Abstract
Introduction
The aim of this prospective and comparative study was to investigate the association of perimetry parameters on visual acuity and contrast sensitivity in primary open-angle glaucoma (POAG) eyes with diffractive extended depth-of-focus (EDoF) and monofocal intraocular lenses (IOLs).
Methods
In cataract eyes with medicinally controlled POAG with no defects in the central visual field and mean deviation (MD) values of − 10 dB or better, EDoF and monofocal IOLs with the same platform except for echelette optics for EDoF were implanted in 22 and 24 eyes, respectively. Corrected distance visual acuity (CDVA), contrast sensitivity at 3 to 18 cycles per degree (cpd), and automated perimetry using 30-2 and 10-2 Swedish Interactive Threshold Algorithm programs were examined 3 months postoperatively. The influences of perimetry parameters including MD, foveal sensitivity (FS), and the means of the central four points (central MD and central FS) on CDVA and contrast sensitivity were evaluated using linear and multiple regression analyses.
Results
In POAG eyes with EDoF IOLs, contrast sensitivities at 12 and 18 cpd were associated with 30-2 and 10-2 perimetry parameters. In POAG eyes with monofocal IOLs, associations of 30-2 parameters were found in CDVA and 3-cpd contrast sensitivity.
Conclusions
The visual function of POAG eyes with EDoF IOLs was associated with perimetry parameters in high spatial frequency contrast sensitivity, which was different from that of POAG eyes with monofocal IOL.
Trial Registration
Japan Registry for Clinical Research: jRCTs032200218.
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Why carry out this study? |
Latest clinical evaluation revealed that the use of extended depth-of-focus (EDoF) intraocular lenses (IOLs) in eyes with mild-to-moderate primary open angle glaucoma (POAG) provided visual function comparable with the use in eyes with monofocal IOLs. |
The degradation of visual field in POAG eyes influences visual function, but the associations of the perimetry condition with visual function in POAG eyes with EDoF and monofocal IOLs have not been investigated. |
What was learned from the study? |
In POAG eyes with EDoF IOLs, contrast sensitivity at high spatial frequency was degraded with lower central perimetry parameters, although such associations were found in visual acuity and contrast sensitivity at low spatial frequency in eyes with monofocal IOLs. |
Prominent associations were observed with central retinal sensitivities in eyes with EDoF IOLs. The association with contrast sensitivity at high spatial frequencies demonstrates the significant influence of EDoF IOL. |
Introduction
Various types of presbyopia-correcting intraocular lenses (IOLs) are available, and the benefits of their use in cataract eyes without ocular comorbidities have been well reviewed and compared with the use of monofocal IOLs [1]. Owing to the presence of photic phenomena and decreased contrast sensitivity, implantation of a bifocal IOL is not recommended for eyes with ocular diseases such as glaucoma [1, 2]. Glaucoma is a common complication in cataractous eyes [3], and its progression increases the risk of contrast sensitivity degradation.
Diffractive extended depth-of-focus (EDoF) IOLs allow visual acuity at far-to-intermediate distances with the fewest photic phenomena. Optical bench tests and clinical studies have demonstrated that the optical properties and contrast sensitivities of eyes with EDoF IOLs are comparable to those of eyes with monofocal IOLs [4, 5]. Hence, it was anticipated that EDoF IOLs could be used in eyes with early-stage glaucoma. A case series reported implantations of diffractive EDoF IOLs in 16 early-stage normal tension glaucoma (NTG) eyes with satisfactory postoperative visual function [6]. Our recent prospective comparative study showed no differences in visual function after implantation of diffractive EDoF and monofocal IOLs in eyes with mild-to-moderate primary open-angle glaucoma (POAG) [7], together with comparison of automated perimetry results. To our knowledge, the influence of the visual field on visual function has not precisely evaluated. As a secondary endpoint of the previous study [7], this exploratory analysis aimed to investigate the association of perimetry parameters on the visual acuity and contrast sensitivity of POAG eyes with monofocal and diffractive EDoF IOL.
