Abstract
The kidneys in people living with HIV (PLWH) are constantly exposed to highly antiretroviral therapy (HAART) which may cause renal dysfunction. Cystatin C (CystC), a biomarker of renal function, is well associated with renal impairment in various populations, however, it is underexplored in the South African population of PLWH, as compared to creatinine-based measurements. Creatinine-based measurements for assessing kidney function in people with HIV can be limited due to the influence of muscle wasting, altered creatinine metabolism, and the potential for certain antiretroviral medications to impact creatinine secretion. Therefore, the current study aimed to explore the effect of HAART on renal function among PLWH with the use of Cyst C-based measures. We conducted a cross-sectional study of 111 adults PLWH, 84 on HAART, and 27 on HAART-naïve. The cluster of differentiation 4 (CD4 +) count, plasma CystC, as well as the estimated glomerular filtration rate (eGFR) using the chronic kidney disease-epidemiology collaboration (CKD-EPI) formula, were determined. In the present study, no significant differences were observed between HAART-treated and HAART-naïve groups in terms of plasma CystC and eGFRCystC. Participants who were on HAART for > 5 years were significantly associated with eGFRCystC < 90 mL/min/1.73 m2, as indicated by substantial odds ratio of 4.39 (confidence interval: 1.60–12.02, p = 0.004) in the unadjusted analysis and even after adjustment for age, sex and smoking status, with odds ratio of 4.10 (confidence interval: 1.41–11.86, p = 0.009). Advanced World Health Organization (WHO) clinical stage significantly associated with eGFRCystC < 90 mL/min/1.73 m2 as indicated by odds ratio of 11.73 (confidence interval: 2.27–60.75, p = 0.003) in the unadjusted analysis. Even after adjusting for confounders which included age, sex, and smoking status, the association persisted, with an odds ratio of 16.71 (confidence interval: 2.57–108.75, p = 0.003). In conclusion, CystC and eGFRCystC levels were not different between PLWH on HAART and not on HAART but prolonged HAART use for over five years and advanced HIV disease were associated with reduced renal function in this population of PLWH on HAART, as assessed using CystC-based measures. These findings may suggest that HAART had initial beneficial effects and long-term adverse effects. The future risk of progressive eGFR decline may be increased in this HIV population on HAART due to the cumulative effect of prolonged tenofovir disoproxil fumarate (TDF) use. These findings remain important to guide future research with larger populations on the management of conditions related to renal function decline in PLWH.
Highlights.
Highlights
-
There was no difference in eGFRCystC between the HAART-treated and HAART-naïve groups.
-
Prolonged HAART use (especially with TDF) for more than five years and advanced HIV disease were associated with reduced renal function.
-
These findings may reflect the initial benefit of HAART on renal function and long-term harmful effects due to the potential adverse effects of TDF.
-
The PLWH on HAART may be at an increased risk of progressive renal function decline.
-
This highlights the need for regular monitoring of renal function in this population and consideration of less nephrotoxic treatment options.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The burden of renal dysfunction in people living with HIV (PLWH) on highly active antiretroviral therapy (HAART) is escalating and has serious clinical and economic implications [1]. The kidneys are pivotal in the metabolism and excretion of drugs [2] and are constantly exposed to antiretroviral drugs among PLWH. Several factors which include HIV infection, low CD4 + counts, chronic inflammation, older age, comorbidities, co-infections, genetic predisposition, and HAART have been implicated in renal dysfunction among PLWH [3]. The impact of HAART on renal function has been studied extensively, with substantial evidence demonstrating its beneficial and nephrotoxic effects [4].
