Abstract
Introduction
Photobiomodulation (PBM) has become a promising approach for slowing the progression of early and intermediate dry age-related macular degeneration (dAMD) to advanced AMD. This technique uses light to penetrate tissues and activate molecules that influence biochemical reactions and cellular metabolism. This preliminary analysis is aimed at assessing the safety, tolerability, and short-term effectiveness of the EYE-LIGHT®PBM treatment device in patients with dAMD.
Methods
The EYE-LIGHT® device employs two wavelengths, 590 nm (yellow) and 630 nm (red), in both continuous and pulsed modes. Patients over 50 years of age with a diagnosis of dAMD in any AREDS (Age-Related Eye Disease Study) category were randomly assigned to either the treatment group or the sham group. The treatment plan consisted of an initial cycle of two sessions per week for 4 weeks. Safety, tolerability, and compliance outcomes, along with functional and anatomical outcomes, were assessed at the end of the fourth month.
Results
This preliminary analysis included data from 76 patients (152 eyes). All patients were fully compliant with treatment sessions, and only one fifth of patients treated with PBM reported mild ocular adverse events, highlighting exceptional results in terms of tolerability and adherence. Changes in best-corrected visual acuity (BCVA) from baseline to month 4 differed significantly between the sham and PBM-treated groups, favoring the latter, with a higher proportion achieving a gain of five or more letters post-treatment (8.9% vs. 20.3%, respectively; p = 0.043). No significant differences in central subfield thickness (CST) were observed between the two groups over the 4-month period. The study also found a statistically significant disparity in mean drusen volume changes from baseline to month 4 between the groups in favor of patients treated with PBM (p = 0.013).
Conclusion
These preliminary results indicate that PBM treatment using the EYE-LIGHT® system is safe and well tolerated among patients with dAMD. Furthermore, both functional and anatomical data support the treatment’s short-term efficacy.
Trial Registration
ClinicalTrials.gov identifier NCT06046118.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Why carry out this study? |
Age-related macular degeneration (AMD) is a condition characterized by impaired mitochondrial function, oxidative stress, and inflammation affecting the retinal pigment epithelium (RPE), choriocapillaris, and neuroretina. |
Previous research indicated that photobiomodulation (PBM) therapy may play a beneficial role in enhancing mitochondrial metabolism, regulating inflammation pathways, and mitigating oxidative stress. |
What was learned from the study? |
This randomized controlled double-blind study investigated the safety, tolerability, patient compliance, and short-term efficacy of a novel PBM device employed in patients with non-exudative AMD. |
Preliminary results collected at 4 months show that PBM therapy using the EYE-LIGHT® system was safe and well tolerated in patients with dry AMD (dAMD). Patients were fully compliant. Additionally, short-term efficacy was confirmed by the significant improvement of both functional and anatomical data obtained in the group of treated patients compared to sham. |
Introduction
Age-related macular degeneration (AMD) is a common ocular condition characterized by the degeneration of the macula, eventually leading to a progressive and severe vision loss. AMD represents the major cause of legal blindness in developed countries, impacting approximately 20 million individuals in the United States and 196 million worldwide, with a significant impact on quality of life in affected patients [1]. Given the progressive aging of the population, the number of patients with AMD is projected to rise significantly in the near future [2].
The clinical classification of AMD includes various stages [3]. While early or intermediate AMD is identified by the presence of drusen with or without pigmentary changes, late AMD can be complicated by macular neovascularization (MNV) or geographic atrophy (GA). Notably, any stage without MNV is considered dry AMD, encompassing early/intermediate AMD and GA. The dry stage of the disease (dAMD) affects approximately 85–90% of patients with AMD.
The pathogenesis of AMD is multifactorial, involving older age, genetic predisposition, and several environmental risk factors that can ultimately trigger increased inflammation, mitochondrial dysfunction, and oxidative stress. As a result, waste products from the photoreceptor outer segment such as lipofuscin, lipids, lipoproteins, cellular debris, and pigment accumulate underneath the retina. These deposits may form drusen, which typically localize between the basement membrane of the retinal pigment epithelium (RPE) and the inner collagenous layer of Bruch’s membrane [4].
