Abstract
Introduction
The aim of this study was to investigate the predictive factors for persistent disease activity following anti-vascular endothelial growth factors (anti-VEGF) and their long-term effects in patients to be treated for neovascular age-related macular degeneration (nAMD) under real-world conditions.
Methods
Retrospective data analysis of the PROOF study, a multi-center real-world retrospective chart review conducted across Korea in patients with nAMD included treatment-naive patients with nAMD who received first anti-VEGF (ranibizumab, bevacizumab, or aflibercept) between January 2017 and March 2019 was performed. All 600 patients (cohort 1) had a minimum follow-up of 12 months of which 453 patients (cohort 2) were followed-up for 24 months from baseline.
Results
At month 12 after anti-VEGF therapy, 58.10% (95% confidence interval [CI]: 54.09, 62.12) of patients and at month 24, 66.02% of patients continued to have persistent retinal fluid. At both months 12 and 24, predictive factors for persistent disease activity were fibrovascular pigment epithelial detachments (PED) (P = 0.0494) and retinal fluid at month 3 after loading phase (P = 0.0082). The mean changes in visual acuity were + 6.2, + 10.1, and + 13.3 letters and in the central subfield thickness were − 79.1 µm, − 96.3 µm, and − 134.4 µm at 12 months from baseline, in the bevacizumab, aflibercept, and ranibizumab groups, respectively.
Conclusions
The presence of retinal fluid after loading phase and fibrovascular PED were predictors of persistent disease activity after at least 1 year of anti-VEGF treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Why carry out this study? |
In a real-world setting, it has been observed that patients with neovascular age-related macular degeneration (nAMD) display lower treatment frequencies and poorer vision outcomes compared to clinical trials. |
The PROOF study was conducted to understand the real-world treatment patterns and outcomes in patients with nAMD treated with various anti-vascular endothelial growth factors (anti-VEGF) therapies in Korea. |
In this study, we analyzed the data from the PROOF study to evaluate predictive factors for persistent disease activity following treatment with anti-VEGFs in patients with nAMD. |
What was learned from the study? |
Results from this analysis suggest that the presence of retinal fluid after loading phase and fibrovascular pigment epithelial detachments (PED) were predictors of persistent disease activity at both year 1 and 2. |
Such analysis is helpful in predicting the needs and treatment results that are not satisfied with the current treatment approaches. |
Introduction
Neovascular age-related macular degeneration (nAMD) is a chronic, progressive disease and a leading cause of vision loss. Anti-vascular endothelial growth factor (anti-VEGF) therapy, considered the standard of care, depends on several variables, including the patient's age, the lesion's features, its size, baseline visual acuity (VA), and the presence of specific genetic risk alleles [1, 2].
However, in a real-world setting, patients with nAMD may not achieve the best possible long-term outcomes, with lower treatment frequencies and poorer vision outcomes compared to clinical trials [3]. Suboptimal long-term outcomes may be attributable to lack of patient adherence, high treatment burden in terms of frequent clinic visits and injections, lack of monitoring, high costs, and lapses in physician regimentation of anti-VEGF injections and monitoring [4]. In Korea, ranibizumab and aflibercept are approved anti-VEGFs to treat visual impairment due to nAMD. Bevacizumab is also commonly used as an off-label treatment option for nAMD because of its low cost compared to other available treatments [5, 6]. Simultaneously, diagnostic technologies, such as optical coherence tomography (OCT) have revolutionized the diagnosis and treatment algorithm. Morphological signs of disease activity on OCT are given primary importance because they correspond to early signs of recurrence, usually observed before visual acuity loss [7]. Using an OCT-guided anti-VEGF treatment regimen, the PrONTO study found that the qualitative assessment of OCT B scans was better at detecting fluid in the macula than waiting for changes in visual acuity [8]. The presence of fluid has been included as a key retreatment criterion across the nAMD landmark clinical trials and current society guidelines recommend that treatment decisions be based on the presence of fluid. However, there is dissociation between real-world practice and best practices recommended in the guidelines [1, 9,10,11].