Methods
Participants
This investigator-initiated, prospective, open-label comparative study was approved by a certified local review board, Hattori Clinic (CRB3180027 - approval ref 731ET), and registered with the Japan Registry for Clinical Research (ID jRCTs032200218). The study was conducted in accordance with the tenets of the Declaration of Helsinki and the Clinical Trials Act of Japan (Act No. 16, 2017). Written informed consent was obtained from all participants after explaining the study protocol.
Patients with POAG and cataracts were recruited at two sites (Tokyo Dental College Suidobashi Hospital and Ryuundo Eye Clinic). The inclusion criteria were that POAG was medically controlled for over 6 months with no central visual field defect and a mean deviation (MD) of − 10 dB or better on the 30-2 test grid of the Swedish Interactive Threshold Algorithm (SITA) standard program (Humphrey Field Analyzer III; Carl Zeiss Meditec, Dublin, CA, USA). Stable and controlled intraocular pressure (IOP) was confirmed using Goldmann applanation records from two previous visits. Patients with eye diseases other than cataracts (such as uncontrollable glaucoma, progressive diabetic retinopathy, uveitis, retinal detachment, iris neovascularization, or corneal degeneration), severe visual field defects (such as diffuse, central, or progressive defects), or a risk of serious postoperative complications (such as zonular rupture, posterior capsule rupture, vitreous prolapse, hyphema, or incomplete in-the-bag implantation) were excluded.
Sample Size
The original sample size of the study was determined for examining non-inferiority of contrast sensitivity of eyes with EDoF IOLs compared to eyes with monofocal IOLs. Twenty eyes in each group were necessary with a margin of 0.20 logarithm contrast sensitivity, a significance level of 0.05, detection power of 0.85 (TrialSize package version 1. 3, R version 3.6.1), and a sample size of 25 eyes was obtained to allow for a 5% dropout rate [7].
For the exploratory correlation analysis, 23 eyes in each group were necessary under linear correlation coefficient of 0.55, significance level of 0.05, detection power of 0.75 (pwr package version 1. 3–0).
Preoperative Examination and Intraocular Lenses
Preoperative examinations included IOP (Goldman applanation), axial length (IOLMaster 700, Carl Zeiss Meditec), automated perimetry (30-2 SITA program), and optical coherence tomography (CIRRUS, Carl Zeiss Meditec). Perimetry of 10-2 SITA program was not examined preoperatively because of the lens opacity due to cataract. To ensure the reliability of the automated perimetry results, the rates of fixation loss, false positive errors, and false negative errors were confirmed to be under 20%, 15%, and 33%, respectively.
Implanted IOLs were diffractive EDoF IOL ZXR00V (Johnson & Johnson Surgical Vision, J&J, Santa Ana, CA, USA) and its toric models (ZXV150-375 and ZXW150-375), and a monofocal IOL ZCB00V (J &J) and its toric models (ZCV150-375 and ZCW150-375, J&J). Both types of IOLs had an identical platform consisting of a hydrophobic acrylic material, designs of the optics and haptics, and violet light-blocking chromophore, except that the EDoF IOL was implemented with echelette optics with the addition of + 1.75 D to extend the range of vision to 0.7 m and farther. The implanted IOL was selected according to patient preferences. IOL power was determined to achieve emmetropia of postoperative refraction using EDoF IOLs. When monofocal IOLs were used, the power was determined according to the preferred distance of vision for each patient.
After the cataract was removed using a phacoemulsification and aspiration technique through a 2.4-mm temporal corneal incision, IOLs were inserted into the capsular bag using specific injectors.
Postoperative Examinations
Three months postoperatively, automated perimetry, corrected distance visual acuity (CDVA), and contrast sensitivity were examined. Automated perimetry was performed in the same manner as preoperative examination using the 30-2 and 10-2 SITA standard programs. CDVA was measured using a Landolt ring chart at 5 m and converted to the logarithm of the minimum angle of resolution (logMAR). Contrast sensitivity at 3, 6, 12, and 18 cycles per degree (cpd) without glare was measured using a CSV-1000 (Vector Vision, Fairfield, CT, USA) under distance-corrected and photopic illumination (85 cd/m2).