Given the significant impact on quality of life and the related social costs of AMD, diagnosing, monitoring, and treating the disease are poised to become major challenges for the global ophthalmology community in the coming years. Consequently, research efforts and innovative therapeutic approaches are focusing on slowing the progression of the disease and preventing the advanced stages, characterized by either outer retinal and RPE atrophy, or development and exudation of MNV.
Currently, the primary strategy to delay early and intermediate AMD progression includes maintaining a healthy lifestyle and taking oral nutritional supplements [5]. More recently, a noninvasive biotechnology known as photobiomodulation (PBM) has emerged as a promising strategy to reduce the progression of early and intermediate dAMD to late AMD. Previous studies employing a three-wavelength PBM device (Valeda Light Delivery System, LumiThera, Inc., Poulsbo, WA, USA) have shown that this technology is effective for the treatment of intermediate dAMD, demonstrating improvements in visual acuity and contrast sensitivity, as well as reducing drusen volume [6,7,8,9].
In 2024, the EYE-LIGHT® PBM device (Espansione Group S.p.A., Bologna, Italy) obtained the CE mark in the European Union for the treatment, amongst other destinations of use involving the ocular surface [10,11,12,13], of dAMD, in compliance with the Medical Device Regulation. This device uses medical-grade light-emitting diodes (LEDs) emitting wavelengths of ~590 nm and ~630 nm in both continuous and pulsed modes. This technology, also known as Light Modulation® Low-level Light Therapy (LM® LLLT), involves the penetration of tissues by light that activates molecules modulating biochemical reactions and cellular metabolism.
The purpose of this preliminary analysis was to evaluate the safety, tolerability, and short-term efficacy of LM® LLLT using the EYE-LIGHT® PBM treatment in patients with dAMD.
Methods
This is a preliminary analysis of the safety, tolerability, and short-term efficacy of the first cycle of PBM treatment employing the EYE-LIGHT® device. This randomized controlled study was conducted across five European centers (University Magna Graecia of Catanzaro, Catanzaro, Italy; University of Genoa, Genoa, Italy; University of Ferrara, Ferrara, Italy; İstanbul Retina Institute, İstanbul, Turkey; Ankara University Faculty of Medicine, Ankara, Turkey), received the Ethics Committee approval from the University Magna Graecia of Catanzaro, and was conducted in accordance with the tenets of the Declaration of Helsinki. All study participants provided written informed consent. Patients were informed of their right to withdraw from the study at any time and for any reason, without any impact on their medical care.
Patients were included if they were aged > 50 years and had a diagnosis of dAMD belonging to any AREDS (Age-Related Eye Disease Study) category, and were randomly assigned to either the treatment or sham group. The AREDS stage was ascertained based on color picture and fundus autofluorescence imaging [14]. Specifically, AREDS category 1 was defined as no AMD, including patients with no or only a few small drusen (i.e., < 63 microns in diameter). AREDS category 2 was defined as early AMD, characterized by multiple small drusen, a few intermediate drusen (i.e., 63 to 124 microns in diameter), or abnormalities in the RPE abnormalities. AREDS category 3 was defined as intermediate AMD, characterized by extensive intermediate drusen, including at least one large drusen (> 125 μm in diameter) or geographic atrophy not involving the center of the fovea. Lastly, AREDS category 4 was defined as advanced/late AMD [15]. In the case of bilateral disease, both eyes of the patient were included in the analysis. Patients were excluded in the case of macular neovascular membrane (MNV), herpes infection, dense cataract not permitting high-quality imaging examinations, or any other significant ocular and/or retinal diseases in either eye. Additional exclusion criteria were concomitant epilepsy, neurological diseases, psychiatric comorbidities, and pregnancy.
