Abstract
Introduction
Regorafenib is a multikinase inhibitor approved for the treatment of metastatic colorectal cancer (mCRC). Despite providing a statistically significant survival benefit, a substantial number of patients fail to respond to or continue with treatment, which has resulted in an unmet clinical need for a biomarker of regorafenib efficacy.
Methods
The JACCRO CC-12 study was a prospective, multicenter, single-arm phase II trial designed to evaluate the usefulness of [18F]fluorodeoxyglucose positron emission tomography (FDG-PET) as an imaging biomarker of regorafenib in patients with mCRC that progressed after standard chemotherapies. FDG-PET and contrast-enhanced computed tomography (CT) were performed before and after treatment with regorafenib 160 mg once daily 3 weeks on/1 week off. The primary end point was the change in the maximum standardized uptake value in the lesion with the highest uptake at pre-treatment FDG-PET. The secondary end points included overall survival (OS), progression-free survival (PFS), the objective response rate (ORR), safety, and the correlation between FDG-PET and CT.
Results
Twenty patients were enrolled from November 2014 to March 2016, 17 of whom were evaluated for metabolic and morphological changes. Metabolic response with FDG-PET was partial response (PR) in one case (5.9%), stable disease (SD) in four (23.5%), and progressive disease (PD) in 12 (70.6%). The metabolic response rate was 5.9%. On CT imaging, no complete response or PR was observed, and the ORR was 0%. Median PFS and OS were 1.7 and 9.8 months, respectively. The median PFS of patients who achieved PR or SD by FDG-PET was 3.7 months, whereas that of those assessed as PD was 1 month (p = 0.13). The median OS of patients who achieved PR or SD by FDG-PET was 13.0 months, whereas that of patients assessed as PD was 10.6 months (p = 0.43). Frequent adverse events were palmar–plantar erythrodysesthesia syndrome, hypertension, loss of appetite, and fatigue.
Conclusions
In this study, FDG-PET failed to demonstrate usefulness as an early imaging biomarker of regorafenib in patients with mCRC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Regorafenib is a multikinase inhibitor used as late-line therapy for metastatic colorectal cancer. Despite being associated with various adverse events, including hand–foot skin reactions, the objective response rate is 1%, and a potential biomarker is required. |
What did the study ask?/What was the hypothesis of the study? |
We investigated the utility of FDG-PET as an imaging metabolic biomarker for the efficacy of regorafenib to detect responders/nonresponders. |
What were the study outcomes/conclusions? |
The observed metabolic response rate using centrally reviewed FDG-PET was 5.9%, below our expected early detection rate of 10%. Therefore, the JACCRO CC-12 study failed to demonstrate the usefulness of FDG-PET as an imaging biomarker of regorafenib. |
What has been learned from the study? |
The observed metabolic response (5.9%) was higher than the objective response rate (0%) using RECIST, and patients who were diagnosed with partial response or stable disease using FDG-PET showed better outcomes than those diagnosed with progressive disease. These data suggest the potential for early metabolic diagnosis using FDG-PET. To evaluate the metabolic response, whole-body total lesion glycolysis might be more useful than the SUVmax of target lesions. |
Introduction
Regorafenib, an oral multikinase inhibitor that inhibits the activity of angiogenic (VEGFR1, VEGFR2, VEGFR3, TIE2), stromal (PDGFR, FGFR), and oncogenic (KIT, RET, RAF-1, BRAF, BRAFV600E) receptor tyrosine kinases, has been approved for the treatment of metastatic colorectal cancer (mCRC), gastrointestinal stromal tumor, and hepatocellular carcinoma [1, 2]. Two randomized phase III trials, CORRECT and CONCUR, demonstrated a clinically modest prolongation of overall survival (OS) ranging from 1.4 to 2.5 months in patients treated with regorafenib combined with best supportive care compared with placebo combined with best supportive care [3, 4]. In the CORRECT trial, even though the objective response rate (ORR) was only 1%, treatment-related adverse events, including hand–foot skin reactions, fatigue, diarrhea, hypertension, and rash, occurred in 93% of the patients assigned to receive regorafenib [3]. Therefore, the identification of potential biomarkers might help select those patients most likely to benefit from regorafenib and avoid unnecessary adverse effects in patients who are not likely to respond.
