Abstract
Lithium metal is considered a highly promising anode material because of its low reduction potential and high theoretical specific capacity. However, lithium metal is prone to irreversible side reactions with liquid electrolytes, resulting in the consumption of metallic lithium and electrolytes due to the high reactivity of lithium metal. The uneven plating/stripping of lithium ions leads to the growth of lithium dendrites and battery safety risks, hindering the further development and commercial application of lithium metal batteries (LMBs). Constructing solid-state electrolyte (SSE) systems with high mechanical strength and low flammability is among the most effective strategies for suppressing dendrite growth and improving the safety of LMBs. However, the structural defects, intrinsic ionic conductivity, redox potential and solid-solid contacts of SSEs can cause new electrochemical problems and solid-phase dendrite growth drawbacks in the application of solid-state batteries (SSBs). In this review, the mechanisms of lithium dendrite growth in SSEs are comprehensively summarized. Strategies to suppress lithium dendrite growth, stabilize the interface, and enhance ion transport in organic, inorganic and composite SSEs are emphasized. We conclude with not only relevant experimental findings but also computational predictions to qualitatively and quantitatively characterize the ionic conductivity, interfacial stability and other properties of SSEs based on both chemical and physical principles. The development direction and urgent problems of SSEs are summarized and discussed.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
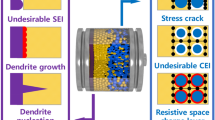
Lithium-ion batteries (LIBs) with high energy densities, high output voltages, and long lifespans are widely used in electric vehicles, mobile wearable devices, smart power grids and so forth. To date, traditional nonaqueous LIBs are based on flammable and explosive organic solvents, which pose a great safety hazard for practical applications. Furthermore, the limited specific capacity of widely used graphite anodes (theoretical specific capacity: 372 mAh g−1) cannot effectively support the step-forward development of energy-dense rechargeable batteries. In addition to LIBs, lithium metals with a high theoretical specific capacity (3 860 mAh g−1), extremely low reduction potential (− 3.04 V vs. a standard hydrogen electrode) [1,2,3,4,5,6] and excellent mechanical flexibility are acknowledged as ideal anode materials to meet the requirements of high energy density batteries. However, the practical application of lithium metal anodes is greatly hindered by the dramatic growth of dendrites and the cascade thermal runaway process. The dendrite filaments formed during electrochemical deposition can penetrate through the separator and induce short circuits in cells. Dendritic lithium is prone to detach from lithium electrodes and becomes a well-known “dead Li”, causing electrical departure, the loss of active materials and shortening of the cycle lifespan of lithium metal batteries (LMBs). Due to the high reactivity of lithium metals, side reactions continuously occur to deplete the organic electrolyte and compromise interfacial stability. Such interface problems increase the resistance and reduce the Coulombic efficiency and the cycle stability. Furthermore, the repeated lithium deposition/stripping process causes a large volume variation with infinite volume expansion, which destroys the solid-electrolyte interface (SEI), thickens the SEI layer, lowers the Coulombic efficiency and exaggerates the interfacial issues. Due to the inherent thermodynamic instability of lithium metal anodes in organic electrolytes, SSEs and SSBs have been highlighted to eliminate the drawbacks of lithium deposition in liquid electrolytes and can potentially be the ultimate solution. SSEs permit a high lithium ion transference number, a wide electrochemically stable voltage window (up to 5 V), thermal stability and separator-free cell stacking, which allow for a higher energy density and better safety than those of their liquid counterparts. However, issues of dendrite growth and interfacial instability in conjunction with lithium metal anodes remain challenging in SSBs, limiting the development of LMBs.
In recent years, a great deal of attention has been given to deepen the understanding of the fading mechanism of lithium metal anodes and better inhibit the growth of lithium dendrites in SSBs. This paper summarizes recent studies on the mechanism of lithium dendrite growth in SSEs and SSBs. Various approaches developed to solve key challenges in organic, inorganic, and composite SSEs are reviewed and discussed, as are the advances and improvements for inhibiting lithium dendrite growth (Figs. 1, 2). An outlook for an in-depth understanding of the dendrite problem in solid-state lithium metal batteries and the development of practical batteries is essentially provided.
1.1 Growth Mechanisms and Strategies for the Suppression of Lithium Dendrites
Dendritic filament formation during the electrodeposition of lithium metals is a result of multiple factors, and a step-by-step understanding of dendrite growth mechanisms is accompanied by parallel explorations among liquid-based, semisolid-state and all-solid-state LIBs, which can be traced back to the 1990s (Fig. 3). Due to its high reactivity, lithium metal undoubtedly undergoes a series of side reactions with liquid electrolytes, which irreversibly consume lithium and electrolytes, reducing battery utilization [7]. Different from the interstitial intercalation mechanism, lithium ions are continuously plated/stripped onto a lithium metal anode substrate during the charging and discharging processes. An ideal Li plating/exfoliation process should be uniform, consistent and preferably two-dimensional. As expected, such plating/stripping processes are affected by various factors [8]. The deposition of lithium ions at the interface is often uneven, which is the root of lithium dendrite growth and electrochemical performance deterioration (capacity fading, lower Coulomb efficiency, etc.), and the main reason hindering the overall application of lithium metal anodes. Due to the “tip effect”, once a lithium dendrite is generated, more charges accumulate at the tip, which triggers selective lithium deposition at the tip and results in continuous dendrite growth. The morphologies of dendrites generally include needle-like, mossy, dendritic, fibrous, granular, and amorphous structures. On the one hand, when lithium dendrites break from the surface of lithium metal, the electron transport pathway is cut off and an isolated “dead lithium” structure is formed, which makes it difficult to participate in subsequent charge-discharge reactions, reducing the Coulomb efficiency, specific capacity, reversibility and other electrochemical properties of batteries [9]. On the other hand, the spreading growth of dendrites may penetrate into separators, causing short circuits inside batteries and triggering cascade thermal runaway behavior, which greatly decreases the safety and lifespan of LMBs [10, 11].

(1) Reproduced with permission from Ref. [15]. Copyright © 1998, Elsevier. (2) Reproduced with permission from Ref. [16]. Copyright © 2002, IOP Publishing. (3) Reproduced with permission from Ref. [17]. Copyright © 2003, IOP Publishing. (4) Reproduced with permission from Ref. [18]. Copyright © 2013, IOP Publishing. (5) Reproduced with permission from Ref. [14]. Copyright © 2015, arXiv. (6) Reproduced with permission from Ref. [19]. Copyright © 2015, IOP Publishing. (7) Reproduced with permission from Ref. [20]. Copyright © 2015, Elsevier. (8) Reproduced with permission from Ref. [21]. Copyright © 2018, American Chemical Society. (9) Reproduced with permission from Ref. [22]. Copyright © 2018, Elsevier. (10) Reproduced with permission from Ref. [23]. Copyright © 2017, Elsevier. (11) Reproduced with permission from Ref. [24]. Copyright © 2019, American Association for the Advancement of Science. (12) Reproduced with permission from Ref. [25]. Copyright © 2019, Elsevier. (13) Reproduced with permission from Ref. [26]. Copyright © 2020, Elsevier. (14) Reproduced with permission from Ref. [27]. Copyright © 2020, John Wiley and Sons. (15) Reproduced with permission from Ref. [28]. Copyright © 2021, The Royal Society of Chemistry. (16) Reproduced with permission from Ref. [29]. Copyright © 2021, John Wiley and Sons. (17) Reproduced with permission from Ref. [30]. Copyright © 2023, Springer Nature. (18) Reproduced with permission from Ref. [31]. Copyright © 2023, Springer Nature. (19) Reproduced with permission from Ref. [32]. Copyright © 2024, John Wiley and Sons. (20) Reproduced with permission from Ref. [33]. Copyright © 2024, American Chemical Society
Chronology of critical achievements toward understanding the growth mechanisms and suppression strategies for lithium metal dendrites.
1.1.1 Models for Lithium Dendrite Formation
Understanding and clarifying the mechanism of lithium dendrite growth are particularly valuable to effectively suppress the growth of lithium dendrites during battery operation. A long period of development underlies a variety of mechanisms and models for lithium dendrite growth, which are insufficient for tackling lithium dendrite issues [12]. Currently, the reason for the insufficient understanding of dendrite growth is not only the complexity of chemical and electrochemical liquid environments but also the significant interface deterioration, corrosive side reactions, dead Li generation and volume expansion during repeated Li+ dissolution/deposition.
-
(a)
Surface nucleation and diffusion model. A prior understanding of the formation of lithium filaments was derived from first-principle calculations by Ling et al. [13], who studied the free energy difference between Mg/Li metals from one- to three-dimensional nucleation. This difference in dimension is more prominent for Mg metals than for lithium metals due to the stronger Mg–Mg bond strength, as a result of which the Mg metal is prone to deposition through a high-dimensional pathway rather than one-dimensional (1D) whiskers. For the same reason, lithium prefers to form a 1D morphology, generating lithium filaments and becoming a well-known obstacle to the implementation of liquid-based LMBs. The surface nucleation and diffusion model was further explained by Ozhabes et al. [14], who performed density functional theory (DFT)-based calculations to determine the surface diffusion barriers and surface energy among several solid-electrolyte interphase (SEI) components [Fig. 3(5)]. From a thermodynamic perspective, an SEI component with a higher surface energy offers greater resistance to dendrite formation, and a component with fast Li+ ion diffusion, for instance, a component with a low diffusion barrier, is less likely to form dendrites. Based on these DFT results, a lithium-halide SEI layer is subsequently suggested, which leads to an improved interface stability with a higher surface energy and a lower surface diffusion barrier, both of which are expected to enable a dendrite-free deposited morphology.
-
(b)
Heterogeneous nucleation model. The heterogeneous nucleation model describes the lithium plating behavior at the initial stage [12]. In this regard, Ely et al. [18] systematically analyzed the heterogeneous nucleation and growth of electrodeposited lithium metal anodes and defined five regimes, including the nucleation suppression, short incubation time, long incubation time, early growth, and late growth regimes [Fig. 3(4)]. Embryos in the nucleation suppression regime are thermodynamically unstable. They thus were redissolved into the electrolyte. Thermodynamically favored embryos persist and grow after thermal fluctuations in the long incubation time regime, which results in a kinetically favorable growth process. Above a critical overpotential, a short incubation time regime induces a narrow size distribution of embryos. In the early and late growth regimes, thermodynamically and kinetically stable nuclei grow and shape gradually [34].
-
(c)
Space charge model. The heterogeneous nucleation theory is no longer applicable after the initial heterogeneous nucleation, where homogeneous lithium deposition dominates the surface behavior. Therefore, Chazalievl [35] proposed the space charge model in 1990, which describes surface cation and anion migration in low-concentration electrolytes or under fast lithium deposition conditions. As expected, electric fields push directional anion and cation migration toward the positive and negative electrodes, respectively. However, when lithium ions are rapidly deposited, the anion concentration on the anode surface decreases rapidly, and this drastic concentration change creates a large electric field and space charge at the interface between the anode and the electrolyte, which induces dendrite growth [12, 36, 37]. The calculation of the Li+ ion concentration gradient based on the polarization of binary polymer Li symmetric cells was simulated by Rosso et al. [38]:
$$\frac{\partial C}{\partial x}\left(x\right)=\frac{J{\mu }_{\text{a}}}{De\left({\mu }_{\text{a}}+{\mu }_{\text{c}}\right)}$$(1)where \({\mu }_{\text{a}}\) and \({\mu }_{\text{c}}\) represent the anionic and cationic mobility, respectively; \(e\) is the elementary charge; \(D\) represents the ambipolar diffusion constant, \(D=\left({\mu }_{\text{a}}{D}_{\text{c}}+{\mu }_{\text{c}}{D}_{\text{a}}\right)/\left({\mu }_{\text{a}}+{\mu }_{\text{c}}\right)\); and \(J\) is the current density. According to Eq. (1), different lithium deposition conditions can be predicted by the interelectrode distance \(l\), initial concentration \({C}_{0}\), diffusion coefficient \(D\) and current density \(J\): when \(\text{d}c/\text{d}x<2{C}_{0}/l\), the ion concentration distribution near the negative electrode is in a stable state and exhibits a flat lithium deposition morphology; when \(\text{d}c/\text{d}x>2{C}_{0}/l\), the negative electrode ion concentration drops to zero at a particular moment (Sand’s time \({\tau }_{\text{S}}\)), while the potential tends to diverge, and the local space charge causes instability at the lithium anode to generate dendrites.
-
(d)
Solid-electrolyte interface (SEI) model. The formation of nanoscale SEI layers passivates the surface from continuous parasitic reactions, and they compete with lithium metal deposition to consume the Faradaic current, which directly impacts the root vs. tip growth mechanism. Kushima et al. [23] separated the SEI model process into four distinct stages (stage 1: hindered surface growth; stage 2: fast root growth; stage 3: hindered root growth; and stage 4: fast root growth) based on a quantitative TEM analysis [Fig. 3(10)]. They noted that a denser and thicker initial SEI was also conducive to an increase in stress, as the SEI patches tended to connect together and completely passivate the electron-donating surfaces, accelerating the root growth mode-dominated lithium deposition. Once the breakdown of the initial SEI occurs in stages 1–2, a hole forms, similar to the “fumarole” of a volcano, and the kinetics facilitate the direct deposition of lithium metal at the root, thus causing fast lithium deposition at the fumarole.
-
(e)
Sand’s time model. The initiation time of lithium dendrites in dilute solutions is usually defined as Sand’s time (\({\tau }_{\text{S}}\)). In Sand’s early studies, when a cell was polarized with a binary electrolyte at a high current density, the ionic concentration near the anode tended toward zero at Sand’s time (\({\tau }_{\text{S}}\)) [39]. Rosso et al. [38] have related Sand’s time to parameters in cells. The Sand’s time varies as follows:
$${\tau }_{\text{S}}=\uppi D{\left(\frac{{Z}_{\text{c}}e{C}_{\text{c}0}}{2J}\right)}^{2}{\left(\frac{{\mu }_{\text{a}}+{\mu }_{\text{c}}}{{\mu }_{\text{a}}}\right)}^{2}$$(2)where \({\mu }_{\text{a}}\) and \({\mu }_{\text{c}}\) represent the anionic and cationic mobility, respectively; \(e\) is the elementary charge; \(D\) is the ambipolar diffusion constant, \(D=\left({\mu }_{\text{a}}{D}_{\text{c}}+{\mu }_{\text{c}}{D}_{\text{a}}\right)/\left({\mu }_{\text{a}}+{\mu }_{\text{c}}\right)\); \({D}_{\text{a}}\) and \({D}_{\text{c}}\) are the anionic and cationic diffusion constants, respectively; \({C}_{\text{c}0}\) represents the initial cationic concentration; \(J\) is the current density; and \({Z}_{\text{c}}\) is the cationic charge number. Sand’s time model shows better predictability under a larger current density.
1.1.2 Strategies for Suppressing Lithium Dendrite Growth
Due to their inherent advantages, extensive investigations in the past few decades have been devoted to solving the problem of “how to suppress the growth of lithium dendrites” for the sake of lithium anode recovery. In this study, we have briefly summarized dendrite suppression strategies into three categories.
-
(i)
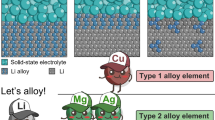
Li alloy structures. The adoption of alloying structures, such as LiAl, LiB, LiSi, LiSn and LiC [40,41,42,43], constrains the deposition and exfoliation behavior of lithium ions, thus not only reducing lithium metal side reactions but also effectively suppressing the generation of lithium dendrites [46]. Combined with nanostructure manipulation, the surface geometry configuration and charge state of lithium metal anodes are regulated and strengthened to promote an even better distribution and diffusion of lithium ions [47].
-
(ii)
Reformulating electrolytes. By changing the formula of lithium salts [40, 48], organic solvents [49, 50], and electrolyte additives [51,52,53], the interface can be modulated to some degree to form a component-favorable and electrochemistry-stable SEI layer [54,55,56], thus stabilizing the deposition and exfoliation behavior of lithium ions. One problem that should be carefully addressed is that once the additives are fully consumed, the cells will expire. Therefore, developing electrolytes to achieve an in situ self-healable SEI is critical for extending the long-term operational lifespan of LMBs.
-
(iii)
Implementing SSEs for LMBs. Taking advantage of the high Young’s modulus [57, 58], the growth and penetration of lithium dendrites can be theoretically suppressed in SSEs [59, 60]. However, recent investigations have suggested that the growth of dendrites can also occur in SSE LMB systems. Therefore, clarifying the dendrite growth mechanism, regulating the electrolyte composition and structure, and improving the ionic conductivity at room temperature and at solid-solid contacts are urgent problems that need to be solved.
1.2 Lithium Dendrite Growth Mechanisms in Solid-State LMBs
Although extensive efforts are still underway to fully understand the fundamental mechanism of lithium dendrite formation in solid electrolytes (SEs), we are attempting to provide a panoramic survey of the key parameters that determine solid-state dendrite growth (Fig. 4). Because of these inherent differences, dendrite growth in organic electrolytes is not entirely analogous to that in inorganic SEs; therefore, we discuss them separately. In solid polymer electrolytes (SPEs), the inherently low room-temperature ionic conductivity and poor mechanical properties are the main reasons for dendrite growth and penetration. Three principles to explain dendrite issues are summarized below [61].
-
(i)
Spiry or lateral dendrite growth. The electric field near the protruding spherical tip is the strongest on the electrode surface [62]. According to the constitutive model established by Monroe, lithium ions are inclined to deposit at the dendrite tip due to the effects of the surface energy and tip curvature [Fig. 3(3)] [17], and these dendrites in turn penetrate the polymer electrolyte and eventually lead to short circuits in the cell. A scheme ascribed to tip-induced dendrite growth was proposed by Dolle et al. in 2002 [Fig. 3(2)] [16]. As a supplement tip-induced dendrite growth, Dolle et al. [16] also reported that lithium dendrites can laterally propagate [Fig. 3(2)]. As deposition continues, the dendrites can even penetrate into the SEI layer and separate from both the electrode and the SE. As a result, delamination between the electrode and polymer electrolyte occurs due to the lateral growth of lithium dendrites. Poor electrolyte-electrode contact is considered the primary reason for such lateral growth of dendrites.
-
(ii)
Impurities cause uneven deposition. Inhomogeneities at local surfaces in SEs play an important role in the formation of dendritic lithium as well. The impurity particles in the electrolyte or at the interface could cause irregular Li deposition and the generation of voids. Harry et al. [19] noted that the particles are electronically insulating, preventing the deposition of lithium metal on top of these impurities. In turn, the SEI is interrupted at the edges of these impurities, leading to the deposition of lithium metal at the corners of the particles [Fig. 3(6a)]. These impurities deactivate local lithium deposition and thereafter generate voids at interface and induce inhomogeneous lithium deposition nearby [Fig. 3(6b)].
-
(iii)
Space charge layer. The potential difference between the electrolyte and electrode leads to the redistribution of lithium ions [63], and this space charge layer creates additional interfacial impedance [35]. Due to the low lithium ion transference number (\({t}_{{\text{Li}}^{+}}\)) of polymer electrolytes, anions in the electrolyte are consumed rapidly. These anions in dual-ion SPEs can move freely and then away from lithium electrodes, which leads to a large electric field and subsequently induces the growth of dendrites. In comparison, Cao et al. [25] prepared a solid-state single-ion polymer electrolyte with high ionic conductivity for dendrite-free LMBs [Fig. 3(12)]. Anions are immobilized in single-ion SPEs, effectively eliminating the electric field caused by charge redistribution and thus minimizing dendrite growth.

Reproduced with permission from Refs. [19,20,21, 25, 44, 45]. Copyright © 2015, IOP Publishing. Copyright © 2015, Elsevier. Copyright © 2018, American Chemical Society. Copyright © 2019, Elsevier. Copyright © 2022, John Wiley and Sons. Copyright © 2023, Springer Nature
Overview diagram of the factors influencing lithium metal dendrites in solid electrolytes.
Dendrite growth in inorganic SSEs is dominantly induced by undesirable wettability, unstable electrode/electrolyte interfaces, defects in grain boundaries and voids, and the unacceptable electronic conductivity of SSEs. The growth mechanisms of lithium dendrites in inorganic solid ceramic electrolytes can be summarized into four categories.
-
(i)
Poor interface wetting. Despite the promise of a high mechanical strength and a high Li+ ion transference number (close to 1), inorganic SEs suffer from poor wettability with lithium metal anodes, and point-to-point contacts are accompanied by distinct interface voids [Fig. 3(8)] [21, 64], which results in uneven Li+ ion flexibility and greatly disrupts the plating/stripping process, as well as the critical current density. Earlier studies [65, 66] have shown nonwetting behavior on a contaminated LLZO surface due to the presence of LiOH and Li2CO3, which results in inhomogeneous electrodeposition and the subsequent penetration of lithium metal into SEs.
-
(ii)
Grain boundary. Most inorganic ceramic SSEs are polycrystalline, and their grain boundaries cannot be ignored during Li+ ion transfer due to their significant ion transport resistance, low shear modulus, and different phase compositions [67]. A locally excessive Li+ ion resistivity and irregular interface shape at the grain boundaries hinder the migration of lithium ions and promote the growth of lithium dendrites [Fig. 3(8)] [21]. Manipulating the grain size of ceramic SEs is recognized as an efficient method to regulate the number of grain boundaries and mitigate interfacial resistance [68]. The grain boundary energy can be quantified using Eq. (12). One study [69] showed that more compact boundaries allow for fast diffusion, while higher-energy grain boundaries with less compact structures decrease the Li+ ion conductivity to a greater degree.
-
(iii)
High electronic conductivity. An ideal SSE should provide a high ionic conductivity and an extremely low electronic conductivity, fully ensuring the requirement of lithium ion permeability but electron inhibition. Driven by the electric potential, Li+ ions are reduced to metallic lithium only at the lithium-electrolyte interface [70]. However, the electronic conductivity of SSEs is excessively high, resulting in a strong local electric field. Li+ ions tend to be deposited in the presence of a strong electric field and are reduced to metallic lithium, thus forming lithium dendrites inside the SE [Fig. 3(9)] [22, 71].
-
(iv)
Interphase effect. Wendel et al. [20] distinguished three types of lithium metal/SE interface formations in detail [Fig. 3(7)]. In the first case [Fig. 3(7a)], a thermodynamically stable interface is formed, and the lithium metal and SE in contact are in thermodynamic equilibrium without any parasitic reaction. In the other two cases [Fig. 3(7b, c)], chemical reactions occur between the SE and lithium metal, and both materials form a thermodynamically unstable interface. In Fig. 3(7b), the final reaction products are mixed ionic and electronic conductors, allowing undesirable electron transport and finally resulting in battery self-discharging. In contrast to the case shown in Fig. 3(7c), a stable interphase is generated when the reaction products are ionically conducting but electronically insulating.
Based on the analysis of the growth mechanism of lithium dendrites in organic and inorganic SSEs, dendrite formation in SE can be suppressed by increasing the ionic conductivity, electrochemical stability window, \({t}_{{\text{Li}}^{+}}\), mechanical strength, interfacial contact and stability of SEs, further protecting the lithium anode and stabilizing the interface. Here, the ion conductivities and electrochemical windows of representative SEs for LMBs are thoroughly summarized in Fig. 5, which is helpful for obtaining an overall understanding of organic, inorganic, and hybrid SEs (more data are summarized in Tables 1, 3, 4, and 6, respectively). Specifically, Fig. 6 summarizes their ionic conductivities at room temperature, and the ionic conductivities of ISEs are generally higher than those of SPEs but are still far from those of liquid electrolytes. Figure 7 shows their electrochemical stability windows. Only if the electrochemical stability window is above the operating voltage range of the cathode material is it possible for the assembled full battery to function properly. Clearly, the low electrochemical window of ISEs cannot match that of some typical cathode materials commonly used today. We will discuss these issues in more detail in subsequent sections (Fig. 7).