Treatment
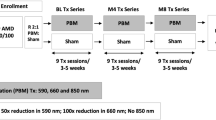
Patients were randomly assigned in a 1:1 ratio to receive either PBM treatment using the EYE-LIGHT® device (Espansione Group S.p.A., Bologna, Italy) or a sham treatment. The sham treatment used the same device but emitted a very low light power (i.e., less than 30% of the standard output), which has no biological effects on target tissues. The complete treatment plan for patients with dAMD with the EYE-LIGHT® device involves two cycles: cycle 1 includes eight sessions, with two sessions per week over 4 weeks, while cycle 2, conducted 4 to 6 months after cycle 1, comprises six sessions, with two sessions per week over 3 weeks. No pupil dilation is required during the treatment. Each session lasts 12 min and has two phases: in phase I, yellow light is delivered continuously with eyes closed for 300 s, followed by pulsed delivery with eyes open for 60 s; in phase II, red light is delivered continuously with eyes closed for 300 s, followed by pulsed delivery with eyes open for 60 s. Figure 1 shows a diagram representing each treatment session and their schedule over time.
Representative image showing the mask and the treatment modality. A Schedule of each cycle and session when used in the setting of dry age-related macular degeneration. B Subsequent phases performed during each treatment session. Specifically: (1) 300 s of continuous yellow light delivered with eyes closed and 60 s of pulsed yellow light delivered with eyes opened; (2) 300 s of continuous red light delivered through eyes closed and 60 s of pulsed red light delivered with eyes opened. LM® LLLT Light Modulation® Low-level Light Therapy
Ophthalmic Examination
All patients were evaluated at baseline (BL) and at 4 months (M4) after the competition of the cycle 1. A complete ophthalmic examination, including best-corrected visual acuity (BCVA) using Early Treatment for Diabetic Retinopathy Study (ETDRS) charts, slit lamp examination, fundus examination by indirect ophthalmoscopy, and intraocular pressure (IOP) measurement, was performed before patient enrollment (BL) and at M4. All patients were also evaluated using spectral-domain optical coherence tomography (SD-OCT) (Heidelberg Engineering, Heidelberg, Germany), using a 20 × 20 high-resolution (HR) horizontal dense volume scan (49 sections each). All scans were tracked with the follow-up function. The mean drusen volume was evaluated within the first ETDRS ring using the Heidelberg software followed by manual segmentation correction if needed. The central subfield thickness was measured using the automated measure provided by the Heidelberg software, and manual adjustments were adopted only in cases of segmentation errors. Drusenoid RPE detachments, defined as RPE elevations ≥ 350 μm, were also included in the drusen volume measurements.
Study Outcomes
The primary outcome was the evaluation of the safety, tolerability, and compliance outcomes of patients with dAMD undergoing one cycle of eight sessions of sham or PBM using the EYE-LIGHT® device. The secondary outcome was the assessment of short-term functional and anatomical outcomes at M4.
Safety, Tolerability, and Compliance
The safety of the treatment was assessed at 4 months by monitoring for signs of retinal phototoxicity including any indications of exudation, inflammation, or new-onset MNV as evaluated by fundus photography, SD-OCT, and optical coherence tomography angiography (OCTA). Additionally, the occurrence of potentially treatment-related ocular-specific adverse events (AEs) was also evaluated, including dry eye-like symptoms, superficial punctate keratitis, visual perseveration (i.e., persistence or reappearance of the glare image), and warmth at the application site.
The tolerability of the treatment was measured at M4 through the Brief Ocular Discomfort Inventory (BODI) questionnaire [16], an 11-point scale where 0 indicates no discomfort and 10 indicates extreme discomfort (Fig. 2).
The compliance was defined as the adherence to the treatment schedule, measured as the percentage of treatment sessions attended and entirely completed over the treatment sessions expected.
Functional and Anatomical Outcomes
Changes from BL to M4 in mean BCVA, mean drusen volume, and mean central subfield thickness were also evaluated in both the sham and PBM groups.
Statistical Analysis
Given the bilateral simultaneous application of the treatment, both eyes of each patient were included, and all analyses were based on individual eyes rather than on individual patients, unless specified otherwise. Categorical variables are expressed as counts and percentages, whereas quantitative variables are expressed as mean ± standard deviation (SD).
