Abstract
Background and Objective
In Italy, the management of metastatic non-small cell lung cancer and melanoma leads to significant healthcare challenges, necessitating cost-effective treatment strategies and offering valuable insights for healthcare policymakers and stakeholders. This study was designed to assess the costs, quality-adjusted life-years (QALYs) and disability-adjusted life-years (DALYs) associated with the health and economic outcomes of (1) pembrolizumab-combined chemotherapy administered as a first-line treatment for metastatic non-squamous and squamous non-small cell lung cancer (NSCLC) where the tumour presents with a programmed death-ligand 1 expression level < 50% and of (2) adjuvant pembrolizumab treatment for stage III melanoma.
Methods
Three cost-effectiveness models developed by MSD were investigated for each treatment indication. A unique model was built to assess the overall effect of pembrolizumab versus chemotherapy or watchful waiting in patients with lung cancer or melanoma, respectively. Theoretical cohorts of patients with metastatic squamous and non-squamous NSCLC were followed over time using a partitioned survival model with weekly cycles. A weekly cycle Markov model was employed for melanoma. The analysis was conducted from the Italian National Health Service perspective, considering a time horizon of 40 years (lifetime). A single closed cohort of treatable patients was followed over time for each indication (4000, 7000 and 900 for NSCLC squamous, non-squamous and melanoma, respectively). The costs evaluated included those for adverse drug events, non-drug disease management, subsequent treatment and terminal care. Drug acquisition and administration costs were excluded.
Results
For each treatment indication assessed, pembrolizumab produced downstream direct cost offsets (− €122,498,568, − €133,369,076 and − €32,993,242 for NSCLC squamous, non-squamous and melanoma indications, respectively), increased quality of life (+2088, +5317 and +2307 QALYs for NSCLC squamous, non-squamous and melanoma indications, respectively) and reduced disability (− 2658, − 7202 and − 3029 DALYs for NSCLC squamous, non-squamous and melanoma indications, respectively). Across indications, the total cost offsets of pembrolizumab were − €288,860,885, with 9712 QALYs gained and 12,889 DALYs avoided.
Conclusions
The analysis demonstrated that, compared with chemotherapy, pembrolizumab is more cost effective in Italy as a first-line treatment in patients with metastatic squamous or non-squamous NSCLC and, if compared with watchful waiting, as adjuvant treatment in patients with stage III melanoma. The present analysis suggested that pembrolizumab use could lead to important health benefits for patients while offsetting a portion of cancer care costs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The analysis assesses health and economic outcomes of pembrolizumab versus chemotherapy or watchful waiting in patients with metastatic non-squamous and squamous non-small cell lung cancer or stage III melanoma in Italy. |
Three cost-effectiveness models were investigated for each treatment indication. The analysis was conducted from the Italian National Health Service perspective considering a 40-year time horizon (lifetime). The costs included were those for adverse drug events, non-drug disease management, subsequent treatment and terminal care. |
For each treatment indication, pembrolizumab was cost effective in Italy as a first-line treatment in patients with metastatic squamous or non-squamous non-small cell lung cancer compared with chemotherapy alone and, if compared with watchful waiting, as adjuvant treatment in patients with stage III melanoma. It produced downstream direct cost offsets and had meaningful health benefits for patients increasing quality of life and reducing disability. |
1 Introduction
Melanoma is one of the leading cancers among young individuals and ranks as the third most common cancer in Italy among individuals under the age of 50 years (AIOM-AIRTUM 2022 [1]). In Italy, in 2022, approximately 169,900 individuals (80,100 male, 89,800 female) were living with a diagnosis of cutaneous melanoma, and approximately 12,700 new melanoma cases were diagnosed [1]. The overall 5-year survival rate after diagnosis is 88% for male individuals and 91% for female individuals, while for those surviving the initial post-diagnosis year, the survival rate for an additional 4 years is 91% among male patients and 93% among female patients [1].