2 Challenges Facing Organic SSEs
Polymers have been pioneered as SEs dating back to the 1970s and are still a class of active candidates for application in SSBs. SPEs conduct Li+ ions through segmental relaxation within noncrystallized regions and a free volume above the glass transition temperature (Tg). Subdiffusive motion, intersegmental hopping, and entire chain movements are three main Li+ ion diffusion pathways that contribute to the overall ionic conductivity. The major benefit of SPEs is the easily tangled interface solid-solid contact, which stems from the flexibility and processability of polymer chains. Fenton et al. [72] discovered the ability of poly(ethylene oxide) (PEO) to complex with alkali metal salts through oxygen atoms along the backbone. This discovery has provided an opportunity for PEO-based commercial batteries to achieve greater safety and a higher energy density practically in the absence of separators and liquid electrolytes, which are typically employed in conventional Li ion cells. More recent studies have shown that although polymer chain movement is necessary for ion migration, crystalline domains are also reported to be capable of ion conduction or are even better than those in amorphous regions. Instead of undergoing chain relaxation, polymers may fold into cylindrical tunnels that conduct Li+ ions through hopping routes while anions are fixed outside tunnels. Depending on their heteroatomic organic functional groups, SPEs can be categorized into polyethers, polyesters, polyamides, etc., and the structural formulas of their repeating units are shown in Fig. 8a–c. Additionally, lithium metal salts of different solubilities and stabilities affect the ionic conductivity of SPEs, and the structural formulas of common lithium salt anions are summarized in Fig. 8d. In addition, some polymers with specific functionalities are also a key direction of current research. Here, as shown in Fig. 9, we summarize the representative structures of reported single-ion polymers and self-healing polymers, which will also be discussed in detail in Sects. 2.1.1 and 2.3.1.
At present, widely studied polymer electrolytes include PEO [73], poly(acrylonitrile) (PAN) [74], poly(methyl methacrylate) (PMMA) [75], and poly(vinylidene fluoride) (PVDF) [76]. However, polymer electrolytes still suffer from well-known challenges, such as sluggish chain segment motion, low room-temperature ionic conductivity, insufficient stable potential windows and insufficient ionic conductivity (10−4 S cm−1), even under high-temperature conditions. With respect to LMBs, the mechanical properties of SPEs are greatly deteriorated at high temperature, which renders the protection of lithium metal anodes difficult. Moreover, the contact and stability of electrode-electrolyte interfaces must be improved. As a target, we have focused on studies on refining the ionic conductivity, mechanical properties, electrochemical performance, and thermal stability of organic SEs through polymer cross-linking, copolymerization, grafting, the formation of heterogeneous structures, and the preparation of self-healing and single-ion conducting polymer electrolytes. Table 1 provides a detailed summary of the ionic conductivity and electrochemical window of basic SPEs.
In PEO-based SPEs, at the polymeric chain level, Li+ ions are transferred from interchain diffusion, shift, or intrachain diffusion, and the latter contributes to the majority of Li+ ion conductivity [109]. Theoretical evidence suggests that ion transfer in polymers is intrinsically linked to polymer motion [110,111,112,113]. The diffusion coefficients can be calculated by evaluating the ions’ mean squared displacement of the corresponding particles [110, 113,114,115]:
where \({x}_{i}\left(t\right)\) is the instantaneous position of particle i at time \(t\), \({t}^{\prime}\) is the lag time, and < ··· > denotes an ensemble average over the particles. The diffusivity of the polymer is calculated by averaging the diffusivities of O, N, S, etc., atoms in the polymer chains. The ionic conductivity and transference number of the entire polymer electrolyte were calculated using the cluster Nernst-Einstein (cNE) approach to obtain values closer to the exact fully interactive approach [110, 113,114,115]:
where \({z}_{ij}\), \({\alpha }_{ij}\), and \({D}_{ij}\) are the charge number, population, and diffusion coefficient of a cluster composed of i cations and j anions, respectively. Finally, one can also define a transference number for the cation as follows [110, 113, 114]:
Based on the understanding above, Xie et al. [110] conducted an amorphous polymer electrolyte screen and accelerated the calculation by machine learning to reduce errors in molecular dynamics. They achieved relatively accurate predictions of the ionic conductivity and \({t}_{{\text{Li}}^{+}}\) of SPEs and screened up to 6 247 polymers (Fig. 10a–c). Finally, they extracted several design principles and provided an open dataset for the community. Similarly, France-Lanord et al. [113] constructed polymers by alternating ethylene oxide monomers and secondary sites (Fig. 10). The authors investigated the relationship between secondary sites and the ion transport properties of polymer electrolytes via theoretical calculations. Two different secondary sites were considered, including a carbonate group (CAR) and a sulfonyl group (SUL). The investigation suggested that the carbonate groups in PEO backbones can significantly restrain ion pairing due to the enhanced interaction with Li+ ions, while a sulfonyl secondary site improves the cation transference number due to its strong affinity with TFSI− (Fig. 10d–i).

Reproduced with permission from Ref. [110]. Copyright © 2022, Springer Nature. d–i Transport properties of the three investigated polymer electrolytes. Left column: chemical structures. Time evolution of the mean squared displacement for (d) Li+ and (e) TFSI− ions. (f) Conductivity results for the Nernst-Einstein (NE) and cluster NE (cNE) approximations. (g) Haven ratio. (h) Cation transference number as defined in both approximations. (i) Ionic diffusion coefficients. Reproduced with permission from Ref. [113]. Copyright © 2019, American Chemical Society
a–c Screening of polymer electrolytes. (a) Distribution of the conductivities of the top 50 polymers after each iteration, showing the quartiles of the conductivity distributions. (b) Predictions of 50 ns and 5 ns conductivities for 6 247 polymers in the search space. The green line denotes the top 50 conductivities from both predictions. (c) CPU hours that were actually used, required to screen the entire 6 247 search space, and required to screen the 53 362 candidate space.
2.1 Single-Ion Conducting Design
In most dual-ion conducting polymer electrolytes (DIC-PEs), both Li+ ions and anions move freely. Due to the Lewis coordination between Li+ ions and polymer chains, Li+ ions move much slower than anions, resulting in a low \({t}_{{\text{Li}}^{+}}\) (typically below 0.5) [116]. A lower \({t}_{\text{Li}^{+}}\) induces stronger concentration polarization, as a result of which Li+ ions are depleted at the surface of the lithium metal electrode and thereafter begin to deposit and grow in the form of dendrites.
Therefore, pursuing \({t}_{{\text{Li}}^{+}}\) values of up to unity can lessen such concentration polarization and suppress one route of dendrite growth, which is the initial target for developing single-ion conducting polymer electrolytes (SIC-PEs) [117]. In response, several strategies have been applied for anion immobilization: (1) chemically linking anions into the polymer backbone; (2) attaching anions to the inorganic backbone; and (3) introducing anion-trapping agents in DIC-PEs. SIC-PEs can be solid-state or gel-type polymers (containing large amounts of plasticizers). Li+ ions are mainly transported through the amorphous region via an interchain or intrachain hopping process in SIC-SSPEs [116]. Generally, because of their high \({t}_{{\text{Li}}^{+}}\), SIC-PEs homogenize the ion concentration distribution and indeed achieve uniform lithium deposition in the absence of lithium dendrites [118].
Various anion acceptors, such as carboxylates, sulfonates [118], tetrahedral borates [119, 120], bis(sulfonyl)imides [117, 121,122,123], other neutral anion acceptors or even a set of hydrogen bonds [124], have been utilized as anchoring groups through interactions between the anion acceptor and the target small anion to realize optimized SIC-PE configurations. Some representative structures of reported SIC-PEs with ionic conductivity at room temperature are summarized in Fig. 9a, and \({t}_{{\text{Li}}^{+}}\) is shown in Table 2.
-
(i)
Carbonate-based and sulfonate-based SIC-PEs have been developed due to their easy synthesis process and moderate negative charge distribution. However, one problem of sulfonate-based SIC-PEs is the strong attraction between Li+ ions and carboxylates or sulfonates, which results in a low concentration of free Li+ ions [117]. Recent investigations have suggested the incorporation of nitrogen atoms into sulfonate to reduce the anion-cation binding energy and thus favor ionic dissociation. For instance, by covalently attaching propylsulfonate motifs to nitrogen atoms in linear poly(ethyleneimine) (LPEI), Doyle et al. [118] generated PEG-grafted lithium solvating matrices and propylsulfonate-bearing linear poly(ethyleneimine) (LPEI) as single-ion lithium conductors, which promoted ion pair dissociation, stabilized the macromolecular mixture and succeeded in improving the dissociation of polymer-bound lithium salts (Fig. 11a). The prepared PEI-based SSPEs exhibited decent ionic conductivity (4 × 10−4 S cm−1) and showed no electrochemical degradation in the ± 5 V potential range.
-
(ii)
Tetrahedral borate-based lithium salts enable high ionic conductivity, facile preparation, thermal stability, cost effectiveness, environmental friendliness and favorable SE interfaces. Inspired by these advantages, various boron-based SIC-PEs have been carefully designed. Recently, Liu et al. [119] proposed a Li-containing boron-centered fluorinated single-ion conducting gel polymer electrolyte (LiBFSIE). In the LiBFSIE design, Li+ ions were anchored on the delocalized borate anion centers (Fig. 11b). Anion immobilization endows the polymer electrolyte with a high \({t}_{{\text{Li}}^{+}}\) (0.93).
-
(iii)
Bis(sulfonyl)imides are acknowledged as versatile anions in SIC-PEs due to their high degree of Li+ ion dissociation and stability. Meziane et al. [121] synthesized and studied two types of single-ion lithium poly(4-styrenesulfonyl(trifluoromethylsulfonyl)imide) (PSTFSI) associated with CF3–SO2–N− − SO2 anionic groups (Fig. 11c). When mixed with PEO, the polymers formed an electrolyte membrane to achieve improved Li+ ion solvation without any addition of extra lithium salts. The ionic conductivity of PSTFSI/PEO is approximately ten times higher than that of PEO. In these polymers, \({t}_{{\text{Li}}^{+}}\) was at unity, while the ionic conductivity was maintained at a high level. However, the presence of sulfonate groups is suggested to decrease the conductivity.
However, preparing SSPEs that can offer both mS cm–1-level ionic conductivity and high \({t}_{{\text{Li}}^{+}}\) (> 0.8) under ambient conditions remains arduous and is currently limited by (1) the strong Li–O− interactions between lithium salts and polymer backbones and (2) the low dissociation of lithium salts in polymer electrolytes. Therefore, methods to address this trade-off and achieve a balanced enhancement of the ionic conductivity and \({t}_{{\text{Li}}^{+}}\) for SSPEs are urgently needed. In response to this issue, Sun et al. [122] reported a polyamide-based single-ion gel-polymer electrolyte (LiPA) using bis(4-carboxy phenyl sulfonyl)imide and 2,4-diamino-benzene sulfonic acid precursors (Fig. 11d). The PVDF-LiPA membrane has an ionic conductivity of 3.8 × 10−4 S cm−1, which chiefly arises from channels provided by the bis(sulfonyl)imide anions in the polymer chains and moderate interfacial resistance. The high \({t}_{{\text{Li}}^{+}}\) of the membrane (0.88) significantly reduces the polarization potential of the battery, which enhances the electrochemical stability of the electrolyte membrane. Borzutzki et al. [123] studied a polysulfonylamide homopolymer tailored by a –C(CF3)2 functional group. An optimized gel-type polymer electrolyte membrane was produced as a 1:3 blend of PVDF-HFP and SIC polymers, which exhibited a high ionic conductivity of 5.2 × 10−4 S cm−1, corresponding \({t}_{{\text{Li}}^{+}}\) of 0.9, a 7Li self-diffusion coefficient of 4.6 × 10−11 m2 s−1 and an electrochemically stable voltage window up to 4.6 V vs. Li/Li+.

Reproduced with permission from Ref. [118]. Copyright © 2014, American Chemical Society. b Synthesis of the single-ion polymer electrolyte LiBFSIE. Reproduced with permission from Ref. [119]. Copyright © 2020, American Chemical Society. c Synthesis of PSTFSI. Reproduced with permission from Ref. [121] Copyright © 2011, Elsevier. d Schematic of the synthesis of EG-P[SSPSILi-alt-MA]. Reproduced with permission from Ref. [117]. Copyright © 2019, American Chemical Society. e Schematic illustration of anion immobilization by boron in 3D-BGPE. Reproduced with permission from Ref. [126]. Copyright © 2019, The Royal Society of Chemistry. f Chemical structure of the anion acceptor CBP. Reproduced with permission from Ref. [127]. Copyright © 2010, John Wiley and Sons
a Synthesis of homopolymers and the single-ion transport mechanism of PEI-based SSPEs.
-
(iv)
Another possible solution for partial anionic immobilization to improve \({t}_{{\text{Li}}^{+}}\) is to use neutral anion acceptors. Anion acceptors are mainly Lewis acids, which can react with counter anions via Lewis acid-base interactions and promote electron delocalization. Thus, they not only immobilize the counter anions but also enhance the dissociation of Li+ ions. Specifically, the anion acceptor may interact with anions via hydrogen bonds. Dai et al. [126] obtained a cross-linked SIC-PE (3D-BGPE) through the in situ polymerization of both linear and branched cross-linkers (Fig. 11e). Lewis acid boron esters in 3D-BGPE act as anion acceptors. The 3D-BGPE exhibited high ionic conductivity (8.4 × 10−4 S cm−1) and near-single-ion conduction (\({t}_{{\text{Li}}^{+}}\) of 0.76). Moreover, Stephan et al. [127] proposed a new anion-trapping component, calix[2]-p-benzo[4]pyrrole (CBP), with hydrogen bonds (Fig. 11f). The \({t}_{{\text{Li}}^{+}}\) increased from 0.23 to 0.78 upon the incorporation of CBP in the polymer electrolyte. Although the CBP did not play a significant role in the ionic conductivity, it improved the interfacial properties significantly.
2.2 Molecular Structure Modification
In response to most polymers that are highly crystalline and unavailable for Li+ ion transfer, methods based on copolymerization [94, 128,129,130], cross-linking [131,132,133], hyperbranching [84, 134], and adding ionic liquids [135,136,137] for molecular structure design are often reported to increase the room-temperature ionic conductivity of polymer electrolytes.
Quasisolid star brush block copolymer electrolytes (SBBCEs) were proposed by Gang et al. [128] and were designed with a BAB-repeating structure composed of a 2-arm star polymer of (poly[poly(ethylene glycol) methyl ether acrylate]b-polystyrene)2 [(PPEGMEA-b-PS)2] (Fig. 12a). CH3O-PEG-PC, which contains a carbonate terminal group that is highly dissociative to lithium salts, results in SBBCEs with ionic conductivities up to 2.1 × 10−4 S cm−1 at room temperature. Combining LiBF2C2O4 (LiODFB) as an additive, Fu et al. [131] designed ultrathin double-salt PEO-based gel polymer electrolytes (DPPEs) by introducing tetraethylene glycol dimethyl ether (TEGDME). By generating a cross-linked network under UV irradiation, the DPPEs showed a superior ionic conductivity of 0.57 mS cm−1 (30 °C) (Fig. 12b). Zhang et al. [84] designed a novel ion-dipole-reinforced polyether electrolyte (PHMP) through the cationic ring-opening polymerization (CROP) of 3-hydroxymethyl-3′-methyloxetane (HMO). The original PHMP was characterized by unique ion solvation cages composed of regionally condensed hyperbranched ether groups with enhanced chain segmental movements (Fig. 12c). First-principle simulations characterized seven representative ion solvation cage configurations, which were assembled with different ether group numbers (n = 3–5) and types (linear, binary, or ternary). The binding energy between the Li+ ions and ion solvation cages varied from − 4.47 to − 5.66 eV, and the Li+ ion transfer energy among the different ion solvation cages ranged from 0.1–0.4 eV. Therefore, such a hyperbranched structure restrained the dehydrogenation reaction of ether-based SEs, broadening the stable potential window to ~ 4.7 V. Therefore, such “ion-dipole” configurations not only enhanced salt dissociation and ion conduction but also restrained the proton-initiated decomposition process at high voltage, which synergistically achieved high ion conduction (1.26 × 10−4 S cm−1 at 25 °C) and high compatibility with high-voltage cathodes. Zhou et al. [135] proposed a novel cross-linked gel polymer electrolyte (GPE) consisting of an ionic liquid with a fluorinated alkyl side chain (F-IL), di-pentaerythritol penta-/hexa-acrylate (DPEPA) and poly(ethylene glycol) methacrylate (PEGMA) (Fig. 12d). PEGMA achieves high ionic conductivity through glycol chains; DPEPA is able to generate a highly cross-linked gel network through abundant acrylate functional groups. The side chain of F-IL can effectively immobilize the lithium salt anion and thus reduce the affinity of Li+ for oxygen atoms on the ethylene glycol chain and improve the electrochemical stability of cross-linked gel polymer electrolytes (CGPEs).

Reproduced with permission from Ref. [128]. Copyright © 2019, American Chemical Society. b Schematic diagram of DPPE chain interconnections. Reproduced with permission from Ref. [131]. Copyright © 2022, Elsevier. c Synthetic scheme of the PHMP solid electrolyte and uniform Li+ diffusion and deposition enabled by PHMP. Reproduced with permission from Ref. [84]. Copyright © 2021, John Wiley and Sons. d Schematic representation of the synthesis of gel polymer electrolytes containing ionic liquid end groups. Reproduced with permission from Ref. [135]. Copyright © 2021, John Wiley and Sons
a Schematic of the synthesis of SBBCEs.
2.3 Mechanical Strength
Grazioli et al. [138] quantitatively expressed the stress-strain relation of SPE, which is defined as:
where \({\varvec{u}}({\varvec{x}}, t)\) is the displacement vector. The stress tensor \({\varvec{\sigma}}\) is defined as the sum of its deviatoric component and pressure.
The constant G represents the bulk modulus, \(\mathbf 1\) represents the identity tensor, and \({\varvec{p}}\) is the pressure. The effects of the ionic concentration and electrostatic forces on the mechanical properties of the material are neglected in the above equation.
A fundamental understanding indicates that the mechanical properties of polymer electrolytes play critical roles in resisting dissolution and preventing punctures, thus increasing overall battery safety. The inferior mechanical strength of SPEs is among the primary causes of the growth and penetration of lithium dendrites. Chen et al. [107] synthesized a new microporous gel polymer electrolyte by blending superior rigid PVDF with PEO to enhance the mechanical strength of GPEs. The composite electrolyte presented a mechanical strength up to 30 MPa and favorable liquid absorption capacity with a room-temperature ionic conductivity of 1.96 × 10−3 S cm−1. Zhang et al. [102] prepared a flexible PEO-based SPE (PTT) via a UV-derived dual reaction. An SPE with a high ionic conductivity of 2.7 × 10−4 S cm−1 and enhanced mechanical strength was obtained. A study of Li//Li symmetrical cells revealed the suppression of dendrites with lithium and a stable voltage profile for over 400 h. Bouchet et al. [103] reported a new SIC-PE based on self-assembled polyanionic “BAB”-type triblock copolymers [P(STFSILi)-PEO-P(STFSILi)] with finely tuned mechanical properties, ionic conductivity and \({t}_{{\text{Li}}^{+}}\). This SIC-PE exhibited unprecedented performance for LMBs in terms of ionic conductivity (1.3 × 10−5 S cm−1 at 60 °C), \({t}_{{\text{Li}}^{+}}\) > 0.85 and improved mechanical strength (10 MPa at 40 °C). Kimura et al. [104] developed an ambient temperature-operating highly concentrated PEC-based composite polymer electrolyte (PI-PEC-LiFSI) by employing a “pore-filling” technique. The highly concentrated poly(ethylene carbonate) (PEC) electrolyte was filled into a three-dimensionally ordered microporous (3D-OM) polyimide (PI) matrix to support the electrolyte and ensure its mechanical stability so that the problem where PEC cannot independently self-support film formation was solved. The 3D porous structure and high concentration of PEC jointly promoted the transport of lithium ions inside the electrolyte, including a reasonable conductivity of 1.6 × 10−4 S cm−1 and a high Li transference number greater than 0.5, while ensuring its mechanical strength. Cyanoethyl polyvinyl alcohol (PVA-CN) is a polymer compound with a high dielectric constant and good adhesion. Using a nitrile material (SEN), Zhou et al. [105] prepared a hierarchical SSE through the in situ polymerization of PVA-CN in a succinonitrile (SN)-based SE. The crosslinked PVA-CN polymer framework strongly enhanced the mechanical strength of the composite electrolyte. The obtained SEN electrolyte exhibited excellent favorable mechanical strength (15.31 MPa).
2.4 Self-Healing Polymer Electrolytes (SHPEs)
The complex application scenario requires lithium batteries to possess a certain self-healing capability to address volume changes, interface instability or even breakage during utilization [139]. Self-healing materials usually self-repair through interfacial interactions such as covalent bonds (including disulfide bonds [139,140,141], esters [142], imines [143], Diels-Alder reactions [144], etc.) or noncovalent interactions (including hydrogen bonds [141, 145], host-guest interactions [146], metal-ligand interactions [147], etc.). Some representative structures of reported SHPEs are summarized in Fig. 9b. In recent years, research on self-healing electrolytes has received increasing interest. With the self-healing effect of electrolytes, the interfacial stability of the electrode-electrolyte can be effectively improved, and the growth of lithium dendrites arising from electrolyte damage can be prevented, thus realizing the protection of lithium metal anodes. Hence, SHPEs can improve the safety, reliability and cycling life of batteries [143].
Reversible dynamic covalent bonds have a higher bonding energy and thus better stability, which endows SHPEs with good mechanical strength [142]. Sun et al. [139] prepared a rigid-flexible self-healing polymer electrolyte (RFSPE) with a flexible-rigid epoxy resin as the backbone and disulfide bonds as reversible cross-linking points. Figure 13a depicts the self-healing mechanism of RFSPEs, where disulfide bonds, as crosslinking points, undergo an exchange reaction to heal damage. The epoxy resin offered superior mechanical abilities with tensile strength greater than 20 MPa, while the disulfide bonds provided excellent self-healing efficiency (> 95%). In particular, RFSPEs play an essential role in lithium dendrite suppression. Compared to that of the non-self-healing RFCPE, the surface of the lithium metal electrode with RFSPE as the electrolyte was smoother (Fig. 13b), suggesting a stabler interface and stronger inhibition of lithium dendrite growth. With the function of disulfide bonds, the broken molecular chains due to the destruction of the electrolyte by lithium dendrites were reconnected, and the cracks were healed (Fig. 13c). Zhou et al. [143] developed a self-healing polymer electrolyte (IBshPE) cross-linked by two types of synergetic dynamic bonds. Boroxine bonds with B-N coordination and imine bonds can synchronously allow for fast bond exchange reactions, supporting the fast self-healing response of IBshPE, as shown in Fig. 13d. IBshPE showed a high self-healing efficiency of 97% within 4 h and a favorable ionic conductivity of 5.08 × 10−3 S cm−1 at 30 °C. The IBshPE-modified LiFePO4/Li cells exhibited better cycling performance (98.6% capacity retention after 80 cycles) and an improved rate capability, with a specific capacity of 130.5 mAh g−1 at 2 C.

Reproduced with permission from Ref. [139]. Copyright © 2022, Elsevier. d Schematic diagram of the self-healing mechanism of IBshPE. Reproduced with permission from Ref. [143]. Copyright © 2022, Elsevier. e Schematic illustration of self-healing between the cut surfaces of the PP-PU. Reproduced with permission from Ref. [145]. Copyright © 2022, American Chemical Society
a Schematic illustration of the self-healing mechanism of RFSPE-3 and the bond exchange reaction of disulfide bonds. b SEM images of pristine Li metal, Li from Li/RFSPE-3/Li, and Li from Li/RFCPE-3/Li after lithium plating/stripping. c Interfacial self-healing process and lithium dendrite-suppressing mechanism of RFSPE-3.
One supramolecular system containing a quadruple hydrogen-bonded structure of a ureidopyrimidinone structure, the UPy system, has been widely used in the field of SHPEs [145]. Chen et al. [145] proposed a multifunctional gel polymer electrolyte with high ionic conductivity and self-healing ability by incorporating brush-like PEG chains with urea-pyrimidinone (PEG-UPy) into a PVDF-HFP matrix to form an inorganic cross-linked network. When the electrolyte film broke, hydrogen bonds formed between UPy units at the interface rejoined, allowing a return to its pre-damaged condition without external stimulation (Fig. 13e). The electrolyte exhibited a good self-healing capability at 25 °C. The scraping of electrolytes and the puncturing caused by lithium dendrite growth were eliminated during charging and discharging, thus enhancing the safety of LMBs.