RadioCORRECT, a post hoc analysis of a cohort of patients within the CORRECT trial, investigated the association between tumor response and early radiological parameters [5]. The results of multivariate analyses and baseline and week 8 lung cavitation were found to be significant predictors of progression-free survival (PFS), and baseline lung cavitation, RECIST (Response Evaluation Criteria in Solid Tumours) 1.1 responses, and change in the sum of target lesion diameters (≥ 9% vs. < 9%) were found to be predictors of OS. Assessment of liver metastases did not predict clinical outcomes. That analysis suggested that response to multikinase inhibitors depended not only on changes in the sum of target lesion diameters, but also on non-morphological changes.
Here, we focused on [18F]fluorodeoxyglucose (FDG) positron emission tomography (PET) as an imaging biomarker for the efficacy of regorafenib. The standard definition of tumor response to cytotoxic agents is based on the measurement of changes in tumor size as determined using morphological imaging methods such as contrast-enhanced (CE) computed tomography (CT). Despite the widely accepted practice of using these criteria, the introduction of molecularly targeted agents such as angiogenesis inhibitors, including regorafenib, requires a new modality for monitoring antitumor effects, because they have different biological mechanisms compared with classic cytotoxic chemotherapy. These antiangiogenic agents inhibit the growth of new blood vessels in cancer tissue, which does not immediately result in the dissolution of tumor masses. Considering these mechanisms, FDG-PET has recently been widely introduced not only for preoperative examinations and diagnosis, but also for the prediction and evaluation of treatment response in various solid tumors [7, 8]. Regarding colorectal cancer, the usefulness of FDG-PET as a predictive/prognostic biomarker after locoregional therapies, including tumor ablation, has already been reported [9, 10].
Therefore, we conducted a prospective multicenter phase II trial to examine whether FDG-PET can aid the early prediction of the efficacy of regorafenib.
Methods
Study design
The JACCRO CC-12, a single-arm, prospective, open-label phase II study, was conducted at five centers in Japan in accordance with the Ethical Guidelines for Medical and Health Research Involving Human Subjects and the Declaration of Helsinki. The institutional review board and/or ethics committee at each site approved the protocol, and all patients provided written informed consent to participate.
Patients
The eligibility criteria were as follows: histologically confirmed colorectal cancer; mCRC or inoperable locally advanced colorectal cancer (regardless of RAS mutation); measurable lesions according to RECIST (ver. 1.1); assessable target lesion by FDG-PET/CT; refractory to fluorinated pyrimidine, oxaliplatin, irinotecan, bevacizumab, and anti-EGFR antibody therapy; Eastern Cooperative Oncology Group performance status 0–1; age ≥ 20 years; life expectancy of more than 3 months; able to take oral medications; adequate organ function for study treatment within 14 days before enrollment (white blood cell count ≥ 3000/mm3, < 12,000/mm3, neutrophil count ≥ 1500/mm3, platelet count ≥ 100,000/mm3, hemoglobin level ≥ 9.0 g/dL, total bilirubin ≤ 1.5 × upper limit of normal (ULN), aspartate aminotransferase ≤ 2.5 × ULN, alanine aminotransferase ≤ 2.5 × ULN, creatinine ≤ 1.5 × ULN, prothrombin time-international normalized ratio ≤ 1.5 × ULN); and provision of written informed consent.
The main exclusion criteria were as follows: synchronous or metachronous multiple malignancy within 5 years of disease-free survival; serious infection; any of the following severe comorbidities: (1) diabetes mellitus requiring insulin, (2) uncontrolled hypertension (systolic blood pressure [BP] > 150 mmHg, and/or diastolic BP > 90 mmHg), (3) unstable angina, myocardial infarction, cerebral infarction, or pulmonary embolism within 6 months, (4) abnormal cardiac rhythm requiring antiarrhythmic agent, (5) congestive heart failure (New York Heart Association ≥ class 2), (6) active or chronic hepatitis B and/or hepatitis C, (7) active interstitial pneumonia, (8) hemorrhage (Common Terminology Criteria for Adverse Events Grade ≥ 3) within 4 weeks before enrollment, or (9) nonhealing wound, ulcer, or bone fracture, brain metastases, history of treatment with regorafenib, history of allergy to regorafenib, extended-field radiotherapy within 4 weeks or local radiotherapy within 2 weeks before enrollment, major surgery, skin-open biopsy, or severe injury within 4 weeks before enrollment, sustained proteinuria (3+), unwilling to avoid pregnancy, pregnancy or breastfeeding, a positive pregnancy test, and any other patients regarded as inadequate for study enrollment by the investigators.