Statistical Analysis
Preoperative and postoperative MD and foveal sensitivity (FS) values were used for the analysis because they are related to visual function [8, 9]. Central MD and central FS were calculated from the postoperative MD and FS values at the four central points [10, 11] by taking the anti-log of the raw values, averaging them, and converting the average to dB values [10]. Associations of the four kinds of perimetry parameters with CDVA and contrast sensitivity at each spatial frequency were examined using linear regression analysis. Stepwise multiple regression analysis was also performed to identify significant factors. For each objective variable of CDVA and contrast sensitivity at four spatial frequency, preoperative (MD and central MD) and postoperative (MD, central MD, FS, and central FS) perimetric parameters of the 30-2 grid were use as explanatory variables. In a similar manner, stepwise multiple regression analysis was performed with explanatory variables of postoperative perimetry parameters of the 10-2 grid. Analyses were performed using SAS software (version 9.4; SAS Institute Inc., Cary, NC, USA). Statistical significance was set at P < 0.05.
Results
Of the 50 eyes from 50 patients, four eyes were excluded before and during surgery. Therefore, 22 eyes with EDoF IOLs and 24 eyes with monofocal IOLs were eligible for analysis. Table 1 lists the demographic data of the eligible participants. The mean preoperative IOP, MD, and axial length were significantly different between the two groups. The mean differences were 1.3 mmHg in IOP (< 2 mmHg) and 1.8 dB in MD, so that they were clinically negligible. The mean difference in axial length was 1.5 mm and both values were in the normal range, so that the difference was also clinically negligible. IOL power was lower in eyes with EDoF IOLs owing to slightly longer axial lengths.
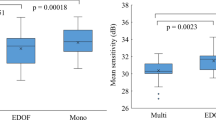
Table 2 lists the means of preoperative MD values and postoperative perimetry parameters, CDVA, and contrast sensitivity. Although there were significant differences in the preoperative MD, no differences were found in postoperative perimetry parameters (P > 0.09). CDVA values of all eyes were 0.0 logMAR (20/20 in Snellen) or better, while the mean CDVA values of eyes with EDoF IOLs were better (P = 0.002, Mann–Whitney test) compared with the eyes with monofocal IOLs. No differences were found in contrast sensitivity (P > 0.094) between eyes with EDoF and monofocal IOLs. No IOL opacifications were observed, so that the influence of posterior capsule opacification was neglectable.
Table 3 shows the P values from the linear regression analysis with 30-2 SITA perimetry parameters. In the POAG eyes with EDoF IOLs, associations with the perimetric parameters were not found in CDVA (P > 0.31) and contrast sensitivity at 3 and 6 cpd (P > 0.079). In contrast, the parameters were associated with degradation in the contrast sensitivity at 12 cpd (P < 0.015), except for postoperative MD and FS. Furthermore, the 18-cpd contrast sensitivity was associated with all parameters (P < 0.044). The central MD and FS were more sensitive, compared with the MD and FS. In the eyes with monofocal IOLs, associations were found between the CDVA and postoperative central MD (P = 0.019) and between the 3-cpd contrast sensitivity and postoperative MD value (P = 0.023). Associations due to the central MD and FS were not observed. Table 4 shows the linear regression analysis results with postoperative 10-2 SITA perimetry parameters. In the POAG eyes with EDoF IOLs, associations were found in 12- and 18-cpd contrast sensitivity (P < 0.015), while such associations were not found in eyes with monofocal IOLs.
Figure 1 shows the associations between postoperative perimetry parameters and contrast sensitivity at 12 and 18 cpd (upper and lower, respectively) in POAG eyes with EDoF IOLs. Regression equations indicated that the 18-cpd contrast sensitivity was more influenced by the perimetry parameters than at 12 cpd. Both the central MD and FS represented changes in contrast sensitivity at 12 and 18 cpd. Figure 2 shows the associations of the postoperative perimetry parameters with CDVA and contrast sensitivity at 3 cpd (upper and lower, respectively) in eyes with monofocal IOLs. Contrast sensitivity at 3 cpd correlated with MD values, whereas the slope (0.0286) was lower than one observed at 18 cpd in eyes with EDoF IOLs (0.0796).