2.5 Electrode-Electrolyte Interfacial Contact
A stable lithium metal-electrolyte interface is a prerequisite for long-term cycling of all-solid-state batteries and for suppressing dendrite growth. In situ self-polymerization of electrolytes or polymers is among the most effective approaches for obtaining a stable interface. Huang et al. [85] reported a poly(tetrahydrofuran) (PTHF)-based polymer electrolyte (PTSPE) using boron trifluoride diethyl ether (BF3·OEt2) through the in situ polymerization of THF in a LiFePO4/Li cell (Fig. 14a). The in situ polymerization technique markedly enhanced the contact features and interfacial stability. The in situ formed PTSPE exhibited an ionic conductivity of 2.3 × 10−4 S cm−1 and a 4.5 V electrochemically stable voltage window at 60 °C. The LiFePO4/Li SSBs obtained using this PTSPE showed stable cycling ability and delivered a high discharge capacity of 142.3 mAh g−1 after 100 cycles. Zheng et al. [108] designed a cross-linking gel copolymer electrolyte containing various alkyl acrylates, triethylene glycol dimethacrylate (TEGDMA), and a liquid electrolyte using in situ thermal polymerization, as depicted in Fig. 14b. The as-assembled LiFePO4/Li cell and graphite/Li cell employing this in situ formed GPE revealed that the prepared GPE had good compatibility with the battery cathode and anode materials. The flexibility and liquid absorption rate of the electrolyte were boosted by copolymerization, with a room-temperature ionic conductivity of 5 × 10−3 S cm−1. Moreover, ionic liquids, which act as nonflammable, nonvolatile electrolytes with high ionic conductivity, have been investigated for the preparation of SSEs by in situ polymerization. Zhou et al. [106] fabricated a nesting doll-like hierarchical poly(ionic liquid)-based SE (HPILSE) in situ (Fig. 14c), which provided an excellent ionic conductivity greater than 10−3 S cm−1.

Reproduced with permission from Ref. [85]. Copyright © 2019, Elsevier. b The synthesis of chemically cross-linked GPEs. Reproduced with permission from Ref. [108]. Copyright © 2014, Springer Nature. c Schematic illustration of the in situ synthesis route of nesting doll-like HPILSE. Reproduced with permission from Ref. [106]. Copyright © 2017, Elsevier
a Schematic diagram of the in situ polymerization process of the PTHF electrolyte.
Due to the substantial difference in the electrochemical environments of the cathode and anode, achieving simultaneous stability for both the cathode and anode using a single-component solid-state polymer electrolyte is practically infeasible. For example, the PEO SSE interfaces favorably with the lithium metal anode, but the relatively narrow electrochemical window (3.9 V) renders it unavailable to match with the high-voltage cathode. Constructing a double-layered heterogeneous structure is an ideal treatment for the high-voltage intolerance of PEO. Zhou et al. [78] combined two different functional polymers to constitute a double-layer polymer electrolyte. The PEO-LiTFSI layer was in contact with only the lithium metal anode to prevent dendrite growth, and the poly(N-methyl-malonic amide) (PMA)-LiTFSI layer was used to contact only the cathode to stabilize the cathode interface. A dendrite-free Li-metal anode interface and a stable polymer/oxide cathode interface were produced with 100 charge/discharge cycles at 4 V and 65 °C for an as-assembled all-solid-state LiCoO2/Li cell. The oxidation potential, mechanical properties, Li+ ion conductivity, thickness of the electrolyte membrane, and interpolymer compatibility are identified as the critical considerations for advancing double-layer organic composite SSEs.
3 Challenges Facing Inorganic SSEs
Inorganic SSEs are typically oxides, sulfides, halides, hydrides, and nitrides [148]. In terms of crystallinity, inorganic SSEs are segmented into crystalline and amorphous structures. The dominant crystalline structures include the lithium superionic conductor (LISICON) structure (Li1−xZn1−xGeO4) and its sulfide (thio-LISICON) (Li4−xGe1−xPxS4) [149], sodium superionic conductor (NASICON) structure [Li1−xMxTi2−x(PO4)3, M = Al, Y, In, …] [150], garnet structure [Li7La3Zr2O12 (LLZO)] [151], Li6.4La3Zr1.4Ta0.6O12 (LLZTO) [152], perovskite structure [La2/3−xLi3xTiO3 (LLTO)] [153, 154], anti-perovskite structure (Li3OX, X = Cl, Br) [155], layer-structured hydrides [Li2(NH), Li(BH4)] [156], AlCl3-type halogen compounds (Li3MCl6, M = Mg, Mn, Fe, Cd) [157], and argyrodite structure (Li6PS5X, X = Cl, Br, I) [158, 159]. The amorphous structures predominantly consist of sulfide glasses (Li2S-P2S5 [160] and Li2S-SiS2 [161]) and nitrogenous compounds (Li3N [162] and LiPON [163]). Inorganic SEs suffer from solid–solid contact, dendrite growth, and excessive grain boundaries. Investigators aim to ameliorate these problems by modulating the microstructure (compactness, grain boundaries, grain size, etc.) and enhancing the interfacial contact. The electrochemical properties of some inorganic SSEs that have been studied are shown in Table 3. In this review, we propose that interfacial and dendrite problems, such as interfacial stability, interface contact/wettability and interfacial Li stripping/plating processes, are the main drawbacks of inorganic SSEs in achieving high-performance cells. This section reviews the drawbacks and dominant solutions of inorganic SSEs in practical investigations.
3.1 Interfacial Chemical/Electrochemical Stability
High interfacial impedance severely hinders the transport of Li+ ions and leads to poor performance of all-solid-state lithium batteries (ASSLMBs). The main reasons for the high interfacial impedance of the cell originate from the decomposition of the SE and the formation of the interfacial phase. The narrow electrochemical window of the electrolyte may lead to redox reactions at the electrolyte/electrode interface when lower or higher voltages are exerted on the SE. The poor chemical stability between the electrolyte and electrode can also cause interfacial side reactions and changes in the chemical structure at the interface. Therefore, the interfacial stability remains decisive for the development of high-energy and high-power density ASSLMBs. Among inorganic SEs, the interfacial stability of sulfide SSEs is particularly problematic. In this section, we attribute the problem of interfacial stability of inorganic SSEs (ISSEs) to two aspects: (1) the electrochemical stability of the ISSE itself and (2) the side reactivity with the lithium metal.
During the operation of LIBs, the low voltage of the anode drives mobile cations into the electrolyte and vice versa for the cathode, inducing the depletion or accumulation of charge carriers (Fig. 15a) [164]. The migration of internally mobile species will inevitably cause a change in the electrochemical potential. The evolution of the electrochemical potential leads to a concentration gradient of the mobile cation, which directly triggers the decomposition of the SE and/or reaction with the electrodes [164]. The applied voltage can be directly converted to a lithium chemical potential \({\mu }_{\text{Li}}\) using Eq. (8) [165], where \({\mu }_{\text{Li}}^{0}\) is the lithium chemical potential in lithium metal and e is the elementary charge.

Reproduced with permission from Ref. [164]. Copyright © 2019, Springer Nature. b Experimental setup of the in situ XPS experiment to monitor the reaction between sulfide and Li. c XPS spectra recorded during deposition of 31 nm Li metal on Li10GeP2S12. Detailed S 2p, Ge 3d, and P 2p/Ge 3p spectra are shown for different deposition states. d S 2p, Ge 3d, and P 2p XPS spectra and model fits for the pristine LGPS sample and the after deposition of 31 nm Li metal. Reproduced with permission from Ref. [169]. Copyright © 2016, American Chemical Society. e Contour plots of operando 7Li NMR spectra and their corresponding charge/discharge curves for LGPS, LPS, LSiPSCl, and LPSCl. Reproduced with permission from Ref. [172]. Copyright © 2023, Springer Nature
a Evolution of the chemical potential across the solid electrolyte in contact with an anode and a cathode.
Energy-dense batteries necessarily have anodes and cathodes with very different \({\mu }_{\text{Li}}\) values. We must consider subjecting the electrolyte to these extreme lithium potentials without other parasitic reactions [166]. The electrochemically stable voltage window is the voltage range that an electrolyte can sustain without redox decomposition. The intrinsic electrochemical stability of most SEs is often lower than that reported. In particular, for some sulfide electrolytes, their low electrochemical stability windows limit further progress [167]. Zhu et al. [168] investigated the electrochemical window and decomposition products of several common inorganic SE materials (Table 4). The electrochemical window for most sulfide electrolytes is very narrow, approximately 1.7–2.1 V, which means that they are extremely susceptible to decomposition during battery operation. Sulfide materials are generally not thermodynamically stable when in contact with lithium anodes, whereas the reduction of most sulfide materials starts at 1.6–1.7 V in the presence of Li+/Li, and even LLZO starts to be reduced at 0.05 V. Wenzel et al. [169] used in situ XPS and electrochemical measurements to observe chemical reactions at the Li/LGPS interface. They confirmed that LGPS decomposed into Li2S, Li3P and Ge metal or Li-Ge alloy when in contact with Li metal (Fig. 15b–d). Similar phenomena were discovered for Li7P3S11 and Li6PS5X (X = Cl, Br, or I) [170, 171]. The authors confirmed that Li7P3S11 decomposed into Li2S and Li3P, and Li6PS5X decomposed into Li2S, Li3P, and LiX. Liang et al. [172] proposed operando in situ NMR measurements for the real-time quantification and evolution tracking of inactive lithium formed in solid-state LMBs with four different sulfide-based SEs, namely, Li10GeP2S12 (LGPS), Li9.54Si1.74P1.44S11.7Cl0.3 (LSiPSCl), Li6PS5Cl (LPSCl) and Li7P3S11 (LPS). They revealed the evolution of dead Li formation and the amount of the Li-containing SEI component via operando NMR spectroscopy. Figure 15e shows the contour plots of operando 7Li NMR spectra and corresponding charge/discharge curves of four sulfide-based SEs. The study indicated that LGPS is prone to react with deposited lithium metals immediately, converting all active lithium into SEI-Li, the formation of which is severer than that of dead Li in LSiPSCl.
Building a protective layer at the Li-electrolyte interface is the most well-recognized method to inhibit side reactions and stabilize the interface [173,174,175]. Ye et al. [176] took advantage of the contraction susceptibility of the lithiation reaction of micron-sized Si at the interface to add a SiG (silicon, graphite, and polytetrafluoroethylene composite) protective layer on the lithium metal anode, and the assembled Li|SiG| Li5.5PS4.5Cl1.5 (LPSCl1.5)-Li10SnP2S12 (LSnPS)-Li5.5PS4.5Cl1.5 (LPSCl1.5)|NMC83 solid-state cell exhibited an 80% capacity retention after 2 000 cycles at a high current density.
In addition, experimental and simulation results indicated that the balance of lithiophobicity, electronic and ionic conductivity, and interlayer porosity are the key factors for the stable deposition/stripping of lithium at high capacity. Therefore, Wang et al. [177] designed a porous lithiophobic ion/electron-conducting L7N2I-CNT interlayer and an electron-conducting gradient L7N2I-Mg interlayer between a Li6PS5Cl (LPSC) electrolyte and a Li metal anode. This configuration enables Li to be deposited on the Li/interlayer interface and reversibly permeate into the porous interlayer. The configured Li4SiO4@NCM811|LPSC|Li full cell maintains 82.4% of its capacity after 350 cycles at 60 °C.
3.2 Interfacial Contact/Wettability
The point-to-point contact between the inorganic SSE particles and the lithium metal anode radically leads to high interfacial resistance, which is highly predisposed to an uneven distribution of Li+ ion flux and provides a gap for lithium dendrite growth. Various coating materials, such as alloying reaction coatings (Au, Si, Al, Ge, Ag, etc.), inactive coatings (ZnO, Al2O3, SiO2, SnO2, SnNx, and Li-C composite), a polymeric interlayer and artificial SEI layer for lithium anode [173, 202,203,204,205,206], have been developed for the electrode/ISSE interface to improve the electrochemical performance of ASSLMBs, to suppress the side reactions, and to stabilize the electrode/o interface.
On the anodic side of lithium metal, alloy coatings such as Au [64] and Ge [207] and inactive coatings such as ZnO [208], Al2O3 [209], and Li-C composites [202] are commonly used as buffer coatings to enhance interfacial contact. For example, based on the Au-Li alloying reaction, Tsai et al. [64] sputtered a Au buffer layer on a polished Al-contaminated Ta-substituted Li7La3Zr2O12 (LLZ:Ta) electrolyte surface. The use of a Au buffer layer improved the contact between LLZ:Ta and the lithium electrode and promoted the homogenization of Li+ ions at the interface, which efficiently prevented lithium dendrite growth (Fig. 16a). The interface resistance decreased dramatically (from 3 000 to 380 Ω cm2). Xu et al. [208] designed a novel all-in-one porous-dense-porous trilayer garnet SSE for lithium-sulfur batteries (Fig. 16b). Both the lithium anode and the sulfur cathode were infiltrated into the porous garnet framework, and a ZnO surface treatment was introduced to achieve seamless contact between the electrolyte and lithium metal anode. The all-in-one battery design provided continuous pathways for Li+ ions and electrons that led to a lower resistance, which effectively inhibited lithium polysulfide shuttling, lithium dendrite penetration, and volume expansion. Han et al. [209] also solved the problem of large interfacial impedance between lithium metal anodes and garnet electrolytes via atomic layer deposition (ALD) with ultrathin Al2O3 to effectively improve the wettability and stability of the interface between the garnet SSE and lithium metal (Fig. 16c), which significantly reduced the interfacial impedance from 1 710 to 1 Ω cm2.

Reproduced with permission from Ref. [64]. Copyright © 2016, American Chemical Society. b Schematic diagram of the structure and working principle of an all-in-one solid-state Li-S battery based on a trilayer garnet electrolyte. Reproduced with permission from Ref. [208]. Copyright © 2018, Elsevier. c Schematic diagram of the wetting behavior of a garnet surface with molten Li and SEM images of the garnet solid-state electrolyte/Li metal interface. Reproduced with permission from Ref. [209]. Copyright © 2016, Springer Nature. d Schematic diagram of casting lithium-graphite (Li-C) composites on garnet SSEs. The Li-C composite can spread well on garnet like a paste and provide intimate contact. Reproduced with permission from Ref. [202]. Copyright © 2019, John Wiley and Sons. e Schematic diagram of Li deposition behavior using an LLZTO solid-state electrolyte and an LLZTO-2wt%Li3OCl composite solid-state electrolyte. Reproduced with permission from Ref. [179]. Copyright © 2018, Elsevier. f Schematic illustration of the preparation and role of the PPF40 interlayer. Reproduced with permission from Ref. [216]. Copyright © 2023, John Wiley and Sons. g Schematic diagram of lithium deposition behavior in a cell. Reproduced with permission from Ref. [214]. Copyright © 2021, John Wiley and Sons. h Schematic illustration of dendrite growth in an unmodified Li anode and smooth lithium deposition in a modified LNA-Li anode. Reproduced with permission from Ref. [217]. Copyright © 2023, American Chemical Society. i Schematic representation of the pretreatment process for the formation of a LiF-rich SEI layer between Li metal and LPS solid-state electrolytes. Reproduced with permission from Ref. [218]. Copyright © 2018, American Association for the Advancement of Science
a Schematic diagram of the lithium ion transport process and impedance spectra of Li/LLZ:Ta/Li cells with and without Au buffer layers.
Moreover, temperature also considerably affects the interfacial contact properties of lithium metal. Studies have shown that the interface formed between molten lithium metal and SSE exhibits a rather low interfacial resistance [210]. However, the chemical stability between the SSE and molten lithium requires special attention, as some SSEs will react violently with molten lithium metal at high temperature [211]. Duan et al. [202] achieved an intimate garnet interface by casting a lithium-graphite (Li-C) composite onto a garnet-type SSE. Like a paste, Li-C composites with lower fluidity and higher viscosity can be cast onto garnet and exhibit intimate contact (Fig. 16d). They also found that the Li-C composite and garnet showed a minor interfacial reaction with a small reaction energy, delivering a decreased interfacial resistance from 381 to 11 Ω cm2. The origin of the large interfacial resistance of the garnet electrolyte is intimately related to the Li+-ion-insulating Li2CO3 surface layer on the garnet surface [212]. Li et al. [212] effectively removed the impurity Li2CO3 in the garnet electrolyte Li6.5La3Zr1.5Ta0.5O12 (LLZTO) by reacting the garnet electrolyte with carbon, which enhanced the interfacial wettability.
A similar approach was implemented in garnet electrolytes by Tian et al. [179]. As shown in Fig. 16e, Li3OCl, which has an excellent affinity for lithium metals in the voids and boundaries of Li6.75La3Zr1.75Ta0.25O12 (LLZTO) particles, was introduced using the melting-quenching method. By acting as a binder, amorphous Li3OCl promoted the formation of a stable and dense interfacial layer and continuous ionic conductive network among LLZTO particles, improving surface wetting not only with lithium metal but also among LLZTO particles. The investigation indicated that the interfacial resistance between the composite electrolyte and lithium metal decreased from 1 850 to 90 Ω cm−2.
The current density at which Li penetrates an SSE is often referred to as the critical current density (CCD). Interfacial contact and the electrolyte microstructure (pores, grain boundaries, etc.) are the main factors affecting the CCD of SSE [213]. The CCD of the SSE can be critical for its ability to inhibit the growth of lithium dendrites. Charging batteries at current densities above a critical value can result in lithium-filled cracks, commonly referred to as “dendrites”. Figure 17 and Table 5 summarize representative SEs and the corresponding CCDs, including both organic and inorganic SEs [214, 215]. The CCDs used in most of the studies (\(\leqslant\) 1 mA cm−2, most of which were \(\leqslant\) 0.5 mA cm−2) were far below the practically acceptable values (\(\geqslant\) 3 mA cm−2) [215].
At Li-SSE interfaces with poor electrical contacts or discontinuities, electrochemical currents wind around defects or voids, leading to an increase in the local current density near the edges of defects/voids. If the current density in the defects is sufficiently high, the SSE will rupture due to pressure accumulation at the defects, which further leads to the propagation of Li filaments and the formation of Li dendrites. Therefore, the surface modification of lithium metal or SSE is one of the key methods to improve the CCD of SSE.
Polymers provide favorable safety, chemical stability, and flexibility, and thus using polymers as an anode interface buffer layer may greatly enhance contact. Zheng et al. [216] designed an extensible elastic Li+-conducting polymer interlayer, poly(ethylene glycol methyl ether acrylate) (PEGMEMA), with a poly(vinylidene fluoride)-hexafluoropropylene (PVDF-HFP) elastomeric skeleton formed with perfluoropolyether (PFPE) additives for the lithium/garnet interface (Fig. 16f). The elastic network builds a continuous Li+ transport path, and the good viscoelasticity of the middle layer buffers the change in the lithium volume and results in tight interfacial contact. The PFPE additive is used to promote the formation of LiF-containing SEIs. Due to the specific structural and compositional integration, this strategy endows the lithium symmetric battery with a high CCD (3.6 mA cm−2) and an excellent cycle life (stable for more than 400 h at a current density of 1.0 mA cm−2). Peng et al. [214] combined a liquid lithium metal cathode (Li-Bp-DME), a sulfide SSE (Li7P3S11) and an interfacial protective layer (PEO), resulting in the highest CCD (17.78 mA cm−2) currently available for sulfide solid electrolyte-based batteries (Fig. 16g).
LiF is one of the key components for stabilizing the SEI layer and is the preferred choice for lithium anode protection. Cheng et al. [217] fabricated an artificial SEI layer (consisting of LiF and nano-Ag) through a substitution reaction between AgF and Li (Fig. 16h). On the one hand, LiF can guide the horizontal uniform deposition of Li due to its high surface energy; on the other hand, nano-Ag enables alloying with Li, which compensates for the electronic insulating property and low ionic conductivity of LiF, resulting in uniform and dense lithium deposition. The symmetric cell can be stably cycled for 600 h at 10 mA cm−2 with low and constant voltage hysteresis. Fan et al. [218] infiltrated a drop of highly concentrated 6 mol L−1 LiFSI dimethoxyethane (DME) (~ 20 mL) between the Li metal anode and the SSE of Li3PS4 and then dried it under vacuum overnight at 120 °C to evaporate the DME solvent. A LiF-rich SEI layer was constructed between Li3PS4 and Li metal (Fig. 16i), which successfully suppressed the formation of lithium dendrites and could increase the CCD of Li3PS4 to more than 2 mA cm−2.
Additives in liquid electrolyte systems can serve to improve the SEI film composition and alleviate the generation of dendrites [219,220,221]. SEI components such as LiF, LiI and Li3N are considered to play such important roles. Similarly, this principle has been applied to LMBs. Han et al. [222] incorporated LiI as an additive into the Li2S-P2S5 SSE, which has high ionic conductivity but electronic insulation in the SEI while increasing the lithium ion transference number, promoting the homogeneity of lithium deposition at the interface, and increasing the electrochemical stability of sulfide electrolytes (Fig. 17).
3.3 Microstructure Modulation
Granular inorganic SEs inevitably feature defects, such as inhomogeneities, porosity, cracks, grain boundaries, and impurity precipitation, which are all nucleation sites for dendrite precipitation. An ideal single-crystal SSE is the fundamental remedy for the grain boundary issue. More recent studies have shown that dendrites can penetrate through stiff SSE membranes along grain boundary networks, resulting in unexpected cell failure [69, 210, 243, 244]. This surprising result suggests that microstructural features should be rationally regulated in the design of practical SEs.
Kataoka et al. [232] successfully grew a centimeter-sized single-crystal garnet-type SE Li6.5La3Zr1.5Nb0.5O12 (LLZNb05) without grain boundaries and pores using the floating zone method. Figure 18a shows an optical image of an LLZNb05 single-crystal plate polished on the surface and an SEM image. From the SEM image, only polishing scratches were observed on the crystal surface, and voids or grain boundaries were not observed. The single-crystal SE featured an extremely high lithium ion conductivity of 1.39 × 10−3 S cm−1 at room temperature and a favorable CCD of 0.5 mA cm−2. However, the costly fabrication and complicated synthesis techniques are key issues that hinder the further development and application of single crystals at this stage. The tendency of single-crystal SEs to simplify the synthesis processes and constitute innovative SE interface structures has been demonstrated.

Reproduced with permission from Ref. [232]. Copyright © 2016, Springer Nature. b X-ray tomographic reconstructions of the void phase in the interior of LLZO electrolytes and the changes in pore size distribution between the pristine and failed electrolytes sintered at 1 050, 1 100, and 1 150 °C. Reproduced with permission from Ref. [245]. Copyright © 2018, American Chemical Society. c Proposed mechanism for pore formation in Li metal anodes. Reproduced with permission from Ref. [246]. Copyright © 2018, Elsevier. d, e Schematic of the synthesis process of Li6PS5Cl. Reproduced with permission from Ref. [238]. Copyright © 2020, American Chemical Society
a Optical and SEM images of a polished LLZNb05 single-crystal plate.
In polycrystalline SSEs, the porosity exerts significant effects on various properties, such as morphology, ionic conductivity, and mechanical strength. When Li+ ions form dendrites at the lithium-electrolyte interface, the pore size and distribution substantially contribute to the expansion of lithium dendrites inside the electrolyte. Using synchrotron X-ray tomography, Shen et al. [245] explored the relationship between the porosity and the critical current density (CCD) of the garnet-type material Li7La3Zr2O12 (LLZO) at different temperatures. As illustrated in Fig. 18b, morphological transformations were observed via X-ray tomography. They found that the connectivity of the pore region increases while the porosity decreases with increasing sintering temperature. SEs with interconnected pores were prone to short circuits at lower critical current densities. As a result, as the sintering temperature increased from 1 050 to 1 150 °C, despite the decrease in porosity, the connectivity among the pores increased significantly, which accelerated the growth of lithium dendrites and diminished the CCD.
When lithium metal is used in liquid battery systems, the effect of the microstructure and defects may be minimal due to the fluidity. However, when it is matched with a rigid SSE, dislocations within the lithium metal, as well as metal microstructures and intrinsic defects therein, can form pores during lithium stripping, leading to unstable, high-impedance interfaces. Because strain regions exhibit higher free energy, the strain field around dislocations often influences electrochemical behavior by (1) decreasing the charge transfer resistance, (2) increasing the local transport kinetics, and (3) thermodynamically favoring stripping (Fig. 18c) [246]. These factors promote localized stripping of regions with dislocations on the original lattice region, which tends to accelerate the formation of voids at the dislocations. As voids grow during stripping, they become annihilation sites for dislocations, leading to roughening of the pore surface [247].