The distribution of continuous variables was inspected graphically and then verified with the Shapiro–Wilk test. Given the lack of a normal distribution of the data, non-parametric tests were used. Between-group comparisons were performed with either the Wilcoxon rank-sum (Mann–Whitney U) test or the Fisher’s exact test according to the variable type. The Wilcoxon signed-ranked test for paired data was used to compare changes from BL to M4 within the same patient group (sham or PBM).
All statistical analyses were performed using Stata 18.0 software (StataCorp, College Station, TX, USA). A two-sided p-value of less than 0.05 was considered statistically significant.
Results
This preliminary analysis included data from 76 patients for a total of 152 qualifying eyes. The mean patient age was 68.6 ± 10.2 years, and 76% (n = 58) were female.
Most patients were phakic (81.8%) and had early/intermediate-stage dAMD, classified as AREDS categories 2 (40.0%) and 3 (46.7%). No statistically significant differences between the sham and the PBM groups were found at BL for demographic and clinical parameters, as shown in Table 1.
Safety, Tolerability, and Compliance Outcomes
No signs of retinal phototoxicity were observed in either group at M4. A total of 46 eyes (30.3% of the total) had at least one ocular AE. The number of eyes with ocular-specific AEs was higher in the sham group (40.8%; n = 31) than in the PBM group (19.7%; n = 15) (p = 0.008): specifically, dry eye-like symptoms in seven eyes (9.2% of the total) in the sham group versus six eyes (7.9%) in PBM (p = 1.00); visual perseveration in nine eyes (11.8%) in the sham group versus three eyes (3.9%) in PBM (p = 0.130); warmth at the application site in 17 eyes (22.4%) in the sham group versus eight eyes (10.5%) in the PBM (p = 0.079). Conversely, superficial punctate keratitis was never diagnosed after any mask session in either group. No ocular-specific AEs led to study discontinuation.
At M4, the mean BODI score was 1.22 ± 2.0 in the sham group and 1.6 ± 2.5 in the PBM group, with no significant differences between the two groups (p = 0.50).
Overall, all patients (100%) of both the sham and PBM groups were fully compliant with all treatment sessions. Three patients were withdrawn from the study due to the development of MNV in one eye; interestingly, all these patients belonged to the sham group (7.8% vs. 0% in the treatment group; p = 0.240).
Functional and Anatomical Outcomes
The values of all functional and anatomical parameters for the sham and PBM groups at both time points are reported in Table 2.
Best-Corrected Visual Acuity
Sham- and PBM-treated groups had similar BCVA at BL (66.2 ± 14.7 vs. 64 ± 15.5 ETDRS letters, respectively, p = 0.367). At M4, neither the sham nor the PBM group experienced significant BCVA changes compared to BL; however, a non-statistically significant decrease in ETDRS letters was noted in the sham group (1.13 ± 5.0, p = 0.071), while a non-statistically significant improvement was detected in the PBM group (0.48 ± 5.31, p = 0.184) (Fig. 3A). The comparison of BCVA changes from BL to M4 detected in the two groups was significantly different in favor of patients treated with PBM (p = 0.026) (Fig. 3B). Additionally, stratified analysis showed that a significantly higher percentage of patients in the PBM group gained five or more letters (one-line improvement or better) compared to the sham group (20.3% vs. 8.9%, respectively; p = 0.043).
A, C, E Changes from baseline (BL) to month 4 (M4) in the sham- and PBM-treated groups for best-corrected visual acuity (BCVA), mean drusen volume (MDV), and central subfield thickness (CST), respectively. P-value refers to the comparison within each group. B, D, F Comparison of changes between baseline and month 4 in the sham- and PBM-treated groups for BCVA, MDV, and CST, respectively. Δ delta of change, PBM photobiomodulation
Mean Drusen Volume
Mean drusen volume increased significantly from BL to M4 by an average of 0.25 ± 5.20 mm3 in the sham group (p = 0.048), while a nonsignificant reduction by a mean of −0.97 ± 16.74 mm3 was found in the PBM group (p = 0.10) (Fig. 3C). A statistically significant difference was detected in the change in mean drusen volume between the two groups in favor of patients treated with PBM (p = 0.013) (Fig. 3D). Figure 4 shows the reduction of macular drusen from BL to M4 in a representative patient belonging to the PBM group.