Associations between postoperative 30-2 SITA perimetry parameters on the contrast sensitivity at spatial frequencies of 12 (upper) and 18 (lower) cycles per degree (cpd) of primary open-angle glaucoma eyes with diffractive extended depth-of-focus intraocular lenses (EDoF IOLs). SITA Swedish Interactive Threshold Algorithm, MD mean deviation, FS foveal sensitivity, dB decibel. Solid lines denote significant linear regression
Associations between postoperative 30-2 SITA perimetry parameters on the corrected distance visual acuity (CDVA, upper) and contrast sensitivity at spatial frequencies of 3 cycles per degree (cpd, lower) of primary open-angle glaucoma eyes with monofocal intraocular lenses (IOLs). SITA Swedish Interactive Threshold Algorithm, MD mean deviation, FS foveal sensitivity, dB decibel, logMAR logarithm of the minimum angle of resolution. Solid lines denote significant linear regression
Table 5 shows the results of the stepwise multiple regression analysis. In eyes with EDoF IOLs, significant regressions of contrast sensitivity at 12 and 18 cpd were found with postoperative central FS in the 30-2 grid and central MD and FS in the 10-2 grid. In contrast, such associations were found in the eyes with monofocal IOLs, while there were associations in 3-cpd contrast sensitivity and CDVA in 30-2 SITA perimetry parameters.
Discussion
In the linear regression analysis of 22 POAG eyes with diffractive EDoF IOLs, contrast sensitivities at 12 and 18 cpd were associated with the perimetry parameters. In contrast, similar associations were found in the CDVA and 3-cpd contrast sensitivity with two 30-2 SITA parameters in POAG eyes with monofocal IOLs. To the best of our knowledge, this is the first study to evaluate the influence of perimetry parameters on contrast sensitivity in eyes with EDoF and monofocal IOLs. Fatehi et al. evaluated the structural and functional factors associated with visual acuity and contrast sensitivity in 105 glaucomatous eyes [9], revealing a prominent association with contrast sensitivity at 6 cpd. A recent cross-sectional study of 103 POAG eyes indicated that contrast sensitivity at low spatial frequency notably changes with glaucomatous structural damage, such as radial peripapillary capillaries and macular ganglion cell complexes (GCCs) [12]. A similar reduction at low spatial frequencies was observed in POAG eyes with monofocal IOLs. On the other hand, decreased contrast sensitivities at high spatial frequencies are frequently observed in eyes with presbyopia-correcting IOLs [1]. Although the contrast sensitivities of eyes with EDoF IOLs are comparable to those with monofocal IOLs [5], there is a risk of a decrease in contrast sensitivity in POAG eyes at high spatial frequencies, such as 12 and 18 cpd.
In the eyes with EDoF IOLs, only contrast sensitivities at 12 and 18 cpd were associated with the perimetry parameters, and there was no influence on CDVA or 3- and 6-cpd contrast sensitivities. The current results for monofocal IOLs coincided with those in phakic POAG eyes [9, 12]. It has been assumed that there would be a similar degradation in eyes with EDoF IOLs. The slopes of the regression in Figs. 1 and 2 indicated that the effect of EDoF IOLs was more intense, and the multivariate regression analysis results demonstrated that the associations were dominant. Since such a phenomenon commonly occurs in eyes with presbyopia-correcting IOLs [1], at this moment, we could not conclude whether POAG facilitates contrast sensitivity degradation.
In the eyes with EDoF IOLs, the central MD and FS were prominently associated with 12- and 18-cpd contrast sensitivities. In glaucoma eyes, contrast sensitivity is associated with the central visual field [9, 12, 13], and low spatial frequency contrast sensitivity is affected by the function of the central visual field in POAG phakic eyes [9]. In a previous investigation of MD and FS reductions by implantation of EDoF IOLs [11], the mean difference in the central FS between EDoF and monofocal IOLs is 0.17 dB, which would be least influenced. From these findings, it was speculated that preoperative examination of central FS is important.