The main challenge with ISSE for the CCD is poor electrochemical or mechanical stability, which leads to increased interfacial resistance during cycling. This property typically contributes to the formation of an unstable interface between the ISSE and the Li metal, causing the Li dendrites to form when the current density increases. When the current density exceeds the CCD during battery cycling, voids begin to appear at the Li-electrolyte interface, resulting in an increase in the local current density until the dendrite formation threshold is reached, leading to a short circuit. Thus, the external stack pressure ensures better interfacial contact and prevents the formation of voids during lithium stripping, which greatly reduces interfacial issues and increases the CCD. Wang et al. [248] measured an increase in the CCD with increasing cell stack pressure. In addition, a flat potential response was recorded at higher cell stack pressures, indicating that the total cell resistance was negligible, while a potential ramp-up was observed at lower cell stack pressures.
Wang et al. [238] successfully suppressed the growth of lithium dendrites in a Li6PS5Cl solid electrolyte by improving the surface flatness and densification of Li6PS5Cl electrolyte sheets, which in turn increased the CCD. They obtained nanorod-shaped Li6PS5Cl solid electrolytes by meticulously tuning the sintering time, which eliminated the pores between Li6PS5Cl particles during the densification and sintering process, while the grain boundaries were filled with amorphous phases to improve the contact between the particles (Fig. 18d), which further inhibited the growth of lithium dendrites. The room-temperature CCD of the prepared densified Li6PS5Cl electrolyte reached 1.05 mA cm−2.
3.4 Lithium Infiltration Mechanism in ISSEs
In Sect. 1.2, we discussed in detail the possible mechanisms of lithium dendrite growth in SPEs and ISSEs. Previous studies have shown that dendrite growth in an SPE would be inhibited if the SPE had a sufficiently high shear modulus. Unlike mechanical puncturing in SPEs, the penetration and propagation of lithium filaments in ISSEs is a complex process involving multiple disciplines [249]. As researchers’ awareness and research techniques continue to advance, grain boundaries (GBs), random pores, and cracks are considered the main pathways for lithium penetration in ISSEs. A typical example is cubic Li7La3Zr2O12 (LLZO). Lithium dendrites penetrate LLZO along the GBs once cycling exceeds the CCD. The GBs of approximately half of the LLZO possess a reduced bandgap of approximately 1–3 eV, which makes it a potential conduit for leakage currents (Fig. 19a–e) [250]. As a result, instead of combining with electrons at the anode, Li+ ions are prematurely reduced by electrons at grain boundaries, forming localized lithium filaments. These lithium filaments are interconnected through the GBs, leading to a short circuit. Therefore, optimizing the electronic conductivity at GBs must be a primary consideration in future SSB designs.

Reproduced with permission from Ref. [250]. Copyright © 2021, Springer Nature. f − h In situ phase-contrast XCT virtual cross-sections during single plating of a Li/Li6PS5Cl/Li cell and analysis of lithium deposition in the cracks showing that cracks propagate ahead of Li. Reproduced with permission from Ref. [253]. Copyright © 2021, Springer Nature
a − e Differences in the atomic and electronic structures at the grain bulk and GBs of pristine LLZO.
Biao et al. [251] injected LiAlO2 (LAO) with a large energy band gap (4.6 eV) into the GBs of LLZO and doped F atoms in the GBs of LLZO (LAO-LLZOF) to construct a continuous intergranular phase with high ionic conductivity (7.69 × 10−4 S cm−1) and low electronic conductivity (1.27 × 10−8 S cm−1), effectively suppressing the infiltration of lithium metal in LLZO. The assembled all-solid-state LiFePO4/LAO-LLZOF/Li battery was stably cycled for 5 500 cycles under 3 C conditions. Jia et al. [252] proposed the “detour and buffer” strategy. They designed and prepared an SSE with a bimodal distribution of grain sizes by adopting a particle grading method in which fine grains with an average size of approximately 5 µm surrounded coarse grains (average size of 50–60 µm). The driving force for lithium penetration is continuously consumed by the high-density fine GBs and finely distributed pores; at the same time, the large grains can effectively increase the tortuosity of the lithium dendritic growth paths, which can effectively inhibit and delay the failure of the SSE.
Li6PS5Cl, a sulfur-based SSE, not only has a high electrical conductivity but also does not have a continuous formation of growing interfacial phases accompanied by volume changes. However, the entry of lithium into the plated surface leads to the formation of scattered cracks (pits) adjacent to the interface with the plated electrode. This crack formation occurs due to the uneven stresses generated by lithium deposition. Due to the higher localized electric field and current density, cracks first form at the edge of the lithium electrode and then gradually form transverse cracks that propagate through the electrolyte. Further studies showed that the cracks propagated through the electrolyte before the lithium dendrites, rather than the lithium metal driving the crack tip forward (Fig. 19f–h) [253]. Therefore, we suggest that more attention should be focused on stopping dry crack propagation to prevent dendrite propagation, e.g., through ceramic toughening and crack blocking.
Wang et al. developed a multiphase field model to elucidate the failure mechanism of ASSLMBs [254]. This model reveals the role of voids leading to cracks and dendrites. The growth of voids is a result of the stripping of different diffusivities in the SSE surface layer and lithium blocks. Due to the presence of voids, Li+ ions are unevenly distributed on the SSE surface near the voids during the charging process, leading to nonuniform stresses, which ultimately induce SSE cracks and Li penetration. However, a high stacking pressure cannot inhibit cracking and Li infiltration; in contrast, appropriate lateral compressive stresses can prevent SSE cracking and thus inhibit lithium dendrite generation.
Generally, lithium infiltration in SSEs is a complex process involving multiple disciplines. The experimental results also suggest that the growth and penetration of lithium dendrites may be influenced by multiple mechanisms rather than a single mechanism. Despite the various theories proposed in the literature, researchers still do not have a widely accepted explanation for how the low yield strength of lithium metal leads to the penetration of dendritic grains into ISSEs with high fracture toughness. The main mechanism of lithium penetration into ISSEs remains ambiguous, as it is more complicated than that in polymer electrolytes due to the presence of GBs, random voids, and cracks in ISSEs, and the various mechanisms are not always absolute because they do not exist independently of each other. In conclusion, more in-depth research and an understanding of lithium permeation mechanisms in ISSEs are needed before practical and scalable strategies can be developed.
3.5 Computational Methodology Predictions
Computational methods enable the efficient screening of high-performance SSEs by predicting the desired system with specific properties, such as interfacial stability. However, conventional synthesis methods require extensive experimental work while wasting raw materials. Therefore, computationally assisted interfacial property studies and computationally directed SE discovery are considered promising strategies for the rapid screening and optimization of high-performance SSEs [255].
3.5.1 Surface Energy
Several procedures have been proposed to suppress lithium dendrite formation and growth in inorganic SEs. However, we still note that the lithium deposited at interlayers or lithium metal/solid-electrolyte interface does not readily remain defect-free. Volume change-induced defects form inside the interlayer in terms of cracks or pores. Thus, ensuring that the defect surfaces are robust enough to prevent metallic lithium nucleation inside the interlayer is also important. The surface energy values are computed to distinguish which surface is energetically stabler. The surface energy values of various slab structures of organic SEs are determined according to Eq. (9) [22]:
where \({E}_{\text{slab}}\) is the total energy of the slab, A is the cross-sectional area, \({n}_{\text{formula}}\) represents the integer number of stoichiometric formula units, \({\mu }_{\text{bulk}}\) is the energy of one formula unit of the corresponding bulk structure, \({n}_{i}\) is the number of atoms of type i in the slab in excess of the stoichiometric amount, and \({\mu }_{i}\) is the chemical potential of element i. The DFT research of Tian et al. [22] on the calculation of Li7La3Zr2O12 (LLZO) suggested that the La-Li cotermination and Li termination have the lowest energy for the (110) and (001) surfaces, respectively. Furthermore, the calculated surface energy of Li2PO2N (0.46 J m−2) showed that it is a stable surface structure and that an interlayer of Li2PO2N at the Li/cubic phase LLZO (c-LLZO) interface would be efficient and defect-tolerant to suppress Li dendrite formation, while a tetragonal LLZO (t-LLZO) interlayer would not.
3.5.2 Interfacial Wettability
From Sect. 3.2.1, we understand that surface coatings contribute to reducing the Li/electrolyte interfacial impedance and enhancing the interfacial wettability. However, these views on interfacial wettability are largely limited to empirical observations and understanding, and a quantitative evaluation of lithium wettability as a function of SSE surface chemistry is currently lacking. In this regard, the relationships among interfacial chemistry, lithium wettability, and facile charge transport were quantitatively investigated by Sharafi et al. [65]. The interfacial work of adhesion (\({W}_{\text{ad}}\)) of the Li/electrolyte interface is evaluated as follows:
where \({E}_{\text{int}}\) refers to the energy of the interface cell and \({E}_{x{\text-}\text{slab}}\) is the energy of an isolated lithium or electrolyte slab. The contact angle \(\theta\) for Li/electrolyte interfaces is calculated by combining the Young-Dupré Eq. (11) with DFT calculations of \({W}_{\text{ad}}\) and the surface energy of lithium (\({\sigma }_{\text{Li}}=0.45\text{ J }{\text{m}}^{-2}\)).
Through the above equations, Sharafi et al. [65] predicted the wetting angle for the Li-Li2CO3 interface and Li-LLZO interface. Combining the experimental results, the presence of common LLZO surface contaminants, Li2CO3 and LiOH, led to poor wettability and high interfacial impedance. The removal of LLZO surface contamination effectively enhanced the Li wetting of LLZO.
3.5.3 Grain Boundary Energy
The properties of materials are strongly influenced by interfaces between individual crystallites and grain boundaries [69]. In the case of ISSEs, grain boundary-related phenomena have been used in the transport of Li+ ions and short circuiting [69, 210, 243, 244]. Grain boundary resistance can decrease Li+ ion conductivity, which can be partially alleviated by optimizing synthesis conditions to increase porosity, increase the grain size, and/or improve the contact at the grain boundaries. Moreover, lithium dendrites can penetrate stiff ISSE membranes along grain boundary networks, leading to the cell short circuits. Understanding the relationship between the grain boundary structure and ion transport is important for accelerating the development of SEs.
Predicting the energetics, composition, and transport properties of grain boundaries with the aid of a certain computational methodology bridges the knowledge gap of ISSEs in this area. The energy of the grain boundaries of ISSEs can be evaluated using Eq. (12) [69]:
where \({E}_{\text{GB}}\) is the total energy of the grain boundary-containing cell, n is the number of formula units in that grain boundary-containing cell, \({E}_{\text{Bulk}}\) is the total energy per formula unit of the bulk electrolyte, and A is the area of the grain boundary plane. Lithium ion transport is generally reduced in the grain boundary region. However, Li+ ion diffusion is enhanced in grain boundaries at high temperatures but is still slower than bulk diffusion. The study also showed that more compact boundaries allow for faster diffusion, while higher-energy grain boundaries with less compact structures penalize Li+ ion conductivity to a greater degree [69].
4 Composite SSEs
Currently, composite SSEs (CSSEs) are a hotspot in the field of SSBs. Composite SSEs are derived from mixing inorganic fillers into a polymer matrix in the form of nanoparticles, nanofibers and 3D skeletons or adding polymers into an inorganic electrolyte matrix by infiltration, mechanical mixing, etc. The purpose of complementing the advantages of inorganic and organic SSEs is achieved through diverse inorganic-organic composite forms so that the composite SSEs present high ionic conductivity, ideal electrochemical stability, favorable mechanical properties and good contact with the electrode interface.
In 1983, Hashin [256] had already analyzed composite materials in detail. The following properties of various types of composite materials were considered: elasticity, thermal expansion, moisture swelling, viscoelasticity, conductivity, and static strength. A multiphase approach was applied to estimate the effective conductivity [257]. Several analytical formulations for the prediction of composite conductivity are widely available [258]. The well-known formulations in this regard are the brick-layer model, Maxwell theory, and McLachlan’s generalized EMT (GEMT). Several representative computational models proposed by Kalnaus et al. [257] are summarized in Table 6. The reported ionic conductivities and electrochemical windows of some organic-inorganic composite SEs are summarized in Table 7.
4.1 Mechanical Strength of the SEI
The SEI formed on the lithium anode is mechanically unstable under large interfacial fluctuations and morphological changes because it will undergo repeated interfacial displacements during lithium electrochemical stripping and plating, risking the formation of cracks or even fracture. Fresh lithium will be exposed to the electrolyte and continuously react with the electrolyte, leading to electrolyte depletion, the loss of active lithium, and ultimately battery failure. Therefore, the mechanical strength of the SEI membrane is critical for the electrochemical performance of the LMBs.
Liu et al. [281] built an electro-chemo-mechanical model to understand the correlation between the mechanical properties of an artificial SEI and lithium deposition. As shown in Figs. 20k and 18l, a significant stress concentration is observed due to the low Young’s modulus (0.5 and 0.1 GPa) in the neck region of the teeth-like deposited lithium. This property induces the breakdown of the deposited lithium layer, which is a significant risk of safety in LMBs. With increasing mechanical strength (1.0–4.0 GPa), the morphology of the deposited lithium changes to bulb-like, with the region of stress concentration shifting to the top corners of the pillars (Fig. 20m–o). For SEIs with high mechanical strength (> 4.0 GPa), a uniform stress distribution induces much evener lithium electrodeposition. However, if the SEI is very rigid (\(\geqslant\) 5.0 GPa), the overall rate of lithium growth will decrease, thus resulting in less efficient lithium plating. Therefore, SEIs with a moderate Young’s modulus of approximately 4.0 GPa can effectively mitigate stress concentrations by suppressing SEI breakdown and promoting uniform electrodeposition.

Reproduced with permission from Ref. [281]. Copyright © 2022, John Wiley and Sons. p–w Cryo-EM image revealing an emergent SEI nanostructure formed at elevated temperature. Reproduced with permission from Ref. [282]. Copyright © 2019, Springer Nature
a–o Morphological evolution and distribution of physical fields on electrodeposited Li covered by an SEI with different mechanical strengths.
The mechanical properties of SEIs and their growth characteristics are also temperature dependent. Wang et al. [282] observed by cryo-electron microscopy that the size of lithium metal particles deposited at high temperature (60 °C) is larger than that of those deposited at low temperature (20 °C) (Fig. 20p, t), contributing to the decreased overpotentials and faster charge transfer. Similarly, because of the increase in reaction kinetics induced at elevated temperature, the SEI layer becomes thicker (Fig. 20q, u). In addition, a low temperature induces an amorphous polymeric SEI, which is fragile and easy to dissolve, provides limited passivation and leads to the mechanical instability of the interface due to continuous SEI formation (Fig. 20r, s). A high temperature generates an ordered layered SEI (Li2O) at 60 °C (Fig. 20v, w), which stabilizes the anode with sustainably high mechanical properties, thus suppressing later-cycle SEI formation.
For decades, researchers have typically addressed the poor mechanical properties of SEIs via two approaches. The conventional strategy is to replace electrolyte-derived SEIs with in situ fabricated protective layers (e.g., inorganic salts, lithium alloys, and polymers). However, these layers may breakdown during cycling and fail to reorganize, thus triggering lithium-electrolyte reactions on the exposed lithium surface. Another approach is to design new electrolytes (e.g., concentrated electrolytes, ionic liquids, and fluorinated electrolytes) or to use sacrificial additives in conventional electrolytes. The formed SEI can reduce lithium consumption but still continues to consume the electrolyte [283]. Therefore, a method for rationally designing stabilized SEIs using functional SEI precursors rather than electrolytes is needed to address the problem of unstable SEIs. To this end, an organic-inorganic composite SEI layer for stable LMBs was designed by Gao et al. [283]. They used a reactive polymer composite (RPC) as the precursor. The SEI mainly consisted of polymeric lithium salts embedded with LiF nanoparticles and GO nanosheets. This layered composite provided excellent SEI stability and effective electrolyte cycling retention. The polymer-nanoparticle composite was dense and showed good passivation properties, which ensured the formation of a homogeneous SEI. GO nanosheets imparted the mechanical strength of the SEI and helped prevent lithium dendrite growth. The NCM523|Li cell had a 77.1% capacity retention after 600 cycles due to the incorporation of the RPC-derived SEI.
4.2 Inorganic-in-Organic Composite
4.2.1 Inert Inorganic Fillers
A large number of inert inorganic fillers, including SiO2, TiO2, Al2O3, hydrophobic clay and other mesoporous particles, have been developed [59, 284]. Inorganic nanoparticles promote the dissociation of lithium salts via Lewis acid-base interactions. Moreover, inorganic components act as cross-linking sites to reduce the crystallinity of the polymers and enhance the motion of polymer chain segments. They can increase the ionic conductivity of polymer electrolytes by 1–2 orders of magnitude, as well as the thermal stability and mechanical properties. The distribution uniformity and particle size of the inorganic fillers directly affect the ionic conductivity of composite SEs. At this stage, the agglomeration of ceramic particle fillers and weak polymer-ceramic interactions hinder the improvement of ionic conductivity. Lin et al. [260] reported an in situ synthesis of ceramic filler particles inside a polymer electrolyte, which is different from the conventional blending method. Monodisperse ultrafine SiO2 (MUSiO2) nanospheres with 12 nm in diameter were produced inside PEO polymers via in situ hydrolysis of tetraethyl orthosilicate (TEOS) to form a composite polymer electrolyte (PEO-MUSiO2CPE). As illustrated in Fig. 21a, the MUSiO2 nanospheres and PEO chains were firmly connected to each other through an in situ hydrolysis reaction, resulting in strong chemical bonding and mechanical entanglement. On the one hand, the crystallization of PEO was inhibited, and thus, the polymer segmental motion for ionic conduction was promoted. On the other hand, the dissociation degree of LiClO4 and the ionic conductivity increased (1.2 × 10−3 S cm−1 at 60 °C and 4.4 × 10−5 S cm−1 at 30 °C) with an electrochemical window up to 5.5 V. The as-assembled all-solid-state LiFePO4/CPE/Li cells did not exhibit any short circuit due to lithium dendrite growth after 80 cycles, indicating satisfactory lithium dendrite suppression. The in situ composite approach provides a new approach to address the challenges of ceramic filler agglomeration and weak polymer-ceramic interactions.

Reproduced with permission from Ref. [260]. Copyright © 2016, American Chemical Society. b Schematic diagram of the synthetic procedures for the SiO2-aerogel-reinforced CPE. Reproduced with permission from Ref. [96]. Copyright © 2018, John Wiley and Sons. c Schematic illustration of the synthesis of ceramic nanowire-filled polymer-based composite electrolytes, together with a comparison of possible lithium ion conduction pathways in nanowire-filled and nanoparticle-filled composite electrolytes, and an illustration of the electrode configuration for AC impedance spectroscopy measurements. Reproduced with permission from Ref. [272]. Copyright © 2015, American Chemical Society. d Schematic illustration of the Li ion conduction pathways of nanoparticles, random nanowires and aligned nanowires in composite polymer electrolytes. Reproduced with permission from Ref. [273]. Copyright © 2017, American Chemical Society. e Schematic diagram of the 3D continuous framework formed by the prepercolated LLTO network. Reproduced with permission from Ref. [267]. Copyright © 2018, John Wiley and Sons. f Conductivity of composites with porous particles. g, h Conductivity of composites containing dense particles. Reproduced with permission from Ref. [285]. Copyright © 2022, Springer Nature
a Schematic diagram of the procedure for in situ hydrolysis and interaction mechanisms among PEO chains and MUSiO2.
Lin et al. [96] designed and prepared a composite polymer electrolyte (CPE) containing a three-dimensional continuous skeleton by introducing a stiff mesoporous SiO2 aerogel as the backbone for a polymer-based electrolyte to further enhance the ionic conductivity and mechanical properties of composite SEs (Fig. 21b). The interconnected SiO2 aerogel not only functioned as a strong backbone to enhance the mechanical strength but also provided large and continuous surfaces for strong anion adsorption, which promoted more pronounced Lewis acid-base interactions with anions and thus increased the ionic conductivity. As a consequence of these merits, a SiO2-aerogel-reinforced CPE with a high elastic modulus of 0.43 GPa and an unexceptionable ionic conductivity of 6 × 10−3 S cm−1 at 30 °C was achieved. In addition, it contributed to a notable dendrite suppression effect at prolonged cycles. Full cells with LFP cathodes exhibited stable cycling performance and improved rate capability. Apparently, compounding a quantity of inert inorganic fillers in organic SEs decreases the crystallinity of the polymers, increases the chain segment motility, and ultimately enhances the ionic conductivity. In addition, it also plays a positive role in improving the mechanical strength. However, since inert inorganic fillers contribute little to ionic conductivity, the amounts of inert components incorporated need to be carefully optimized to significantly influence the overall ionic conductivity.
4.2.2 Active Inorganic Fillers
The active inorganic particles include lithium superionic conductor (LISICON) structures, sodium superionic conductor (NASICON) structures, garnet structures, perovskite structures, etc. The addition of active inorganic particles is beneficial for promoting the dissociation of lithium salts and reducing the crystallinity of polymer SSEs while providing a pathway for Li+ ion transport. However, when the amount of active inorganic particles is relatively low, polymer electrolytes still play a major role in composite SSEs, while active inorganic particles play a role similar to that of inert inorganic particles. Therefore, how to fully transport ions by increasing the active inorganic particle ratio has emerged as a research focus to further enhance the ionic conductivity and mechanical strength properties of composite electrolytes. The incorporation of inorganic particles can effectively reduce the Tg and crystallinity of polymer electrolytes, which greatly increases the ionic conductivity of SSEs. However, when the proportion of inorganic particles in the composite electrolyte is exceedingly high (> 20%), the inorganic particles in the polymer matrix will inevitably agglomerate, and the agglomeration and uneven distribution of inorganic particles will lead to a uniform current distribution inside the electrolyte [266]. Therefore, eliminating the agglomeration of inorganic particles in the polymer matrix through general mechanical mixing is difficult.
A wide range of investigations have been implemented to address the agglomeration of inorganic particles. Employing ceramic nanofibers as fillers is an effective tactic to address the problem of ceramic particle agglomeration and enable ceramic fillers to form ion channels. Liu et al. [272] fabricated fibrous Li0.33La0.557TiO3 (LLTO) nanowires by electrospinning and dispersed them into a polyacrylonitrile (PAN)-LiClO4 polymer. In Fig. 21c, the schematic illustration shows the procedure for the synthesis of LLTO nanowire-filled PAN-LiClO4 composite electrolytes. An unprecedented conductivity of 2.4 × 10−4 S cm−1 at room temperature with an electrochemical stability up to 5.0 V was achieved for the composite electrolyte containing 15% weight content of LLTO nanowires. The inorganic ceramic nanowires were dispersed into the PAN matrix by electrospinning to form a three-dimensional linked conductive network, which diminished the activation energy of lithium ion transport in the electrolyte and facilitated lithium ion transport, thus substantially increasing the ionic conductivity of the composite electrolyte and suppressing the formation of lithium dendrites. When ceramic nanowires are irregularly arranged in the polymer matrix, crossover coincidences between the nanowires are unavoidable. LLTO nanowires well aligned along the normal direction of the electrode were incorporated into the PAN polymer electrolyte by Liu et al. to investigate the effect of the direction of the nanowire arrangement on the ionic conductivity and ion transfer number of the composite electrolytes [273]. The composite polymer electrolyte with well-aligned LLTO nanowires exhibited an ionic conductivity that was one order of magnitude higher than that of polymer electrolytes with randomly aligned nanowires, which was attributed to a fast ion-conducting pathway without crossing junctions on the surfaces of the aligned nanowires. As shown in Fig. 21d, the surface region of the inorganic nanoparticles (NPs) and nanowires (NWs) served as a highway for lithium ion conduction, and the nanowires with a large aspect ratio provided a long and fast pathway for lithium ion transport. The interactions between nanowires and polymer chain segments stimulated the local relaxation and chain segment motion of the polymers, which increased the lithium ion transference number from 0.27 to 0.42. The calculations revealed that the surface ionic conductivity of nanowires in polymer electrolytes (6.05 × 10−5 S cm−1 at 30 °C) was comparable to that of liquid electrolytes. The use of nanowires enhances the long-term stability of polymer electrolytes. The exploration of the nanowire arrangement direction doped in polymers provides fresh insights into the structural design of composite SSEs. Bae et al. [267] described a three-dimensional nanostructured hydrogel-derived prepercolated LLTO framework for high-performance composite electrolytes. Compared with conventional nanofiller dispersion systems, this artificial 3D percolated network effectively avoided the agglomeration of nanofiller particles. The prepercolated LLTO network can provide a continuous interphase that serves as a pathway for Li ion conduction and delivers a 3D interconnected structure (Fig. 21e). When the LLTO framework was compounded with a PEO-LiTFSI polymer electrolyte, an enhanced lithium ion conductivity close to 10−4 S cm−1 was achieved at room temperature. The relatively low proportions of polymers in this composite electrolyte assisted in enhancing the electrochemical stability, thermal stability and battery safety. This novel methodology for constructing three-dimensional nanohydrogel percolated structures provides an alternative approach for preparing high-performance composite polymer electrolytes.
