Abstract
Introduction
Clinical remission is the main target in the management of patients with rheumatoid arthritis (RA). However, several authors found synovitis in patients with RA in clinical remission at ultrasonography (US). Upadacitinib is a selective Janus kinase 1 inhibitor that achieved significantly higher remission rates than adalimumab and abatacept in patients with RA. Here we present the 24-week data of the UPAdacitinib Rheumatoid Arthritis REmission UltraSonography (UPARAREMUS) study.
Methods
This is a longitudinal multicenter observational study, enrolling bio-naïve and bio-inadequate responder patients affected by RA. The primary endpoint was the proportion of patients achieving both clinical and US remission at week 24. The proportion of patients achieving clinical remission with different composite indexes at week 12 and 24 was also evaluated. US of four target joints (wrists and second metacarpophalangeal bilaterally) was performed at baseline and weeks 12/24, and US remission was defined as the absence of power Doppler (PD) signal ≥ 2 in one target joint, or PD ≥ 1 in two target joints.
Results
After 12 weeks and 24 weeks, 40% and 63.6% of patients achieved US plus clinical remission. The following parameters were associated with US plus clinical remission: being bio-naïve and having a shorter disease duration, although at multivariate analysis significant odds ratio (OR) was found only for being bio-naïve.
Conclusions
UPARAREMUS is the first study evaluating the efficacy of upadacitinib in reaching both clinical and US remission in patients with RA. At 24 weeks, 63.6% of patients reached the primary endpoint, the only baseline associated parameter was being bio-naïve.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This is the first study evaluating the efficacy of upadacitinib in reaching clinical plus ultrasonographic remission in patients with rheumatoid arthritis, in a real-life scenario. |
A 24 weeks upadacitinib treatment led to clinical plus ultrasonographic remission in almost two-thirds of enrolled patients. |
The main baseline parameter associated with reaching the primary endpoint was being bio-naïve; whereas monotherapy, higher body mass index, and comorbidities did not affect the chance of reaching this target. |
Introduction
Remission and low disease activity represent the main targets in the management of patients with rheumatoid arthritis (RA) [1]. Different indexes are used to assess remission in clinical trials and in clinical practice. These include Boolean-based and index-based remission definitions such as the simplified disease activity index (SDAI) or clinical disease activity index (CDAI) as well as the less stringent Disease Activity Score on 28 joints (DAS28), which is still commonly used in everyday practice. The reported range of patients with RA achieving clinical remission with different biologic disease-modifying antirheumatic drugs (bDMARDs) such as the anti-tumor necrosis alpha (TNFα) is highly variable depending on several factors, including the remission indexes adopted, the population setting analyzed (long-standing vs early RA), the study design (clinical trials vs real-life studies), and the time frame considered [2,3,4]. Whatever the clinical remission definition applied, and the rates obtained, it is generally recognized that it does not always reflect a true remission condition, considered as the lack of joint inflammation and the interruption of structural damage progression. In fact, different authors have found active synovitis in a relevant proportion (up to 50%) of patients with RA in clinical remission, by using high-sensitivity imaging techniques such as ultrasonography (US) [5,6,7,8,9,10]. In particular, the application of power Doppler (PD) can allow the detection of pathologic synovial vascularization and it is considered a valid tool for monitoring joint disease activity and subclinical synovitis [11,12,13,14,15,16,17]. For this reason, the European League Against Rheumatism (EULAR) recommendations for the use of imaging in the clinical management of RA suggest the application of US to assess persistent inflammation in patients affected by RA even when clinical remission is present [18].
There is no consensus yet about which target joints should be included in the US assessment of patients with RA in clinical remission and, consequently, several sets of joints have been evaluated so far [19,20,21,22,23]. As one might conceive, to be applicable in a real-life scenario, the assessment of target joints in patients affected by RA should be both representative of global disease activity and feasible. In this regard, restricted scores have demonstrated superior performance compared to clinical examination in detecting residual synovitis, to a similar degree as extensive scores [24]. We already demonstrated that the US evaluation of only 3–4 target joints (wrists and second metacarpophalangeal (MCP) joints) allows one to reach a high sensitivity in detecting subclinical active synovitis in patients with RA in clinical remission [25].
Recently Janus kinase (JAK)-STAT signaling has emerged as a key pathway for RA and, therefore, a possible therapeutic target [26, 27]. Upadacitinib is a selective JAK1 and JAK3 inhibitor that was demonstrated to be superior to placebo and achieved significantly higher remission rates at week 12 than adalimumab in patients with active RA and inadequate response to methotrexate (MTX) by using different indexes such as DAS28, CDAI, SDAI, and Boolean criteria [28,29,30]. However, real-life data evaluating the clinical and US effects of upadacitinib in patients affected by RA are still lacking.