The PROOF study was conducted to understand the real-world treatment patterns and outcomes in patients with nAMD treated with various anti-VEGF therapies in Korea. The results from the primary analysis revealed that > 50% of patients with nAMD had retinal fluid even after 12 months of treatment with their current anti-VEGF and presence of retinal fluid was associated with relatively worse VA outcomes [12]. Of note, some patients might be at higher risk for persistent disease activity [13]. Thus, it is warranted to find markers which could help in identifying such patients. These markers or predictors will also be useful for determining course of treatments, managing side effects, and decreasing financial costs by reducing unnecessary monitoring visits and improved prognosis. To this end, we analyzed real-world data obtained from the PROOF study to evaluate predictive factors for persistent disease activity following anti-VEGFs. We also evaluated the long-term effects of these factors in patients with nAMD under real-world conditions.
Methods
Study Design
This is a retrospective data analysis of the PROOF study, the methods for which have been published previously [12]. Briefly, the PROOF study was a retrospective chart review of patients with nAMD who had received their first anti-VEGF treatment between January 1, 2017, and March 31, 2019 in ten ophthalmology clinics across South Korea.
The study period of this extension was between July 1, 2016, and March 31, 2020, which allowed a 6-month pre-index period and at least a 12-month follow-up period. Data were collected at every 3 months (± 1 month until 9 months and ± 2 months until 24 months thereafter). The index/baseline date was defined as the date of the first anti-VEGF injection. Patients were given anti-VEGF treatment which included ranibizumab, bevacizumab, or aflibercept; the choice of treatment and the decision to discontinue treatment were at the discretion of the prescribing investigator and the patient. Patients were followed up for over 1 year from their date of first injection and outcomes were assessed every 3 months. A window period of ± 1 month was considered during the entire study period.
This study was conducted in accordance with the Guidelines for Good Pharmacoepidemiology Practices issued by the International Society for Pharmacoepidemiology and the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline and adhered to the ethical principles derived from the Declaration of Helsinki. Each center's Institutional Review Board/Independent Ethics Committee (IRB/ IEC) reviewed and approved the study protocol. As this is a retrospective RWE (real-world evidence) study, we applied for a waiver of patient consent from IRB and approved to perform when we submit protocol approval to IRB. However, IRB/IEC of Samsung Medical Center recommended to proceed with the consent form for personal information. Thus, Samsung Medical Center received the subjects’ consents for personal information. Samsung Medical Center Institutional Review Board approved the study (approval number 2020-05-070). A list of all ethics committees that approved the study protocol are provided in Supplementary Table 1.
Eligibility Criteria
Treatment-naive patients with nAMD who (1) had received their first anti-VEGF treatment between January 1, 2017, and March 31, 2019; (2) had at least one record of nAMD for diagnosis, treatment indication, or clinical findings on or within 6 months prior to the pre-index date and at least a 12-month follow-up period; (3) were ≥ 50 years of age at baseline. Patients who received other anti-VEGF and/or PDT in the study eye, prior to baseline with concurrent progressive retinal disease were excluded from the study. Detailed eligibility criteria were published previously [12].
Endpoints and Assessments
The main objective of this retrospective analysis was to identify the predictive factors for persistent disease activity following 1 year of anti-VEGF treatment in patients with nAMD. The other objectives were the changes in visual acuity (VA) and central subfield thickness (CST) from baseline, number of injections, number of visits, treatment pattern (continuation, switch or discontinuation) in nAMD treatment. All patient eyes were included in the analysis in a pooled fashion. Details of these assessments were published previously [12]. Predictive factors of disease activity at 1 year (analyzed using multivariate regression model) including impact of index therapy, nAMD subtypes and switch treatment on functional and anatomical outcomes (including retinal fluid defined as either presence of intra-retinal fluid (IRF), and/or sub-retinal fluid (SRF) and/or sub- retinal pigment epithelium fluid (sub-RPE fluid)) were assessed during this analysis. Persistent disease activity is defined as presence of fluid and CST of at least 200 μm. Switch treatment is defined as patients with only the first switching from the index therapy to another anti-VEGF therapy for each patient who underwent the switch. Frequent injection group is defined as the patients who received at least seven injections in the first year while the infrequent injection group is defined as patients receiving < 7 injections in first year.
OCT images were assessed for pathoanatomical subcompartment features relevant to the exudative process including IRF, SRF, and sub-RPE fluid within the PED. IRF was considered as the hyporeflective space within the retina, not including those spaces with a hyperreflective border that corresponded to outer retinal tubulation. SRF was considered as the hyporeflective space bounded internally by the photoreceptor outer segment tips and externally by the retinal pigment epithelium. PED thickness was considered from the RPE inner border to Bruch’s membrane. Sub-RPE fluid was measured from inner to outer borders of the hyporeflective space and recognized by its hyporeflectivity. Drusenoid PEDs were excluded and were differentiated by predominantly medium homogenous internal reflectivity. Thus, PEDs were differentiated as fibrovascular, serous, hemorrhagic, or mixed by the graders.