In fact, the factors governing the conductivity of dispersed inorganic particles in conductive organic matrices are not clear; therefore, a physical description of effective conductivity is highly desirable [285]. Isaac et al. [285] used a model system for composite electrolytes in which ceramic electrolytes (CEs) were dispersed in liquid electrolytes (LEs) to measure the effect of ceramic addition on conductivity. By introducing an effective porous particle conductivity (\({\sigma }_{\text{p},\text{ eff}}\)), the aggregated grains and internal porosity are considered effective media, resulting in a modified Maxwell Eq. (13) for porous particles with a porosity \({\varepsilon }_{\text{p}}\):
where \({\sigma }_{\text{eff}}\), \({\sigma }^{0}\), and \({\sigma }_{\text{p}}\) are the effective conductivity of the mixture, the conductivity of the continuous phase (namely, the LE or SPE), and the conductivity of the dispersed CE phase, respectively; \({\varepsilon }_{\text{LE}}\) is the volume fraction of the continuous LE domain; and \({\varepsilon }_{\text{ext}}\) is the external porosity.
The model predicted that adding porous particles to a viscoelastic electrolyte would lead to a systematic decrease in conductivity (Fig. 21f). In contrast, the addition of dense ceramic particles resulted in increased conductivity, as long as the conductivity of the ceramic electrolyte was higher than that of the LE (Fig. 21g,h).
4.3 Organic-in-Inorganic and Multilayer Heterogeneous Structures
The enhancement of polymer SSEs in terms of low ionic conductivity and poor mechanical strength can be accomplished by integrating inorganic fillers into the polymer matrix. From one-dimensional nanoparticles to two-dimensional nanofibers and to three-dimensional skeletons, the proportion of inorganic phases in composite electrolytes has gradually increased. However, the rigidity of the composite electrolytes gradually increases with increasing inorganic component content, decreasing the interfacial contact between the electrode and the electrolyte. The excess use of inorganic components results in particulate agglomerates and precipitates, which drastically damages the overall performance of the composite electrolyte. In addition, ion transport through dispersed inorganic fillers is inefficient due to the high interparticle contact resistance and insufficient particle-particle contact area. Therefore, the effective utilization of the high ionic conductivity of inorganic SEs through conventional methods of fabricating discrete particle composites is difficult.
A common strategy for overcoming these problems is to fabricate interconnected inorganic networks with significantly reduced interparticle resistance. Fu et al. [286] filled LiTFSI-PEO polymer into a porous 3D ceramic (Li6.4La3Zr2Al0.2O12, LLZO) network to form 3D garnet-polymer composite membranes. Unlike conventional polymer-based composite electrolyte preparation methods, 3D garnet-polymer composite membranes do not require mechanical mixing of the filler with the polymer. Instead, the prefabricated 3D ceramic structure can be directly immersed in a lithium salt-polymer solution to obtain the desired composite electrolyte heterostructure (Fig. 22a), thus simplifying the fabrication process and avoiding the agglomeration of fillers. This composite structure further provided structural enhancement to improve the mechanical properties of the polymer matrix. The ionic conductivity of the composite electrolyte was 2.5 × 10−4 S cm−1 at room temperature. Second, the composite electrolyte can effectively block dendrites in symmetric lithium batteries with a current density of 0.2 mA cm−2 for approximately 500 h and those with a current density of 0.5 mA cm−2 for more than 300 h. Palmer et al. [185] loaded a crosslinked PEO polymer into a 3D interconnected structure of LATP with a dense backbone (Fig. 22b). The composite exhibited a ceramic loading up to 77% in weight percentage (61% in volume percentage) and an ionic conductivity of 3.5 × 10−5 S cm−1 at 20 °C. The main transport pathway for ions passed through the ceramic network. Due to the interconnected structure of the ceramics, the composite electrolyte exhibited greatly improved mechanical strength (equibiaxial strength of 19.5 MPa). In fact, in most of the above composite structures, the thin excess polymer layer on the surface of the composite electrolyte is always present either as an indirect consequence of the permeation process or as a deliberate design.

Reproduced with permission from Ref. [286]. Copyright © 2022, Proceedings of the National Academy of Sciences. b Schematic illustration of the procedure for fabricating the composite electrolyte film. Reproduced with permission from Ref. [185]. Copyright © 2020, Elsevier. c Schematic diagram of an all-solid-state Li-PEO(LiTFSI)/LAGP-PEO(LiTFSI)/LiMn0.8Fe0.2PO4 cell. Reproduced with permission from Ref. [268]. Copyright © 2017, American Chemical Society. d Illustration of the all-solid-state battery design with the PCPSE electrolyte and the structure of the polymer CPMEA. Reproduced with permission from Ref. [87]. Copyright © 2016, American Chemical Society. e Schematic diagram of a sulfide-based all-solid-state LMB and a schematic diagram of a sulfide-based all-solid-state LMB with a PCE interlayer. Reproduced with permission from Ref. [287]. Copyright © 2019, John Wiley and Sons
a Schematic diagram of the hybrid solid-state composite electrolyte, where ceramic garnet nanofibers function as reinforcements and lithium-ion-conducting polymers function as the matrix. The interwelded garnet nanofiber network provides a continuous ion-conducting pathway in the electrolyte membrane.
Another effective approach is to design multilayer heterogeneous structures. In this regard, polymeric protective layers are the commonest. On the one hand, the organic layer on the external side can ameliorate the poor contact between the inorganic SE and the electrode, protecting the inorganic SE and preventing the lithium dendrites from growing along the grain boundaries of the inorganic SE. On the other hand, the lithium ions are homogeneously distributed on the surface of the composite electrolyte to suppress lithium dendrite nucleation. The internal inorganic layer provides mechanical strength to the composite electrolyte on the one hand and enhances the ionic conductivity on the other hand. Wang et al. [268] established an LAGP-PEO composite SE and a PEO (LiTFSI)-modified lithium metal anode for all-solid-state lithium batteries, employing different SEs in contact with the cathode and anode, respectively, to suppress lithium dendrite formation and protect the lithium anode. As depicted in Fig. 22c, the PEO electrolyte buffer layer was in contact with the lithium metal anode, which improved the interfacial contact between the electrolyte and lithium anode and prevented direct contact between LAGP and lithium metal, inhibiting the onset of side reactions. The LAGP-PEO (LiTFSI) electrolyte came in contact with the cathode, and the use of LAGP decreased the PEO crystallinity and increased the PEO decomposition potential while diminishing the activation energy of lithium ion migration and increasing the ionic conductivity. PEO remained stable even at a high potential of 5.12 V (vs. Li/Li+). The fabricated composite SSE suppressed lithium dendrite growth, and the as-assembled Li-PEO (LiTFSI)/LAGP-PEO/LiMn0.8Fe0.2PO4 all-solid-state cell exhibited an initial discharge capacity of 160.8 mAh g−1. Similarly, Zhou et al. [87] synthesized a cross-linked polymer/ceramic membrane/polymer sandwich electrolyte (PCPSE) for an all-solid-state LMB (Fig. 22d). Cross-linked poly(ethylene glycol) methyl ether acrylate (CPMEA) was employed as a ceramic layer and a ceramic membrane of NASICON Li1.3Al0.3Ti1.7(PO4)3 (LATP) with high lithium ion conductivity and chemical stability in air was used as the ceramic layer. The ceramic layer immobilized the anions in the polymer salt, which facilitated lithium ion migration and decreased the number of positive charges transferred to the polymer interface and the interfacial electric field. This property improved the stability of the polymer electrolyte and weakened the lithium-polymer interfacial electric double layer, thus suppressing the nucleation of lithium dendrites and the decomposition of the polymer electrolyte. The polymer layers were shown to be wetted by a metallic lithium anode such that the migration resistance of lithium ions decreased and the efficiency of lithium ion plating/stripping increased, which homogenized the ion distribution and unified the lithium deposition. With the PCPSE, the capacity retention of all-solid-state Li/LiFePO4 cells was still approximately 102 mAh g−1 at 0.6 C after 640 cycles.
Sulfide SE is considered the most promising SE at present because of its superior ionic conductivity. However, the use of lithium metal anodes in sulfide-based all-solid-state LMBs has rarely been successful. The main challenges are the interfacial reaction between Li and the sulfide electrolyte and the formation of Li dendrites. Wang et al. [287] engineered a solid-state plastic crystal electrolyte (PCE) as an interlayer in sulfide-based all-solid-state LMBs. The PCE interlayer can prevent interfacial reactions and Li dendrite formation between the sulfide SE and Li metal (Fig. 22e). As a result, the assembled Li|LiFePO4 cell exhibited a high initial capacity of 148 mAh g−1 at 0.1 C and 131 mAh g−1 at 0.5 C (1 C = 170 mA g−1), which remained at 122 mAh g−1 after 120 cycles at 0.5 C.
Although the studies above did, to some degree, enhance the interfacial compatibility of SSEs, the underlying mechanisms still require further exploration. For example, Chen et al. [288] used a poly(propylene carbonate) (PPC)-based polymer electrolyte as an initiator to generate an ultrastable SEI between the LPSC and the Li anode. They noted the potential sustained-release effects of the PPC during the cycling process: (1) the PPC serves as a convenient coating that avoids direct contact between the Li anode and the SSE; (2) propylene carbonate (PC), produced by the gradual degradation of the PPC, swells the coating, which comes into full contact with the Li anode and forms a uniform lithium deposition; and (3) the slow decomposition of solid PPC into liquid PC not only significantly reduces the interfacial resistance but also facilitates the formation of a stable LiF-enriched SEI with the participation of solvated LiTFSI and polysulfide species. They also identified the pincer mechanism (bilateral bond coupling mechanism) of LixSiOy coatings between Li and LPSC interfaces. A strong Li/artificial SEI/LPSC structure is formed by binding the Li anode via Li − S and Li − O − Si bonds and the LPSC via Si − S − P bonds [289].
Profound investigations into the mechanisms of interfacial action have led to in-depth insights into interfacial enhancement and the underlying mechanisms, which has prompted interfacial modification to no longer be limited to superficial properties. Proposals such as the sustained-release effect and the pincer effect provide new ideas for future research on interfacial enhancement, which will be helpful for people to delve into the mechanism and learn from the past. Additionally, while embedding inorganic SEs into organic polymers combines the advantages of high conductivity and flexibility, SSEs must be thin with excellent mechanical properties and the ability to form a low-resistance interface with the electrode material to achieve all-solid-state batteries (ASSBs) with a high energy density [290]. In general, ultrathin SSEs typically have a lower impedance, shorter ion migration paths, and more efficient ion mobility [86, 290, 291]. However, designing and fabricating ultrathin SSEs with excellent electrochemical properties, mechanical properties, and stability is still a major challenge.
A large number of studies have focused on the electrolyte structure design to solve these problems. The special structure of the electrolyte not only optimizes the Li+ transport path but also helps to solve the interfacial issues of ASSLMB, which is vital for improving the energy density of the battery. Zhang et al. [291] designed a trilayer ultrathin asymmetric electrolyte for stabilizing the anode and cathode interfaces. The middle layer of the ultrathin PE diaphragm serves as a flexible support, and LLZO and metal-organic framework (MOF) layers are coated on both sides of the PE diaphragm by casting. The MOF layer is used to stabilize the lithium anode and to form a homogeneous lithium flux. The LLZO layer provides high voltage stability and forms an ultradurable CEI layer. The asymmetric electrolyte design meets the different needs of both the anode and cathode. The extended flow method minimizes the electrolyte thickness (12.6 µm). The weight/volume energy density of the assembled pouch cell reaches 344.0 Wh kg−1/773.1 Wh L−1.
While multilayer asymmetric electrolyte structures can accurately meet the performance requirements of different electrodes, multilayer structures are cumbersome and costly to prepare and are not conducive to large-scale fabrication. Therefore, designing single-layer electrolytes that can satisfy the needs of both the anode and cathode is particularly important. Bao et al. [292] prepared 12 µm-thick CSEs with 3D interconnected structures through the in situ curing of ethoxylated trimethylolpropane triacrylate (ETPTA) in an inorganic ceramic skeleton (LLZO). The sintered LLZO ceramic avoids the inhomogeneous distribution of the inorganic phases, and the crosslinked ETPTA polymer electrolyte contributes to the enhancement of the interfacial contacts; in addition, the continuous two-phase interface can provide a fast transport channel for Li+. The room-temperature ionic conductivity of the ultrathin, flexible CS-CSSE can reach 1.19 × 10−3 S cm−1. CS-CSSE-based flexible pack batteries can achieve high energy densities of 376 Wh kg−1 and 1 186 Wh L−1.
Compared to the ex situ fabrication and assembly of electrolytes/electrodes, the integrated electrolyte-electrode structure enhances the interfacial properties to a greater extent. Zhang et al. [86] proposed a poly(ethylene glycol methacrylate)-Li1.5Al0.5Ge1.5(PO4)3-lithium (PEGMA-LAGP-Li) integrated hierarchy through the in situ copolymerization of an organic-inorganic hybrid SE onto lithium foil, achieving a composite integrated SE-lithium anode structure. Through a meticulous scraping process, the thickness of the electrolyte layer is only 8.5 µm. This integrated hierarchy provided favorable room-temperature ionic conductivity (2.37 × 10−4 S cm−1) and flexible-rigid mechanical properties (Young’s modulus 3 GPa). More promising, the PEGMA-LAGP-Li-assembled LiFePO4|Li ASSLMB maintained a high reversibility with a specific capacity of 159.2 mAh g−1 after exposure to air for 30 min.
In conclusion, ultrathinning is an emerging trend in the field of CSSE. On the one hand, the lower mass/thickness of the electrolyte enhances the weight/volume energy density of the ASSLMB. On the other hand, the ultrathin electrolyte helps to reduce the ohmic resistance of the cell, which is favorable for improving the overall performance of the cell. CSSE membranes also need to have high ionic conductivity, high interfacial stability, and good mechanical properties to meet the growing market demand. These properties are critical for the stability and safety of solid-state batteries. To achieve these goals, electrolyte formulations and preparation processes need to be continuously optimized to improve the performance and stability of CSSE membranes. The performance of CSSE membranes under various application conditions needs to be further investigated to ensure their reliability and safety in various application scenarios.
5 Summary and Outlook
The rapid development of electrification has led to an increasing demand for high-energy density batteries in electric devices. Lithium metal has been considered the ideal material for batteries due to its high specific energy, but the growth of lithium dendrites has been a major obstacle to its development as an anode material. Here, we provide a detailed summary and review of the model and mechanism of lithium dendrite growth in SSEs. We aim to provide a comprehensive understanding of the factors contributing to this issue. A comprehensive review and analysis of the key issues governing the development of organic, inorganic, and composite SSEs and their strategies are presented, providing significant literature references and experimental guidance for solving the lithium dendrite issue in SSEs. We also present our views on and insights into the interface/structure concerns of SSEs and the future development of ASSLMBs (Fig. 23).
-
(i)
Regarding the growth mechanism of lithium dendrites, numerous studies have been conducted by investigators over the past few decades. These studies have proposed various models, including the surface nucleation and diffusion model, heterogeneous nucleation model, space-charge model, SEI model, and Sand’s time model. Based on these models, a sequence of strategies have been developed to suppress dendrite growth. One effective strategy is the use of SSEs, which can effectively suppress the growth of lithium dendrites and enhance the safety performance of lithium secondary batteries. In SSEs, the growth of lithium dendrites can be provoked or exacerbated by factors such as inhomogeneous contact at the electrolyte-lithium metal interface, defects inside the electrolyte, grain boundaries, porosity, low ionic conductivity, and other factors. Therefore, stabilizing the lithium-SE interface, redesigning the structure of the lithium anode and electrolyte, and improving the comprehensive performance of the SE are often employed to suppress dendrite growth in lithium metal anodes.
-
(ii)
Ionic conductivity, mechanical strength, and interfacial stability are significant challenges for organic polymer electrolytes. Some studies have attempted to address these issues by engineering the molecular structures of polymers. By employing techniques such as copolymerization, cross-linking, hyperbranching, twinning, and the addition of ionic liquids, the room-temperature ionic conductivities of polymer electrolytes have dramatically increased to over 10−4 S cm−1. However, these values are still significantly lower (by 1–2 orders of magnitude) than those of inorganic SSEs. While the complete elimination of the safety concerns associated with LMBs is theoretically impossible, the focus for industrialization is currently on gel electrolytes that provide a more comprehensive and balanced performance. In these gel electrolytes, the active substances and conductive agents in the cathode materials can potentially catalytically decompose organic SSEs. Therefore, the design of self-healing polymer electrolytes and the construction of in situ self-polymerizing electrolytes are viable approaches for achieving stable electrode–electrolyte interfaces.
-
(iii)
ISSEs exhibit superior ionic conductivity. In practical applications, the densification, single crystallization, and thinning of ISSEs are considered key factors for regulation. Minimizing manufacturing costs and simplifying the synthesis process are also important considerations for the commercialization of ISSEs. Additionally, special interface designs are necessary to prevent side reactions with lithium metal and promote interfacial contact and wettability. In terms of the microstructure, defects, grain boundaries, and pores are inevitable in inorganic SEs, serving as nucleation sites for dendritic lithium precipitation. Therefore, modulating the microstructure is an effective approach for enhancing the performance of ISSEs. For complex issues, such as interfaces and grain boundaries in ISSEs, theoretical calculations can provide valuable insights. These calculations can be used to predict interfacial energy, interfacial compatibility, and grain boundary energy effectively, thereby guiding the practical application of ISSEs.
-
(iv)
Organic-inorganic composite SSEs possess a combination of rigidity and flexibility, which can effectively increase the ionic conductivity of polymer SSEs and enhance the interfacial stability of inorganic SSEs, making them the preferred choice for organic and inorganic SSEs to complement each other’s strengths. The construction of highly ionically conductive composite SSEs with interactive lithium ion transport networks, good interfacial contact and stability, and favorable mechanical strength through the structural layout is a hot topic for the industrialization of SSEs. In this regard, the use of inorganic fillers in polymer matrices and the design of multilayer heterogeneous structures are prospective endeavors.
-
(v)
Artificial intelligence and materials genomics have emerged as material discovery methods in recent years, and the key is to realize the “high throughput” of materials R&D, i.e., to complete the computational simulation, preparation, and characterization of “a batch” instead of “one” material sample in parallel as a method for accelerating the process from materials discovery to application. While traditional screening processes are lengthy and complex, through artificial intelligence and material genomics, we are able to more efficiently discover potential new materials and optimize existing materials to fit the specific needs of lithium anodes. Furthermore, artificial intelligence and material genomics are being used in the intelligent design of lithium metal anode artificial interface layers for stabilizing lithium metal anodes. Artificial intelligence accelerates the material screening process by identifying the correlations between material properties and performance through machine learning algorithms. Material genomics, meanwhile, provides a rich resource for new material development through genotype-phenotype association databases. The combination of the two is expected to provide more reliable solutions for lithium metal anode protection in SSBs.
Solid-state LMBs are promising next-generation energy devices, but commercialization of these devices is difficult. Further optimization of the structural design, synthesis method and preparation procedure of SSE is recommended to develop an ideal SSE with good mechanical properties, high ionic conductivity, a wide electrochemical window, satisfactory safety and low cost. In addition, combined with advanced characterization tools and intelligent technologies, we will further investigate the growth mechanism of lithium dendrites in SSE systems and explore the transport mechanism and interfacial behavior of Li+ ions in various SSEs to facilitate the early commercialization of all-solid-state LMBs.