Here we present the 24-week data of the UPAdacitinib Rheumatoid Arthritis REmission UltraSonography (UPARAREMUS) study, a multicenter prospective real-life study in bio-naïve and bio-failure patients with RA, evaluating the effectiveness of upadacitinib in reaching clinical plus US remission.
Methods
Patients and Study Design
This is a longitudinal observational study involving nine rheumatology centers. Consecutive patients with RA, classified according to the EULAR/American College of Rheumatology (ACR) 2010 criteria [31], who were bio-naïve (inadequate responders to conventional synthetic disease-modifying antirheumatic drugs, csDMARDs) or bio-failures (inadequate responders to csDMARDs and bDMARDs) were enrolled according to the criteria listed below.
Inclusion Criteria
-
Moderate to severe activity (DAS28crp > 3.2)
-
Male or female; 18–65 years of age, inclusive
-
Inadequate responders, as per EULAR response criteria [1], to at least 6 months treatment with MTX at standard dosage (bio-naïve patients)
-
Inadequate responders, as per EULAR response criteria [1], to at least 6 months treatment with MTX and bDMARDs at standard dosage (bio-failure patients)
Exclusion Criteria
-
Any contraindications to upadacitinib at the time of enrollment as per local label (Summary of Product Characteristic, SmPC)
-
Concomitant use of any bDMARDs and targeted synthetic disease-modifying antirheumatic drugs (tsDMARDs) other than upadacitinib
-
Previous use of any tsDMARDs and bDMARDs (bio-naïve patients)
Ultrasonography
US was performed by rheumatologists, experts in musculoskeletal (MS) US, who had passed an interobserver reliability test against a reference standard (AI), performed on static images and using an e-learning platform. A good to excellent reliability (weighted kappa ≥ 0.7) was required to be approved in participating in the study. Sonographers were blinded to clinical data. Only centers equipped with high-level US machines (e.g., MyLab X8 eXP, MyLab 70XVG, Logiq9, LogiqE9, or other high-end equipment) with high-frequency probes (14–18 MHz) were included. US-detected synovitis and PD synovitis were scored according to a 0–3 semiquantitative simplified score (0 = absent, 1 = mild, 2 = moderate, 3 = severe) [32, 33]. The following settings were used for PD: frequency 8.3–10 MHz, pulse repetition frequency 600 Hz, gain adjusted just below the level that caused the appearance of noise artifacts, low wall filter. For the identification of actively inflamed joints, we focused on PD-positive synovitis (according to the Outcome Measures in Rheumatology (OMERACT) definitions) [34] because it is the reference lesion in most studies analyzing residual activity in patients with RA and has shown the highest predictive value in relation to radiographic damage progression/clinical relapse among elementary US lesions such as tenosynovitis and erosions [33]. In accordance with our previous study, two target joints were examined bilaterally: the second MCP and wrist [25]. Swollen joints at clinical examination were also included in the US evaluation. All joints were assessed following internationally approved guidelines [34].
Endpoints and Definitions
The primary endpoint of the study was the proportion of patients achieving US plus clinical remission at week 24.
Secondary endpoints were:
-
The proportion of patients achieving clinical remission with each composite measure (CDAI, DAS28crp, and SDAI) at weeks 12 and 24 [35,36,37]
-
The changes from baseline to weeks 12 and 24 in the DAS28crp, CDAI, and SDAI
Clinical remission was defined as DAS28crp < 2.6 or CDAI ≤ 2.8 or SDAI ≤ 3.3.
Considering that inflammatory changes can also be found in healthy subjects [38], US remission was defined as the absence of PD signal ≥ 2 in one target joint, or PD ≥ 1 in two target joints.
The study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of the coordinating center (Lazio area 1, Approval numbers 6493_2021 on June 15, 2021) and by each center participating in the study (Supplementary Materials Table S2); written informed consent was obtained from all the patients.
Study Procedures
The following procedures were performed at week 0/enrollment visit (T0), 12 weeks (T1), and 24 weeks (T2):
-
Health and clinical history
-
Clinical examination and assessment of disease activity (DAS28crp, CDAI, SDAI)
-
US of target joints (wrists and second MCP bilaterally) and swollen joints
Upadacitinib treatment was started according to the European Medicines Agency (EMA)’s SmPC [39] and clinical judgment independently of patients’ participation in the study, at the approved standard dose for RA of 15 mg once daily.
Statistical Analysis
The proportions of patients who had achieved clinical remission and clinical plus US remission at weeks 12 and 24 were calculated. Also, median values in DAS28crp, CDAI, and SDAI at baseline, week 12, and week 24 were calculated and compared using the Wilcoxon non-parametric test. Demographic and clinical factors (age, gender, body mass index (BMI), duration of disease, comorbidities, anti-cyclic citrullinated peptides antibodies (ACPA) or rheumatoid factor (RF), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), visual analogue (VAS), immunosuppressive treatment) were evaluated to characterize patients. Proportions, medians, and interquartile ranges (IQR) were calculated as appropriate and comparisons between patients who achieved US plus clinical remission versus those who did not were assessed using chi-squared and Mann–Whitney tests. Logistic regression was used to evaluate if there were baseline factors associated with US plus clinical remission, adjusting for the effect of confounders.
