes were confocal to 2 clusters (Fig. 7A). 81 differentially mutated and differentially expressed genes were enriched to 7 clusters (Fig. 7B). 28 shared differentially methylated and differentially expressed genes were enriched to each cluster (Fig. 7C). 32 shared genes that were both differentially methylated and differentially expressed were enriched to 3 clusters (Fig. 7D).
Gene function enrichment analysis for 11 differentially expressed genes, differentially mutated genes and differentially methylated genes (A), 81 differentially mutated and differentially expressed genes (B), 28 shared differentially methylated and differentially expressed genes (C) and 32 shared genes that were both differentially methylated and differentially expressed (D)
In addition, PPI network was performed on the above 152 genes that were differentially expressed, differentially mutated or/and differentially methylated. As shown in Fig. 8, Trop2 mutate-related genes were mainly concentrated in the PPI network centered on CDC20, CDCA8, KIF2C, EZH2 and FBXO5.
4 Discussion
BC is the most common cancer among women in the world. Trop2 could be expressed in the cytoplasm during metastasis and recurrence. Besides, Trop2 is associated with the ability of cancer cell invasion and proliferation. Sacituzumab govitecan, an ADC drug targeting Trop2, has been proved exhibiting acceptable toxicity and encouraging therapeutic activity in patients with BC. Therefore, Trop2 is an ideal candidate for targeted therapy in BC patients. In our study, Trop2 is overexpressed in BC and more than 85% of TNBC [11, 12]. Trop2-positive patients were identified in 96.1% (74/77) BC patients. In addition, the positive rate of Trop2 was 97.14% (34/35) in TNBC patients, and 93.55% (29/31) in HR + HER2- BC patients. In order to further understand the molecular characteristics of Trop2 in BC, we performed NGS on 52 samples. Then, we found that the top 4 high-frequency mutated genes were TP53 (76.9%), PIK3CA (34.6%), ERBB2 (26.9%) and KMT2D (19.2%), which were consistent with previous studies [20, 21]. Grouping these samples according to the expression of Trop2, and comparing the mutation landscape between Trop2 medium expression group and the high expression group, we revealed that there were significant differences in mutation frequency among MAP3K1, NOTCH2, PTEN and MAGI2, while these four genes were mutated only in the high-Trop2 group. A study elucidated that NOTCH1 or NOTCH2 alterations induced the transcriptional repression of PTEN, which may contribute to poor prognosis in BC [22]. An in vitro study verified that knockdown of Trop2 could increase the expression of total PTEN, p-PTEN and PDK-1 but reduce p-AKT via PI3K/AKT pathway [23], and Trop2 could induce tumor growth through AKT and determines sensitivity to AKT inhibitors [24]. Therefore, our study indicated thatTrop2 overexpression may affect tumorigenesis and growth through AKT signaling pathway.
To further explore the impact of Trop2 expression in BC, we investigated the effect of the Trop2 gene (Trop2 coding gene) mutations on other genes, including co-expressed genes, differentially mutated genes, differentially expressed genes, gene methylation and phosphorylation. Altogether 612 differentially expressed genes, 952 differentially mutated genes, 450 differentially methylated genes and 459 differentially phosphorylated genes were identified by comparing the Trop2 mutant group with the wild group. Eleven genes, MED8, DPH2, KDM4A, EBNA1BP2, USP1, IPO13, CGAS, PRKAA2, NCOA7, ASCC3 and ABRACL, were shared by differentially expressed genes, differentially mutated genes and differentially methylated genes. MED8 was associated with shorter survival and advanced TNM stage and showed higher expression in metastatic than primary tumors in renal cell carcinoma, and in vitro, siRNA mediated MED8 knockdown significantly impaired proliferation and motility in RCC cell lines [25]. MED8 was overexpressed in hepatocellular carcinoma (HCC) tissues and increased expression of MED8 was an indicator of poor outcome in HCC. The knockdown of MED8 weakened the proliferation, colony forming, and migration of HepG2 and Huh7 cells [26]. Bianchini et al. revealed that KDM5A promoted BC cell proliferation [27]. Upregulated USP1 expression in BC tissues associates with malignant progression and poor prognosis of BC patients. USP1 increases BC cell migration and invasion in vitro. ABRACL was highly expressed in BC tissues and cells, and ABRACL knockdown suppressed the proliferation, invasion, migration and epithelial-mesenchymal transition (EMT) of BC cells [28]. In general, the proteins encoded by these genes were overexpressed in BC, thereby promoting the occurrence and development of BC, and the overexpression of these proteins may be related to the mutation of Trop2, which needs further research to verify.