References
Xu, W., Wang, J.L., Ding, F., et al.: Lithium metal anodes for rechargeable batteries. Energy Environ. Sci. 7, 513–537 (2014). https://doi.org/10.1039/c3ee40795k
Peng, H.J., Huang, J.Q., Cheng, X.B., et al.: Review on high-loading and high-energy lithium-sulfur batteries. Adv. Energy Mater. 7, 1700260 (2017). https://doi.org/10.1002/aenm.201700260
Fang, R.P., Zhao, S.Y., Sun, Z.H., et al.: More reliable lithium-sulfur batteries: status, solutions and prospects. Adv. Mater. 29, 1606823 (2017). https://doi.org/10.1002/adma.201606823
Liang, J., Sun, Z.H., Li, F., et al.: Carbon materials for Li-S batteries: functional evolution and performance improvement. Energy Storage Mater. 2, 76–106 (2016). https://doi.org/10.1016/j.ensm.2015.09.007
Manthiram, A., Chung, S.H., Zu, C.X.: lithium-sulfur batteries: progress and prospects. Adv. Mater. 27, 1980–2006 (2015). https://doi.org/10.1002/adma.201405115
Kanamura, K., Shiraishi, S., Takehara, Z.I.: Electrochemical deposition of very smooth lithium using nonaqueous electrolytes containing HF. J. Electrochem. Soc. 143, 2187–2197 (1996). https://doi.org/10.1149/1.1836979
Okamoto, H.: The Li-Si (lithium-silicon) system. Bull. Alloy Phase Diagrams 11, 306–312 (1990). https://doi.org/10.1007/BF03029305
Tan, J., Yao, W., Ye, M.X., et al.: Atomistic insights into the morphology of deposited Li. J. Mater. Chem. A 10, 18577–18591 (2022). https://doi.org/10.1039/d2ta04974k
Wu, H., Cui, Y.: Designing nanostructured Si anodes for high energy lithium ion batteries. Nano Today 7, 414–429 (2012). https://doi.org/10.1016/j.nantod.2012.08.004
Wang, S.L., Wang, R.H., Chang, J., et al.: Self-supporting Co3O4/graphene hybrid films as binder-free anode materials for lithium ion batteries. Sci. Rep. 8, 3182 (2018). https://doi.org/10.1038/s41598-018-21436-4
Wu, H., Yu, G.H., Pan, L.J., et al.: Stable Li-ion battery anodes by in situ polymerization of conducting hydrogel to conformally coat silicon nanoparticles. Nat. Commun. 4, 1943 (2013). https://doi.org/10.1038/ncomms2941
Cheng, X.B., Zhang, Q.: Growth mechanisms and suppression strategies of lithium metal dendrites. Prog. Chem. 30, 51–72 (2018). https://doi.org/10.7536/PC170704
Ling, C., Banerjee, D., Matsui, M.: Study of the electrochemical deposition of Mg in the atomic level: why it prefers the non-dendritic morphology. Electrochim. Acta 76, 270–274 (2012). https://doi.org/10.1016/j.electacta.2012.05.001
Ozhabes, Y., Gunceler, D., Arias, T.A.: Stability and surface diffusion at lithium-electrolyte interphases with connections to dendrite suppression. arXiv 1504, 05799 (2015). https://arxiv.org/abs/1504.05799
Yamaki, J.I., Tobishima, S.I., Hayashi, K., et al.: A consideration of the morphology of electrochemically deposited lithium in an organic electrolyte. J. Power Sources 74, 219–227 (1998). https://doi.org/10.1016/s0378-7753(98)00067-6
Dollé, M., Sannier, L., Beaudoin, B., et al.: Live scanning electron microscope observations of dendritic growth in lithium/polymer cells. Electrochem. Solid-State Lett. 5(12), A286 (2002). https://doi.org/10.1149/1.1519970
Monroe, C., Newman, J.: Dendrite growth in lithium/polymer systems. J. Electrochem. Soc. 150, A1377 (2003). https://doi.org/10.1149/1.1606686
Ely, D.R., García, R.E.: Heterogeneous nucleation and growth of lithium electrodeposits on negative electrodes. J. Electrochem. Soc. 160, A662–A668 (2013). https://doi.org/10.1149/1.057304jes
Harry, K.J., Liao, X.X., Parkinson, D.Y., et al.: Electrochemical deposition and stripping behavior of lithium metal across a rigid block copolymer electrolyte membrane. J. Electrochem. Soc. 162, A2699–A2706 (2015). https://doi.org/10.1149/2.0321514jes
Wenzel, S., Leichtweiss, T., Krüger, D., et al.: Interphase formation on lithium solid electrolytes: an in situ approach to study interfacial reactions by photoelectron spectroscopy. Solid State Ion. 278, 98–105 (2015). https://doi.org/10.1016/j.ssi.2015.06.001
Yu, S., Siegel, D.J.: Grain boundary softening: a potential mechanism for lithium metal penetration through stiff solid electrolytes. ACS Appl. Mater. Interfaces 10, 38151–38158 (2018). https://doi.org/10.1021/acsami.8b17223
Tian, H.K., Xu, B., Qi, Y.: Computational study of lithium nucleation tendency in Li7La3Zr2O12 (LLZO) and rational design of interlayer materials to prevent lithium dendrites. J. Power Sources 392, 79–86 (2018). https://doi.org/10.1016/j.jpowsour.2018.04.098
Kushima, A., So, K.P., Su, C., et al.: Liquid cell transmission electron microscopy observation of lithium metal growth and dissolution: root growth, dead lithium and lithium flotsams. Nano Energy 32, 271–279 (2017). https://doi.org/10.1016/j.nanoen.2016.12.001
Xiao, J.: How lithium dendrites form in liquid batteries. Science 366, 426–427 (2019). https://doi.org/10.1126/science.aay8672
Cao, C., Li, Y., Feng, Y.Y., et al.: A solid-state single-ion polymer electrolyte with ultrahigh ionic conductivity for dendrite-free lithium metal batteries. Energy Storage Mater. 19, 401–407 (2019). https://doi.org/10.1016/j.ensm.2019.03.004
Gao, X.W., Zhou, Y.N., Han, D.H., et al.: Thermodynamic understanding of Li-dendrite formation. Joule 4, 1864–1879 (2020). https://doi.org/10.1016/j.joule.2020.06.016
Shi, Y., Wan, J., Liu, G.X., et al.: Interfacial evolution of lithium dendrites and their solid electrolyte interphase shells of quasi-solid-state lithium-metal batteries. Angew. Chem. Int. Ed. 59, 18120–18125 (2020). https://doi.org/10.1002/anie.202001117
Cha, E., Yun, J.H., Ponraj, R., et al.: A mechanistic review of lithiophilic materials: resolving lithium dendrites and advancing lithium metal-based batteries. Mater. Chem. Front. 5, 6294–6314 (2021). https://doi.org/10.1039/d1qm00579k
Wang, H.C., Gao, H.W., Chen, X.X., et al.: Linking the defects to the formation and growth of Li dendrite in all-solid-state batteries. Adv. Energy Mater. 11, 2102148 (2021). https://doi.org/10.1002/aenm.202102148
Yang, M.H., Liu, Y.S., Mo, Y.F.: Lithium crystallization at solid interfaces. Nat. Commun. 14, 2986 (2023). https://doi.org/10.1038/s41467-023-38757-2
Sadd, M., Xiong, S.Z., Bowen, J.R., et al.: Investigating microstructure evolution of lithium metal during plating and stripping via operando X-ray tomographic microscopy. Nat. Commun. 14, 854 (2023). https://doi.org/10.1038/s41467-023-36568-z
Cao, T.C., Xu, R., Cheng, X.P., et al.: Chemomechanical origins of the dynamic evolution of isolated Li filaments in inorganic solid-state electrolytes. Nano Lett. 24, 1843–1850 (2024). https://doi.org/10.1021/acs.nanolett.3c03321
Li, Y.H., Xu, H., Ning, Q.R., et al.: Visualizing structure, growth, and dynamics of Li dendrite in batteries: from atomic to device scales. Adv. Funct. Mater. (2024). https://doi.org/10.1002/adfm.202401361
Li, B.R., Chao, Y., Li, M.C., et al.: A review of solid electrolyte interphase (SEI) and dendrite formation in lithium batteries. Electrochem. Energy Rev. 6, 7 (2023). https://doi.org/10.1007/s41918-022-00147-5
Chazalviel, J.N.: Electrochemical aspects of the generation of ramified metallic electrodeposits. Phys. Rev. A 42, 7355–7367 (1990). https://doi.org/10.1103/physreva.42.7355
Qiu, X.G., Liu, W., Liu, J.D., et al.: Nucleation mechanism and substrate modification of lithium metal anode. Acta Phys. Chim. Sin. 37, 2009012 (2021). https://doi.org/10.3866/pku.whxb202009012
Fleury, V., Chazalviel, J.N., Rosso, M., et al.: The role of the anions in the growth speed of fractal electrodeposits. J. Electroanal. Chem. Interfacial Electrochem. 290, 249–255 (1990). https://doi.org/10.1016/0022-0728(90)87434-l
Rosso, M., Brissot, C., Teyssot, A., et al.: Dendrite short-circuit and fuse effect on Li/polymer/Li cells. Electrochim. Acta 51, 5334–5340 (2006). https://doi.org/10.1016/j.electacta.2006.02.004
Sand, H.J.S.: On the concentration at the electrodes in a solution, with special reference to the liberation of hydrogen by electrolysis of a mixture of copper sulphate and sulphuric acid. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1, 45–79 (1901). https://doi.org/10.1080/14786440109462590
Yang, L., Smith, C., Patrissi, C., et al.: Surface reactions and performance of non-aqueous electrolytes with lithium metal anodes. J. Power Sources 185, 1359–1366 (2008). https://doi.org/10.1016/j.jpowsour.2008.09.037
Liang, Z., Lin, D.C., Zhao, J., et al.: Composite lithium metal anode by melt infusion of lithium into a 3D conducting scaffold with lithiophilic coating. Proc. Natl. Acad. Sci. U. S. A. 113, 2862–2867 (2016). https://doi.org/10.1073/pnas.1518188113
Rao, B.M.L., Francis, R.W., Christopher, H.A.: Lithium-aluminum electrode. J. Electrochem. Soc. 124, 1490–1492 (1977). https://doi.org/10.1149/1.2133098
Chenebault, P., Vallin, D., Thevenin, J., et al.: Properties of surface layers formed on lithium in Li-SOCl2 cells: synergetic effect of SO2 and LiAl(SO3Cl)4. J. Appl. Electrochem. 18, 625–632 (1988). https://doi.org/10.1007/BF01022261
Bi, Z.J., Sun, Q.F., Jia, M.Y., et al.: Molten salt driven conversion reaction enabling lithiophilic and air-stable garnet surface for solid-state lithium batteries. Adv. Funct. Mater. 32, 2208751 (2022). https://doi.org/10.1002/adfm.202208751
Ning, Z.Y., Li, G.C., Melvin, D.L.R., et al.: Dendrite initiation and propagation in lithium metal solid-state batteries. Nature 618, 287–293 (2023). https://doi.org/10.1038/s41586-023-05970-4
Jin, S., Ye, Y.D., Niu, Y.J., et al.: Solid-solution-based metal alloy phase for highly reversible lithium metal anode. J. Am. Chem. Soc. 142, 8818–8826 (2020). https://doi.org/10.1021/jacs.0c01811
Yang, C.P., Yin, Y.X., Zhang, S.F., et al.: Accommodating lithium into 3D current collectors with a submicron skeleton towards long-life lithium metal anodes. Nat. Commun. 6, 8058 (2015). https://doi.org/10.1038/ncomms9058
Ma, Q., Fang, Z., Liu, P., et al.: Improved cycling stability of lithium-metal anode with concentrated electrolytes based on lithium (fluorosulfonyl)(trifluoromethanesulfonyl)imide. ChemElectroChem 3, 531–536 (2016). https://doi.org/10.1002/celc.201500520
Jeong, S.K., Seo, H.Y., Kim, D.H., et al.: Suppression of dendritic lithium formation by using concentrated electrolyte solutions. Electrochem. Commun. 10, 635–638 (2008). https://doi.org/10.1016/j.elecom.2008.02.006
Chen, H.N., Zhou, Y.C., Lu, Y.C.: Lithium-organic nanocomposite suspension for high-energy-density redox flow batteries. ACS Energy Lett. 3, 1991–1997 (2018). https://doi.org/10.1021/acsenergylett.8b01257
Ding, F., Xu, W., Graff, G.L., et al.: Dendrite-free lithium deposition via self-healing electrostatic shield mechanism. J. Am. Chem. Soc. 135, 4450–4456 (2013). https://doi.org/10.1021/ja312241y
Yoon, S., Lee, J., Kim, S.O., et al.: Enhanced cyclability and surface characteristics of lithium batteries by Li-Mg co-deposition and addition of HF acid in electrolyte. Electrochim. Acta 53, 2501–2506 (2008). https://doi.org/10.1016/j.electacta.2007.10.019
Stark, J.K., Ding, Y., Kohl, P.A.: Dendrite-free electrodeposition and reoxidation of lithium-sodium alloy for metal-anode battery. J. Electrochem. Soc. 158, A1100 (2011). https://doi.org/10.1149/1.3622348
Xu, R., Xiao, Y., Zhang, R., et al.: Dual-phase single-ion pathway interfaces for robust lithium metal in working batteries. Adv. Mater. 31, 1808392 (2019). https://doi.org/10.1002/adma.201808392
Cheng, X.B., Zhang, R., Zhao, C.Z., et al.: A review of solid electrolyte interphases on lithium metal anode. Adv. Sci. 3, 1500213 (2015). https://doi.org/10.1002/advs.201500213
Xu, K.: Electrolytes and interphases in Li-ion batteries and beyond. Chem. Rev. 114, 11503–11618 (2014). https://doi.org/10.1021/cr500003w
Chen, R.J., Qu, W.J., Guo, X., et al.: The pursuit of solid-state electrolytes for lithium batteries: from comprehensive insight to emerging horizons. Mater. Horiz. 3, 487–516 (2016). https://doi.org/10.1039/c6mh00218h
Sun, Y.Z., Huang, J.Q., Zhao, C.Z., et al.: A review of solid electrolytes for safe lithium-sulfur batteries. Sci. China Chem. 60, 1508–1526 (2017). https://doi.org/10.1007/s11426-017-9164-2
Fan, L., Wei, S.Y., Li, S.Y., et al.: Recent progress of the solid-state electrolytes for high-energy metal-based batteries. Adv. Energy Mater. 8, 1702657 (2018). https://doi.org/10.1002/aenm.201702657
Wu, F., Zhang, K., Liu, Y.R., et al.: Polymer electrolytes and interfaces toward solid-state batteries: recent advances and prospects. Energy Storage Mater. 33, 26–54 (2020). https://doi.org/10.1016/j.ensm.2020.08.002
Cao, D.X., Sun, X., Li, Q., et al.: Lithium dendrite in all-solid-state batteries: growth mechanisms, suppression strategies, and characterizations. Matter 3, 57–94 (2020). https://doi.org/10.1016/j.matt.2020.03.015
Brissot, C., Rosso, M., Chazalviel, J.N., et al.: Dendritic growth mechanisms in lithium/polymer cells. J. Power Sources 81(82), 925–929 (1999). https://doi.org/10.1016/s0378-7753(98)00242-0
Zhao, C.Z., Chen, P.Y., Zhang, R., et al.: An ion redistributor for dendrite-free lithium metal anodes. Sci. Adv. 4, eaat3446 (2018). https://doi.org/10.1126/sciadv.aat3446
Tsai, C.L., Roddatis, V., Chandran, C.V., et al.: Li7La3Zr2O12 interface modification for Li dendrite prevention. ACS Appl. Mater. Interfaces 8, 10617–10626 (2016). https://doi.org/10.1021/acsami.6b00831
Sharafi, A., Kazyak, E., Davis, A.L., et al.: Surface chemistry mechanism of ultra-low interfacial resistance in the solid-state electrolyte Li7La3Zr2O12. Chem. Mater. 29, 7961–7968 (2017). https://doi.org/10.1021/acs.chemmater.7b03002
Sharafi, A., Yu, S., Naguib, M., et al.: Impact of air exposure and surface chemistry on Li-Li7La3Zr2O12 interfacial resistance. J. Mater. Chem. A 5, 13475–13487 (2017). https://doi.org/10.1039/c7ta03162a
Li, Y.Q., Wang, Z., Li, C.L., et al.: Densification and ionic-conduction improvement of lithium garnet solid electrolytes by flowing oxygen sintering. J. Power Sources 248, 642–646 (2014). https://doi.org/10.1016/j.jpowsour.2013.09.140
Nagao, M., Hayashi, A., Tatsumisago, M., et al.: In situ SEM study of a lithium deposition and dissolution mechanism in a bulk-type solid-state cell with a Li2S-P2S5 solid electrolyte. Phys. Chem. Chem. Phys. 15, 18600 (2013). https://doi.org/10.1039/c3cp51059j
Yu, S., Siegel, D.J.: Grain boundary contributions to Li-ion transport in the solid electrolyte Li7La3Zr2O12 (LLZO). Chem. Mater. 29, 9639–9647 (2017). https://doi.org/10.1021/acs.chemmater.7b02805
Chen, Y.T., Jena, A., Pang, W.K., et al.: Voltammetric enhancement of Li-ion conduction in Al-doped Li7−xLa3Zr2O12 solid electrolyte. J. Phys. Chem. C 121, 15565–15573 (2017). https://doi.org/10.1021/acs.jpcc.7b04004
Aguesse, F., Manalastas, W., Buannic, L., et al.: Investigating the dendritic growth during full cell cycling of garnet electrolyte in direct contact with Li metal. ACS Appl. Mater. Interfaces 9, 3808–3816 (2017). https://doi.org/10.1021/acsami.6b13925
Fenton, D.E., Parker, J.M., Wright, P.V.: Complexes of alkali metal ions with poly(ethylene oxide). Polymer 14, 589 (1973). https://doi.org/10.1016/0032-3861(73)90146-8
Gorecki, W., Jeannin, M., Belorizky, E., et al.: Physical properties of solid polymer electrolyte PEO(LiTFSI) complexes. J. Phys. Condens. Matter 7, 6823–6832 (1995). https://doi.org/10.1088/0953-8984/7/34/007
Gao, H.C., Grundish, N.S., Zhao, Y.J., et al.: Formation of stable interphase of polymer-in-salt electrolyte in all-solid-state lithium batteries. Energy Mater. Adv. 2021, 1932952 (2021)
Xiao, Q.Z., Wang, X.Z., Li, W., et al.: Macroporous polymer electrolytes based on PVDF/PEO-b-PMMA block copolymer blends for rechargeable lithium ion battery. J. Membr. Sci. 334, 117–122 (2009). https://doi.org/10.1016/j.memsci.2009.02.018
Saito, Y., Takeda, S., Yamagami, S., et al.: Effect of the morphological features of the poly(vinylidene difluoride)-based gel electrolytes on the ionic mobility for lithium secondary batteries. Macromolecules 52, 2112–2119 (2019). https://doi.org/10.1021/acs.macromol.8b02503
Zhang, H., Liu, C.Y., Zheng, L.P., et al.: Lithium bis(fluorosulfonyl)imide/poly(ethylene oxide) polymer electrolyte. Electrochim. Acta 133, 529–538 (2014). https://doi.org/10.1016/j.electacta.2014.04.099
Zhou, W.D., Wang, Z.X., Pu, Y., et al.: Double-layer polymer electrolyte for high-voltage all-solid-state rechargeable batteries. Adv. Mater. 31, 1805574 (2019). https://doi.org/10.1002/adma.201805574
Wu, X.L., Xin, S., Seo, H.H., et al.: Enhanced Li+ conductivity in PEO-LiBOB polymer electrolytes by using succinonitrile as a plasticizer. Solid State Ion. 186, 1–6 (2011). https://doi.org/10.1016/j.ssi.2011.01.010
Bandara, L.R.A.K., Dissanayake, M.A.K.L., Mellander, B.E.: Ionic conductivity of plasticized (PEO)-LiCF3SO3 electrolytes. Electrochim. Acta 43, 1447–1451 (1998). https://doi.org/10.1016/s0013-4686(97)10082-2
Yang, C.R., Perng, J.T., Wang, Y.Y., et al.: Conductive behaviour of lithium ions in polyacrylonitrile. J. Power Sources 62, 89–93 (1996). https://doi.org/10.1016/s0378-7753(96)02414-7
Forsyth, M., Sun, J.Z., MacFarlane, D.R.: Novel polymer-in-salt electrolytes based on polyacrylonitrile (PAN) lithium triflate salt mixtures. Solid State Ion. 112, 161–163 (1998). https://doi.org/10.1016/S0167-2738(98)00166-0
Chai, J.C., Liu, Z.H., Ma, J., et al.: In situ generation of poly (vinylene carbonate) based solid electrolyte with interfacial stability for LiCoO2 lithium batteries. Adv. Sci. 4, 1600377 (2017). https://doi.org/10.1002/advs.201600377
Zhang, K., Wu, F., Wang, X.R., et al.: An ion-dipole-reinforced polyether electrolyte with ion-solvation cages enabling high-voltage-tolerant and ion-conductive solid-state lithium metal batteries. Adv. Funct. Mater. 32, 2107764 (2022). https://doi.org/10.1002/adfm.202107764
Huang, S.Q., Cui, Z.L., Qiao, L.X., et al.: An in situ polymerized solid polymer electrolyte enables excellent interfacial compatibility in lithium batteries. Electrochim. Acta 299, 820–827 (2019). https://doi.org/10.1016/j.electacta.2019.01.039
Zhang, K., Wu, F., Wang, X.R., et al.: 8.5 µm-Thick flexible-rigid hybrid solid-electrolyte/lithium integration for air-stable and interface-compatible all-solid-state lithium metal batteries. Adv. Energy Mater. 12, 2200368 (2022). https://doi.org/10.1002/aenm.202200368
Zhou, W.D., Wang, S.F., Li, Y.T., et al.: Plating a dendrite-free lithium anode with a polymer/ceramic/polymer sandwich electrolyte. J. Am. Chem. Soc. 138, 9385–9388 (2016). https://doi.org/10.1021/jacs.6b05341
Yuan, F., Chen, H.Z., Yang, H.Y., et al.: PAN-PEO solid polymer electrolytes with high ionic conductivity. Mater. Chem. Phys. 89, 390–394 (2005). https://doi.org/10.1016/j.matchemphys.2004.09.032
Choudhary, S., Sengwa, R.J.: Effects of different inorganic nanoparticles on the structural, dielectric and ion transportation properties of polymers blend based nanocomposite solid polymer electrolytes. Electrochim. Acta 247, 924–941 (2017). https://doi.org/10.1016/j.electacta.2017.07.051
Liang, B., Tang, S.Q., Jiang, Q.B., et al.: Preparation and characterization of PEO-PMMA polymer composite electrolytes doped with nano-Al2O3. Electrochim. Acta 169, 334–341 (2015). https://doi.org/10.1016/j.electacta.2015.04.039
Zhang, A.L., Cao, F.Y., Na, G.Z., et al.: A novel PEO-based blends solid polymer electrolytes doping liquid crystalline ionomers. Ionics 22, 2103–2112 (2016). https://doi.org/10.1007/s11581-016-1732-z
Tsuchida, E., Ohno, H., Tsunemi, K., et al.: Lithium ionic conduction in poly(methacrylic acid)-poly(ethylene oxide) complex containing lithium perchlorate. Solid State Ion. 11, 227–233 (1983). https://doi.org/10.1016/0167-2738(83)90028-0
Lee, M.: Effect of phase separation on ionic conductivity of poly(methyl methacrylate)-based solid polymer electrolyte. Solid State Ion. 85, 91–98 (1996). https://doi.org/10.1016/0167-2738(96)00046-x
Niitani, T., Shimada, M., Kawamura, K., et al.: Characteristics of new-type solid polymer electrolyte controlling nano-structure. J. Power Sources 146, 386–390 (2005). https://doi.org/10.1016/j.jpowsour.2005.03.102
Morita, M., Fujisaki, T., Yoshimoto, N., et al.: Ionic conductance behavior of polymeric composite solid electrolytes containing lithium aluminate. Electrochim. Acta 46, 1565–1569 (2001). https://doi.org/10.1016/s0013-4686(00)00754-4
Lin, D.C., Yuen, P.Y., Liu, Y.Y., et al.: A silica-aerogel-reinforced composite polymer electrolyte with high ionic conductivity and high modulus. Adv. Mater. 30, 1802661 (2018). https://doi.org/10.1002/adma.201802661
Rajendran, S., Babu, R., Sivakumar, P.: Optimization of PVC-PAN-based polymer electrolytes. J. Appl. Polym. Sci. 113, 1651–1656 (2009). https://doi.org/10.1002/app.30174
Liu, W.Y., Yi, C.J., Li, L.P., et al.: Designing polymer-in-salt electrolyte and fully infiltrated 3D electrode for integrated solid-state lithium batteries. Angew. Chem. Int. Ed. 60, 12931–12940 (2021). https://doi.org/10.1002/anie.202101537
Pradeepa, P., Edwin Raj, S., Sowmya, G., et al.: Optimization of hybrid polymer electrolytes with the effect of lithium salt concentration in PEO/PVdF-HFP blends. Mater. Sci. Eng. B 205(6), 17 (2016). https://doi.org/10.1016/j.mseb.2015.11.009
Li, Y.J., Fan, C.Y., Zhang, J.P., et al.: A promising PMHS/PEO blend polymer electrolyte for all-solid-state lithium ion batteries. Dalton Trans. 47, 14932–14937 (2018). https://doi.org/10.1039/c8dt02904k
Tao, C., Gao, M.H., Yin, B.H., et al.: A promising TPU/PEO blend polymer electrolyte for all-solid-state lithium ion batteries. Electrochim. Acta 257, 31–39 (2017). https://doi.org/10.1016/j.electacta.2017.10.037
Zhang, Y.H., Lu, W., Cong, L.N., et al.: Cross-linking network based on poly(ethylene oxide): solid polymer electrolyte for room temperature lithium battery. J. Power Sources 420, 63–72 (2019). https://doi.org/10.1016/j.jpowsour.2019.02.090