Previous clinical trials, observational and case reports have shown that regardless of the drug, HAART is beneficial in improving renal function among individuals with HIV-associated kidney disease, advanced HIV stage and low CD4 + counts [5]. Some observational studies have demonstrated renal function improvement in HAART-treated individuals on tenofovir, protease inhibitors and other nucleotide reverse transcriptase inhibitor (NRTI)-based regimens in the absence of advanced HIV stage and abnormal baseline creatinine and urea concentrations [6]. Interestingly, renal function remained stable after 16 months of initiating the treatment for up to 7 years [7]. Jaroszewicz et al. (2006) [8] observed a decrease in serum CystC concentration during prolonged HAART use (mean HAART exposure duration of 36 months), which was indicative of renal function improvement. Furthermore, the participants had CD4 + counts > 350 cells/µl, undetectable viral loads and were not using tenofovir-based regimen [8]. Conversely, a plethora of studies also reported that HAART is detrimental on the kidneys, causing renal function decline and increased incidence of mild-to-moderate and severe renal dysfunction [9]. The use of tenofovir disoproxil fumarate (TDF) is mostly implicated in nephrotoxicity among HAART users, with research findings demonstrating its association with reductions in estimated glomerular filtration rate (eGFR) and progression to chronic kidney disease (CKD) [10, 11]. Similar associations were also observed with ritonavir-boosted atazanavir or lopinavir use [12, 13] in addition to the reports of crystalluria, nephrolithiasis, and tubulointerstitial nephritis [10, 14,15,16]. Indinavir has been associated with nephrolithiasis, crystalluria, and obstructive acute renal failure [17]. Renal loss occurred during the first 12 months on HAART onwards up to 10 years [11], with cumulative exposure to TDF for over 5 years strongly correlating with tubular dysfunction [18] and eGFR decrement [11, 19]. These findings underscore the need to continuously monitor and update renal function among PLWH on HAART, especially because the recently approved prodrug of TDF, tenofovir alafenamide fumarate (TAF), is generally unavailable in Africa and all PLWH starting ART are placed on TDF-based regimen.
Although considered one of the epicentres of HIV [20], there is still a paucity of data reporting on the impact of HAART on renal function by CystC-based measures among PLWH in South Africa. Plasma CystC is now a widely recognized alternative marker for creatinine, with superiority over creatinine as a marker of glomerular filtration rate [21, 22]. Unlike creatinine which has several limitations, CystC is produced at a constant rate and is not affected by muscle mass or protein intake [8, 22]. This biomarker has also been implemented in clinical practice in some high-income European countries [23]. The current study aimed to assess the impact of HAART on renal function using plasma CystC-based measures.
2 Materials and methods
2.1 Study design, setting and population
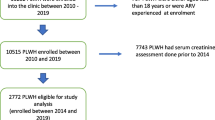
This study employed a cross-sectional design and was conducted at the Mankweng Hospital situated in rural setting in Sovenga, Limpopo Province, between 02 October 2019 and 02 October 2020. The hospital provides a range of healthcare services including counselling, family planning, diagnostic testing, management, and treatment of various diseases. A formula developed by Cochran (1963) [24], with a 5% error and a 95% confidence level, was used to determine the sample size (n = 111) of this study. Subsequently, HAART-treated (n = 84) and HAART-naïve (n = 27) PLWH were included.
2.2 Inclusion and exclusion criteria
The inclusion criterion was based on HIV status, whether patients were on HAART or not, which was followed by the consent to participate in the study. To be included, the patients had to have an HIV diagnosis and be treated or not treated with HAART. The study encompassed both male and female adults over the age of 18 years. Upon screening their medical files, participants were excluded if they presented with renal dysfunction (defined as an estimated glomerular filtration < 60 mL/min/1.73m2 for at least 3 months with or without proteinuria), chronic conditions such as hypertension (defined as a persistent elevated of blood pressure above 140/90 mmHg), diabetes (defined as fasting plasma glucose ≥ 7.0 mmol/L, 2-h oral glucose tolerance test plasma glucose ≥ 11.1 mmol/L or glycated haemoglobin ≥ 48 mmol/mol) and dyslipidaemia (defined as the presence of abnormal serum lipid levels that increase the risk of cardiovascular events) [25,26,27,28]. This further encompasses other cardiovascular-related conditions and co-infections such as tuberculosis at the time of enrolment based on their medical records. Participants were also excluded if they presented with thyroid dysfunction and were taking treatments that affect renal function such as cimetidine, nonsteroidal anti-inflammatory drugs, diuretics, angiotensin-converting enzyme inhibitors, antimicrobials or adrenocorticosteroids at the time of enrolment. Breastfeeding and pregnant women were also excluded. A structured questionnaire was used to collect demographic and medical information including age, gender, HIV status, smoking status, HAART use, type of HAART regimen and duration on the treatment.