For treating stage III melanoma, there are numerous approved anti-programmed cell death protein 1, BRAF and/or MEK inhibitors. Italian Medicines Agency (AIFA) reimbursement for pembrolizumab adjuvant treatment of patients with stage III melanoma with lymph node involvement who undergo complete resection was achieved in 2019 [2]. Lung cancer is among the five most common cancers in the general population, with an estimated incidence of 43,900 new cases in 2022 [1]. It is the second most common neoplasm in men (15% of cancers) and the third most common neoplasm in women (6% of cancers). In Italy, approximately 117,800 individuals are living with a lung cancer diagnosis (77,200 male, 40,600 female) [1]. The overall 5-year survival rate after diagnosis is 16% for male individuals and 23% for female individuals, while for those surviving the initial post-diagnosis year, the survival rate for an additional 4 years is 37% for male individuals and 44% for female individuals [1]. Non-small cell lung cancer (NSCLC) accounts for approximately 85% of lung cancer cases, while small cell lung cancer represents the remaining cases [3, 4]. The advent of molecularly targeted drugs and immunotherapy has revolutionised medical oncology therapies, replacing the limited efficacy of chemotherapy as the only available option up to approximately a decade ago.
Pembrolizumab is a high-affinity monoclonal antibody that binds to and consequently blocks the interaction between the programmed cell death protein 1 receptor and the programmed cell death ligands 1 and 2. This reaction activates tumour-specific cytotoxic T lymphocytes, which destroy tumour cells and subsequently restore anti-tumour immunity in affected patients.
The purpose of this study was to estimate the benefits of treatment with pembrolizumab in terms of downstream cost offsets, quality-adjusted life-years (QALYs) and disability-adjusted life-years (DALYs) that might be gained from treatment compared with therapeutic alternatives evaluated in regulatory trials involving patients with NSCLC with squamous and non-squamous histology and for melanoma in the stage III adjuvant setting.
2 Methods
For each treatment approach, three cost-effectiveness models developed by MSD were analysed, and a unique model was built to evaluate the overall effect of pembrolizumab compared with that of chemotherapy or watchful waiting in terms of costs, QALYs and DALYs. Quality-adjusted life-years are a widely used health indicator in economic assessment analyses of health interventions [5]. The basic concept of this indicator lies in the term “utility”, which represents the health gain that is achieved through a health intervention. Otherwise, DALYs measure the number of years of healthy life lost because of a disease and can be represented as the sum of years lost because of premature mortality caused by that specific disease (years of life lost [YLL]) and disease-free years lost through disability (years lived with a disability).
The cost-effectiveness models developed for squamous and non-squamous NSCLC adopt a partitioned-survival model approach, which directly estimates the proportions of patients in each health state at each timepoint. Using the partitioned-survival approach has the advantage of being able to use the trial progression-free survival and overall survival data directly, without a separate estimation of transition probabilities. Here, the model effectiveness parameters use KN189 and KN407 patient-level data for modelling progression-free survival and overall survival. The curves are reported in the published article redacted by Bensimon et al. [6]. In contrast, the cost-effectiveness model for melanoma was developed using a Markov cohort structure, in which transition probabilities between health states are needed. A parametric multi-state modelling approach was used to estimate transition probabilities, in which different parametric functions were fitted to each individual transition. Health state transition probabilities are the same as those used by Insinga et al. [7].
2.1 Model Structure by Treatment Indication
The first step of the analysis consisted of the exploration of three cost-effectiveness models of pembrolizumab treatment previously developed for the USA and adapted to the Italian context for the following treatment approaches:
-
adjuvant therapy for patients with stage III melanoma with lymph node involvement who underwent complete resection (stage III melanoma) [6];
-
first-line therapy for metastatic squamous NSCLC in adults in which the tumour presented with a programmed death-ligand 1 expression level < 50% (squamous NSCLC) [7];
-
first-line therapy for metastatic non-squamous NSCLC in adults is defined as a tumour that presents with a programmed death-ligand 1 expression level < 50% and is not positive for EGFR or ALK mutations (non-squamous NSCLC) [7].