Treatment Procedures
Patients received regorafenib at a standard dose of 160 mg once daily for the first 3 weeks, followed by 1 week of rest until disease progression, death, occurrence of unacceptable toxic effects, withdrawal of consent by the patient, or a decision by the treating physician that discontinuation would be in the patient’s best interest. Reduction of dosage to 120 mg/day or 80 mg/day due to adverse effects was permitted.
FDG-PET/CT Imaging
Pre-treatment FDG-PET/CT imaging was performed ≤ 21 days before the start of regorafenib. Subsequent FDG-PET/CT imaging was performed on day 28 (ideal range: days 24–32). All FDG-PET images were centrally reviewed by an independent monitoring committee. The lesion with the highest maximum standardized uptake value (SUVmax) at baseline FDG-PET was defined as the target site for the primary end point.
Response Criteria of FDG-PET/CT
The response criteria assessed using FDG-PET in this study were defined based on EORTC (European Organisation for Research and Treatment of Cancer) and PERCIST (Positron Emission Tomography Response Criteria in Solid Tumors) criteria [11, 12]. The FDG-PET criteria used in this study were as follows: complete response (CR) (complete resolution of FDG uptake in the main target lesion); partial response (PR) (a > 25% reduction in the SUVmax in the main target lesion); stable disease (SD) (non-progressive disease [PD] or CR/PR); PD corresponds to (1) an increase of > 20% in the longest diameter of the main target lesion on CT, (2) an increase of > 25% in the SUVmax in at least one of the top five target lesions, or (3) new FDG uptake in metastatic lesions (new lesions and/or the progression of existing non-target lesions).
CE-CT
Change in tumor diameter was assessed using CE-CT. CE-CT scans were performed ≤ 21 days before the start of regorafenib and on day 28 of the first course. All CE-CT images were centrally reviewed by an independent monitoring committee using RECIST version 1.1 [12].
Statistical Considerations
The primary end point was the change in the SUVmax of the lesion with the highest uptake on baseline FDG-PET. The secondary end points were PFS, OS, ORR, correlation between changes in SUVmax and PFS/OS, and safety. The severity of adverse events was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, v.4 [13].
Based on previous studies, we assumed a response rate by CE-CT to regorafenib of 0%, and expected a metabolic early detection rate of 10% by FDG-PET with a clinically meaningful response rate. Given a two-sided alpha of 0.05 and statistical power of 95%, with about 20% dropout from adverse events and/or early tumor progression, we set 20 patients as the target sample size in this study. The full analysis set was defined as patients who fulfilled the eligibility criteria and received protocol therapy at least once. The per-protocol set was defined as patients who received protocol therapy at least once and in whom FDG-PET/CT was performed after one course of regorafenib. The safety population was defined as all eligible patients who received treatment at least once. PFS and OS curves were calculated using the Kaplan–Meier method, and 95% confidence intervals (CIs) were estimated using Greenwood’s formula.
Results
Patient Background Characteristics
From November 2014 to March 2016, 20 patients were registered from three institutions. Intention-to-treat and safety analyses were carried out for all 20 patients. Table 1 shows the patients’ background characteristics. Thirteen patients were male, and seven were female, with a median age of 66 years (range: 42–79 years). All patients had received more than two prior regimens of chemotherapy, including oxaliplatin, irinotecan, and 5-fluorouracil. Four patients (20%) had received four prior therapies before accrual. The median duration from initiation of first-line chemotherapy to enrollment was 26.7 months (range: 11.5–44.5 months). The most common metastatic sites were lung and liver (13 patients, 65%). The data cutoff was November 2017.
Treatment Exposure
Table 2 shows the treatment exposure. Treatment ranged from one to seven cycles. The total dose of regorafenib administered ranged from 320 to 16,800 mg, and the median total dose was 2080 mg. The calculated relative dose intensity of regorafenib was 59.3%.