In BC, Trop2 promoted cell migration and invasion by inducing EMT, leading to lymph node involvement and distant metastasis, portending poor prognosis and reducing OS [7]. Elevated level of membrane Trop2 is an unfavorable prognostic factor and increased tumor growth in BC, including decreased survival [10, 29]. In our study, survival analysis of public databases showed that BC patients with high expression of Trop2 had shorter RFS. BC patients with Trop2 mutation had shorter DFS.
In this study, there are some limitations should be acknowledged. Firstly, future studies should be used to explore the predictive value of the Trop2 via independent large cohort. Second, molecular mechanism of the Trop2 need to be fully investigated in the future. Laboratory-based validation would implement into future work to build on the insights gained from this study. Third, the sample of this study is limited.
5 Conclusions
In conclusion, our study deeply investigated the clinical and genetic mutation characteristics of BC patients with different Trop2 expression levels. Given the epithelial origin of BC, the high positive rate of Trop2 levels with only 3 Trop2-negative cases highlights the potential as therapeutic target furtherly. Herein, it is potential to guide individualized targeted therapy in BC patients.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
The codes used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BC:
-
Breast cancer
- DFS:
-
Disease-free survival
- RFS:
-
Relapse-free survival
- TNBC:
-
Triple-negative breast cancer
- ER:
-
Estrogen receptor
- PR:
-
Progesterone receptor
- HER2:
-
Human epidermal growth receptor 2
- OS:
-
Overall survival
- ADC:
-
Antibody–drug conjugate
- GDPH:
-
Guangdong Provincial People's Hospital
- IHC:
-
Immunohistochemistry
- TMB:
-
Tumor mutational burden
- FFPE:
-
Formalin-fixed paraffin-embedded
- SNV:
-
Single nucleotide variant
- CNV:
-
Copy number variation
- GO:
-
Gene Ontology
- ASCP:
-
American society for clinical pathology
- TCGA:
-
The Cancer Genome Atlas
- PPI:
-
Protein–protein interaction
- SNP:
-
Single nucleotide polymorphism
- HCC:
-
Hepatocellular carcinoma
- EMT:
-
Epithelial-mesenchymal transition
References
Bray F, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–63.
Lai J, et al. A radiogenomic multimodal and whole-transcriptome sequencing for preoperative prediction of axillary lymph node metastasis and drug therapeutic response in breast cancer: a retrospective, machine learning and international multi-cohort study. Int J Surg. 2024;110(4):2162–77.
Ferrari P, et al. Molecular mechanisms, biomarkers and emerging therapies for chemotherapy resistant TNBC. Int J Mol Sci. 2022;23:3.
Plasilova ML, et al. Features of triple-negative breast cancer: analysis of 38,813 cases from the national cancer database. Medicine (Baltimore). 2016;95(35): e4614.
Ciccarelli FD, Acciarito A, Alberti S. Large and diverse numbers of human diseases with HIKE mutations. Hum Mol Genet. 2000;9(6):1001–7.
McDougall AR, et al. The oncogene Trop2 regulates fetal lung cell proliferation. Am J Physiol Lung Cell Mol Physiol. 2011;301(4):L478–89.
Zaman S, et al. Targeting Trop-2 in solid tumors: future prospects. Onco Targets Ther. 2019;12:1781–90.
Stepan LP, et al. Expression of Trop2 cell surface glycoprotein in normal and tumor tissues: potential implications as a cancer therapeutic target. J Histochem Cytochem. 2011;59(7):701–10.
Toda S, et al. TROP-2, Nectin-4, GPNMB, and B7–H3 are potentially therapeutic targets for anaplastic thyroid carcinoma. Cancers (Basel). 2022;14:3.
Zeng P, et al. Impact of TROP2 expression on prognosis in solid tumors: a systematic review and meta-analysis. Sci Rep. 2016;6:33658.
Bardia A, et al. Sacituzumab Govitecan-hziy in refractory metastatic triple-negative breast cancer. N Engl J Med. 2019;380(8):741–51.
Goldenberg DM, Stein R, Sharkey RM. The emergence of trophoblast cell-surface antigen 2 (TROP-2) as a novel cancer target. Oncotarget. 2018;9(48):28989–9006.