Statistical Analyses
The analysis set included all patients who were enrolled in this study. The patients were divided into two cohorts: cohort 1 comprised patients who had met the inclusion and exclusion criteria with a minimum follow-up period of 12 months, and cohort 2 comprised patients who had met the inclusion and exclusion criteria and had a minimum follow-up period of 24 months. As this is an exploratory study, comparative analysis was not planned. These analyses were separately conducted for patients with different follow-up periods. The point estimates as well as the corresponding two-sided 95% confidence interval were presented for all the patient population. Testing for finding predictive factors (p value) of treatment outcomes (persistent disease activity) using a multivariate regression (generalized estimating equations) analysis using robust standard errors (Huber–White) or parameter estimates. Testing for difference between frequent and infrequent injection groups was done using Wilcoxon signed-rank test. Kaplan–Meier method was used for the analysis of time-to-event, such as time from the initiation of anti-VEGF therapy to its discontinuation, time to switch or time to disease activity.
Results
Patient Baseline Characteristics
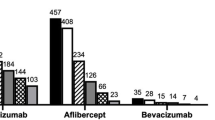
A total of 600 patients met the inclusion/exclusion criteria and were included in the study. Cohort 1 included all 600 patients who had a minimum follow-up period of 12 months, whereas cohort 2 included 453 patients of them who had a minimum follow-up period of 24 months (Fig. 1). The demographic characteristics of all patients were previously presented and determined for all subjects (Supplementary Table 2) [12]. For this analysis, patients were stratified by index therapy into ranibizumab (n = 141), bevacizumab (n = 59), and aflibercept (n = 217) groups. One patient was treated with adjunctive PDT at 3, 6, and 15 months in cohort 1. One patient was treated with PDT at month 6 and two patients were treated with PDT at month 15 in in cohort 2.
Baseline characteristics were similar between the treatment arms with no considerable differences among various parameters, except for age, macular neovascularization (MNV) lesion location, and MNV lesion composition by index therapy. Baseline characteristics demonstrated significant differences in parameters such as age, MNV lesion location, and MNV lesion composition by index therapy (Table 1). The mean (SD) age of the study patients was 75.53 (8.86), 75.58 (9.96), and 71.66 (8.31) years in ranibizumab, bevacizumab, and aflibercept groups, respectively (p < 0.0001). At baseline, the majority of patients had subfoveal lesions (ranibizumab [67.38%], bevacizumab [57.63], and aflibercept [67.28]). MNV lesion composition was predominantly classic in 51 (36.17%), 22 (37.29%), 41 (18.89%) patients in ranibizumab, bevacizumab, and aflibercept groups, respectively (p < 0.0001). Furthermore, some differences were observed in polypoidal choroidal vasculopathy (PCV) and retinal angiomatous proliferation (RAP) status among the subgroups. PCV was seen in 26 (18.44%), 13 (22.03%), and 89 (41.01%) patients while RAP was seen in 27 (19.15%), seven (11.86%), and ten (4.61%) patients in the ranibizumab, bevacizumab, and aflibercept groups, respectively (p = 0.0001 in both PCV and RAP groups).
Proportion of Patients with Presence of Retinal Fluid from Baseline to 24 Months
Approximately 58.10% (95% CI: 54.09, 62.12) of patients at 12 months and 66.02% (95% CI: 60.74%, 71.30%) (Fig. 2) still retain retinal fluid following anti-VEGF treatment at month 24. The proportion of patients with IRF, SRF, and sub-RPE at month 24 were 25.89% (95% CI: 21.01%, 30.77%), 43.69% (95% CI: 38.16%, 49.22%), 23.62% (95% CI: 18.89%, 28.36%), respectively. As reported previously, visual acuity gains were relatively better in patients with absence of retinal fluid versus those with presence of retinal fluid, throughout the follow-up period [12].