Efficacy
Metabolic response assessed using FDG-PET and CE-CT at the main target lesion
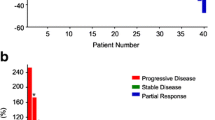
Three patients were excluded from the FDG-PET efficacy analysis because of a need for over 28 days of rest in one patient and mortality in two; therefore, per-protocol analysis for response was performed in 17 patients. Table 3 shows the metabolic response assessed based on the FDG-PET criteria used in this study. The SUVmax of the main target lesion in one patient was changed from 10.47 to 7.58, and this patient was diagnosed as PR by an independent monitoring committee. The response rate using centrally reviewed FDG-PET was 5.9% (95% CI: 0.1%–28.7%). Table 4 shows the morphological change in target lesions evaluated using CE-CT. No patient with CR or PR was found on CE-CT based on RECIST version 1.1, and the ORR based on CE-CT by an independent monitoring committee was 0%.
Figure 1 shows the best percentage change in the SUVmax between before and after regorafenib treatment. Although two of 17 patients showed a greater than 25% decrease in SUVmax, one was assessed as PD because of the appearance of new FDG uptake. The median percentages of change in SUVmax were −8.1% in PR + SD patients and +3.9% in PD patients.
Survival
After a median follow-up of 8.3 months, the median PFS was 1.7 months (95% CI: 0.95–3.68 months) (Fig. 2). Regarding the median PFS among subgroups based on FDG-PET response, patients with PR + SD in the main target lesion showed a better tendency than did patients with PD (3.68 months [95% CI: 1.81–NR] vs. 1.00 months [95% CI: 0.82–3.84], respectively, p = 0.13) (Fig. 3). The median OS was 9.8 months (95% CI: 3.29–19.94) (Fig. 4). Regarding the median OS among subgroups based on FDG-PET response, patients with PR + SD in the main target lesion showed a slightly better tendency than did those with PD (12.98 months [95% CI: 6.77–NR] vs. 10.61 months [95% CI: 2.04–22.21], respectively, p = 0.43) (Fig. 5).
Safety
Adverse events are summarized in Table 5. Toxicity was assessed in all 20 patients. Palmar–plantar erythrodysesthesia syndrome was observed in 12 patients (60%) and grade 3 proteinuria in three (15%). Among all 20 patients, two patients died: one as a result of liver failure and the other because of tumor lysis syndrome.
Discussion
In this study, the ORR using centrally reviewed FDG-PET was 5.9%, below our expected early detection rate of 10%. Therefore, the JACCRO CC-12 study failed to demonstrate the usefulness of FDG-PET as an imaging biomarker of regorafenib.
Possible explanations for the negative results include the small number of patients, the single-arm design, and mortality. In the present study, three patients (15%) could not undergo FDG-PET after regorafenib treatment, so the efficacy analysis was performed using data from 17 patients. Because we had estimated that four patients (20%) would drop out because of adverse events and/or other reasons when setting the sample size in the study protocol, this decrease did not affect the statistical robustness of the results.
Two patients died during the treatment period: one from liver failure and the other from what was believed to be tumor lysis syndrome. The mortality rate of 10% was much higher than the reported 2% in the CORRECT trial [3]. This high mortality rate is partly as a result of the small number of patients. Liver failure is known to be a severe adverse event related to regorafenib treatment [3, 4]. A post-marketing survey in Japan noted that fatal outcomes resulting from serious hepatic-related adverse reactions were reported in 27 (0.3%) of an estimated 8380 patients with exposure to regorafenib [14]. The multivariate analysis in that survey revealed that the baseline total bilirubin level (1.2 vs. < 1.2 mg/dL) and the starting dose (< 160 vs. 160 mg) significantly affected the occurrence of serious adverse reactions in the liver. Regarding the patient who died because of liver failure in the present study, the total bilirubin level before regorafenib treatment was 1.54 mg/dL and the starting dose was 160 mg/day. This patient was considered to be at high risk for liver failure. As a future measure, a dose-escalation strategy starting with a lower dose could reduce these adverse events in such populations [15, 16].