Central Subfield Thickness
No significant differences in central subfield thickness were noted in either the sham or PBM group at any time point, nor did the amount of change from BL to M4 differ significantly between the two groups (all p > 0.05) (Fig. 3E, F).
Discussion
This preliminary analysis after the first cycle of PBM treatment with the EYE-LIGHT® system in patients with dAMD showed a favorable safety profile with no signs of retinal phototoxicity observed in either the sham or PBM group. About one fifth of treated patients experienced mild ocular AEs, mainly warmth at the application site, that did not influence patient adherence to treatment.
Safety results align with findings obtained in the LIGHTSITE III study involving patients with dAMD treated with a different PBM device (LumiThera Valeda Light Delivery System), which reported ocular-specific AEs in about one fourth of treated patients [17].
PBM therapy typically acts in an optical window with three wavelengths ranging from the red to near-infrared regions of the light spectrum (500–1000 nm) delivered by a light source [18]. EYE-LIGHT® LLLT uses light-emitting diodes (LED) emitting two wavelengths of 590 nm (yellow) and 630 nm (red) in continuous and pulsed mode. On the other hand, the Valeda Light Delivery System delivers through LED three wavelengths in the yellow (590 nm–5 mW/cm2), red (660 nm–65 mW/cm2) and near-infrared (NIR) (850 nm–8 mW/cm2) in a total exposure time of 250 s subdivided into four phases. Phase 1 delivers pulsed yellow and NIR wavelengths for 35 s with the patient’s eyes open. Phase 2 delivers a continuous red wavelength for 90 s with closed eyes. Phase 3 and phase 4 repeat the first two phases, respectively. Specifically, red light (630 nm) targets cytochrome c oxidase (CcO), a crucial chromophore within mitochondria responsible for the electron transport mechanism, restoration of mitochondrial membrane potential (MMP), and ATP production; yellow light (590 nm) inhibits the expression of vascular endothelial growth factor (VEGF), a protein involved in the formation of blood vessels in the advanced exudative stages of the disease [19]. Additionally, yellow light promotes the release of nitric oxide (NO) from intracellular stores, reducing oxidative stress-mediated injury and enhancing oxygen delivery. Furthermore, photon absorption activates numerous transcription factors, leading to increased expression of antioxidant enzymes and anti-apoptotic and anti-inflammatory signaling proteins supporting the vitality of retinal cells [19,20,21]. These effects suggest that PBM treatment could have a beneficial role in AMD, a condition marked by mitochondrial dysfunction, oxidative stress, and inflammation within the RPE, choriocapillaris, and neuroretina.
Our evaluation of procedure tolerability showed a similar BODI score between the two study groups, confirming the good level of patient satisfaction with treatment. All patients were fully compliant except for three who were withdrawn from the study due to the development of MNV in one eye. Interestingly, all three belonged to the sham group, suggesting the beneficial effects of PBM therapy in counteracting the natural progression of dAMD to the advanced wet stages. This result contrasts with the higher conversion rate from dAMD to wet AMD reported in the LIGHTSITE III study in patients treated with the Valeda Light Delivery System versus controls (5.4% vs. 1.8%) after a longer follow-up (13 months) [17].
Given the short-term follow-up of this preliminary analysis, functional and anatomical parameters were considered secondary outcomes. The BCVA changes from BL to M4 experienced by sham and treated groups were significantly different in favor of patients treated with PBM who, in a statistical higher percentage, reached a post-treatment gain of five or more letters in comparison to the sham group. These functional improvements are in line with data reported in the LIGHTSITE III study, in which the average increase in BCVA was 5.4 letters in the PBM group and 3.0 letters in sham-treated eyes at month 13 [17].