Predictive Factors of Persistent Disease Activity
As shown in Fig. 3, the statistically significant predictive factors of treatment outcomes in terms of persistent disease activity at year 1 were: staying-on index therapy of bevacizumab (P = 0.0206), fibrovascular pigment epithelial detachments (PED) (P = 0.0001), injection rate during maintenance (P < 0.0001) and retinal fluid at month 3 after loading phase (P = 0.0001). Similarly at year 2, statistically significant predictive factors of treatment outcomes were fibrovascular PED (P = 0.0494) and retinal fluid at month 3 after loading phase (P = 0.0082). The presence of retinal fluid after loading phase and fibrovascular PED were predictors of persistent disease activity at both years 1 and 2.
Predictive factors of treatment outcomes (persistent disease activity) at year 1 and 2. AFL aflibercept, BL baseline, BVZ bevacizumab, CI confidence interval, DA disease activity, Inj injection, M month, PCV polypoidal macular vasculopathy, PED pigment epithelial detachment, VA visual acuity. Testing for finding predictive factors of treatment outcomes (persistent disease activity) using GEE analysis using robust standard errors or parameter estimates
Functional and Anatomical Outcomes by Anti-VEGF Agent
Mean injection numbers were comparable between bevacizumab (4.68; n = 59) and aflibercept (4.55; n = 217) groups, while slightly lesser than ranibizumab (5.47; n = 147) groups at year 1 and similar trend was observed at year 2 (Table 2). Gain in VA was relatively higher in patients staying on index therapy of ranibizumab (13.3 ETDRS letters) vs. bevacizumab (6.2 ETDRS letters) and aflibercept (10.1 ETDRS letters) (Fig. 4A). A similar result was observed for CST reduction. Reduction in CST was relatively higher in patients on index therapy of ranibizumab vs. bevacizumab and aflibercept during year 1 (Fig. 4B). The switch from bevacizumab to ranibizumab or aflibercept resulted in an increase in VA, while the switch from aflibercept to ranibizumab and ranibizumab to aflibercept did not show a significant increase in VA (Fig. 4C). The switch from bevacizumab to ranibizumab and bevacizumab to aflibercept showed a decrease in CST (Fig. 4D).
A VA gain in patients staying on index therapy. B CST reduction in patients staying on index therapy. C VA before and after index therapy switch. D CST before and after index therapy switch. *Testing for difference among staying-on index therapy groups at each timepoint (Kruskal–Wallis test); **Testing for difference between ranibizumab and each staying-on index therapy groups at each timepoint (Wilcoxon signed-rank test). A aflibercept, B bevacizumab, BL baseline, CST central subfield thickness, EDTRS Early Treatment Diabetic Retinopathy Study, R ranibizumab, VA visual acuity
Functional and Anatomical Outcomes by Injection Frequency and PCV Status
VA gains (ETDRS letters from baseline) were significantly higher with frequent injection group (n = 105); 10.73 and 10.88 at year 1 (P = 0.018) and year 2 (P = 0.019), respectively, compared to infrequent injection group (n = 191); 8.08 and 4.83. Reduction in CST was similar between groups in year 1 (frequent injection group; − 107.24 and infrequent injection group − 106.16). However, in year 2, a trend of increase in CST was seen in infrequent injection group (frequent injection group; − 102.3 and infrequent injection group − 90.86) (Fig. 5A, B). With an initial reduction in retinal fluid in both patients with PCV and non PCV, substantial number of patients still retain fluid by month 12 and 24 (Supplementary Fig. 1A and 1B). VA gains (ETDRS letters from baseline) were higher in patients with PCV; 10.95 and 8.08 at year 1 and year 2, respectively, compared to patients with non-PCV); 8.22 and 7.24. (Supplementary Fig. 1B) Reduction in CST was also better in patients with PCV; − 117.55 and − 96.83 at year 1 and year 2, respectively, compared to 98.59 and − 90.85 at year 1 and year 2, respectively, in patients with non-PCV. (Supplementary Fig. 1B).