Although JACCRO CC-12 ended as a negative study, some interesting findings were observed. First, an FDG-PET response of 5.9% was observed despite the fact that CE-CT showed an objective response of 0% based on RECIST. The PFS and OS of the patient diagnosed as PR using FDG-PET were 4.7 and 13.0 months, respectively. These values were about twice as high as those reported in the CORRECT trial, in which the PFS and OS were 1.9 and 6.4 months, respectively [3]. Also, in the present study, patients diagnosed as PR or SD using FDG-PET showed a better tendency than did patients diagnosed as PD, although this difference was not significant. These facts may allow us to consider that metabolic changes in the target lesion could help justify the continuation of regorafenib treatment. A second interesting finding is related to the definition of metabolic response. In one patient in this study, the SUVmax of the target lesion was dramatically decreased, from 13.3 to 7.1, but a new lesion was detected and this patient was diagnosed as PD using FDG-PET. This result suggests that the FDG-PET response should be assessed based not only on the SUVmax of one target lesion, but also on whole-body total lesion glycolysis (TLG). Recently, metabolic tumor volume (MTV) and TLG have been widely used as tumor metabolic and volumetric parameters in FDG-PET. MTV represents the volume of a tumor with active FDG uptake, and TLG is calculated by multiplying MTV by the mean SUV; these parameters indicate the volume of burden and metabolic activity of tumors, respectively [18,19,20], and should be used in future FDG-PET studies.
The first limitation to note is the large dropout rate of patients. JACCRO CC-12 was designed in 2013, when the dosage of regorafenib was limited to 160 mg/day, and frequent adverse events were observed at that dosage. In recent years, low-dose administration of regorafenib has been proposed [15]. ReDOS study, an open-label phase II study comparing a dose-escalation strategy group (a starting dose of 80 mg/day with weekly escalation, in 40 mg increments, to 160 mg/day regorafenib) and a standard-dose strategy group (160 mg/day orally) showed non-inferiority of the dose-escalation strategy, with less toxicity. Starting with a lower dose of regorafenib might prevent dropout, increase continuity, and more reliably confirm the efficacy of FDG-PET. A secondary point of limitation is the timing of the FDG-PET. In many studies, FDG-PET was performed 7 to 14 days after the initiation of each therapy. In this study, we set the second PET at 28 days after the start of treatment. This setting was dependent on the Japanese medical insurance system, which does not allow two FDG-PET scans in the same month. Considering that the reported PFS of patients treated with regorafenib was 1.7 months, the timing of an FDG-PET-based diagnosis should be earlier.
Conclusion
In summary, JACCRO CC-12 failed to demonstrate the usefulness of FDG-PET as an early imaging biomarker of regorafenib in patients with mCRC. Therefore, more effective methods need to be established for the early detection of responders and nonresponders to regorafenib.
References
Mross K, Frost A, Steinbild S, et al. A phase I dose-escalation study of regorafenib (BAY 73–4506), an inhibitor of oncogenic, angiogenic, and stromal kinases, in patients with advanced solid tumors. Clin Cancer Res. 2012;18:2658–67.
Strumberg D, Scheulen ME, Schultheis B, et al. Regorafenib (BAY 73–4506) in advanced colorectal cancer: a phase I study. Br J Cancer. 2012;106:1722–7.
Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–12.
Li J, Qin S, Xu R, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015;16:619–29.
Ricotta R, Verrioli A, Ghezzi S, et al. Radiological imaging markers predicting clinical outcome in patients with metastatic colorectal carcinoma treated with regorafenib: post hoc analysis of the CORRECT phase III trial (RadioCORRECT study). ESMO Open. 2017;1:e000111.
Pinker K, Riedl C, Weber WA. Evaluating tumor response with FDG PET: updates on PERCIST, comparison with EORTC criteria and clues to future developments. Eur J Nucl Med Mol Imaging. 2017;44(Suppl 1):55–66.
Avallone A, Aloj L, Pecori B, et al. (18)F-FDG PET/CT Is an early predictor of pathologic tumor response and survival after preoperative radiochemotherapy with bevacizumab in high-risk locally advanced rectal cancer. J Nucl Med. 2019;60:1560–8.
Cornelis FH, Petre EN, Vakiani E, et al. Immediate postablation 18 F-FDG injection and corresponding SUV are surrogate biomarkers of local tumor progression after thermal ablation of colorectal carcinoma liver metastases. J Nucl Med. 2018;59:1360–5.
Cornelis F, Sotirchos V, Violari E, et al. 18F-FDG PET/CT Is an immediate imaging biomarker of treatment success after liver metastasis ablation. J Nucl Med. 2016;57:1052–7.
Young H, Baum R, Cremerius U, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European organization for research and treatment of cancer (EORTC) PET study group. Eur J Cancer. 1999;35:1773–82.