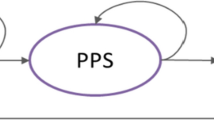
The two cost-effectiveness analyses conducted to evaluate pembrolizumab combined with chemotherapy as a first-line therapy in patients with metastatic squamous and non-squamous NSCLC versus chemotherapy were conducted, taking into account data from the KEYNOTE-407 (KN407; database cut-off date of 3 April, 2018) and KEYNOTE-189 (KN189; database cut-off date of 8 November, 2017) clinical trials, respectively. Theoretical cohorts of patients with metastatic squamous and non-squamous NSCLC were followed over time using a partitioned survival model with weekly cycles (Fig. 1). This model resulted in three mutually exclusive states of health as follows:
-
progression-free state;
-
progressive disease;
-
death.
Diagram of the partitioned survival model for the theoretical cohort of patients with lung cancer [7]. There are three mutually exclusive health states in the model: a progression-free state (PF) is the starting health state and defined as the time from the start of the regime use to disease progression or death (whichever comes first), b progressive-disease state (PD), which encompasses time after the first progression and c death. Patients in each cohort start in the “PF” state. At the end of each cycle, patients who are “PF” may stay in “PF”, transition to the “PD” state or die. Patients in the “PD” state may stay in “PD” or die at the end of each cycle. Patients in the “PD” state cannot go back to the “PF” state
Each patient cohort enters the model starting from the progression-free state. At the end of each cycle, patients could remain in the progression-free state, transition to the progressive disease state or die. Patients in the progressive disease state could remain in this state of health or die at the end of each cycle. Patients in the progressive disease state were unable to return to the progression-free state.
A cost-effectiveness analysis was conducted to evaluate pembrolizumab as adjuvant therapy versus observation (watchful waiting) in patients with stage III melanoma who underwent complete resection; the results were developed using data from the KEYNOTE-054 (KN054) clinical trial. To monitor the course of the disease and patient survival over time, a Markov model with weekly cycles was used; this model (Fig. 2) is characterized by four mutually exclusive states of health as follows [6]:
-
recurrence free;
-
locoregional recurrence;
-
distant metastasis;
-
death.
Markov model for the theoretical cohort of patients with melanoma [6]. The model consists of four mutually exclusive health states (i.e. recurrence free, locoregional recurrence, distant metastases and death). In a subgroup of patients, locoregional recurrence and distant metastasis may happen as simultaneous events, while in a minority of cases, locoregional recurrence may develop without simultaneous presence of distant metastatic tumour cell spread. This type of recurrence may potentially progress to distant metastasis at a later point in time
The model differentiates states of health according to the type of recurrence (locoregional recurrence or distant metastasis), as the type of recurrence experienced by patients is one of the most important prognostic factors in stage III melanoma. In a subset of patients, locoregional recurrence and distant metastasis could occur simultaneously, while in a minority of cases, locoregional recurrence could develop without the simultaneous occurrence of distant metastatic tumour cell dissemination [8, 9]. This type of recurrence could potentially progress to distant metastasis at a later time [8]. The economic models have been adapted to the Italian context using the input parameters reported in Tables S1–S3 of the Electronic Supplementary Material (ESM).
2.2 Ex-Novo Analysis
The second step of analysis was characterized by the development of an ex-novo model to assess the impact of pembrolizumab in terms of QALYs, DALYs and costs for each treatment indication as well as at the combined aggregate level for the three treatment options described in the previous paragraph. To obtain an estimate of the QALYs and DALYs associated with each treatment option in relation to each individual treatment indication as well as to the aggregate total of the treatment indications (NSCLC and melanoma), a new model was developed containing all the necessary information regarding the patient cohorts simulated in the three previously illustrated models.
In particular, for each treatment indication, simulation tracings of each cohort between different health states (with “half cycle correction”) and their associated costs were acquired (see Figs. S4–S15 in the ESM). In addition, health-state utility values were acquired for the purpose of calculating QALYs and derived from clinical trials (Table 1) [10]. The analysis was conducted using a lifetime time horizon (40 years).