Structural OCT has significantly enhanced our ability to explore and accurately measure drusen volume thanks to the development of various algorithms designed to automatically segment drusen volume, enabling precise quantification [22, 23]. Abdelfattah and colleagues [24] were the first to use structural OCT to investigate the role of drusen volume in the progression to late AMD among individuals with early/intermediate AMD. Their findings revealed that patients with a drusen volume exceeding 0.03 mm3 had more than a fourfold higher likelihood of advancing to late AMD than those with lower drusen volumes. Subsequently, Hirabayashi et al. [25] used structural OCT to determine the frequency of multiple biomarkers of intermediate AMD and their relationship with the development of complete retinal pigment epithelium and outer retinal atrophy (cRORA) after 2 years. Their study, involving 330 consecutive patients with intermediate AMD in at least one eye, indicated that drusen volume is a risk factor for the onset of cRORA within a 2-year period. More recently, Liu et al. [26] conducted a longitudinal study on 171 eyes with intermediate AMD to design a clinical trial for therapies aimed at slowing the progression from intermediate AMD to GA. Using persistent choroidal hypertransmission defects (hyperTDs) as the clinical trial endpoint, they found that a drusen volume higher than 0.25 mm3 within a 5-mm fovea-centered circle could predict that approximately 68% of intermediate AMD eyes would develop a hyperTD within 12 months. Therefore, drusen volume is an important risk factor for disease progression, although it should be noted that changes in drusen volume are dynamic and not consistently progressive throughout the course of the disease.
In the present study, a statistically significant difference in the changes in mean drusen volume from BL to M4 was observed between the two groups. This may suggest that PBM has the potential to slow the accumulation of drusenoid material or improving the RPE function, leading to partial resorption of drusen. Although our findings should be considered preliminary, it is important to note that these results are in contrast to those reported in the LIGHTSITE III study, in which no significant change in mean drusen volume was reported in patients treated with PBM, while an increase was detected in those receiving sham treatment [17].
As mentioned above, changes in drusen volume are dynamic and not consistently progressive throughout the course of the disease. For instance, a reduction in drusen volume may precede drusen-associated RPE atrophy. Therefore, a reduction in drusen volume is not always associated with a positive outcome, as it may indicate a biomarker of progressive late AMD development. We recognize that the short-term follow-up of this preliminary analysis does not allow us to draw any definite conclusions. However, it is noteworthy that none of the treated eyes developed new onset of interdigitation and ellipsoid zone irregularities or new formation of RPE loss, indicating that the regression of drusen did not contribute to new GA formation.
Concerning CST, no significant differences were noted in either group over 4 months. Anatomical findings reported in this study regarding thickness measurements are consistent with structural OCT data reported in the LIGHTSITE II study (LumiThera Valeda), where at 9 months no significant difference was observed between the treatment and the sham groups [27].
This preliminary analysis, focused mainly on safety and tolerability outcomes, suffers from limitations related to the short-term follow-up and the lack of strict inclusion criteria that will be overcome by a prospective, interventional, randomized, sham-controlled, double-masked multicentric clinical trial including adult patients suffering from dAMD (DRUSEN study, part of the broader LIGHTWAVE I effort) currently ongoing across the same centers. Another limitation is related to the study of retinal phototoxicity that was evaluated solely through multimodal imaging rather than using functional tests, such as electroretinography. However, multimodal imaging is frequently used to detect even subtle signs of retinal toxicity.
Conclusion
In conclusion, these preliminary results show that PBM therapy using the EYE-LIGHT® system is safe and well tolerated in patients with dAMD. Furthermore, functional and anatomical data appear to suggest the short-term efficacy of the procedure.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Fleckenstein M, Schmitz-Valckenberg S, Chakravarthy U. Age-related macular degeneration. JAMA. 2024;331:147.
Li JQ, Welchowski T, Schmid M, Mauschitz MM, Holz FG, Finger RP. Prevalence and incidence of age-related macular degeneration in Europe: a systematic review and meta-analysis. Br J Ophthalmol. 2020;104:1077–84.