Patients who discontinued the study treatment before the first timepoint were considered not to have had a response (non-responder imputation) for all clinical composite measures. Patients who discontinued the study treatment before the second timepoint were considered as dropouts. p values ≤ 0.05 were considered statistically significant.
Results
Demographic and Clinical Characteristics
This is an analysis of patients enrolled from December 2021 to March 30, 2023.
A total of 60 patients affected by RA were enrolled. Each patient completed 12-week follow-up (T1), and 55 patients completed 24-week follow-up (T2) (Table 1).
The median age was 56 years (IQR 52–62), 43/60 (71.7%) were women, and the median disease duration was 9 years (IQR 4.8–13.7). A higher proportion of the patients (58.3%; 35/60) were bio-experienced, whereas 41.7% (25/60) were bio-naïve.
Upadacitinib was used in combination with csDMARDs in 31/60 (51.7%) of patients, whereas 29/60 (48.3%) patients were receiving upadacitinib monotherapy.
Most of the patients were positive for ACPA (78.2%; 43/55) or RF (82.5%; 47/57).
Regarding disease activity, the median baseline DAS28crp was 4.7 (IQR 3.9–5.17), CDAI 23.00 (IQR 15.75–30.00), and SDAI 24.39 (IQR 17.25–31.63).
Primary Endpoint
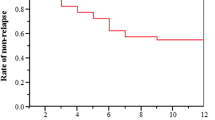
After 12 weeks and 24 weeks respectively, 40% (24/60) and 63.6% (35/55) of patients achieved the primary endpoint (Fig. 1). In particular, between 12 and 24 weeks of treatment, 13 patients more achieved US plus clinical remission while 2 patients lost it.
Looking at the baseline parameters we found that patients achieving the primary endpoint at 24 weeks presented the following significant differences in comparison to the group who did not achieve it: lower BMI (p = 0.03), shorter disease duration (p = 0.05), lower VAS patient activity and pain (p = 0.01 and p = 0.03, respectively), fewer tender joints (p = 0.04), and a lower CDAI and SDAI (p = 0.02 and p = 0.03, respectively) (Table 2).
Among the baseline parameters included at univariate analysis, the variables associated with a higher probability of reaching the primary endpoint were being bio-naïve (p = 0.039) and having a shorter disease duration (p = 0.036) (Table 3). After the data were adjusted according to sex, age, corticosteroids use, monotherapy, and disease duration, a statistically significant odds ratio (OR) for primary endpoint was found only for being bio-naïve [(aOR 4.50 (95% CI 1.12–18.08) p = 0.034].
Secondary Endpoints
After 12 weeks, 45% (27/60) of patients achieved clinical remission with at least one of the remission indices; three out of these 27 patients did not reach US remission (11%). At 24 weeks, the percentage of patients achieving clinical remission improved to 65.5% (36/55) and the proportion of patients without an associated US remission further decrease to 3% (1/36) (Fig. 1).
A significant reduction in disease activity was registered during the follow-up with each composite measure (Fig. 2). In particular, the median DAS28crp significantly decreased from 4.7 at T0 to 2.9 at T1 (p < 0.01) and 2 at T2 (p < 0.01); CDAI significantly reduced from 23 at T0 to 10 at T1 (p < 0.01) and 6 at T2 (p < 0.01); and SDAI reduced from 24.4 at T0 to 10.6 at T1 (p < 0.01) and 6.1 at T2 (p < 0.01). Looking at the single components of the composite remission score, we observed a significant improvement from T0 to T1 for median ESR (from 30 to 17.5; p < 0.01) and tender joints (from 6 to 2; p < 0.01). Whereas the other variables significantly reduced both at T1 and T2: CRP (from 1.05 to 0.28 to 0.17; p < 0.01), swollen joints (from 4 to 1 to 0; p < 0.01), VAS pain from 70 to 40 and 15 (p < 0.01), VAS PGA from 60 to 35 and 20 (p < 0.01), and VAS EGA from 70 to 30 and 20 (p < 0.01) (Supplementary Materials Table S1). At T1, 45% of patients reached clinical remission by using DAS28crp, 15% SDAI and 10% CDAI. At T2 this proportion changed as follows: 65.4% DAS28crp, 30.9% SDAI, and 27.3% CDAI (Fig. 2).