The current study had several limitations. First, only photopic contrast sensitivity was evaluated, but glaucomatous eyes have an increased risk for reduced mesopic contrast sensitivity [14]. The scotopic contrast sensitivity of eyes with EDoF IOLs is worse than that of eyes with monofocal IOLs [15], so it was anticipated that a more obvious influence would be found. Future studies should examine mesopic and scotopic contrast sensitivities. Next, analysis of optical coherence tomography images was not included. The structure parameters such as GCC associate with perimetric parameters [16]. For high myopic PAOG eyes, which would correspond to the use of toric IOLs with an axial length over 31 mm in eyes with EDoF IOLs, the inferior temporal macular ganglion cell and inner plexiform layer thickness was associated with mean sensitivity in the 10-2 grid [17]. At this moment, whether there are such associations between OCT and perimetry parameters has not been investigated yet; future investigation is anticipated.
Conclusion
The high spatial frequency contrast sensitivity of POAG eyes with diffractive EDoF IOLs was associated with perimetry parameters, which was different from PAOG eyes with monofocal IOLs.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Schallhorn JM, Pantanelli SM, Lin CC, et al. Multifocal and accommodating intraocular lenses for the treatment of presbyopia: a report by the American Academy of Ophthalmology. Ophthalmology. 2021;128:1469–82.
Braga-Mele R, Chang D, Dewey S, et al. Multifocal intraocular lenses: relative indications and contraindications for implantation. J Cataract Refract Surg. 2014;40:313–22.
Negishi K, Hayashi K, Kamiya K, et al. Nationwide prospective cohort study on cataract surgery with multifocal intraocular lens implantation in Japan. Am J Ophthalmol. 2019;208:133–44.
Lee S, Choi M, Xu Z, Zhao Z, Alexander E, Liu Y. Optical bench performance of a novel trifocal intraocular lens compared with a multifocal intraocular lens. Clin Ophthalmol. 2020;10:1031–8. https://doi.org/10.2147/OPTH.S106646.
Pedrotti E, Bruni E, Bonacci E, Badalamenti R, Mastropasqua R, Marchini G. Comparative analysis of the clinical outcomes with a monofocal and an extended range of vision intraocular lens. J Refract Surg. 2016;32:436–42.
Bissen-Miyajima H, Ota Y, Yuki K, Minami K. Implantation of diffractive extended depth-of-focus intraocular lenses in normal tension glaucoma eyes: a case series. Am J Ophthalmol Case Rep. 2022;29:101792. https://doi.org/10.1016/j.ajoc.2022.101792.
Bissen-Miyajima H, Ota Y, Taira Y, Takemura R, Minami K. Visual function after implantation of diffractive extended depth-of-focus intraocular lenses in eyes with primary open-angle glaucoma. Ophthalmol Ther. 2023;12:3099–108. https://doi.org/10.1007/s40123-023-00801-1.
Hawkins AS, Szlyk JP, Ardickas Z, Alexander KR, Wilensky JT. Comparison of contrast sensitivity, visual acuity, and Humphrey visual field testing in patients with glaucoma. J Glaucoma. 2003;12:134–8.
Fatehi N, Nowroozizadeh S, Henry S, Coleman AL, Caprioli J, Nouri-Mahdavi K. Association of structural and functional measures with contrast sensitivity in glaucoma. Am J Ophthalmol. 2017;178:129–39.
Aychoua N, Junoy Montolio FG, Jansonius NM. Influence of multifocal intraocular lenses on standard automated perimetry test results. JAMA Ophthalmol. 2013;131:481–5.
Lee J, Mori Y, Nejima R, Minami K, Miyata K. Influence of implantations of extended depth-of-focus on standard automated perimetry. Sci Rep. 2020;10:20153. https://doi.org/10.1038/s41598-020-77214-8.