Ferris FL, Wilkinson CP, Bird A, Chakravarthy U, Chew E, Csaky K, et al. Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120:844–51.
Wong JHC, Ma JYW, Jobling AI, Brandli A, Greferath U, Fletcher EL, et al. Exploring the pathogenesis of age-related macular degeneration: a review of the interplay between retinal pigment epithelium dysfunction and the innate immune system. Front Neurosci. 2022;16:1.
Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration. JAMA. 2013;309:2005.
Markowitz SN, Devenyi RG, Munk MR, Croissant CL, Tedford SE, Rückert R, et al. A double-masked, randomized, sham-controlled, single-center study with photobiomodulation for the treatment of dry age-related macular degeneration. Retina. 2020;40:1471–82.
Merry GF, Munk MR, Dotson RS, Walker MG, Devenyi RG. Photobiomodulation reduces drusen volume and improves visual acuity and contrast sensitivity in dry age-related macular degeneration. Acta Ophthalmol. 2017;95:5.
Ivandic BT, Ivandic T. Low-level laser therapy improves vision in patients with age-related macular degeneration. Photomed Laser Surg. 2008;26:241–5.
Fantaguzzi F, Tombolini B, Servillo A, Zucchiatti I, Sacconi R, Bandello F, et al. Shedding light on photobiomodulation therapy for age-related macular degeneration: a narrative review. Ophthalmol Ther. 2023;12:2903–15.
Giannaccare G, Pellegrini M, Carnovale Scalzo G, Borselli M, Ceravolo D, Scorcia V. Low-level light therapy versus intense pulsed light for the treatment of meibomian gland dysfunction: preliminary results from a prospective randomized comparative study. Cornea. 2023;42:141–4.
Cannas C, Pintus B, Corgiolu L, Borrelli E, Boscia G, Toro MD, et al. Current applications and future perspectives of photobiomodulation in ocular diseases: a narrative review. Appl Sci. 2024;14:2623.
Giannaccare G, Vaccaro S, Pellegrini M, Borselli M, Carnovale Scalzo G, Taloni A, et al. Serial sessions of a novel low-level light therapy device for home treatment of dry eye disease. Ophthalmol Ther. 2023;12:459–68.
Giannaccare G, Rossi C, Borselli M, Carnovale Scalzo G, Scalia G, Pietropaolo R, et al. Outcomes of low-level light therapy before and after cataract surgery for the prophylaxis of postoperative dry eye: a prospective randomised double-masked controlled clinical trial. Br J Ophthalmol. 2023;2:5.
The age-related eye disease study (AREDS). Control Clin Trials. 1999;20:573–600.
Randomized A. Placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss. Arch Ophthalmol. 2001;119:1417.
Pistilli M, Peskin E, Brader H, Dentone P, Maguire M, Asbell P. Evaluation of a modification of the brief pain inventory (BODI) as a measure of severity of dry eye disease. Invest Ophthalmol Vis Sci. 2013;54:8.
Boyer D, Hu A, Warrow D, Xavier S, Gonzalez V, Lad E, et al. LIGHTSITE III. Retina. 2024;44:487–97.
Dompe C, Moncrieff L, Matys J, Grzech-Leśniak K, Kocherova I, Bryja A, et al. Photobiomodulation—underlying mechanism and clinical applications. J Clin Med. 2020;9:1–17.
de Freitas LF, Hamblin MR. Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J Sel Top Quantum Electron. 2016;22:348–64.
Martignago CCS, Oliveira RF, Pires-Oliveira DAA, Oliveira PD, Pacheco Soares C, Monzani PS, et al. Effect of low-level laser therapy on the gene expression of collagen and vascular endothelial growth factor in a culture of fibroblast cells in mice. Lasers Med Sci. 2015;30:203–8.
McDaniel DH, Weiss RA, Geronemus RG, Mazur C, Wilson S, Weiss MA. Varying ratios of wavelengths in dual wavelength LED photomodulation alters gene expression profiles in human skin fibroblasts. Lasers Surg Med. 2010;42:540–5.