Considering US synovitis, a significant reduction both in the total number and total PD grading of active target joints was observed both at T1 and T2. The median number of total involved joints reduced from 2 joints per patient to 0 joints per patient at T1 and at T2. The total median PD grading in the target joints significantly reduced from 2 per patient at T0 to 0 per patient at T2 (Fig. 3 and Supplementary Materials Table S1).
Representatives examples of PD synovitis at T0 and T1 in two patients. a T0 severe synovitis of the second metacarpophalangeal (MCP) joint of right hand, PD grade 2. b T0 severe synovitis of the left radiocarpal joint, PD grade 3. c T1 moderate synovitis of the second MCP joint of right hand, PD negative. d T1 mild synovitis of the left radiocarpal joint, PD negative. PD power Doppler, T1 12-week follow-up, T2 24-week follow-up
Safety
In the follow-up, 5/60 patients (8%) were considered as dropouts. All these patients stopped upadacitinib between T1 and T2.
Non-serious adverse events were registered in 4/60 (6.7%) of patients (one optic neuritis, one respiratory infection, one neutropenia, and one recurrent herpes zoster infection), whereas one patient interrupted the drug for inefficacy.
Discussion
UPARAREMUS is the first real-life observational study aimed at evaluating the efficacy of upadacitinib in reaching clinical plus US remission in patients with RA.
According to the latest EULAR recommendations, the treatment of RA should aim at clinical remission, to prevent joint damage and consequent disability [1]. However, it is known that a relevant proportion of patients in clinical remission still have joint inflammation observed at US and MRI, a condition that makes them more susceptible to the risk of radiographic progression and disease flare-up [6, 40, 41]. In particular PD-positive synovitis has emerged as the US lesion with the best value for predicting flares in patients with RA in clinical remission [42]. In addition, earlier studies have shown that the lack of PD synovitis is more predictive of reduced radiographic progression at 12–24 months follow-up than the absence of clinical inflammation in the setting of clinical remission [42]. These observations have a significant impact in guiding therapeutic decisions, suggesting that patients with RA in clinical remission but with PD-positive synovitis should not reduce ongoing treatment. In this regard, a comprehensive target represented by both clinical and imaging remission would be more reliable and accurate.
The efficacy of upadacitinib has been demonstrated by six randomized clinical trials (RCTs) involving 3233 bio-naïve and bio-failure patients with RA, both in association with csDMARDs and monotherapy and two head to head (H2H) trials versus adalimumab and abatacept [43, 44]. On the other hand, real-life longitudinal studies evaluating upadacitinib efficacy in patients with RA are lacking, and there are still no reports on its effect on joint inflammation analyzed by US.
The data of this UPARAREMUS cohort showed a rapid and high efficacy of upadacitinib in control of both clinical and US activity in patients affected by RA. Indeed, the primary endpoint of US plus clinical remission was achieved by 40% of patients at 12 weeks with a further significant increase to 63.6% at 24 weeks. The only baseline parameter associated with a higher probability of reaching the primary endpoint, when adjusted for possible confounders, was being bio-naïve. Notably no significant difference in the chance of reaching the primary endpoint emerged for patients with higher BMI, comorbidities, longer disease duration, and the use of upadacitinib monotherapy.
Regarding clinical remission, 45% of patients achieved this target after 12 weeks and 65% at 24 weeks. Most of the patients at T1 and, to a lesser extent, at T2 reached clinical remission with DAS28crp, followed by SDAI and CDAI, confirming that the last indices are more stringent compared to DAS28. On the other hand, the fact that DAS28 remission correlates better than CDAI and SDAI with US remission may seem unexpected. However, this result has been previously reported by other authors. Indeed, Ben Abdelghani et al. recently showed that DAS28 was the most valid score for assessing remission when the absence of a PD signal is taken as a reference [45].
Our data on clinical remission rates are similar, although higher, to those reported by clinical trials of upadacitinib. In particular in the SELECT-COMPARE and SELECT-CHOICE, two randomized double-blind H2H trials in patients with moderate-to-severe active RA who had an inadequate response to csDMARDs and bDMARDs respectively, the percentage of patients in remission at 24 weeks was 41% and 46% with DAS28crp, 24% and 21% with SDAI, 23% and 21% with CDAI, respectively [29, 46]. The higher percentage observed in the present study probably reflects the differences in population setting and study design. Among the most evident differences, our patients at baseline presented a lower clinical disease activity (i.e., median DAS28crp 4.6 in our study vs 5.8 and 5.7 in the COMPARE and CHOICE trial, respectively) and had far fewer affected joints (6 tender and 4 swollen joints in the present study vs 26 and 17 in the COMPARE trial and 14 and 10 in the CHOICE), probably a patient picture closer to that usually found in real-life practice.
A marked improvement was observed also for US synovitis, with a significant reduction in both the number and severity of the involved active target joints from baseline to T1 and T2, and a relevant percentage of patients reaching US remission at both timepoints. These results show the efficacy of upadacitinib in controlling disease activity at a deeper level than clinical examination, thus reducing the risk of disease relapse and damage progression.