Pang R, Peng J, Cao K, et al. Association between contrast sensitivity function and structural damage in primary open-angle glaucoma. Br J Ophthalmol. 2024;108:801–6.
Zulauf M, Flammer J. Correlation of spatial contrast sensitivity and visual fields in glaucoma. Graefes Arch Clin Exp Ophthalmol. 1993;231:146–50.
Bambo MP, Ferrandez B, Güerri N, et al. Evaluation of contrast sensitivity, chromatic vision, and reading ability in patients with primary open angle glaucoma. J Ophthalmol. 2016;2016:7074016. https://doi.org/10.1155/2016/7074016.
Liu J, Dong Y, Wang Y. Efficacy and safety of extended depth of focus intraocular lenses in cataract surgery: a systematic review and meta-analysis. BMC Ophthalmol. 2019;19:198. https://doi.org/10.1186/s12886-019-1204-0.
Bambo MP, Guerri N, Ferrandez B, et al. Evaluation of the macular ganglion cell-inner plexiform layer and the circumpapillary retinal nerve fiber layer in early to severe stages of glaucoma: correlation with central visual function and visual field indexes. Ophthalmic Res. 2017;57:216–23.
Wen W, Zhang Y, Zhang T, Sun X. Consistency between optical coherence tomography and Humphrey visual field for evaluating glaucomatous defects in high myopic eyes. BMC Ophthalmol. 2020;20:460. https://doi.org/10.1186/s12886-020-01724-2.
Acknowledgements
We thank the participants of the study.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Funding
This investigator-initiated trial study was supported by Johnson & Johnson Surgical Vision, including partial cost of the journal’s Rapid Service Fee. The remainder of the publication fees were funded by the authors.
Author information
Authors and Affiliations
Contributions
Conceptualization & Funding acquisition: Hiroko Bissen-Miyajima, Keiichiro Minami; Investigation: Hiroko Bissen-Miyajima, Yuka Ota, Yoko Taira; Project administration: Hiroko Bissen-Miyajima; Methodology: Yuka Ota, Yoko Taira; Formal analysis: Ryo Takemura; Validation and Writing – original draft: Keiichiro Minami; Writing – review, editing, and approval: all authors.
Corresponding author
Ethics declarations
Conflict of Interest
Hiroko Bissen-Miyajima reports grant, consultant, and speaker honorarium from Alcon and Johnson & Johnson Surgical Vision, consultant from BVI and Zeiss, speaker honorarium from BVI and Hoya. Hiroko Bissen-Miyajima is an Editorial Board member of Ophthalmology and Therapy. Hiroko Bissen-Miyajima was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions. Yuka Ota reports speaker honorarium from Johnson & Johnson Surgical Vision. Keiichiro Minami reports Patent pending from Tomey. Yoko Taira and Ryo Takemura declare that they have no competing interests.
Ethical Approval
This investigator-initiated study was approved by the Certified Review Board, Hattori Clinic (CRB3180027 - approval ref 731ET), and registered in the Japan Registry for Clinical Research (identifier jRCTs032200218). The study was conducted in accordance with the tenets of the Declaration of Helsinki and the Clinical Trials Act of Japan (Act No. 16, 2017). Written informed consent was obtained from all participants after explaining the study protocol.
Additional information
Prior Presentation: The American Academy of Ophthalmology Annual Meeting, 2023, San Francisco, November 3–6, 2023—Poster number PO034. The ARVO Annual Meeting, 2024, Seattle, May 5–9, 2024—Poster number 6337. The 42nd Congress of the European Society of Cataract and Refractive Surgeons, Barcelona, September 6–11 2024—Poster number PP02.04.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Bissen-Miyajima, H., Ota, Y., Minami, K. et al. Influence of Visual Field on Visual Acuity and Contrast Sensitivity in Open-Angle Glaucoma Eyes with Monofocal and Extended Depth-of-Focus Intraocular Lenses. Ophthalmol Ther (2024). https://doi.org/10.1007/s40123-024-01035-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40123-024-01035-5