2.3 Ethics
This study was approved by the University of Limpopo Turfloop Research and Ethics Committee (TREC) (project number TREC/315/2019: PG), as part of the initial project approved by TREC (project number TREC/119/2016: PG). The Department of Health, Primary Health Care Division and Social Development (Limpopo Province) also granted permission to conduct the study. The study was conducted in accordance with the principles of the Declaration of Helsinki [29]. Prior to commencing data collection, the purpose of the study was carefully explained to all participants and written informed consent was obtained.
2.4 Blood collection, HIV status confirmation and CD4 + count determination
Fasting venous blood was collected from all participants and centrifuged to obtain serum and plasma using the Allegra X-30 benchtop centrifuge (Beckman Coulter, Indianapolis, United States) according to manufacturer instructions. The HIV test was performed to confirm the status of participants using the Alere Determine HIV-1/2 kit (Alere to Abbott Medical Co Ltd., Japan), following the manufacturer’s instructions. The CD4 + count was determined in whole blood samples using a factory-calibrated Alere PIMA analyzer (Alere Technologies GmbH, Germany) according to the manufacturer’s instructions. World Health Organization (WHO) classification criteria were used to classify the CD4 + count within the study population, based on different clinical stages [30].
2.5 Renal function assessment
A bead-based Luminex 200™ analyzer (Merck KGaA, Germany) was used to quantify the levels of cyst C in plasma according to the manufacturer’s instructions. Cyst C was used to determine eGFR using the CKD-EPI formula as described below [31] and was classified using the reference ranges as previously reported [32, 33]. The eGFRCystC was also classified into various stages according to the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF KDOQI) criteria [34]. Reduced renal function was defined as an eGFRCystC < 90 mL/min/1.73m2 in this study.
eGFR = 133 × min (Cyst C/0.8, 1)−0.499 × max (Cyst C/0.8, 1)−1.328 × 0.996Age × 0.932 [if female].
eGFR (estimated glomerular filtration rate) = mL/min/1.73 m.2
Cyst C (standardized cyst C) = mg/l.
min = indicates the minimum of Cyst C/0.8 or 1.
max = indicates the maximum of Cyst C/0.8 or 1.
age = years.
2.6 Statistical analysis
All statistical analysis was performed using the International Business Machine (IBM) statistical package for the social science (SPSS) software version 29 (IBM corporation, United States of America). The normality of the data was assessed by using Shapiro–Wilk test. The normally distributed continuous variables were expressed as mean ± standard deviation (SD) while median (interquartile range) were used to express not normally distributed data. Chi-square test was employed to compare percentages for categorical variables. Independent samples t-test was employed to compare means of normally distributed variables. Mann–Whitney and Kruskal–Wallis tests were used for variables that were not normally distributed. Binary logistic regression analysis was performed to assess the association between HAART exposure, WHO clinical stage and renal function outcomes. The significance differences and association levels were assumed at a p-value < 0.05.
3 Results
3.1 3.1. An overview of characteristic features of participants, comparing age, CD4 + count, sex and WHO clinical stage in PLWH on HAART and HAART-naïve individuals
Table 1 gives an overview of information regarding age, sex, CD4 + count, duration on HAART regimens, type of HAART regimen, and smoking status in PLWH on HAART versus HAART-naïve individuals. The study population consisted of adult black African PLWH (n = 111), who were subdivided into two groups of PLWH on HAART (n = 84) and those not on HAART or HAART-naïve individuals (n = 27). The results showed that the total population had a mean age of 41.86 ± 10.54 years. The mean age for PLWH on HAART and HAART-naïve were 42.96 ± 10.51 years and 38.44 ± 10.09 years, respectively. The HAART-treated group consisted mostly of participants from the age group 40–49 years (34.5%) while the HAART-treated group consisted mostly of participants from the age group 30–39 years (33.3%). The study population consisted of a high proportion of females (66.7%) compared to males (33.3%). The overall mean CD4 + count was 412.41 ± 241.52 cells/µL while the PLWH on HAART had a significantly higher mean CD4 + count (440.25 ± 221.60 cells/µL) compared to the HAART-naïve group (319.22 ± 284.72 cells/µL) (p = 0.034). A significant difference in WHO clinical stages was observed between the PLWH on HAART and HAART-naïve (p = 0.008).