Two prospective single-center observational studies on small populations recently evaluated the effectiveness of baricitinib and tofacitinib in obtaining US improvement in patients with RA, without specifically focusing on the achievement of US remission [47, 48]. In the first study, the authors evaluated 59 consecutive patients with RA under baricitinib therapy, over 12 months of follow-up, and reported a significant reduction in DAS28, SDAI, and CDAI associated with a significant US improvement. In particular, the percentage of patients having a positive PD signal in at least one joint decreased from 61% to 23% at week 24 [47]. In the other study, 29 patients with RA under tofacitinib during a 12-month follow-up were enrolled. CDAI, DAS28, and US score improved significantly over 12 weeks; furthermore, baseline US score correlated with 12-week changes in CDAI and DAS28 [48]. In addition to the aforementioned trials, studies of some TNF inhibitors, such as adalimumab and certolizumab pegol, have reported improvements in US outcomes. In one study assessing wrist synovitis via US examination, adalimumab demonstrated a reduction and complete absence of PD signal at 12 weeks in 79% and 39% of patients, respectively [49]. In another study, the US effectiveness of certolizumab pegol was longitudinally observed in up to 24 joints, by using an activity index calculated for synovial hypertrophy, effusion, and PD signal. At 12 weeks, certolizumab pegol demonstrated significant reductions in synovial hypertrophy, effusion, and PD indices by 7, 6.6, and 4.3 points, respectively [50]. However, since the concept of US remission was not defined in these studies, it was not possible to estimate the proportion of patients achieving it at each timepoint, thereby limiting the comparability of these data to our study. As reported in the Methods section, we decided to consider patients with grade 1 PD synovitis in one target joint as being in US remission, due to evidence that mild inflammatory changes can also be found in healthy subjects; if we had used a more stringent definition of US remission, the percentage of patients reaching this criterion would have decreased. Indeed, a further analysis of the data revealed that defining remission as the absence of PD-positive synovitis in each target joint would have led to an 18% reduction at both T1 and T2 (data not shown). We believe that widespread adoption of a consistent definition of US remission in real-life settings would be necessary in the future to enhance the reproducibility of results from real-life trials.
Some limitations of the current study need to be considered. First, it has a relatively small sample size and short-term 24-week follow-up, thus we could not infer data on radiographic progression. In addition, considering the real-life design of the study, reporting or investigator bias cannot be ruled out. Conversely, this study has several strengths such as its multicenter, prospective design, the presence of a homogeneous cohort of patients with RA, and the US assessment of synovitis.
Conclusions
This is the first study evaluating the efficacy of upadacitinib in reaching clinical plus US remission in patients with RA, in a real-life scenario. A 24-week treatment with upadacitinib led to US plus clinical remission in almost two-thirds of enrolled patients. The main baseline parameter associated with reaching the primary endpoint was being bio-naïve; whereas monotherapy, higher BMI, and comorbidities did not affect the chance of reaching this target. These data could have great clinical relevance to optimize and personalize the therapeutic management of patients affected by RA.
Data Availability
The data that support the findings of this study are available from the authors databases upon reasonable request.
References
Smolen JS, Landewé R, Bergstra SA, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann Rheum Dis. 2023;82:3–18. https://doi.org/10.1136/ard-2022-223356.
Verhoeven MMP, Welsing PMJ, Bijlsma JWJ, et al. Effectiveness of remission induction strategies for early rheumatoid arthritis: a systematic literature review. Curr Rheumatol Rep. 2019;21:24. https://doi.org/10.1007/s11926-019-0821-1.
Hamann P, Holland R, Hyrich K, et al. Factors associated with sustained remission in rheumatoid arthritis in patients treated with anti-tumor necrosis factor. Arthritis Care Res (Hoboken). 2017;69(6):783–93. https://doi.org/10.1002/acr.23016.
Ajeganova S, Huizinga T. Sustained remission in rheumatoid arthritis: latest evidence and clinical considerations. Ther Adv Musculoskel Dis. 2017;9(10):249–62. https://doi.org/10.1177/1759720X17720366.
Aletaha D, Smolen JS. Diagnosis and management of rheumatoid arthritis a review. JAMA. 2018;320(13):1360–72. https://doi.org/10.1001/jama.2018.13103.
Ten Cate DF, Luime JJ, Swen N, et al. Role of ultrasonography in diagnosing early rheumatoid arthritis and remission of rheumatoid arthritis - a systematic review of the literature. Arthritis Res Ther. 2013;15(1):R4. https://doi.org/10.1186/ar4132.