Change in (A) VA and (B) CST from baseline stratified by frequency of injection over 24 months. Frequent injection group: patients who were treated with at least seven injections in the first year. * Testing for difference between frequent and infrequent injections at each timepoint (Wilcoxon signed-rank test). BL baseline, CST central subfield thickness, EDTRS Early Treatment Diabetic Retinopathy Study, VA visual acuity
Discussion
The PROOF study has previously shown that despite their current anti-VEGF treatment, a high proportion of patients with nAMD in Korea continued to have retinal fluid at year 1 as well as year 2 of treatment [12]. Proper fluid control is necessary to achieve favorable visual outcomes, which is a substantial unmet need in clinical settings in Korea. This retrospective analysis provides further evidence on predictors of disease activity along with long-term outcomes by each anti-VEGF therapy. The presence of retinal fluid after loading phase and fibrovascular PED were predictors of persistent disease activity at both year 1 and 2 [2]. Similarly, in previous real-world studies using ranibizumab or aflibercept, the treatment response of IRF at month 3 was strongly associated with the persistence of IRF at month 12 [14]. Fibrovascular PED was reported to be significantly associated with nonresponse to anti-VEGF therapy as judged by both BCVA and fundus findings [15]. These results suggest that more intensive treatment needs to be considered in patients with persistent disease activity on existing anti-VEGF therapy. Emerging anti-VEGF therapies including longer-acting anti-VEGF drugs, drugs with multiple mechanisms, and sustained-release devices offer promising efficacy and safety in patients with nAMD. Anti-VEGFs such as brolucizumab demonstrated better disease control including superior fluid resolution, with > 50% of patients treated on a q12w interval after loading up to week 48 versus aflibercept 2 mg dosed q8w in phase 3 studies, HAWK and HARRIER [3], and can provide sustained disease control with a longer duration of action. Such therapies may potentially fulfill the unmet needs of patients with nAMD in Korea.
A number of study eyes treated with three anti-VEGF agents differed (ranibizumab, bevacizumab, and aflibercept), and some baseline parameters showed significant differences among each anti-VEGF agent including age or MNV composition. The occult MNV type rate for ranibizumab was lower than aflibercept. When there is a lesion above the retinal pigment epithelium (RPE) that penetrates both the RPE and the photoreceptors to form subretinal MNV, anti-VEGF therapy provides significant protection. Typical MNV or RAP shows favorable results in any anti-VEGF therapy. Additionally, ranibizumab is preferred among other anti-VEGFs due to progression of RPE atrophy in RAP [16, 17]. Conversely, the response to anti-VEGFs is poor in the case of sub-RPE MNV, including PCV with type 1 MNV variant. It has been reported that occult MNV requires a relatively high number of injections below the RPE for neovascularization [18], but in the case of the variant PCV, aflibercept has shown good results in polyp regression [19]. Therefore, clinicians have used a preferential approach to select appropriate treatments based on these factors. Hence, physicians tend towards selecting aflibercept for PCV, and ranibizumab for classic MNV and RAP types. This can suggest that physician’s preference exists in the choice of the first anti-VEGF agent in treatment-naive patients with nAMD.
In switch patients, there was no significant improvement after switch between approved anti-VEGF (ranibizumab and aflibercept). However, switching from bevacizumab to ranibizumab or aflibercept showed improvement in both VA and CST. This indicates that the efficacy and durability of ranibizumab and aflibercept were comparable in terms of both VA gain and CST reduction [20]. However, the perception of longer durability of aflibercept can lead to less injection frequency, resulting in under-treatment and suboptimal outcomes in real-world practice. These results are comparable to the recent 4-year long-term Korean study, where there was no difference between ranibizumab and aflibercept in VA gain and number of injections (2.9 ± 1.7 in ranibizumab and 3.0 ± 1.5 in aflibercept group, p = 0.692). The mean VA when the treatment was switched from ranibizumab to aflibercept and mean VA at 1 year after switching did not differ significantly (from 69.28 ± 9.58 to 66.93 ± 9.16 letters; p = 0.425) [21].
As seen in the previous publications [5, 6], when we analyzed functional and anatomical outcomes based on frequency of injection, 185 patients were identified as the frequent group, and 415 were identified as the infrequent group. VA gains were relatively higher with the frequent injection group. The reduction in CST was similar between groups in year 1 but a trend of increase in CST was seen in the infrequent group in year 2. Numerous real-world studies have shown that more frequent anti-VEGF injections resulted in better functional and anatomical outcomes in patients with nAMD [22,23,24].