The median duration of HAART treatment was 53 months, while those on the TDF-based regimen were 51 months, with the 25th and 75th percentiles ranging from 20 to 90 months. The majority of the HAART-treated group was under the HAART duration of < 5 years (53.2%). Most HAART users were on tenofovir disoproxil fumarate (TDF) plus emtricitabine and efavirenz (TDF + FTC + EFV) regimen (77%). All the tenofovir used in the study population was TDF and not TAF. First-line therapy consisted of TDF + FTC + EFV (95%), AZT + 3TC + NVP (3.2%) and ABC + 3TC + EFV (1.6%). Second-line therapy consisted of AZT + 3TC + LPV/r (93.8%) and TDF + FTC + LPV/r (6.3%). There was no significant difference in smoking status between the PLWH on HAART and HAART-naïve (p = 0.770).
3.2 A comparative analysis of HAART-treated vs HAART-naïve groups in terms renal function markers
Table 2 gives an overview of comparative analysis of Cyst C and eGFRCystC between PLWH on HAART and HAART-naïve individuals. The median Cyst C levels were 0.72 (0.54–0.97) mg/L in the overall population, however, no significance difference was observed in terms of the median Cyst C levels between the PLWH on HAART versus HAART-naïve (p = 0.053). The HAART-treated group did not show any significance difference compared to the HAART-naïve group in terms of proportions of altered Cyst C and eGFRCystC levels. Majority of participants in both the HAART-treated group (67.9%) and HAART-naïve group (81.5%) had normal eGFRCystC levels.
3.3 Assessing the association of reduced renal function with WHO clinical stage and duration on HAART
A binary logistic regression analysis was performed to determine whether the WHO clinical stage and duration on HAART are associated with poor renal function or not in PLWH (Table 3). WHO clinical stage IV, also called advanced WHO clinical stage (defined as a CD4 + count of < 200 cells/µL), significantly associated with eGFRCystC < 90 mL/min/1.73 m2 as indicated by odds ratio of 11.73 (confidence interval: 2.27–60.75, p = 0.003) in the crude analysis. Even after adjusting for confounders which included age, sex and smoking status, the association persisted, with odds ratio of 16.71 (confidence interval: 2.57–108.75, p = 0.003). The duration on HAART > 5 years significantly associated with eGFRCystC < 90 mL/min/1.73 m2 in the crude analysis with odds ratio of 4.39 (confidence interval: 1.60–12.02, p = 0.004) and even after adjustment for age, sex and smoking status, with an odds ratio of 4.10 (confidence interval: 1.41–11.86, p = 0.009).
4 Discussion
Our initial findings revealed no significant difference in both Cyst C and eGFRCystC levels between the HAART-treated and HAART-naïve groups, although further analysis showed that being on HAART for more than 5 years and advanced WHO clinical stage associated with low eGFRCystC among HAART-treated PLWH. This may reflect the initial beneficial effect of HAART on improving renal function and long-term detrimental effect due to cumulative adverse effects of nephrotoxic regimens, particularly TDF. HAART is generally beneficial on renal function, especially among people with HIV-associated renal disease due to viral suppression and attenuation of HIV-induced damage [35], albeit this beneficial effect may not occur among individuals with poor treatment adherence. Effective HAART is also known to reduce HIV-related inflammation and immune activation [36, 37], which can protect against HIV-associated renal disease [38]. This inflammation often contributes to increased Cyst C levels in virally unsuppressed or HAART-naïve individuals. It was previously reported that serum cystatin C levels are elevated during inflammation and PLWH usually experience increased inflammation due to the virus prior HAART initiation, which persists at low-grade level during viral suppression [39, 40]. The finding that the duration on HAART for more than 5 years is associated with poor renal function suggests that while the overall group comparison (HAART-treated vs. HAART-naive) may not show a difference, a subgroup analysis indicates that prolonged exposure to HAART (more than 5 years) is linked to a decline in renal function. This implies that the duration of HAART use may have a cumulative adverse effect on renal function, and this may be attributed to TDF use, since the majority of HAART users in the present study were on TDF-based regimen which is most commonly associated with renal dysfunction. Previous studies have reported similar findings, that prolonged HAART exposure (especially with TDF) is associated with renal impairment [10, 11, 18]. The long-term use of TDF has been associated with declines in renal function, including tubular dysfunction and a decrease in glomerular filtration rate (GFR) [41, 42] and this is due to intracellular tenofovir accumulation causing renal tubular mitochondrial damage and subsequently, tubular dysfunction and