Wahl RL, Jacene H, Kasamon Y, et al. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl 1):122S-150S.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47.
Common Terminology Criteria for Adverse Events v4.0 (CTCAE). (2009) (https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5x11.pdf).
Uetake H, Sugihara K, Muro K, et al. Clinical features of regorafenib-induced liver injury in Japanese patients from postmarketing experience. Clin Colorectal Cancer. 2018;17:e49–58.
Bekaii-Saab TS, Ou FS, Ahn DH, et al. Regorafenib dose-optimisation in patients with refractory metastatic colorectal cancer (ReDOS): a randomised, multicentre, open-label, phase 2 study. Lancet Oncol. 2019;20:1070–82.
Ogata M, Kotaka M, Ogata T, et al. Regorafenib vs trifluridine/tipiracil for metastatic colorectal cancer refractory to standard chemotherapies: a multicenter retrospective comparison study in Japan. PLoS ONE. 2020;15:e0234314.
Larson SM, Erdi Y, Akhurst T, et al. Tumor treatment response based on visual and quantitative changes in global tumor glycolysis using PET-FDG imaging: the visual response score and the change in total lesion glycolysis. Clin Positron Imaging. 1999;2:159–71.
Moon SH, Hyun SH, Choi JY. Prognostic significance of volume-based PET parameters in cancer patients. Korean J Radiol. 2013;14:1–12.
Arslan N, Miller TR, Dehdashti F, Battafarano RJ, Siegel BA. Evaluation of response to neoadjuvant therapy by quantitative 2-deoxy-2-[18F]fluoro-D-glucose with positron emission tomography in patients with esophageal cancer. Mol Imaging Biol. 2002;4:301–10.
Rahim MK, Kim SE, So H, et al. Recent trends in PET image interpretations using volumetric and texture-based quantification methods in nuclear oncology. Nucl Med Mol Imaging. 2014;48:1–15.
Acknowledgements
Acknowledgements
We are particularly grateful to Atsushi Kono and Hiroshi Takahashi as members of the independent monitoring committee. We thank the participants of the study.
Funding
This study was supported by Bayer Yakuhin, Ltd. and JACCRO (Japan Clinical Cancer Research Organization). JACCRO is also funding the journal’s Rapid Service Fees.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authors’ contributions
Masato Nakamura, Masahiro Takeuchi, Wataru Ichikawa, and Masashi Fujii contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Masato Nakamura, Hironaga Satake, Tamotsu Sagawa, Akinori Takagane, Takashi Sekikawa, Kazuhiro Oguchi, and Tomohito Kaji. The first draft of the manuscript was written by Masato Nakamura. All authors commented on previous versions of the manuscript. All authors read and approved the final version of the manuscript.
Disclosures
Masato Nakamura has received honoraria from Bayer Yakuhin, Ltd., Bristol-Myers Squibb Co., Ltd., Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Eli Lilly Japan Co., Ltd., Merck Bio Pharma Co., Ltd., Ono Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Sanofi Co., Ltd., Taiho Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., and Yakult Honsha Co., Ltd. Hironaga Satake has received research funding from Ono Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., and Takeda Pharmaceutical Co., Ltd., and honoraria from Bayer Co., Ltd., Bristol-Myers Squibb Co., Ltd., Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Eli Lilly Japan Co., Ltd., Merck Bio Pharma Co., Ltd., MSD Co., Ltd., Ono Pharmaceutical Co., Ltd., Sanofi Co., Ltd., Taiho Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., and Yakult Honsha Co., Ltd. Wataru Ichikawa has received honoraria and fees for promotional materials from Bayer Co., Ltd. All remaining authors declare no conflicts of interest.
Compliance with ethics guidelines
The protocol for this study was approved by all relevant regulatory authorities and ethics committees at the participating institutions. This study was conducted in accordance with the Ethical Guidelines for Medical and Health Research Involving Human Subjects and the Declaration of Helsinki. Each patient provided written informed consent before study enrollment.
Data availability
All data generated or analyzed during this study are included in the published article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Nakamura, M., Satake, H., Sagawa, T. et al. 18F-FDG-PET/CT as an imaging biomarker for regorafenib efficacy in metastatic colorectal cancer (JACCRO CC-12). Oncol Ther 9, 635–645 (2021). https://doi.org/10.1007/s40487-021-00173-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40487-021-00173-1