Lin H, et al. Significantly upregulated TACSTD2 and Cyclin D1 correlate with poor prognosis of invasive ductal breast cancer. Exp Mol Pathol. 2013;94(1):73–8.
Starodub AN, et al. First-in-human trial of a novel anti-trop-2 antibody-sn-38 conjugate, sacituzumab govitecan, for the treatment of diverse metastatic solid tumors. Clin Cancer Res. 2015;21(17):3870–8.
Bardia A, et al. Efficacy and safety of anti-trop-2 antibody drug conjugate sacituzumab govitecan (IMMU-132) in heavily pretreated patients with metastatic triple-negative breast cancer. J Clin Oncol. 2017;35(19):2141–8.
Bardia A, et al. Biomarker analyses in the phase III ASCENT study of sacituzumab govitecan versus chemotherapy in patients with metastatic triple-negative breast cancer. Ann Oncol. 2021;32(9):1148–56.
Aslan M, et al. Oncogene-mediated metabolic gene signature predicts breast cancer outcome. NPJ Breast Cancer. 2021;7(1):141.
Chen B, et al. Genetic and immune characteristics of sentinel lymph node metastases and multiple lymph node metastases compared to their matched primary breast tumours. EBioMedicine. 2021;71: 103542.
Bu D, et al. KOBAS-i: intelligent prioritization and exploratory visualization of biological functions for gene enrichment analysis. Nucleic Acids Res. 2021;49(W1):W317–25.
Chung JH, et al. Hybrid capture-based genomic profiling of circulating tumor DNA from patients with estrogen receptor-positive metastatic breast cancer. Ann Oncol. 2017;28(11):2866–73.
Callens C, et al. Molecular features of untreated breast cancer and initial metastatic event inform clinical decision-making and predict outcome: long-term results of ESOPE, a single-arm prospective multicenter study. Genome Med. 2021;13(1):44.
Pappas K, et al. NOTCH and EZH2 collaborate to repress PTEN expression in breast cancer. Commun Biol. 2021;4(1):312.
Li X, et al. TROP2 promotes proliferation, migration and metastasis of gallbladder cancer cells by regulating PI3K/AKT pathway and inducing EMT. Oncotarget. 2017;8(29):47052–63.
Guerra E, et al. Trop-2 induces tumor growth through AKT and determines sensitivity to AKT inhibitors. Clin Cancer Res. 2016;22(16):4197–205.
Syring I, et al. Comprehensive analysis of the transcriptional profile of the Mediator complex across human cancer types. Oncotarget. 2016;7(17):23043–55.
Jin X, et al. A predictive model for prognosis and therapeutic response in hepatocellular carcinoma based on a panel of three MED8-related immunomodulators. Front Oncol. 2022;12: 868411.
Bianchini G, et al. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol. 2016;13(11):674–90.
Li J, Chen H. Actin-binding Rho activating C-terminal like (ABRACL) transcriptionally regulated by MYB proto-oncogene like 2 (MYBL2) promotes the proliferation, invasion, migration and epithelial-mesenchymal transition of breast cancer cells. Bioengineered. 2022;13(4):9019–31.
Ambrogi F, et al. Trop-2 is a determinant of breast cancer survival. PLoS ONE. 2014;9(5): e96993.
Acknowledgements
We thank the TCGA database and Guangdong Provincial People's Hospital for providing the data.
Funding
This work was supported by the National Natural Science Foundation of China (grant number 82372601, 82002928), Medical Scientific Research Foundation of Guangdong Province (grant number A2023035). The funding body had no role in the design of the study and collection, analysis and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
All authors designed this study. JGL, SXD, JYC and YQR provided study materials and patients. JGL, SXD, JYC and YQR collected data. ZMX, XFQ and MX analyzed data. All authors drafted the manuscript and NL, JGL reviewed the manuscript. All authors read and approved the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All methods were carried out in accordance with the Declaration of Helsinki and Ethics regulations of the GDPH Ethics Committee. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Ethics Committee of the GDPH (No. GDREC2014122H). Informed consent was signed by each patient before study.
Competing interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lai, J., Deng, S., Cao, J. et al. Identification of biomarker associated with Trop2 in breast cancer: implication for targeted therapy. Discov Onc 15, 413 (2024). https://doi.org/10.1007/s12672-024-01261-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12672-024-01261-0