Kawashiri SY, Suzuki T, Nakashima Y, et al. Ultrasonographic examination of rheumatoid arthritis patients who are free of physical synovitis: power Doppler subclinical synovitis is associated with bone erosion. Rheumatology (Oxford). 2014;53:562–9. https://doi.org/10.1093/rheumatology/ket405.
Ozgocmen S, Ozdemir H, Kiris A, et al. Clinical evaluation and power Doppler sonography in rheumatoid arthritis: evidence for ongoing synovial inflammation in clinical remission. South Med J. 2008;101(3):240–5. https://doi.org/10.1097/SMJ.0b013e318164e16a.
Saleem B, Brown AK, Keen H, et al. Should imaging be a component of rheumatoid arthritis remission criteria? A comparison between traditional and modified composite remission scores and imaging assessments. Ann Rheum Dis. 2011;70:792–8. https://doi.org/10.1136/ard.2010.134445.
Ben Abdelghani K, Miladi S, Souabni L, et al. Role of ultrasound in assessing remission in rheumatoid arthritis. Diagn Intervent Imaging. 2015;96:3–10. https://doi.org/10.1016/j.diii.2014.07.006.
Elkhouly T, Elnady BM, Rageh EMH. Validity of Doppler subclinical synovitis as an activity marker associated with bone erosions in rheumatoid arthritis patients during clinical remission. Egypt J Radiol Nuclear Med. 2016;47:985–90. https://doi.org/10.1136/annrheumdis-2020-eular.2059.
Naredo E, Bonilla G, Gamero F, et al. Assessment of inflammatory activity in rheumatoid arthritis: a comparative study of clinical evaluation with grey scale and power Doppler ultrasonography. Ann Rheum Dis. 2005;64:375–81. https://doi.org/10.1136/ard.2004.023929.
Dougados M, Jousse-Joulin S, Mistretta F, et al. Evaluation of several ultrasonography scoring systems for synovitis and comparison to clinical examination: results from a prospective multicentre study of rheumatoid arthritis. Ann Rheum Dis. 2010;69:828–33. https://doi.org/10.1136/ard.2009.115493.
Mandl P, Kurucz R, Niedermayer D, et al. Contributions of ultrasound beyond clinical data in assessing inflammatory disease activity in rheumatoid arthritis: current insights and future prospects. Rheumatology (Oxford). 2014;53:2136–42. https://doi.org/10.1093/rheumatology/keu211.
Terslev L, von der Recke P, Torp-Pedersen S, et al. Diagnostic sensitivity and specificity of Doppler ultrasound in rheumatoid arthritis. J Rheumatol. 2008;35:49–53.
Filippucci E, Iagnocco A, Salaffi F, et al. Power Doppler sonography monitoring of synovial perfusion at the wrist joints in patients with rheumatoid arthritis treated with adalimumab. Ann Rheum Dis. 2006;65:1433–7. https://doi.org/10.1136/ard.2005.044628.
Naredo E, Moller I, Cruz A, et al. Power Doppler ultrasonographic monitoring of response to anti-tumor necrosis factor therapy in patients with rheumatoid arthritis. Arthritis Rheum. 2008;58:2248–56. https://doi.org/10.1002/art.23682.
Colebatch AN, Edwards CJ, Østergaard M, et al. EULAR recommendations for the use of imaging of the joints in the clinical management of rheumatoid arthritis. Ann Rheum Dis. 2013;72:804–14. https://doi.org/10.1136/annrheumdis-2012-203158.
Naredo E, Valor L, De La Torre I, et al. Ultrasound joint inflammation in rheumatoid arthritis in clinical remission: how many and which joints should be assessed? Arthritis Care Res (Hoboken). 2013;65:512–7. https://doi.org/10.1002/acr.21869.
Janta I, Valor L, De La Torre I, et al. Ultrasound-detected activity in rheumatoid arthritis on methotrexate therapy: which joints and tendons should be assessed to predict unstable remission? Rheumatol Int. 2016;36:387–96. https://doi.org/10.1007/s00296-015-3409-8.
Rosa J, Ruta S, Saucedo C, et al. Does a simplified 6-joint ultrasound index correlate well enough with the 28-joint disease activity score to be used in clinical practice? J Clin Rheumatol. 2016;22:179–83. https://doi.org/10.1097/RHU.0000000000000415.
De Miguel E, Pecondón-Español A, Castaño-Sánchez M, et al. A reduced 12-joint ultrasound examination predicts lack of X-ray progression better than clinical remission criteria in patients with rheumatoid arthritis. Rheumatol Int. 2017;37:1347–56. https://doi.org/10.1007/s00296-017-3714-5.
Aydin SZ, Gunal EK, Ozata M, et al. Six joint ultrasound in rheumatoid arthritis: a feasible approach for implementing ultrasound in remission. Clin Exp Rheumatol. 2017;35:853–6.