progressive renal deterioration [43]. The association between long-term HAART and advanced HIV disease with poor renal outcomes has been observed in various studies [44, 45]. The risk of renal impairment due to cumulative effects of TDF is reportedly higher in individuals on HAART with advanced HIV disease because they may already have compromised renal function [44]. Thus, both the duration of HAART (especially with TDF) and the severity of HIV infection remain important predictors of renal outcomes, as demonstrated in the current study. Advanced HIV disease, characterized by low CD4 + counts and high viral loads, can cause significant damage to various organs and seems to also cause significant damage to kidneys. With most of our HAART-treated group being under the treatment duration of less than 5 years, it is evidently clear from the finding that PLWH on long-term HAART for over 5-year period may be at increased risk of progressive eGFR decline which may lead to poor health outcomes. Our findings also show that with the ageing of the HIV population on TDF-based HAART, renal function deteriorates, which is even worse in those with advanced HIV disease. These findings highlight the need for regular monitoring of kidney function in patients on HAART and consideration of less nephrotoxic regimens, especially those who have been on the treatment for longer periods.
This study has several limitations. It is a cross-sectional study which makes it impossible to infer causation. The sample size was small and unequal between the groups which may have affected the outcomes of the statistical tests in terms of detecting significant difference. The study was conducted in a single district in the Limpopo Province, which potentially limits the generalisability of the results. The study also acknowledges lack of proteinuria/urine markers and creatinine measurements in the data set which potentially hinders the use of sensitive diagnostic criteria and comparison of eGFRs calculated from creatinine with those calculated from Cyst C. This study population is also ethnically homogenous, consisting exclusively of black South Africans. An inclusion of racial or ethnic diversity would provide variation in the outcomes of the study. Furthermore, future research should consider gold standard measures of GFR such as inulin or iohexol clearance or body composition as well as creatinine-based measures and proteinuria, which are necessary to understand the diagnostic value of Cyst C. Although the study is not without limitations, the cross-sectional nature of the study provides a snapshot of the current state of kidney function and allows for the comparison of cystatin C levels across different individuals, highlighting its potential as a clinical marker to determine the risk of kidney disease in a rural cohort of PLWH on HAART.
5 Conclusion
The CystC and eGFRCystC levels were not different between PLWH on HAART and not on HAART but prolonged HAART use for over five-year period and advanced HIV disease were associated with poor renal outcome in this population of PLWH on HAART, as assessed using CystC-based measures. These findings may suggest that HAART had initial beneficial effect and long-term adverse effect. The future risk of progressive eGFR decline may be increased in this HIV population on HAART due to cumulative effect of prolonged TDF use. Future studies incorporating larger populations are needed to confirm the findings and assess the utility of creatinine-cystatin C-based measures in this population.
Data availability
Data can be accessed from the corresponding author upon request.
Code availability
Not applicable.
Abbreviations
- 3TC:
-
Lamivudine
- ARV:
-
Antiretroviral
- AZT:
-
Atazanavir
- CD4 + :
-
Cluster of differentiation 4
- CKD-EPI:
-
Chronic kidney disease epidemiology collaboration
- Cyst C:
-
Cystatin C
- EFV:
-
Efavirenz
- eGFR:
-
Estimated glomerular filtration rate
- eGFRCystC :
-
Cystatin C-based estimated glomerular filtration rate
- FTC:
-
Emtricitabine
- GFR:
-
Glomerular filtration rate
- HAART:
-
Highly active antiretroviral therapy
- HIV:
-
Human immunodeficiency virus
- LPV/r:
-
Ritonavir-boosted lopinavir
- NKF:
-
National kidney foundation
- NKF KDOQI:
-
National kidney foundation kidney disease outcomes quality
- NVP:
-
Nevirapine
- PLWH:
-
People living with HIV
- TAF:
-
Tenofovir alafenamide fumarate
- TDF:
-
Tenofovir disoproxil fumarate
- TREC:
-
Turfloop research ethics committee
- WHO:
-
World Health Organization
References
Heron JE, Bagnis CI, Gracey DM. Contemporary issues and new challenges in chronic kidney disease amongst people living with HIV. AIDS Res Ther. 2020;17(1):11–3.
da Rocha IM, Gasparotto AS, Lazzaretti RK, Notti RK, Sprinz E, Mattevi VS. Polymorphisms associated with renal adverse effects of antiretroviral therapy in a Southern Brazilian HIV cohort. Pharmacogenet Genomics. 2015;25(11):541–7.