The key strength of this study is the large sample size (n = 600), and the findings are clinically meaningful, reflecting the actual clinical settings and providing insights into fluid-management strategies. The demographics and clinical characteristics of the study population were consistent with those reported in previous real-world studies from Korea [5, 12, 25]. The main limitation of this retrospective study was that the study cohort was heterogeneous compared to clinical trials. Patients who received ranibizumab, aflibercept, or bevacizumab and who had received two or more different anti-VEGF agents were pooled in the same cohort. Therefore, the variation in efficacy also serves as a major limitation. Decisions on treatment regimens and schedules were made based on the physicians’ discretion (pro-re-nata or treat and extend) and continuation/discontinuation/switch of treatment. ‘In Korea, most clinicians are performing indocyanine green angiography (ICGA) due to the high prevalence of PCV, but ICGA is not mandated by insurance standards. Asian populations in which ICG is utilized or careful non-ICG parameters are evaluated have shown a much higher percentage of PCV, up to over 50% [26,27,28]. Considering the retrospective nature of this study, some cases in which ICGA was not performed may have been included, and the prevalence rate may have been underestimated accordingly.’ Assessment variables, such as collection and interpretation of retinal images, may also have differed based on the institutions’ practice. Furthermore, the present study evaluated the influence of each fluid compartment on visual outcomes separately. For this reason, we could not evaluate the influence of co-existing two or more fluid compartments. In addition, the difference in the impact of different fluid locations (foveal vs. extrafoveal) was not evaluated. An assessment of fluids was performed by multiple graders across multiple sites. The assessment process involved each grader independently evaluating the presence and characteristics of IRF, SRF, and sub-RPE fluid in the retinal images. While we did not follow the exact criteria like other RCT studies, the graders utilized their expertise and familiarity with the well-known and widely accepted definition of fluid to identify and grade the retinal fluids. Thus, there might be a degree of variability in assessment process owing to multiple graders across multiple sites. Lastly, since the current study focused on real-world anti-VEGF treatment patterns, we did not include safety data, which are well known and reported in the prior literature [12].
Conclusions
In summary, this observational study analyzed the current treatment pattern and treatment results for a number of patients with nAMD and confirmed that retinal fluid after loading phase and fibrovascular PED are predictive factors for disease activity defined as persistent retinal fluid. These results are similar to previously reported studies and showed that medical needs and treatment results that are not satisfied with the current treatment approach can be predicted. These results may suggest the need for new anti-VEGF therapies that can provide sustained disease control to fulfill the unmet needs of patients with nAMD.
Data Availability
Novartis is committed to sharing with qualified external researchers, access to patient-level data, and supporting clinical documents from eligible studies. These requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided are anonymized to respect the privacy of the patients who have participated in the trial in line with applicable laws and regulations. This trial data availability is according to the criteria and process described on www.clinicalstudydatarequest.com
References
Schmidt-Erfurth U, Chong V, Loewenstein A, Larsen M, Souied E, Schlingemann R, et al. Guidelines for the management of neovascular age-related macular degeneration by the European Society of Retina Specialists (EURETINA). Br J Ophthalmol. 2014;98:1144–67.
Amoaku WM, Chakravarthy U, Gale R, Gavin M, Ghanchi F, Gibson J, et al. Defining response to anti-VEGF therapies in neovascular AMD. Eye (Lond). 2015;29:721–31.
Dugel PU, Koh A, Ogura Y, Jaffe GJ, Schmidt-Erfurth U, Brown DM, et al. HAWK and HARRIER: Phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127:72–84.
Wykoff CC, Eichenbaum DA, Roth DB, Hill L, Fung AE, Haskova Z. Ranibizumab induces regression of diabetic retinopathy in most patients at high risk of progression to proliferative diabetic retinopathy. Ophthalmol Retina. 2018;2:997–1009.
Baek SK, Kim JH, Kim JW, Kim CG. Increase in the population of patients with neovascular age-related macular degeneration who underwent long-term active treatment. Sci Rep. 2019;9:13264.
Woo SJ, Cho GE, Cho JH. Short-term efficacy and safety of ranibizumab for neovascular age-related macular degeneration in the real world: a post-marketing surveillance study. Korean J Ophthalmol. 2019;33:150–66.
Androudi S, Dastiridou A, Pharmakakis N, Stefaniotou M, Kalogeropoulos C, Symeonidis C, et al. Guidelines for the management of wet age-related macular degeneration: recommendations from a panel of Greek experts. Adv Ther. 2016;33:715–26.
Lalwani GA, Rosenfeld PJ, Fung AE, Dubovy SR, Michels S, Feuer W, et al. A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO Study. Am J Ophthalmol. 2009;148(43–58): e1.
Busbee BG, Ho AC, Brown DM, Heier JS, Suner IJ, Li Z, et al. Twelve-month efficacy and safety of 05 mg or 20 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology. 2013;120:1046–56.