Only direct costs were taken into consideration, namely, the costs incurred by the Italian National Health Service (NHS). Specifically, disease management costs, adverse event costs, costs of follow-up therapies, end-of-life costs and other treatment costs were taken into consideration. Cost data were mainly derived from Italian national tariffs [11, 12] and, in some cases, from the literature [13,14,15,16,17], as shown in Tables S1–S3 in the ESM. Costs derived from the literature refer to the year of publication. Treatment and administration costs were not included in the ex-novo analysis, both for pembrolizumab and for the standard of care, as they were confidential. In addition, the main focus of the ex-novo analysis was on downstream costs and cost offsets, which are both influenced by treatment utilisation, thus researchers were interested in understanding how certain costs can be reduced or balanced out over time.
For each cycle, QALYs were calculated by multiplying the utility value by the respective state of health. The average ages of the patients with lung cancer included were 65 years and 63 years for patients with squamous and non-squamous cancer, respectively, based on KN189 and KN407 trials eligibility criteria, while in patients with melanoma the average age was 54 years, based on the mean age in the KEYNOTE-054 trial.
The number of patients followed over time with metastatic non-squamous NSCLC eligible for treatment with pembrolizumab combined with chemotherapy was 7000, while the number of patients in the cohort with metastatic squamous NSCLC eligible for treatment with pembrolizumab combined with chemotherapy was 4000. Among patients with stage III melanoma who underwent complete resection, 900 were eligible for adjuvant therapy with pembrolizumab. These values have been obtained from an estimate based on the epidemiological data of the Italian Cancer Registry Association (AIRTUM) and assumption [18].
The QALYs, DALYs and costs associated with each treatment option were estimated for each theoretical patient cohort. The simulation was performed for each indication and globally. The results were reported considering a 3% discount rate for costs and outcomes [19].
3 Results
3.1 Results by Therapeutic Indication
Regarding patients with squamous NSCLC, looking at the theoretical cohorts of patients treated with pembrolizumab combined with chemotherapy or solely with chemotherapy, the model estimated an increase of approximately 33% in terms of QALYs for patients treated with pembrolizumab combined with chemotherapy versus patients treated solely with chemotherapy and a reduction of approximately 4% in terms of DALYs.
Treatment of the entire eligible population (4000 patients) with pembrolizumab combined with chemotherapy would also result in a reduction in NHS expenditure of approximately €122 million across the cost elements evaluated in the present study. In particular, the reduction would be associated mainly with follow-up treatment costs (−€135,168,691) and end-of-life care costs (−€112,457).
Regarding the non-squamous NSCLC population, looking at the theoretical cohorts of patients treated with pembrolizumab combined with chemotherapy or solely with chemotherapy, the model estimated an increase of approximately 56% in terms of QALYs for patients treated with pembrolizumab combined with chemotherapy versus patients treated solely with chemotherapy and a reduction of approximately 5% in terms of DALYs.
Treatment of the entire eligible population (7000 patients) with pembrolizumab combined with chemotherapy would result in a reduction in NHS expenditure of approximately €133 million across the cost elements evaluated in the present study. Even in this case, the reduction would be related mainly to follow-up treatment costs (− €153,778,703) and end-of-life care costs (− €292,982).
For patients with melanoma, considering the theoretical cohort of patients treated with pembrolizumab and the theoretical cohort of patients treated with watchful waiting, the model estimated an increase of approximately 34% in terms of QALYs for pembrolizumab-treated patients versus watchful waiting patients and a reduction of approximately 18% in terms of DALYs. Treatment of the entire eligible population (900 patients) with pembrolizumab would result in a reduction in NHS expenditure of approximately €33 million, mainly because of follow-up treatment costs, end-of-life care costs and disease management costs (− €32,292,844, − €752,042 and − €459,254 respectively).
3.2 Overall Results
Looking at the theoretical cohort of patients treated with pembrolizumab and the theoretical cohort of patients treated with chemotherapy or watchful waiting for a time horizon of 40 years, we found that the aggregate assessment of all the metrics reported above revealed a total increase in terms of QALYs for patients treated with pembrolizumab versus patients treated with chemotherapy or watchful waiting equal to 9712 QALYs (pembrolizumab 32,277; chemotherapy/watchful waiting 22,565) and a reduction in terms of DALYs equal to 12,889 (pembrolizumab 220,371; chemotherapy/watchful waiting 233,260). Figures 3 and 4 show the total QALYs and total DALYs, respectively, estimated under each analysis scenario for the entire eligible population, including the three treatment indications. Treatment with pembrolizumab for the entire eligible population, including the three treatment indications, would result in a reduction in NHS expenditure of approximately €289 million (Table 2) across the cost elements evaluated.