Silvagni E, Zandonella Callegher S, Mauric E, et al. Musculoskeletal ultrasound for treating rheumatoid arthritis to target—a systematic literature review. Rheumatology. 2022;61:4590–602.
Picchianti Diamanti A, Navarini L, Messina F, et al. Ultrasound detection of subclinical synovitis in rheumatoid arthritis patients in clinical remission: a new reduced-joint assessment in 3 target joints. Clin Exp Rheumatol. 2018;36(6):984–9.
Mohamed MF, Beck D, Camp S, et al. Preferential inhibition of JAK1 relative to JAK3 by upadacitinib: exposure-response analyses of ex vivo data from 2 phase 1 clinical trials and comparison to tofacitinib. J Clin Pharmacol. 2020;60(2):188–97. https://doi.org/10.1002/jcph.1513.
McInnes IB, Byers NL, Higgs RE, et al. Comparison of baricitinib, upadacitinib, and tofacitinib mediated regulation of cytokine signaling in human leukocyte subpopulations. Arthritis Res Ther. 2019;21:183. https://doi.org/10.1186/s13075-019-1964-1.
Strand V, Schiff M, Tundia N, et al. Effects of upadacitinib on patient-reported outcomes: results from SELECT-BEYOND, a phase 3 randomized trial in patients with rheumatoid arthritis and inadequate responses to biologic disease-modifying antirheumatic drugs. Arthritis Res Ther. 2019;21:263. https://doi.org/10.1186/s13075-019-2059-8.
Fleischmann R, Pangan AL, Song IH, et al. Upadacitinib versus placebo or adalimumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase III, double-blind randomized controlled trial. Arthritis Rheumatol. 2019;71(11):1788–800. https://doi.org/10.1002/art.41032.
Burmester GR, Kremer JM, Van de Bosch F, et al. Safety and efficacy of upadacitinib in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease-modifying anti-rheumatic drugs (SELECT-NEXT): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391(10139):2503–12. https://doi.org/10.1016/S0140-6736(18)31115-2.
Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2010;69:1580–8. https://doi.org/10.1002/art.27584.
Scheel AK, Hermann K-GA, Kahler E, et al. A novel ultrasonographic synovitis scoring system suitable for analyzing finger joint inflammation in rheumatoid arthritis. Arthritis Rheum. 2005;52:733–43. https://doi.org/10.1002/art.20939.
Szkudlarek M, Court-Payen M, Jacobsen S, et al. Interobserver agreement in ultrasonography of the finger and toe joints in rheumatoid arthritis. Arthritis Rheum. 2003;48:955–62. https://doi.org/10.1002/art.10877.
Bruyn AB, Iagnocco A, Naredo E, et al. OMERACT definitions for ultrasonographic pathology and elementary lesions of rheumatic disorders fifteen years on. J Rheumatol. 2019;46:10. https://doi.org/10.3899/jrheum.181095.
Prevoo ML, Van’t Hof MA, Kuper HH, et al. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–8. https://doi.org/10.1002/art.1780380107.
Felson D. Defining remission in rheumatoid arthritis. Ann Rheum Dis. 2012;71(Suppl 2):i86–8. https://doi.org/10.1136/annrheumdis-2011-200618.
Smolen JS, Breedveld FC, Schiff MH, et al. A simplified disease activity index for rheumatoid arthritis for use in clinical practice. Rheumatology. 2003;42:244–57. https://doi.org/10.1093/rheumatology/keg072.
Nguyen H, Ruyssen-Witrand A, Gandjbakhch F, et al. Prevalence of ultrasound detected residual synovitis and risk of relapse and structural progression in rheumatoid arthritis patients in clinical remission: a systematic review and metaanalysis. Rheumatology (Oxford). 2014;53(11):2110–8. https://doi.org/10.1093/rheumatology/keu217.
https://www.ema.europa.eu/en/medicines/human/EPAR/rinvoq. Accessed 11 Aug 2024
D’Agostino MA, Boers M, Wakefield RJ, et al. Is it time to revisit the role of ultrasound in rheumatoid arthritis management? Ann Rheum Dis. 2017;76(1):7–8. https://doi.org/10.1136/annrheumdis-2016-210453.
Scirè CA, Montecucco C, Codullo V, et al. Ultrasonographic evaluation of joint involvement in early rheumatoid arthritis in clinical remission: power Doppler signal predicts short-term relapse. Rheumatology (Oxford). 2009;48(9):1092–7. https://doi.org/10.1093/rheumatology/kep171.
Silvagni E, Zandonella Callegher S, et al. Musculoskeletal ultrasound for treating rheumatoid arthritis to target—a systematic literature review. Rheumatology. 2022;61:4590–602.
Sanmartí R, Corominas H. Upadacitinib for patients with rheumatoid arthritis: a comprehensive review. J Clin Med. 2023;12(5):1734. https://doi.org/10.3390/jcm12051734.