Kodjikian L, Souied EH, Mimoun G, Mauget-Faysse M, Behar-Cohen F, Decullier E, et al. Ranibizumab versus bevacizumab for neovascular age-related macular degeneration: results from the GEFAL noninferiority randomized trial. Ophthalmology. 2013;120:2300–9.
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–24.
Kim JH, Sagong M, Woo SJ, Kim YC, Cho H, Lee YH, et al. A real-world study assessing the impact of retinal fluid on visual acuity outcomes in patients with neovascular age-related macular degeneration in Korea. Sci Rep. 2022;12:14166.
Bobadilla M, Pariente A, Oca AI, Pelaez R, Perez-Sala A, Larrayoz IM. Biomarkers as predictive factors of anti-VEGF response. Biomedicines. 2022;10:2.
Lai TT, Hsieh YT, Yang CM, Ho TC, Yang CH. Biomarkers of optical coherence tomography in evaluating the treatment outcomes of neovascular age-related macular degeneration: a real-world study. Sci Rep. 2019;9:529.
Suzuki M, Nagai N, Izumi-Nagai K, Shinoda H, Koto T, Uchida A, et al. Predictive factors for non-response to intravitreal ranibizumab treatment in age-related macular degeneration. Br J Ophthalmol. 2014;98:1186–91.
Hata M, Yamashiro K, Oishi A, Ooto S, Tamura H, Miyata M, et al. Retinal pigment epithelial atrophy after anti-vascular endothelial growth factor injections for retinal angiomatous proliferation. Retina. 2017;37:2069–77.
Daniel E, Shaffer J, Ying GS, Grunwald JE, Martin DF, Jaffe GJ, et al. Outcomes in eyes with retinal angiomatous proliferation in the comparison of age-related macular degeneration treatments trials (CATT). Ophthalmology. 2016;123:609–16.
Kodaday K, Kodjikian L, Gadiollet E, Chirpaz N, Loria O, Feldman A, et al. The effects of treatment regimen on the initial management of macular neovascularization subtypes in age-related macular degeneration. Ophthalmologica. 2023;246:113–22.
Lee WK, Iida T, Ogura Y, Chen SJ, Wong TY, Mitchell P, et al. Efficacy and safety of intravitreal aflibercept for polypoidal choroidal vasculopathy in the PLANET study: a randomized clinical trial. JAMA Ophthalmol. 2018;136:786–93.
Horner F, Lip PL, Mohammed BR, Fusi-Rubiano W, Gokhale E, Mushtaq B, et al. Comparing effectiveness of three different anti-VEGF treatment regimens for neovascular age-related macular degeneration: two years’ real-world clinical outcomes. Clin Ophthalmol. 2021;15:1703–13.
Jin KW, Kim JH, Park JY, Park SJ, Park KH, Lee JY, et al. Long-term outcomes of ranibizumab vs. aflibercept for neovascular age-related macular degeneration and polypoidal choroidal vasculopathy. Sci Rep. 2021;11:14623.
Gerding H, Loukopoulos V, Riese J, Hefner L, Timmermann M. Results of flexible ranibizumab treatment in age-related macular degeneration and search for parameters with impact on outcome. Graefes Arch Clin Exp Ophthalmol. 2011;249:653–62.
Holz FG, Tadayoni R, Beatty S, Berger A, Cereda MG, Cortez R, et al. Multi-country real-life experience of anti-vascular endothelial growth factor therapy for wet age-related macular degeneration. Br J Ophthalmol. 2015;99:220–6.
Weber M, Kodjikian L, Coscas F, Faure C, Aubry I, Dufour I, et al. Impact of intravitreal aflibercept dosing regimens in treatment-naive patients with neovascular age-related macular degeneration in routine clinical practice in France: results from the RAINBOW study. BMJ Open Ophthalmol. 2020;5: e000377.
Sagong M, Woo SJ, Lee Y, Korea LS. Real-world effectiveness, treatment pattern, and safety of ranibizumab in Korean patients with neovascular age-related macular degeneration: subgroup analyses from the LUMINOUS study. Clin Ophthalmol. 2021;15:1995–2011.
Wong CW, Wong TY, Cheung CM. Polypoidal choroidal vasculopathy in Asians. J Clin Med. 2015;4(5):782–821. https://doi.org/10.3390/jcm4050782.
Kokame GT, et al. Anti-vascular endothelial growth factor resistance in exudative macular degeneration and polypoidal choroidal vasculopathy. Ophthalmol Retina. 2019;3(9):744–52. https://doi.org/10.1016/j.oret.2019.04.018.