Estimated and cumulative total number of quality-adjusted life-years (QALYs) of the three treatment indications for each year of the time horizon, both for pembrolizumab and comparators (chemotherapy/watchful waiting). As shown graphically, the total number of QALYs gained would be greater for pembrolizumab in all years considered compared with the alternatives
Estimated and cumulative total number of disability-adjusted life-years (DALYs) of the three treatment indications for each year of the time horizon, both for pembrolizumab and comparators (chemotherapy/watchful waiting). As shown graphically, the total number of DALYs would be lower for pembrolizumab in all years considered compared with the alternatives
3.3 Scenario Analysis
To assess the robustness of the baseline results in the aggregated model, a scenario analysis was conducted. To assess how the model parameters affected the baseline results, six different scenarios were considered. Specifically, the utility values and the number of cohorts were changed separately for the three pathologies considered in the analysis. Variability was assumed to be 20%. The results of the aggregated model vary according to the parameters tested in the scenario analysis; however, the variability of each single model was tested in the previously published cost-effectiveness analyses [6, 7]. The results of the scenario analysis are shown in Table 3.
4 Discussion
A study conducted by the Global Burden of Disease Cancer Collaboration estimated that in 2015, cancers caused more than 208 million DALYs, 96% of which were caused by YLLs and 4% by years lived with disability. The analysis focused on estimating not only the mortality rates and incidence of 32 cancers in 195 countries but also the years lived with disability, YLL and DALYs [20]. All of these metrics were found to be important in establishing a correct epidemiological burden for oncological diseases, especially owing to their high prevalence. The impact on patient quality of life is considered to be crucial, and improving not only life expectancy but also quality of life is one of the key steps in establishing the value of therapeutic strategies [20].
The introduction of a new treatment option, which will alter the course of the disease, has a positive impact both on the epidemiological burden (reduction in prevalence) and on quality of life, disability and premature death. To date, the principal metrics for weighing both the quality and quantity of life lived with disease are represented by QALYs and DALYs. While it is true that they represent two sides of the same coin—on one side, the years of life lived with good quality of life, and the years of life lived with disability, on the other side—the two metrics allow us to observe two very different perspectives from a decision-maker’s viewpoint. Quality-adjusted life-years represent a measurement of health outcomes that a more effective procedure might be able to provide for the patients under analysis (widely used in economic assessment analyses), while DALYs allow for disability—caused by a disease and preventable with suitable healthcare procedures—to be weighed. This analysis was also conducted to evaluate the benefits of using pembrolizumab compared to those of the comparators included in clinical trials in patients with the pathologies analysed (squamous NSCLC, non-squamous NSCLC, stage III melanoma) in terms of increased quality of life, reduced years of life lived with disability and reduced costs. The analysis showed that—considering a hypothetical cohort of patients treated for the three diseases over a lifetime time horizon—treatment with pembrolizumab in patients with squamous and non-squamous NSCLC and in patients with stage III melanoma could generate an increase of >9712 QALYs relative to alternative therapies. In addition, pembrolizumab permitted a reduction of approximately 12,889 DALYs among these patients. This means an increase of approximately 43% in terms of QALYs for patients treated with pembrolizumab versus patients treated with the selected standard of care and a reduction of approximately 6% in terms of DALYs.
The relative impact of therapeutic innovation on QALYs versus DALYs was significantly different, particularly for the two indications for NSCLC. This is related to the high mortality rate of the tumour, which overestimates the effects on the YLL. In fact, YLL accounts for more than 95% of the total DALYs left after 40 years. This means that there is an underestimation of the association between the reduction in the disability of survivors and an overestimation of the mortality effects. Conversely, QALYs, by design, allow us to appreciate the increase in quality of life even if linked to a reduced period.