Bouchet, R., Maria, S., Meziane, R., et al.: Single-ion BAB triblock copolymers as highly efficient electrolytes for lithium-metal batteries. Nat. Mater. 12, 452–457 (2013). https://doi.org/10.1038/nmat3602
Kimura, K., Yajima, M., Tominaga, Y.: A highly-concentrated poly(ethylene carbonate)-based electrolyte for all-solid-state Li battery working at room temperature. Electrochem. Commun. 66, 46–48 (2016). https://doi.org/10.1016/j.elecom.2016.02.022
Zhou, D., He, Y.B., Liu, R.L., et al.: In situ synthesis of a hierarchical all-solid-state electrolyte based on nitrile materials for high-performance lithium-ion batteries. Adv. Energy Mater. 5, 1500353 (2015). https://doi.org/10.1002/aenm.201500353
Zhou, D., Liu, R.L., Zhang, J., et al.: In situ synthesis of hierarchical poly(ionic liquid)-based solid electrolytes for high-safety lithium-ion and sodium-ion batteries. Nano Energy 33, 45–54 (2017). https://doi.org/10.1016/j.nanoen.2017.01.027
Xi, J.Y., Qiu, X.P., Li, J., et al.: PVDF-PEO blends based microporous polymer electrolyte: effect of PEO on pore configurations and ionic conductivity. J. Power Sources 157, 501–506 (2006). https://doi.org/10.1016/j.jpowsour.2005.08.009
Zheng, J.Y., Li, X., Yu, Y.J., et al.: Cross-linking copolymers of acrylates’ gel electrolytes with high conductivity for lithium-ion batteries. J. Solid State Electrochem. 18, 2013–2018 (2014). https://doi.org/10.1007/s10008-014-2438-7
Zhao, Y., Wang, L., Zhou, Y.N., et al.: Solid polymer electrolytes with high conductivity and transference number of Li ions for Li-based rechargeable batteries. Adv. Sci. 8, 2003675 (2021). https://doi.org/10.1002/advs.202003675
Xie, T., France-Lanord, A., Wang, Y.M., et al.: Accelerating amorphous polymer electrolyte screening by learning to reduce errors in molecular dynamics simulated properties. Nat. Commun. 13, 3415 (2022). https://doi.org/10.1038/s41467-022-30994-1
Webb, M.A., Jung, Y., Pesko, D.M., et al.: Systematic computational and experimental investigation of lithium-ion transport mechanisms in polyester-based polymer electrolytes. ACS Cent. Sci. 1, 198–205 (2015). https://doi.org/10.1021/acscentsci.5b00195
Savoie, B.M., Webb, M.A., Miller, T.F., III.: Enhancing cation diffusion and suppressing anion diffusion via Lewis-acidic polymer electrolytes. J. Phys. Chem. Lett. 8, 641–646 (2017). https://doi.org/10.1021/acs.jpclett.6b02662
France-Lanord, A., Wang, Y.M., Xie, T., et al.: Effect of chemical variations in the structure of poly(ethylene oxide)-based polymers on lithium transport in concentrated electrolytes. Chem. Mater. 32, 121–126 (2020). https://doi.org/10.1021/acs.chemmater.9b02645
France-Lanord, A., Grossman, J.C.: Correlations from ion pairing and the nernst-einstein equation. Phys. Rev. Lett. 122, 136001 (2019). https://doi.org/10.1103/physrevlett.122.136001
Zhang, S., Ma, J., Dong, S.M., et al.: Designing all-solid-state batteries by theoretical computation: a review. Electrochem. Energy Rev. 6, 4 (2023). https://doi.org/10.1007/s41918-022-00143-9
Deng, K.R., Zeng, Q.G., Wang, D., et al.: Single-ion conducting gel polymer electrolytes: design, preparation and application. J. Mater. Chem. A 8, 1557–1577 (2020). https://doi.org/10.1039/c9ta11178f
Cao, C., Li, Y., Chen, S.S., et al.: Electrolyte-solvent-modified alternating copolymer as a single-ion solid polymer electrolyte for high-performance lithium metal batteries. ACS Appl. Mater. Interfaces 11, 35683–35692 (2019). https://doi.org/10.1021/acsami.9b10595
Doyle, R.P., Chen, X.R., MacRae, M., et al.: Poly(ethylenimine)-based polymer blends as single-ion lithium conductors. Macromolecules 47, 3401–3408 (2014). https://doi.org/10.1021/ma402325a
Liu, K.W., Jiang, S.S., Dzwiniel, T.L., et al.: Molecular design of a highly stable single-ion conducting polymer gel electrolyte. ACS Appl. Mater. Interfaces 12(26), 29162–29172 (2020). https://doi.org/10.1021/acsami.0c03363
Zhang, Y.F., Xu, G.D., Sun, Y.B., et al.: A class of sp3 boron-based single-ion polymeric electrolytes for lithium ion batteries. RSC Adv. 3, 14934 (2013). https://doi.org/10.1039/c3ra41167b
Meziane, R., Bonnet, J.P., Courty, M., et al.: Single-ion polymer electrolytes based on a delocalized polyanion for lithium batteries. Electrochim. Acta 57, 14–19 (2011). https://doi.org/10.1016/j.electacta.2011.03.074
Sun, Y.B., Rohan, R., Cai, W.W., et al.: A polyamide single-ion electrolyte membrane for application in lithium-ion batteries. Energy Technol. 2, 698–704 (2014). https://doi.org/10.1002/ente.201402041
Borzutzki, K., Thienenkamp, J., Diehl, M., et al.: Fluorinated polysulfonamide based single ion conducting room temperature applicable gel-type polymer electrolytes for lithium ion batteries. J. Mater. Chem. A 7, 188–201 (2019). https://doi.org/10.1039/c8ta08391f
Zhang, H., Li, C.M., Piszcz, M., et al.: Single lithium-ion conducting solid polymer electrolytes: advances and perspectives. Chem. Soc. Rev. 46, 797–815 (2017). https://doi.org/10.1039/c6cs00491a
Hou, T.Y., Qian, Y.M., Li, D.G., et al.: Electronegativity-induced single-ion conducting polymer electrolyte for solid-state lithium batteries. Energy Environ. Mater. 6, 12428 (2023). https://doi.org/10.1002/eem2.12428
Stephan, A.M., Prem Kumar, T., Angulakshmi, N., et al.: Influence of calix[2]-p-benzo[4]pyrrole on the electrochemical properties of poly(ethylene oxide)-based electrolytes for lithium batteries. J. Appl. Polym. Sci. 120, 2215–2221 (2011). https://doi.org/10.1002/app.33462
Dai, K., Ma, C., Feng, Y.M., et al.: A borate-rich, cross-linked gel polymer electrolyte with near-single ion conduction for lithium metal batteries. J. Mater. Chem. A 7, 18547–18557 (2019). https://doi.org/10.1039/c9ta05938e
Guan, T.Y., Qian, S.J., Guo, Y.K., et al.: Star brush block copolymer electrolytes with high ambient-temperature ionic conductivity for quasi-solid-state lithium batteries. ACS Mater. Lett. 1, 606–612 (2019). https://doi.org/10.1021/acsmaterialslett.9b00423
Ji, X.Y., Cao, M.X., Fu, X.W., et al.: Efficient room-temperature solid-state lithium ion conductors enabled by mixed-graft block copolymer architectures. Giant 3, 100027 (2020). https://doi.org/10.1016/j.giant.2020.100027
Ren, Z.H., Li, J.X., Cai, M.H., et al.: An in situ formed copolymer electrolyte with high ionic conductivity and high lithium-ion transference number for dendrite-free solid-state lithium metal batteries. J. Mater. Chem. A 11, 1966–1977 (2023). https://doi.org/10.1039/d2ta07516d
Fu, F., Zheng, Y., Jiang, N., et al.: A dual-salt PEO-based polymer electrolyte with cross-linked polymer network for high-voltage lithium metal batteries. Chem. Eng. J. 450, 137776 (2022). https://doi.org/10.1016/j.cej.2022.137776
Zhang, W., Jin, L., Lee, S., et al.: In situ induced crosslinking highly conductive solid polymer electrolyte with intimated electrodes interfacial compatibility for safe Li-ion batteries. J. Power Sources 557, 232568 (2023). https://doi.org/10.1016/j.jpowsour.2022.232568
Zhu, Y.H., Cao, J., Chen, H., et al.: High electrochemical stability of a 3D cross-linked network PEO@nano-SiO2 composite polymer electrolyte for lithium metal batteries. J. Mater. Chem. A 7, 6832–6839 (2019). https://doi.org/10.1039/c9ta00560a
Mardegan, L., Dreessen, C., Sessolo, M., et al.: Stable light-emitting electrochemical cells using hyperbranched polymer electrolyte. Adv. Funct. Mater. 31, 2104249 (2021). https://doi.org/10.1002/adfm.202104249
Zhou, T.H., Zhao, Y., Choi, J.W., et al.: Ionic liquid functionalized gel polymer electrolytes for stable lithium metal batteries. Angew. Chem. Int. Ed. 60, 22791–22796 (2021). https://doi.org/10.1002/anie.202106237
Hoffknecht, J.P., Wettstein, A., Atik, J., et al.: Coordinating anions “to the rescue” of the lithium ion mobility in ternary solid polymer electrolytes plasticized with ionic liquids. Adv. Energy Mater. 13, 2202789 (2023). https://doi.org/10.1002/aenm.202202789
Cho, W.J., Cho, S.K., Lee, J.H., et al.: Solid polymer electrolytes of ionic liquids via a bicontinuous ion transport channel for lithium metal batteries. J. Mater. Chem. A 11, 1676–1683 (2023). https://doi.org/10.1039/d2ta08139c
Grazioli, D., Verners, O., Zadin, V., et al.: Electrochemical-mechanical modeling of solid polymer electrolytes: impact of mechanical stresses on Li-ion battery performance. Electrochim. Acta 296, 1122–1141 (2019). https://doi.org/10.1016/j.electacta.2018.07.234
Sun, Z.F., Wu, J.N., Yuan, H.C., et al.: Self-healing polymer electrolyte for long-life and recyclable lithium-metal batteries. Mater. Today Energy 24, 100939 (2022). https://doi.org/10.1016/j.mtener.2021.100939
Jo, Y.H., Li, S.Q., Zuo, C., et al.: Self-healing solid polymer electrolyte facilitated by a dynamic cross-linked polymer matrix for lithium-ion batteries. Macromolecules 53, 1024–1032 (2020). https://doi.org/10.1021/acs.macromol.9b02305
Rong, J.C., Zhong, J., Yan, W.L., et al.: Study on waterborne self-healing polyurethane with dual dynamic units of quadruple hydrogen bonding and disulfide bonds. Polymer 221, 123625 (2021). https://doi.org/10.1016/j.polymer.2021.123625
Li, S.B., Zuo, C., Zhang, Y., et al.: Covalently cross-linked polymer stabilized electrolytes with self-healing performance via boronic ester bonds. Polym. Chem. 11, 5893–5902 (2020). https://doi.org/10.1039/d0py00728e
Zhou, S.P., Deng, K.R., Xu, Z.L., et al.: Highly conductive self-healing polymer electrolytes based on synergetic dynamic bonds for highly safe lithium metal batteries. Chem. Eng. J. 442, 136083 (2022). https://doi.org/10.1016/j.cej.2022.136083
Goor, O.J.G.M., Brouns, J.E.P., Dankers, P.Y.W.: Introduction of anti-fouling coatings at the surface of supramolecular elastomeric materials via post-modification of reactive supramolecular additives. Polym. Chem. 8, 5228–5238 (2017). https://doi.org/10.1039/c7py00801e
Chen, X.Y., Yi, L.G., Zou, C.F., et al.: High-performance gel polymer electrolyte with self-healing capability for lithium-ion batteries. ACS Appl. Energy Mater. 5, 5267–5276 (2022). https://doi.org/10.1021/acsaem.2c00713
Jin, C.M., Sinawang, G., Osaki, M., et al.: Self-healing thermoplastic polyurethane linked via host-guest interactions. Polymers 12, 1393 (2020). https://doi.org/10.3390/polym12061393
Xia, D.Y., Wang, P., Ji, X.F., et al.: Functional supramolecular polymeric networks: the marriage of covalent polymers and macrocycle-based host-guest interactions. Chem. Rev. 120, 6070–6123 (2020). https://doi.org/10.1021/acs.chemrev.9b00839
Chen, S., Yu, C., Wei, C.C., et al.: Unraveling electrochemical stability and reversible redox of Y-doped Li2ZrCl6 solid electrolytes. Energy Mater. Adv. 4, 0019 (2023)
Kanno, R., Murayama, M.: Lithium ionic conductor thio-LISICON: the Li2S-GeS2-P2S5 system. J. Electrochem. Soc. 148, A742 (2001). https://doi.org/10.1149/1.1379028
Aono, H., Sugimoto, E., Sadaoka, Y., et al.: Ionic conductivity of solid electrolytes based on lithium titanium phosphate. J. Electrochem. Soc. 137, 1023–1027 (1990). https://doi.org/10.1149/1.2086597
Murugan, R., Thangadurai, V., Weppner, W.: Fast lithium ion conduction in garnet-type Li7La3Zr2O12. Angew. Chem. Int. Ed. 46, 7778–7781 (2007). https://doi.org/10.1002/anie.200701144
Guo, Q., Xu, F., Shen, L., et al.: 20 μm-Thick Li6.4La3Zr1.4Ta0.6O1.2-based flexible solid electrolytes for all-solid-state lithium batteries. Energy Mater. Adv. 2022, 9753506 (2022)
Kawai, H., Kuwano, J.: Lithium ion conductivity of A-site deficient perovskite solid solution La0.67−xLi3xTiO3. J. Electrochem. Soc. 141, 78–79 (1994). https://doi.org/10.1149/1.2055043
Bohnke, O.: Mechanism of ionic conduction and electrochemical intercalation of lithium into the perovskite lanthanum lithium titanate. Solid State Ion. 91, 21–31 (1996). https://doi.org/10.1016/s0167-2738(96)00434-1
Zhao, Y.S., Daemen, L.L.: Superionic conductivity in lithium-rich anti-perovskites. J. Am. Chem. Soc. 134, 15042–15047 (2012). https://doi.org/10.1021/ja305709z
Matsuo, M., Remhof, A., Martelli, P., et al.: Complex hydrides with (BH4)− and (NH2)− anions as new lithium fast-ion conductors. J. Am. Chem. Soc. 131, 16389–16391 (2009). https://doi.org/10.1021/ja907249p
Lutz, H.D., Schmidt, W., Haeuseler, H.: Chloride spinels: a new group of solid lithium electrolytes. J. Phys. Chem. Solids 42, 287–289 (1981). https://doi.org/10.1016/0022-3697(81)90142-6
Deiseroth, H.J., Kong, S.T., Eckert, H., et al.: Li6PS5X: a class of crystalline Li-rich solids with an unusually high Li+ mobility. Angew. Chem. Int. Ed. 47, 755–758 (2008). https://doi.org/10.1002/anie.200703900
Zhao, X.L., Shen, L., Zhang, N.N., et al.: Stable binder boosting sulfide solid electrolyte thin membrane for all-solid-state lithium batteries. Energy Mater. Adv. 5, 0074 (2024)
Mizuno, F., Hayashi, A., Tadanaga, K., et al.: New, highly ion-conductive crystals precipitated from Li2S-P2S5 glasses. Adv. Mater. 17, 918–921 (2005). https://doi.org/10.1002/adma.200401286
Kondo, S., Takada, K., Yamamura, Y.: New lithium ion conductors based on Li2S-SiS2 system. Solid State Ion. 53/54/55/56, 1183–1186 (1992). https://doi.org/10.1016/0167-2738(92)90310-l
Alpen, U.V., Rabenau, A., Talat, G.H.: Ionic conductivity in Li3N single crystals. Appl. Phys. Lett. 30, 621–623 (1977). https://doi.org/10.1063/1.89283
Yu, X.H., Bates, J.B., Jellison, G.E., et al.: A stable thin-film lithium electrolyte: lithium phosphorus oxynitride. J. Electrochem. Soc. 144, 524–532 (1997). https://doi.org/10.1149/1.1837443
Famprikis, T., Canepa, P., Dawson, J.A., et al.: Fundamentals of inorganic solid-state electrolytes for batteries. Nat. Mater. 18, 1278–1291 (2019). https://doi.org/10.1038/s41563-019-0431-3
Xiao, Y.H., Wang, Y., Bo, S.H., et al.: Understanding interface stability in solid-state batteries. Nat. Rev. Mater. 5, 105–126 (2020). https://doi.org/10.1038/s41578-019-0157-5
Richards, W.D., Miara, L.J., Wang, Y., et al.: Interface stability in solid-state batteries. Chem. Mater. 28, 266–273 (2016). https://doi.org/10.1021/acs.chemmater.5b04082
Guo, R.Q., Zhang, K., Zhao, W.B., et al.: Interfacial challenges and strategies toward practical sulfide-based solid-state lithium batteries. Energy Mater. Adv. 4, 0022 (2023). https://doi.org/10.34133/energymatadv.0022
Zhu, Y.Z., He, X.F., Mo, Y.F.: Origin of outstanding stability in the lithium solid electrolyte materials: insights from thermodynamic analyses based on first-principles calculations. ACS Appl. Mater. Interfaces 7, 23685–23693 (2015). https://doi.org/10.1021/acsami.5b07517
Wenzel, S., Randau, S., Leichtweiß, T., et al.: Direct observation of the interfacial instability of the fast ionic conductor Li10GeP2S12 at the lithium metal anode. Chem. Mater. 28, 2400–2407 (2016). https://doi.org/10.1021/acs.chemmater.6b00610
Wenzel, S., Weber, D.A., Leichtweiss, T., et al.: Interphase formation and degradation of charge transfer kinetics between a lithium metal anode and highly crystalline Li7P3S11 solid electrolyte. Solid State Ion. 286, 24–33 (2016). https://doi.org/10.1016/j.ssi.2015.11.034
Wenzel, S., Sedlmaier, S.J., Dietrich, C., et al.: Interfacial reactivity and interphase growth of argyrodite solid electrolytes at lithium metal electrodes. Solid State Ion. 318, 102–112 (2018). https://doi.org/10.1016/j.ssi.2017.07.005
Liang, Z.T., Xiang, Y.X., Wang, K.J., et al.: Understanding the failure process of sulfide-based all-solid-state lithium batteries via operando nuclear magnetic resonance spectroscopy. Nat. Commun. 14, 259 (2023). https://doi.org/10.1038/s41467-023-35920-7
Liu, Q.R., Chen, Q.Q., Tang, Y.B., et al.: Interfacial modification, electrode/solid-electrolyte engineering, and monolithic construction of solid-state batteries. Electrochem. Energy Rev. 6, 15 (2023). https://doi.org/10.1007/s41918-022-00167-1
Peng, Y., Xiong, X.S., Fan, W.J., et al.: Strategies to regulate the interface between Li metal anodes and all-solid-state electrolytes. Mater. Chem. Front. 8, 1421–1450 (2024). https://doi.org/10.1039/d3qm01023f
Wang, Q., Lu, T.T., Xiao, Y.B., et al.: Leap of Li metal anodes from coin cells to pouch cells: challenges and progress. Electrochem. Energy Rev. 6, 22 (2023). https://doi.org/10.1007/s41918-023-00185-7
Ye, L.H., Lu, Y., Wang, Y.C., et al.: Fast cycling of lithium metal in solid-state batteries by constriction-susceptible anode materials. Nat. Mater. 23, 244–251 (2024). https://doi.org/10.1038/s41563-023-01722-x
Wang, Z.Y., Xia, J.L., Ji, X., et al.: Lithium anode interlayer design for all-solid-state lithium-metal batteries. Nat. Energy 9, 251–262 (2024). https://doi.org/10.1038/s41560-023-01426-1
He, Z.J., Chen, L., Zhang, B.C., et al.: Flexible poly(ethylene carbonate)/garnet composite solid electrolyte reinforced by poly(vinylidene fluoride-hexafluoropropylene) for lithium metal batteries. J. Power Sources 392, 232–238 (2018). https://doi.org/10.1016/j.jpowsour.2018.05.006
Tian, Y.J., Ding, F., Zhong, H., et al.: Li6.75La3Zr1.75Ta0.25O12@amorphous Li3OCl composite electrolyte for solid state lithium-metal batteries. Energy Storage Mater. 14, 49–57 (2018). https://doi.org/10.1016/j.ensm.2018.02.015
Thompson, T., Sharafi, A., Johannes, M.D., et al.: A tale of two sites: on defining the carrier concentration in garnet-based ionic conductors for advanced Li batteries. Adv. Energy Mater. 5, 1500096 (2015). https://doi.org/10.1002/aenm.201500096
Chi, S.S., Liu, Y.C., Zhao, N., et al.: Solid polymer electrolyte soft interface layer with 3D lithium anode for all-solid-state lithium batteries. Energy Storage Mater. 17, 309–316 (2019). https://doi.org/10.1016/j.ensm.2018.07.004
Zhu, Y.Q., Zhang, Y.F., Lu, L.: Influence of crystallization temperature on ionic conductivity of lithium aluminum germanium phosphate glass-ceramic. J. Power Sources 290, 123–129 (2015). https://doi.org/10.1016/j.jpowsour.2015.04.170
Ma, F.R., Zhao, E.Q., Zhu, S.Y., et al.: Preparation and evaluation of high lithium ion conductivity Li1.3Al0.3Ti1.7(PO4)3 solid electrolyte obtained using a new solution method. Solid State Ion. 295, 7–12 (2016). https://doi.org/10.1016/j.ssi.2016.07.010
Ma, Q.L., Xu, Q., Tsai, C.L., et al.: A novel sol-gel method for large-scale production of nanopowders: preparation of Li1.5Al0.5Ti1.5(PO4)3 as an example. J. Am. Ceram. Soc. 99, 410–414 (2016). https://doi.org/10.1111/jace.13997
Palmer, M.J., Kalnaus, S., Dixit, M.B., et al.: A three-dimensional interconnected polymer/ceramic composite as a thin film solid electrolyte. Energy Storage Mater. 26, 242–249 (2020). https://doi.org/10.1016/j.ensm.2019.12.031
Sun, Y., Zhan, X.W., Hu, J.Z., et al.: Improving ionic conductivity with bimodal-sized Li7La3Zr2O12 fillers for composite polymer electrolytes. ACS Appl. Mater. Interfaces 11, 12467–12475 (2019). https://doi.org/10.1021/acsami.8b21770
Ohta, S., Kobayashi, T., Asaoka, T.: High lithium ionic conductivity in the garnet-type oxide Li7−XLa3(Zr2−X, NbX)O12 (X = 0–2). J. Power Sources 196, 3342–3345 (2011). https://doi.org/10.1016/j.jpowsour.2010.11.089
Liu, Z.C., Fu, W.J., Payzant, E.A., et al.: Anomalous high ionic conductivity of nanoporous β-Li3PS4. J. Am. Chem. Soc. 135, 975–978 (2013). https://doi.org/10.1021/ja3110895
Yamane, H., Shibata, M., Shimane, Y., et al.: Crystal structure of a superionic conductor, Li7P3S11. Solid State Ion. 178, 1163–1167 (2007). https://doi.org/10.1016/j.ssi.2007.05.020
Deiseroth, H.J., Maier, J., Weichert, K., et al.: Li7PS6 and Li6PS5X (X: Cl, Br, I): possible three-dimensional diffusion pathways for lithium ions and temperature dependence of the ionic conductivity by impedance measurements. Z. Für Anorg. Und Allg. Chem. 637, 1287–1294 (2011). https://doi.org/10.1002/zaac.201100158
Zhang, Y.B., Chen, R.J., Liu, T., et al.: High capacity, superior cyclic performances in all-solid-state lithium-ion batteries based on 78Li2S-22P2S5 glass-ceramic electrolytes prepared via simple heat treatment. ACS Appl. Mater. Interfaces 9, 28542–28548 (2017). https://doi.org/10.1021/acsami.7b06038
Whiteley, J.M., Taynton, P., Zhang, W., et al.: Ultra-thin solid-state Li-ion electrolyte membrane facilitated by a self-healing polymer matrix. Adv. Mater. 27, 6922–6927 (2015). https://doi.org/10.1002/adma.201502636
Hayashi, A., Muramatsu, H., Ohtomo, T., et al.: Improved chemical stability and cyclability in Li2S-P2S5-P2O5-ZnO composite electrolytes for all-solid-state rechargeable lithium batteries. J. Alloys Compd. 591, 247–250 (2014). https://doi.org/10.1016/j.jallcom.2013.12.191
Ju, J.W., Wang, Y.T., Chen, B.B., et al.: Integrated interface strategy toward room temperature solid-state lithium batteries. ACS Appl. Mater. Interfaces 10, 13588–13597 (2018). https://doi.org/10.1021/acsami.8b02240
Rao, R.P., Adams, S.: Studies of lithium argyrodite solid electrolytes for all-solid-state batteries. Phys. Status Solidi A 208, 1804–1807 (2011). https://doi.org/10.1002/pssa.201001117
Wang, C.H., Yu, R.Z., Duan, H., et al.: Solvent-free approach for interweaving freestanding and ultrathin inorganic solid electrolyte membranes. ACS Energy Lett. 7, 410–416 (2022). https://doi.org/10.1021/acsenergylett.1c02261
Asano, T., Sakai, A., Ouchi, S., et al.: Solid halide electrolytes with high lithium-ion conductivity for application in 4 V class bulk-type all-solid-state batteries. Adv. Mater. 30, 1803075 (2018). https://doi.org/10.1002/adma.201803075
Liang, J.W., Li, X.N., Wang, S., et al.: Site-occupation-tuned superionic LixScCl3+x halide solid electrolytes for all-solid-state batteries. J. Am. Chem. Soc. 142, 7012–7022 (2020). https://doi.org/10.1021/jacs.0c00134
Tomita, Y.: Ionic conductivity and structure of halocomplex salts of group 13 elements. Solid State Ion. 136(137), 351–355 (2000). https://doi.org/10.1016/s0167-2738(00)00491-4