Cheung CMG, et al. Polypoidal choroidal vasculopathy: consensus nomenclature and non-indocyanine green angiograph diagnostic criteria from the Asia-Pacific ocular imaging society PCV workgroup. Ophthalmology. 2021;128(3):443–52. https://doi.org/10.1016/j.ophtha.2020.08.006.
Acknowledgements
Medical Writing/Editorial Assistance
Medical writing and editorial assistance was provided by Shashank Jain and Aditya Pramod (Novartis Healthcare Pvt. Ltd., Hyderabad, India) in accordance with Good Publication Practice (GPP2022) guidelines. (www.ismpp.org/gpp-2022).
Funding
The funding for this study, writing support, and publication including the journal’s Rapid Service Fee were provided by Novartis Pharma AG.
Author information
Authors and Affiliations
Contributions
Min Sagong, Jae Hui Kim, Se Joon Woo, Yu Cheol Kim, Heeyoon Cho, Young Hoon Lee, Hee Seung Chin, Iksoo Byon, Young Joon Jo, and Se Woong Kang: Conceptualization, methodology, investigation, writing—original draft, writing—review & editing, visualization, and supervision; Jeonghee Kim: Conceptualization, methodology, writing—original draft, writing—review & editing, visualization, and project administration. Jae Eun Chae: Methodology, software, formal analysis, data curation, writing—original draft, writing—review & editing, and visualization.
Corresponding author
Ethics declarations
Conflict of Interest
Min Sagong reports honoraria from Allergan, Bayer, Novartis, and Roche for lecture fees, consultancy, and board membership and reports research grants from Allergan, Bayer, and Novartis. Jae Hui Kim reports grants from Novartis and Bayer. Se Joon Woo is a consultant of Samsung Bioepis, Alteogen, Curacle, Novelty Nobility, Sometech, and Pharmabcine; reports grants from Samsung Bioepis, Curacle, Alteogen, Bayer, Novartis, Geneuintech and Roche; reports lecture fees from Novartis, Bayer, Allergan/AbbVie, Roche and Samil; and owns equity of RetiMark and Panolos Bioscience. Yu Cheol Kim is a consultant for Novartis and Bayer; received honoraria from Allergan, Bayer, and Novartis; and research grants from Chong Kun Dang Pharm., SCD Pharm., Bayer, and Novartis. Heeyoon Cho reports lecture fees and consultancy from Allergan, Bayer, and Novartis. Young Hoon Lee and Hee Seung Chin have no financial disclosures. Iksoo Byon is a consultant/advisor for Novartis, Bayer, and AIinsight Inc and reports grants from Novartis and Busan Technopark. Young Joon Jo reports grants from Bayer and Novartis. Jeonghee Kim is an employee of Novartis Korea Ltd. Jae Eun Chae is an employee of LSK Global Pharma Services, Korea. Se Woong Kang has served on advisory boards for Novartis, Bayer, Allergan, Alcon, and Samchundang and has received consultancy fees and payments for lectures from these companies.
Ethical Approval
This study was conducted in accordance with the Guidelines for Good Pharmacoepidemiology Practices issued by the International Society for Pharmacoepidemiology and the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline and adhered to the ethical principles derived from the Declaration of Helsinki. Each IRB/ IEC reviewed and approved the study protocol. As this is a retrospective RWE study, we applied for a waiver of patient consent from the IRB (institutional review board, IRB) and approved to perform when we submit protocol approval to IRB. However, IRB/IEC of Samsung Medical Center recommended to proceed with the consent form for personal information. Thus, Samsung Medical Center received the subjects’ consents for personal information. Samsung Medical Center Institutional Review Board approved the study (approval number 2020–05-070). A list of the ethics committees that approved the study protocol are provided in Supplementary Table 1.
Additional information
Prior Presentation Sagong et al., Predictors of disease activity after anti-VEGF treatment for neovascular age-related macular degeneration using real-world data from PROOF study; oral presentation, Dec 4, 2021, The Korean Retina Society, Yongsan Dragon city, Seoul, Korea.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Sagong, M., Kim, J.H., Woo, S.J. et al. Predictors of Disease Activity After Anti-VEGF Treatment for Neovascular Age-Related Macular Degeneration Using Real-World Data from the PROOF Study. Ophthalmol Ther (2024). https://doi.org/10.1007/s40123-024-01021-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40123-024-01021-x