Nishijima T, Kawasaki Y, Mutoh Y, Tomonari K, Tsukada K, Kikuchi Y, et al. Prevalence and factors associated with chronic kidney disease and end-stage renal disease in HIV-1-infected Asian patients in Tokyo. Sci Rep. 2017;7(1):14565–x.
Naicker S, Rahmanian S, Kopp JB. HIV and chronic kidney disease. Clin Nephrol. 2015;83(7 Suppl 1):32–8.
Kalayjian RC, Franceschini N, Gupta SK, Szczech LA, Mupere E, Bosch RJ, et al. Suppression of HIV-1 replication by antiretroviral therapy improves renal function in persons with low CD4 cell counts and chronic kidney disease. AIDS. 2008;22(4):481–7.
Kwantwi LB, Obirikorang C, Frempong MA. Nephroprotective effect of highly active antiretroviral therapy among HIV seropositive individuals: a case-control study in Ghana. Int STD Res Rev. 2018;6(3):1–8.
Leport C, Bouteloup V, Rossert J, Garré M, Iordache L, Dellamonica P, et al. Long-term evolution and determinants of renal function in HIV-infected patients who began receiving combination antiretroviral therapy in 1997–1999, ANRS CO8 APROCO-COPILOTE. Clin Infect Dis. 2009;49(12):1950–4.
Jaroszewicz J, Wiercinska-Drapalo A, Lapinski TW, Prokopowicz D, Rogalska M, Parfieniuk A. Does HAART improve renal function? An association between serum cystatin C concentration, HIV viral load and HAART duration. Antivir Ther. 2006;11(5):641–5.
Tan Q, He Y, Yang T, Yan D, Wang Y, Zhao X, et al. Effects of long-term exposure to tenofovir disoproxil fumarate-containing antiretroviral therapy on renal function in HIV-positive Chinese patients. J Microbiol Immunol Infect. 2019;52(5):710–9.
Mocroft A, Lundgren JD, Ross M, Fux CA, Reiss P, Moranne O, et al. Cumulative and current exposure to potentially nephrotoxic antiretrovirals and development of chronic kidney disease in HIV-positive individuals with a normal baseline estimated glomerular filtration rate: a prospective international cohort study. Lancet HIV. 2016;3(1):23.
Nishijima T, Kawasaki Y, Tanaka N, Mizushima D, Aoki T, Watanabe K, et al. Long-term exposure to tenofovir continuously decrease renal function in HIV-1-infected patients with low body weight: results from 10 years of follow-up. AIDS. 2014;28(13):1903–10.
Ryom L, Mocroft A, Kirk O, Worm SW, Kamara DA, Reiss P, et al. Association between antiretroviral exposure and renal impairment among HIV-positive persons with normal baseline renal function: the D:A: D study. J Infect Dis. 2013;207(9):1359–69.
Mocroft A, Kirk O, Reiss P, De Wit S, Sedlacek D, Beniowski M, et al. Estimated glomerular filtration rate, chronic kidney disease and antiretroviral drug use in HIV-positive patients. AIDS. 2010;24(11):1667–78.
Brewster UC, Perazella MA. Acute interstitial nephritis associated with atazanavir, a new protease inhibitor. Am J Kidney Dis. 2004;44(5):e81–4.
Doco-Lecompte T, Garrec A, Thomas L, Trechot P, May T, Rabaud C. Lopinavir–ritonavir (Kaletra) and lithiasis: seven cases. AIDS. 2004;18(4):705–6.
Gallant JE, Moore RD. Renal function with use of a tenofovir-containing initial antiretroviral regimen. AIDS. 2009;23(15):1971–5.