Vallino V, Berni A, Coletto A, Serafino S, Bandello F, Reibaldi M, et al. Structural OCT and OCT angiography biomarkers associated with the development and progression of geographic atrophy in AMD. Graefe’s Arch Clin Exp Ophthalmol. 2024;5:85.
Berni A, Shen M, Cheng Y, Herrera G, Hiya F, Liu J, et al. The total macular burden of hyperreflective foci and the onset of persistent choroidal hypertransmission defects in intermediate AMD. Am J Ophthalmol. 2024;87:88.
Abdelfattah NS, Zhang H, Boyer DS, Rosenfeld PJ, Feuer WJ, Gregori G, et al. Drusen volume as a predictor of disease progression in patients with late age-related macular degeneration in the fellow eye. Investig Opthalmol Vis Sci. 2016;57:1839.
Hirabayashi K, Yu HJ, Wakatsuki Y, Marion KM, Wykoff CC, Sadda SR. OCT risk factors for development of atrophy in eyes with intermediate age-related macular degeneration. Ophthalmol Retina. 2023;7:253–60.
Liu J, Shen M, Laiginhas R, Herrera G, Li J, Shi Y, et al. Onset and progression of persistent choroidal hypertransmission defects in intermediate age-related macular degeneration: a novel clinical trial endpoint. Am J Ophthalmol. 2023;254:11–22.
Burton B, Parodi MB, Jürgens I, Zanlonghi X, Hornan D, Roider J, et al. LIGHTSITE II randomized multicenter trial: evaluation of multiwavelength photobiomodulation in non-exudative age-related macular degeneration. Ophthalmol Ther. 2023;12:953–68.
Funding
No funding or sponsorship was received for this study or publication of this article.
Author information
Authors and Affiliations
Contributions
Enrico Borrelli, Giulia Coco, Marco Pellegrini, Marco Mura, Nicolò Ciarmatori, Vincenzo Scorcia, Adriano Carnevali, Andrea Lucisano, Massimiliano Borselli, Costanza Rossi, Michele Reibaldi, Federico Ricardi, Aldo Vagge, Massimo Nicolò, Paolo Forte, Antonio Cartabellotta, Murat Hasanreisoğlu, Cem Kesim, Sibel Demirel, Özge Yanık, Federico Bernabei, Pierre-Raphael Rothschild, Sarah Farrant, and Giuseppe Giannaccare all contributed to the study conception and design, material preparation, data collection and analysis. All named authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Enrico Borrelli, Giulia Coco, Marco Pellegrini, Marco Mura, Nicolò Ciarmatori, Vincenzo Scorcia, Adriano Carnevali, Andrea Lucisano, Massimiliano Borselli, Costanza Rossi, Michele Reibaldi, Federico Ricardi, Aldo Vagge, Massimo Nicolò, Paolo Forte, Antonio Cartabellotta, Murat Hasanreisoğlu, Cem Kesim, Sibel Demirel, Özge Yanık, Federico Bernabei, Pierre-Raphael Rothschild, Sarah Farrant, and Giuseppe Giannaccare have no conflicts of interest to disclose.
Ethical Approval
This randomized controlled study was conducted across five European centers (University Magna Graecia of Catanzaro, Catanzaro, Italy; University of Genoa, Genoa, Italy; University of Ferrara, Ferrara, Italy; İstanbul Retina Institute, İstanbul, Turkey; Ankara University Faculty of Medicine, Ankara, Turkey), received the Ethics Committee approval from the University Magna Graecia of Catanzaro, and and was conducted in accordance with the tenets of the Declaration of Helsinki. All subjects provided informed consent to participate in the study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Borrelli, E., Coco, G., Pellegrini, M. et al. Safety, Tolerability, and Short-Term Efficacy of Low-Level Light Therapy for Dry Age-Related Macular Degeneration. Ophthalmol Ther (2024). https://doi.org/10.1007/s40123-024-01030-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40123-024-01030-w