More specifically, as confirmed by the published literature, there are no aggregated cost-effectiveness analyses previously conducted in the field of metastatic non-small cell lung cancer and melanoma. For this reason, the current analysis could offer a wider perspective on the potential of pembrolizumab and the impact on clinical practice and decision-making process, fostering its integration into current treatment guidelines.
For all the modelling approaches, this work has different limitations. First, the outcome considered (DALYs and QALYs) is not fully representative of the overall impact of pembrolizumab on these three indications. While these metrics offer a standardised approach, they might not fully capture certain important outcomes, such as patient-reported experiences, long-term impacts, or broader societal benefits and externalities associated with interventions. Consequently, this narrow focus on selected outcomes may overlook critical aspects of healthcare interventions and potentially lead to suboptimal resource allocation decisions.
Second, the effectiveness and cost data used in the three models considered for the analysis were based on controlled clinical trials and the literature, in which strict inclusion and exclusion criteria were not representative of the general population. These trials were designed to demonstrate the effectiveness of the intervention under ideal conditions and might not fully represent the real-world patient population or clinical settings in Italy. As a result, using efficacy data alone could lead to overestimating the intervention’s benefits and cost effectiveness when applied in broader and more diverse healthcare settings. However, the scenario analysis allowed us to test the variability of the results while also considering the effectiveness parameters, demonstrating substantial advantages of the use of pembrolizumab in all the considered scenarios.
Moreover, our model included consideration of the NHS perspective only. However, direct costs sustained by the NHS may not fully capture all relevant healthcare expenses, especially indirect costs and non-medical costs. Indirect costs, such as productivity losses due to illness and absenteeism, and non-medical costs, such as out-of-pocket expenses incurred by patients, may substantially impact economic evaluations but are often challenging to measure accurately. Finally, while our findings offer valuable insights for the Italian healthcare context, clearly further research is needed to ascertain the broader applicability and generalisability of these results across different contexts, especially owing to the large variability in terms of healthcare costs, clinical practices and availability of treatments across different countries.
5 Conclusions
Finally, this analysis assessed the costs, QALYs and DALYs associated with the health and economic outcomes of pembrolizumab-combined chemotherapy administered as a first-line treatment for metastatic non-squamous and squamous NSCLC and of adjuvant pembrolizumab treatment for stage III melanoma, leading to a reduction in the NHS expenditure, an increase of the number of QALYs and a decrease of the number of DALYs, in all therapeutic indications. By providing access to affordable treatments without compromising efficacy, cost-effective drugs represent a decisive factor in achieving the goals of our national health system, improving patients’ outcomes and quality of life.
References
AIOM-AIRTUM. I numeri del cancro in Italia 2022. Last updated 31/03/2024. Available from: https://www.aiom.it/wp-content/uploads/2022/12/2022_AIOM_NDC-web.pdf. Accessed 13 May 2024.
AIFA. GU Serie Generale n.289 del 10-12-2019. http://www.gazzettaufficiale.it/eli/gu/2019/12/10/289/sg/pdf. Accessed 13 May 2024.
Scarpace SL. Metastatic squamous cell non-small-cell lung cancer (NSCLC): disrupting the drug treatment paradigm with immunotherapies. Drugs Context. 2015;4: 212289.
Ettinger DS, Wood DE, Aisner DL, et al. NCCN Guidelines® insights: non-small cell lung cancer, Version 2.2023. J Natl Compr Canc Netw. 2023;21(4):340–50.
Drummond MF, Sculpher MJ, Torrance JW, et al. Methods for the economic evaluation of health care programmes. 3rd ed. Oxford: Oxford University Press; 2005.
Bensimon AG, Zhou ZY, Jenkins M, et al. Cost-effectiveness of pembrolizumab for the adjuvant treatment of resected high-risk stage III melanoma in the United States. J Med Econ. 2019;22(10):981–93.