Panchal V, Vyas HB, Sivasubramanian BP, et al. A meta-analysis evaluating the effectiveness and safety of upadacitinib in treating rheumatoid arthritis in patients with inadequate response to disease-modifying anti-rheumatic drugs. Cureus. 2023;15(1): e34384. https://doi.org/10.7759/cureus.34384.
Abdelghani KB, Miladi S, Makhlouf Y, et al. Validity of remission criteria in rheumatoid arthritis compared to ultrasound-defined remission. Sultan Qaboos University Med J. 2022;22(4):554–60.
Rubbert-Roth A, Enejosa J, Pangan A, et al. Sat0151 efficacy and safety of upadacitinib versus abatacept in patients with active rheumatoid arthritis and prior inadequate response or intolerance to biologic disease modifying anti-rheumatic drugs (select-choice): a double-blind, randomized controlled phase 3 trial. Ann Rheum Dis. 2020;79:1015–6. https://doi.org/10.1136/annrheumdis-2020-eular.2059.
Razmjou AA, Brook J, Elashoff D, et al. Ultrasound and multi-biomarker disease activity score for assessing and predicting clinical response to tofacitinib treatment in patients with rheumatoid arthritis. BMC Rheumatol. 2020;19(4):55.
Spinelli FR, Ceccarelli F, Garufi C, et al. Effectiveness and safety of baricitinib in rheumatoid arthritis: a monocentric, longitudinal, real-life experience. Clin Exp Rheumatol. 2020;39:525–31.
Filippucci E, Iagnocco A, Salaffi F, et al. Power Doppler sonography monitoring of synovial perfusion at the wrist joints in patients with rheumatoid arthritis treated with adalimumab. Ann Rheum Dis. 2006;65(11):1433–7. https://doi.org/10.1136/ard.2005.04462.
Blanco FJ, Rubio-Romero E, Sanmarti R, et al. Clinical, patient-reported, and ultrasound outcomes from an open-label, 12-week observational study of certolizumab pegol in Spanish patients with rheumatoid arthritis with or without prior anti-TNF exposure. Reumatol Clin (Engl Ed). 2020;16(5 Pt):345–52. https://doi.org/10.1016/j.reuma.2018.07.009.
Acknowledgements
We thank the participants of the study.
Funding
This study and the publication fees, including the journal’s Rapid Service Fee, were supported by an unconditional grant from AbbVie srl.
Author information
Authors and Affiliations
Contributions
Conceptualization, Andrea Picchianti Diamanti, Bruno Laganà, Annamaria Iagnocco; methodology Mariasofia Cattaruzza, Andrea Picchianti Diamanti, Marco Canzoni; formal analysis: Mariasofia Cattaruzza; writing—original draft, review and editing: Andrea Picchianti Diamanti, Annamaria Iagnocco, Alen Zabotti, Bruno Laganà; enrolling patients and collecting clinical data: Arianna D’Antonio, Maria Sole Chimenti, Simonetta Salemi, Roberta Di Rosa, Giorgio Sesti, Giandomenico Sebastiani, Alen Zabotti, Ivan Giovannini, Gloria Crepaldi, Michele Maria Luchetti, Gloria Felice, Chiara De Lorenzo, Valentino Paci, Bruno Frediani, Caterina Baldi, Chiara Scirocco, Carlo Perricone. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Andrea Picchianti Diamanti, Bruno Laganà, Annamaria Iagnocco, Mariasofia Cattaruzza, Marco Canzoni, Arianna D’Antonio, Maria Sole Chimenti, Simonetta Salemi, Roberta Di Rosa, Giorgio Sesti, Giandomenico Sebastiani, Chiara Scirocco, Ivan Giovannnini, Michele Maria Luchetti, Gloria Felice, Chiara De Lorenzo, Valentino Paci, Gloria Crepaldi, Bruno Frediani, Caterina Baldi, Carlo Perricone have nothing to disclose. Alen Zabotti is an Editorial Board member of Rheumatology and Therapy. Alen Zabotti was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions.
Ethical Approval
The study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of the coordinating center (Lazio area 1, Approval numbers 6493_2021 on June 15, 2021) and by each center participating in the study (Supplementary Materials Table S2); written informed consent was obtained from all the patients.
Additional information
Prior Presentation: EULAR 2024 Congress, Vienna, Austria; June 12–15. Abstract number AB052.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Picchianti Diamanti, A., Cattaruzza, M.S., Salemi, S. et al. Clinical and Ultrasonographic Remission in Bio-naïve and Bio-failure Patients with Rheumatoid Arthritis at 24 Weeks of Upadacitinib Treatment: The UPARAREMUS Real-Life Study. Rheumatol Ther (2024). https://doi.org/10.1007/s40744-024-00712-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40744-024-00712-y