Tomita, Y., Matsushita, H., Kobayashi, K., et al.: Substitution effect of ionic conductivity in lithium ion conductor, Li3InBr6−xClx. Solid State Ion. 179, 867–870 (2008). https://doi.org/10.1016/j.ssi.2008.02.012
Liu, Z.T., Ma, S., Liu, J., et al.: High ionic conductivity achieved in Li3Y(Br3Cl3) mixed halide solid electrolyte via promoted diffusion pathways and enhanced grain boundary. ACS Energy Lett. 6, 298–304 (2021). https://doi.org/10.1021/acsenergylett.0c01690
Duan, J., Wu, W.Y., Nolan, A.M., et al.: Lithium-graphite paste: an interface compatible anode for solid-state batteries. Adv. Mater. 31, 1807243 (2019). https://doi.org/10.1002/adma.201807243
Abouali, S., Yim, C.H., Merati, A., et al.: Garnet-based solid-state Li batteries: from materials design to battery architecture. ACS Energy Lett. 6, 1920–1941 (2021). https://doi.org/10.1021/acsenergylett.1c00401
Wang, T.R., Luo, W., Huang, Y.H.: Engineering Li metal anode for garnet-based solid-state batteries. Acc. Chem. Res. 56, 667–676 (2023). https://doi.org/10.1021/acs.accounts.2c00822
Zhang, Y., Lu, Y., Jin, J., et al.: Electrolyte design for lithium-ion batteries for extreme temperature applications. Adv. Mater. 36, 2308484 (2024). https://doi.org/10.1002/adma.202308484
Wang, L.C., Wu, J.X., Bao, C.S., et al.: Interfacial engineering for high-performance garnet-based solid-state lithium batteries. SusMat 4, 72–105 (2024). https://doi.org/10.1002/sus2.187
Luo, W., Gong, Y.H., Zhu, Y.Z., et al.: Reducing interfacial resistance between garnet-structured solid-state electrolyte and Li-metal anode by a germanium layer. Adv. Mater. 29, 1606042 (2017). https://doi.org/10.1002/adma.201606042
Xu, S.M., McOwen, D.W., Zhang, L., et al.: All-in-one lithium-sulfur battery enabled by a porous-dense-porous garnet architecture. Energy Storage Mater. 15, 458–464 (2018). https://doi.org/10.1016/j.ensm.2018.08.009
Han, X.G., Gong, Y.H., Fu, K., et al.: Negating interfacial impedance in garnet-based solid-state Li metal batteries. Nat. Mater. 16, 572–579 (2017). https://doi.org/10.1038/nmat4821
Cheng, E.J., Sharafi, A., Sakamoto, J.: Intergranular Li metal propagation through polycrystalline Li6.25Al0.25La3Zr2O12 ceramic electrolyte. Electrochim. Acta 223, 85–91 (2017). https://doi.org/10.1016/j.electacta.2016.12.018
Chung, H., Kang, B.: Mechanical and thermal failure induced by contact between a Li1.5Al0.5Ge1.5(PO4)3 solid electrolyte and Li metal in an all solid-state Li cell. Chem. Mater. 29, 8611–8619 (2017). https://doi.org/10.1021/acs.chemmater.7b02301
Li, Y.T., Chen, X., Dolocan, A., et al.: Garnet electrolyte with an ultralow interfacial resistance for Li-metal batteries. J. Am. Chem. Soc. 140, 6448–6455 (2018). https://doi.org/10.1021/jacs.8b03106
Sarkar, S., Thangadurai, V.: Critical current densities for high-performance all-solid-state Li-metal batteries: fundamentals, mechanisms, interfaces, materials, and applications. ACS Energy Lett. 7, 1492–1527 (2022). https://doi.org/10.1021/acsenergylett.2c00003
Peng, J., Wu, D.X., Song, F.M., et al.: High current density and long cycle life enabled by sulfide solid electrolyte and dendrite-free liquid lithium anode. Adv. Funct. Mater. 32, 2105776 (2022). https://doi.org/10.1002/adfm.202105776
Zhu, J.D., Zhang, Z., Zhao, S., et al.: Single-ion conducting polymer electrolytes for solid-state lithium-metal batteries: design, performance, and challenges. Adv. Energy Mater. 11, 2003836 (2021). https://doi.org/10.1002/aenm.202003836
Zheng, C.J., Lu, Y., Chang, Q., et al.: High-performance garnet-type solid-state lithium metal batteries enabled by scalable elastic and Li+-conducting interlayer. Adv. Funct. Mater. 33, 2302729 (2023). https://doi.org/10.1002/adfm.202302729
Cheng, Z.Z., Chen, Y., Shi, L., et al.: Long-lifespan lithium metal batteries enabled by a hybrid artificial solid electrolyte interface layer. ACS Appl. Mater. Interfaces 15, 10585–10592 (2023). https://doi.org/10.1021/acsami.2c18224
Fan, X.L., Ji, X., Han, F.D., et al.: Fluorinated solid electrolyte interphase enables highly reversible solid-state Li metal battery. Sci. Adv. 4, eaau9245 (2018). https://doi.org/10.1126/sciadv.aau9245
Kanamura, K., Shiraishi, S., Takehara, Z.I.: Electrochemical deposition of uniform lithium on an Ni substrate in a nonaqueous electrolyte. J. Electrochem. Soc. 141, L108–L110 (1994). https://doi.org/10.1149/1.2055169
Kanamura, K., Shiraishi, S., Tamura, H., et al.: X-ray photoelectron spectroscopic analysis and scanning electron microscopic observation of the lithium surface immersed in nonaqueous solvents. J. Electrochem. Soc. 141, 2379–2385 (1994). https://doi.org/10.1149/1.2055129
Takehara, Z.I.: Future prospects of the lithium metal anode. J. Power Sources 68, 82–86 (1997). https://doi.org/10.1016/s0378-7753(96)02546-3
Han, F.D., Yue, J., Zhu, X.Y., et al.: Suppressing Li dendrite formation in Li2S-P2S5 solid electrolyte by LiI incorporation. Adv. Energy Mater. 8, 1703644 (2018). https://doi.org/10.1002/aenm.201703644
Chen, G.P., Niu, C.Q., Chen, Y.B., et al.: A single-ion conducting polymer electrolyte based on poly(lithium 4-styrenesulfonate) for high-performance lithium metal batteries. Solid State Ion. 341, 115048 (2019). https://doi.org/10.1016/j.ssi.2019.115048
Shin, D.M., Bachman, J.E., Taylor, M.K., et al.: A single-ion conducting borate network polymer as a viable quasi-solid electrolyte for lithium metal batteries. Adv. Mater. 32, 1905771 (2020). https://doi.org/10.1002/adma.201905771
Guan, X., Wu, Q.P., Zhang, X.W., et al.: In-situ crosslinked single ion gel polymer electrolyte with superior performances for lithium metal batteries. Chem. Eng. J. 382, 122935 (2020). https://doi.org/10.1016/j.cej.2019.122935
Liu, J., Zhou, J.Q., Wang, M.F., et al.: A functional-gradient-structured ultrahigh modulus solid polymer electrolyte for all-solid-state lithium metal batteries. J. Mater. Chem. A 7, 24477–24485 (2019). https://doi.org/10.1039/c9ta07876b
Wu, N., Li, Y.T., Dolocan, A., et al.: In situ formation of Li3P layer enables fast Li+ conduction across Li/solid polymer electrolyte interface. Adv. Funct. Mater. 30, 2000831 (2020). https://doi.org/10.1002/adfm.202000831
Yang, P., Gao, X.W., Tian, X.L., et al.: Upgrading traditional organic electrolytes toward future lithium metal batteries: a hierarchical nano-SiO2-supported gel polymer electrolyte. ACS Energy Lett. 5, 1681–1688 (2020). https://doi.org/10.1021/acsenergylett.0c00412
Wang, G.X., He, P.G., Fan, L.Z.: Asymmetric polymer electrolyte constructed by metal-organic framework for solid-state, dendrite-free lithium metal battery. Adv. Funct. Mater. 31, 2007198 (2021). https://doi.org/10.1002/adfm.202007198
Le, H.T.T., Ngo, D.T., Ho, V.C., et al.: Insights into degradation of metallic lithium electrodes protected by a bilayer solid electrolyte based on aluminium substituted lithium lanthanum titanate in lithium-air batteries. J. Mater. Chem. A 4, 11124–11138 (2016). https://doi.org/10.1039/c6ta03653h
Huo, H.Y., Gao, J., Zhao, N., et al.: A flexible electron-blocking interfacial shield for dendrite-free solid lithium metal batteries. Nat. Commun. 12, 176 (2021). https://doi.org/10.1038/s41467-020-20463-y
Kataoka, K., Nagata, H., Akimoto, J.: Lithium-ion conducting oxide single crystal as solid electrolyte for advanced lithium battery application. Sci. Rep. 8, 9965 (2018). https://doi.org/10.1038/s41598-018-27851-x
Jiang, Z., Liang, T.B., Liu, Y., et al.: Improved ionic conductivity and Li dendrite suppression capability toward Li7P3S11-based solid electrolytes triggered by Nb and O cosubstitution. ACS Appl. Mater. Interfaces 12, 54662–54670 (2020). https://doi.org/10.1021/acsami.0c15903
Deng, T., Ji, X., Zhao, Y., et al.: Tuning the anode-electrolyte interface chemistry for garnet-based solid-state Li metal batteries. Adv. Mater. 32, 2000030 (2020). https://doi.org/10.1002/adma.202000030
Hitz, G.T., McOwen, D.W., Zhang, L., et al.: High-rate lithium cycling in a scalable trilayer Li-garnet-electrolyte architecture. Mater. Today 22, 50–57 (2019). https://doi.org/10.1016/j.mattod.2018.04.004
Wan, H.L., Liu, S.F., Deng, T., et al.: Bifunctional interphase-enabled Li10GeP2S12 electrolytes for lithium-sulfur battery. ACS Energy Lett. 6, 862–868 (2021). https://doi.org/10.1021/acsenergylett.0c02617
Garcia-Mendez, R., Mizuno, F., Zhang, R.G., et al.: Effect of processing conditions of 75Li2S-25P2S5 solid electrolyte on its DC electrochemical behavior. Electrochim. Acta 237, 144–151 (2017). https://doi.org/10.1016/j.electacta.2017.03.200
Liu, G.Z., Weng, W., Zhang, Z.H., et al.: Densified Li6PS5Cl nanorods with high ionic conductivity and improved critical current density for all-solid-state lithium batteries. Nano Lett. 20, 6660–6665 (2020). https://doi.org/10.1021/acs.nanolett.0c02489
Ji, X., Hou, S., Wang, P.F., et al.: Solid-state electrolyte design for lithium dendrite suppression. Adv. Mater. 32, 2002741 (2020). https://doi.org/10.1002/adma.202002741
Su, Y.B., Ye, L.H., Fitzhugh, W., et al.: A more stable lithium anode by mechanical constriction for solid state batteries. Energy Environ. Sci. 13, 908–916 (2020). https://doi.org/10.1039/c9ee04007b
Shi, X., Pang, Y., Wang, B., et al.: In situ forming LiF nanodecorated electrolyte/electrode interfaces for stable all-solid-state batteries. Mater. Today Nano 10, 100079 (2020). https://doi.org/10.1016/j.mtnano.2020.100079
Wei, Y.Q., Yang, Y.X., Chen, Z.C., et al.: In situ generated electron-blocking lih enabling an unprecedented critical current density of over 15 mA cm−2 for solid-state hydride electrolytes. Adv. Mater. 35, 2304285 (2023). https://doi.org/10.1002/adma.202304285
Suzuki, Y., Kami, K., Watanabe, K., et al.: Transparent cubic garnet-type solid electrolyte of Al2O3-doped Li7La3Zr2O12. Solid State Ion. 278, 172–176 (2015). https://doi.org/10.1016/j.ssi.2015.06.009
Ren, Y.Y., Shen, Y., Lin, Y.H., et al.: Direct observation of lithium dendrites inside garnet-type lithium-ion solid electrolyte. Electrochem. Commun. 57, 27–30 (2015). https://doi.org/10.1016/j.elecom.2015.05.001
Shen, F.Y., Dixit, M.B., Xiao, X.H., et al.: Effect of pore connectivity on Li dendrite propagation within LLZO electrolytes observed with synchrotron X-ray tomography. ACS Energy Lett. 3, 1056–1061 (2018). https://doi.org/10.1021/acsenergylett.8b00249
Singh, D.K., Fuchs, T., Krempaszky, C., et al.: Origin of the lithium metal anode instability in solid-state batteries during discharge. Matter 6, 1463–1483 (2023). https://doi.org/10.1016/j.matt.2023.02.008
Sandoval, S.E., McDowell, M.T.: Lithium metal anodes in solid-state batteries: metal microstructure matters. Matter 6, 2101–2102 (2023). https://doi.org/10.1016/j.matt.2023.05.017
Wang, M.J., Choudhury, R., Sakamoto, J.: Characterizing the Li-solid-electrolyte interface dynamics as a function of stack pressure and current density. Joule 3, 2165–2178 (2019). https://doi.org/10.1016/j.joule.2019.06.017
Yao, J.H., Zhu, G.X., Dong, K., et al.: Progress and perspective of controlling Li dendrites growth in all-solid-state Li metal batteries via external physical fields. Adv. Energy Sustain. Res. 5, 2300165 (2024). https://doi.org/10.1002/aesr.202300165
Liu, X.M., Garcia-Mendez, R., Lupini, A.R., et al.: Local electronic structure variation resulting in Li ‘filament’ formation within solid electrolytes. Nat. Mater. 20, 1485–1490 (2021). https://doi.org/10.1038/s41563-021-01019-x
Biao, J., Han, B., Cao, Y.D., et al.: Inhibiting formation and reduction of Li2CO3 to LiCx at grain boundaries in garnet electrolytes to prevent Li penetration. Adv. Mater. 35, 2208951 (2023). https://doi.org/10.1002/adma.202208951
Jia, Z.H., Shen, H., Kou, J.W., et al.: Solid electrolyte bimodal grain structures for improved cycling performance. Adv. Mater. 36, 2309019 (2024). https://doi.org/10.1002/adma.202309019
Ning, Z.Y., Jolly, D.S., Li, G.C., et al.: Visualizing plating-induced cracking in lithium-anode solid-electrolyte cells. Nat. Mater. 20, 1121–1129 (2021). https://doi.org/10.1038/s41563-021-00967-8
Wang, W., Wang, J.X., Lin, C., et al.: Modeling of void-mediated cracking and lithium penetration in all-solid-state batteries. Adv. Funct. Mater. 33, 2303484 (2023). https://doi.org/10.1002/adfm.202303484
Tufail, M.K., Zhai, P.B., Jia, M.Y., et al.: Design of solid electrolytes with fast ion transport: computation-driven and practical approaches. Energy Mater. Adv. 4, 0015 (2023). https://doi.org/10.34133/energymatadv.0015
Hashin, Z.: Analysis of composite materials: a survey. J. Appl. Mech. 50, 481–505 (1983). https://doi.org/10.1115/1.3167081
Kalnaus, S., Sabau, A.S., Tenhaeff, W.E., et al.: Design of composite polymer electrolytes for Li ion batteries based on mechanical stability criteria. J. Power Sources 201, 280–287 (2012). https://doi.org/10.1016/j.jpowsour.2011.11.020
Ding, Y.Q., He, B., Wang, D., et al.: Software for evaluating ionic conductivity of inorganic-polymer composite solid electrolytes. Energy Mater. Adv. 4, 0041 (2023). https://doi.org/10.34133/energymatadv.0041
Wang, G.X., Yang, L., Wang, J.Z., et al.: Enhancement of ionic conductivity of PEO based polymer electrolyte by the addition of nanosize ceramic powders. J. Nanosci. Nanotech. 5, 1135–1140 (2005). https://doi.org/10.1166/jnn.2005.165
Lin, D.C., Liu, W., Liu, Y.Y., et al.: High ionic conductivity of composite solid polymer electrolyte via in situ synthesis of monodispersed SiO2 nanospheres in poly(ethylene oxide). Nano Lett. 16, 459–465 (2016). https://doi.org/10.1021/acs.nanolett.5b04117
Fan, L.Z., Dang, Z.M., Wei, G.D., et al.: Effect of nanosized ZnO on the electrical properties of (PEO)16LiClO4 electrolytes. Mater. Sci. Eng. B 99, 340–343 (2003). https://doi.org/10.1016/s0921-5107(02)00487-7
Xiong, H.: Elucidating the conductivity enhancement effect of nano-sized SnO2 fillers in the hybrid polymer electrolyte PEO-SnO2-LiClO4. Solid State Ion. 159, 89–95 (2003). https://doi.org/10.1016/s0167-2738(02)00917-7
Chen-Yang, Y.: Polyacrylonitrile electrolytes. 1. A novel high-conductivity composite polymer electrolyte based on PAN, LiClO4 and α-Al2O3. Solid State Ion. 150, 327–335 (2002). https://doi.org/10.1016/s0167-2738(02)00457-5
Lim, Y.S., Jung, H.A., Hwang, H.: Fabrication of PEO-PMMA-LiClO4-based solid polymer electrolytes containing silica aerogel particles for all-solid-state lithium batteries. Energies 11, 2559 (2018). https://doi.org/10.3390/en11102559
Yap, Y.L., You, A.H., Teo, L.L.: Preparation and characterization studies of PMMA-PEO-blend solid polymer electrolytes with SiO2 filler and plasticizer for lithium ion battery. Ionics 25, 3087–3098 (2019). https://doi.org/10.1007/s11581-019-02842-8
Zhao, Y.R., Wu, C., Peng, G., et al.: A new solid polymer electrolyte incorporating Li10GeP2S12 into a polyethylene oxide matrix for all-solid-state lithium batteries. J. Power Sources 301, 47–53 (2016). https://doi.org/10.1016/j.jpowsour.2015.09.111
Bae, J., Li, Y.T., Zhang, J., et al.: A 3D nanostructured hydrogel-framework-derived high-performance composite polymer lithium-ion electrolyte. Angew. Chem. Int. Ed. 57, 2096–2100 (2018). https://doi.org/10.1002/anie.201710841
Wang, C.H., Yang, Y.F., Liu, X.J., et al.: Suppression of lithium dendrite formation by using LAGP-PEO (LiTFSI) composite solid electrolyte and lithium metal anode modified by PEO (LiTFSI) in all-solid-state lithium batteries. ACS Appl. Mater. Interfaces 9, 13694–13702 (2017). https://doi.org/10.1021/acsami.7b00336
Zhang, Y.B., Chen, R.J., Wang, S., et al.: Free-standing sulfide/polymer composite solid electrolyte membranes with high conductance for all-solid-state lithium batteries. Energy Storage Mater. 25, 145–153 (2020). https://doi.org/10.1016/j.ensm.2019.10.020
Fu, X.L., Li, Y.C., Liao, C.Z., et al.: Enhanced electrochemical performance of solid PEO/LiClO4 electrolytes with a 3D porous Li6.28La3Zr2Al02.4O1.2 network. Compos. Sci. Technol. 184, 107863 (2019)
Li, J., Zhu, K.J., Yao, Z.R., et al.: A promising composite solid electrolyte incorporating LLZO into PEO/PVDF matrix for all-solid-state lithium-ion batteries. Ionics 26, 1101–1108 (2020). https://doi.org/10.1007/s11581-019-03320-x
Liu, W., Liu, N., Sun, J., et al.: Ionic conductivity enhancement of polymer electrolytes with ceramic nanowire fillers. Nano Lett. 15, 2740–2745 (2015). https://doi.org/10.1021/acs.nanolett.5b00600
Liu, W., Lee, S.W., Lin, D.C., et al.: Enhancing ionic conductivity in composite polymer electrolytes with well-aligned ceramic nanowires. Nat. Energy 2, 17035 (2017). https://doi.org/10.1038/nenergy.2017.35
Wang, S., Zhang, X., Liu, S.J., et al.: High-conductivity free-standing Li6PS5Cl/poly(vinylidene difluoride) composite solid electrolyte membranes for lithium-ion batteries. J. Materiomics 6, 70–76 (2020). https://doi.org/10.1016/j.jmat.2019.12.010
Hu, J.K., He, P.G., Zhang, B.C., et al.: Porous film host-derived 3D composite polymer electrolyte for high-voltage solid state lithium batteries. Energy Storage Mater. 26, 283–289 (2020). https://doi.org/10.1016/j.ensm.2020.01.006
Zhang, W.Q., Nie, J.H., Li, F., et al.: A durable and safe solid-state lithium battery with a hybrid electrolyte membrane. Nano Energy 45, 413–419 (2018). https://doi.org/10.1016/j.nanoen.2018.01.028
Gai, J.L., Ma, F.R., Zhang, Z.Q., et al.: Flexible organic-inorganic composite solid electrolyte with asymmetric structure for room temperature solid-state Li-ion batteries. ACS Sustain. Chem. Eng. 7, 15896–15903 (2019). https://doi.org/10.1021/acssuschemeng.9b01869
Sun, J.Q., Li, Y.G., Zhang, Q.H., et al.: A highly ionic conductive poly(methyl methacrylate) composite electrolyte with garnet-typed Li6.75La3Zr1.75Nb0.25O12 nanowires. Chem. Eng. J. 375, 121922 (2019)
Chen, L., Li, W.X., Fan, L.Z., et al.: Intercalated electrolyte with high transference number for dendrite-free solid-state lithium batteries. Adv. Funct. Mater. 29, 1901047 (2019). https://doi.org/10.1002/adfm.201901047
Li, J., Chen, H.W., Shen, Y.B., et al.: Covalent interfacial coupling for hybrid solid-state Li ion conductor. Energy Storage Mater. 23, 277–283 (2019). https://doi.org/10.1016/j.ensm.2019.05.002
Liu, Y.Y., Xu, X.Y., Kapitanova, O.O., et al.: Electro-chemo-mechanical modeling of artificial solid electrolyte interphase to enable uniform electrodeposition of lithium metal anodes. Adv. Energy Mater. 12, 2103589 (2022). https://doi.org/10.1002/aenm.202103589
Wang, J.Y., Huang, W., Pei, A., et al.: Improving cyclability of Li metal batteries at elevated temperatures and its origin revealed by cryo-electron microscopy. Nat. Energy 4, 664–670 (2019). https://doi.org/10.1038/s41560-019-0413-3
Gao, Y., Yan, Z.F., Gray, J.L., et al.: Polymer-inorganic solid-electrolyte interphase for stable lithium metal batteries under lean electrolyte conditions. Nat. Mater. 18, 384–389 (2019). https://doi.org/10.1038/s41563-019-0305-8
Lai, H.J., Lu, Y., Zha, W.P., et al.: In situ generated composite gel polymer electrolyte with crosslinking structure for dendrite-free and high-performance sodium metal batteries. Energy Storage Mater. 54, 478–487 (2023). https://doi.org/10.1016/j.ensm.2022.10.032
Isaac, J.A., Devaux, D., Bouchet, R.: Dense inorganic electrolyte particles as a lever to promote composite electrolyte conductivity. Nat. Mater. 21, 1412–1418 (2022). https://doi.org/10.1038/s41563-022-01343-w
Fu, K.K., Gong, Y.H., Dai, J.Q., et al.: Flexible, solid-state, ion-conducting membrane with 3D garnet nanofiber networks for lithium batteries. Proc. Natl. Acad. Sci. U. S. A. 113, 7094–7099 (2016). https://doi.org/10.1073/pnas.1600422113
Wang, C.H., Adair, K.R., Liang, J.W., et al.: Solid-state plastic crystal electrolytes: effective protection interlayers for sulfide-based all-solid-state lithium metal batteries. Adv. Funct. Mater. 29, 1900392 (2019). https://doi.org/10.1002/adfm.201900392
Chen, Y., Li, W.W., Sun, C.Z., et al.: Sustained release-driven formation of ultrastable sei between Li6PS5Cl and lithium anode for sulfide-based solid-state batteries. Adv. Energy Mater. 11, 2002545 (2021). https://doi.org/10.1002/aenm.202002545
Chen, Y., Yao, L., Chen, X.D., et al.: Double-faced bond coupling to induce an ultrastable lithium/Li6PS5Cl interface for high-performance all-solid-state batteries. ACS Appl. Mater. Interfaces 14, 11950–11961 (2022). https://doi.org/10.1021/acsami.1c24506
Wang, Z.Y., Shen, L., Deng, S.G., et al.: 10 μm-Thick high-strength solid polymer electrolytes with excellent interface compatibility for flexible all-solid-state lithium-metal batteries. Adv. Mater. 33, 2100353 (2021). https://doi.org/10.1002/adma.202100353
Zhang, Z., Gou, J.R., Cui, K.X., et al.: 126 μm-Thick asymmetric composite electrolyte with superior interfacial stability for solid-state lithium-metal batteries. Nano Micro Lett. 16, 181 (2024). https://doi.org/10.1007/s40820-024-01389-2
Bao, C.S., Zheng, C.J., Wu, M.F., et al.: 12 µm-Thick sintered garnet ceramic skeleton enabling high-energy-density solid-state lithium metal batteries. Adv. Energy Mater. 13, 2204028 (2023). https://doi.org/10.1002/aenm.202204028
Acknowledgements
This work is supported by the National Key Research and Development Program of China (2022YFB2404400), the National Natural Science Foundation of China (22075025, 22379016), the funding from General Research Institute for Nonferrous Metals (C712620213102034), and the Science and Technology Program of Guangdong Province (Grant No. 32020B0909030004).
Funding
Key Technologies Research and Development Program, (2022YFB2404400), Chuan Wu
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhong, Y., Yang, X., Guo, R. et al. Protecting Lithium Metal Anodes in Solid-State Batteries. Electrochem. Energy Rev. 7, 30 (2024). https://doi.org/10.1007/s41918-024-00230-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41918-024-00230-z