Insinga RP, Feliciano JL, Qiao N, et al. Cost-effectiveness of pembrolizumab + chemotherapy versus chemotherapy and pembrolizumab monotherapy in first line treatment of NSCLC in the US: updated analyses with additional trial follow-up. J Med Econ. 2021;24(1):792–805.
Leiter U, Meier F, Schittek B, et al. The natural course of cutaneous melanoma. J Surg Oncol. 2004;86(4):172–8.
Mervic L. Time course and pattern of metastasis of cutaneous melanoma differ between men and women. PLoS ONE. 2012;7(3): e32955.
Beusterien KM, Szabo SM, Kotapati S, et al. Societal preference values for advanced melanoma health states in the United Kingdom and Australia. Br J Cancer. 2009;101(3):387–9.
Decreto del Ministero della Salute. 18 ottobre 2012. Remunerazione delle prestazioni di assistenza per acuti. Last updated 31 Mar 2024. https://www.trovanorme.salute.gov.it/norme/renderPdf.spring?seriegu=SG&datagu=28/01/2013&redaz=13A00528&artp=1&art=1&subart=1&subart1=10&vers=1&prog=001. Accessed 13 May 2023.
Decreto del Ministero della Salute. 18 ottobre 2012. Nomenclatore tariffario dell’assistenza specialistica ambulatoriale. Available from: http://www.salute.gov.it/portale/temi/p2_6.jsp?lingua=italiano&id=1767&area=programmazioneSanitariaLea&menu=lea. Accessed 13 May 2024.
Scaccabarozzi G, Limonta F, Amodio E. Hospital, local palliative care network and public health: how do they involve terminally ill patients? Eur J Public Health. 2017;27(1):25–30.
Wehler E, Zhao Z, Pinar Bilir S, et al. Economic burden of toxicities associated with treating metastatic melanoma in eight countries. Eur J Health Econ. 2017;18(1):49–58.
Mennini FS, Bini C, Marcellusi A, et al. Cost estimate of immune-related adverse reactions associated with innovative treatments of metastatic melanoma. Clin Drug Investig. 2018;38(10):967–76.
Banz K, Bischoff H, Brunner M, et al. Comparison of treatment costs of grade 3/4 adverse events associated with erlotinib or pemetrexed maintenance therapy for patients with advanced non-small-cell lung cancer (NSCLC) in Germany, France, Italy, and Spain. Lung Cancer. 2011;74(3):529–34.
Ascierto PA, Boccia S, Freguglia V, et al. Valutazione economica dabrafenib. QIJPH. 2014;3(5).
Mennini FS, Marcellusi A, Greco A, et al. Downstream Health and Economic Outcomes of Pembrolizumab in the Treatment of NSCLC and Melanoma in Italy. Last updated: 31 Mar 2024. Available from: https://www.isporitaly.org/wp-content/uploads/2024/04/9.-Downstream-Health-and-Economic-Outcomes-of-Pembrolizumab-in-the-Treatment-of-NSCLC-and-Melanoma-in-Italy.pdf.
Fattore G. Proposta di linee guida per la valutazione economica degli interventi sanitari in Italia. Pharmacoecon Italian Res Articles. 2013;11:83–93.
Global Burden of Disease Cancer, Fitzmaurice C, Allen C, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3(4):524–48.
Funding
Open access funding provided by Università degli Studi di Roma Tor Vergata within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Funding for this research was provided by MSD Italia Srl.
Conflicts of Interests
Martina Paoletti, Chiara Bini, Andrea Marcellusi and Francesco Saverio Mennini have no conflicts of interest that are directly relevant to the content of this article.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
Data archiving is not mandated but data will be made available on reasonable request.
Code Availability
Not applicable.
Authors’ Contributions
MP, CB, AM and FSM contributed equally to the design and implementation of the research, to the analysis of the results and to the writing of the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Paoletti, M., Bini, C., Marcellusi, A. et al. Health and Economic Outcomes of Pembrolizumab in the Treatment of Metastatic Non-small Cell Lung Cancer (mNSCLC) and Melanoma in Italy. Clin Drug Investig 44, 601–609 (2024). https://doi.org/10.1007/s40261-024-01366-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-024-01366-y