Berns JS, Kasbekar N. Highly active antiretroviral therapy and the kidney: an update on antiretroviral medications for nephrologists. Clin J Am Soc Nephrol. 2006;1(1):117–29.
Dauchy F, Lawson-Ayayi S, de La Faille R, Bonnet F, Rigothier C, Mehsen N, et al. Increased risk of abnormal proximal renal tubular function with HIV infection and antiretroviral therapy. Kidney Int. 2011;80(3):302–9.
Overton ET, Nurutdinova D, Freeman J, Seyfried W, Mondy KE. Factors associated with renal dysfunction within an urban HIV-infected cohort in the era of highly active antiretroviral therapy. HIV Med. 2009;10(6):343–50.
Epicentre. HIV/AIDS. 2022. https://epicentre.msf.org/en/our-achievements/hivaids. Accessed 21 Jan 2024.
Grubb A. Cystatin C is indispensable for evaluation of kidney disease. EJIFCC. 2017;28(4):268–76.
Ebert N, Shlipak MG. Cystatin C is ready for clinical use. Curr Opin Nephrol Hypertens. 2020;29(6):591–8.
Benoit SW, Ciccia EA, Devarajan P. Cystatin C as a biomarker of chronic kidney disease: latest developments. Expert Rev Mol Diagn. 2020;20(10):1019–26.
Cochran WG. Methodological problems in the study of human populations. Ann N Y Acad Sci. 1963;107:476–89.
Stevens PE, Ahmed SB, Carrero JJ, Foster B, Francis A, Hall RK, et al. KDIGO 2024 Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2024;105(4):S117–314.
Seedat YK, Rayner BL, Veriava Y. South African hypertension practice guideline 2014. Cardiovasc J Afr. 2014;25(6):288–94.
Pheiffer C, Pillay-van Wyk V, Turawa E, Levitt N, Kengne AP, Bradshaw D. Prevalence of type 2 diabetes in South Africa: a systematic review and meta-analysis. Int J Environ Res Public Health. 2021;18(11):5868.
Ntusi N. Dyslipidaemia in South Africa. S Afr Med J. 2018;108(4):256–7.
World Medical Association. World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–4.
World Health Organization. WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children. Geneva: World Health Organization; 2007.
Lyas C, Zuber K, Davis J. Reevaluating race and the glomerular filtration rate calculator. JAAPA. 2021;34(12):59–61.
Edinga-Melenge BE, Yakam AT, Nansseu JR, Bilong C, Belinga S, Minkala E, et al. Reference intervals for serum cystatin C and serum creatinine in an adult sub-Saharan African population. BMC Clin Pathol. 2019;19:1–9.
Murty MSN, Sharma UK, Pandey VB, Kankare SB. Serum cystatin C as a marker of renal function in detection of early acute kidney injury. Indian J Nephrol. 2013;23(3):180–3.
Estrella MM, Jaar BG, Cavanaugh KL, Fox CH, Perazella MA, Soman SS, et al. Perceptions and use of the national kidney foundation KDOQI guidelines: a survey of U.S. renal healthcare providers. BMC Nephrol. 2013;14:230–230.
Post FA, Campbell LJ, Hamzah L, Collins L, Jones R, Siwani R, et al. Predictors of renal outcome in HIV-associated nephropathy. Clin Infect Dis. 2008;46(8):1282–9.
Nyambuya TM, Dludla PV, Mxinwa V, Nkambule BB. The effect of successful antiretroviral therapy on immune activation and reconstitution in HIV-infected adults: a systematic review and meta-analysis. AIDS Rev. 2021. https://doi.org/10.24875/AIDSRev.20000039.
Babu H, Ambikan AT, Gabriel EE, Svensson Akusjärvi S, Palaniappan AN, Sundaraj V, et al. Systemic inflammation and the increased risk of inflamm-aging and age-associated diseases in people living with HIV on long term suppressive antiretroviral therapy. Front Immunol. 2019;10:1965.
Zicari S, Sessa L, Cotugno N, Ruggiero A, Morrocchi E, Concato C, et al. Immune activation, inflammation, and non-AIDS co-morbidities in HIV-infected patients under long-term ART. Viruses. 2019;11(3):200.
Gagneux-Brunon A, Mariat C, Delanaye P. Cystatin C in HIV-infected patients: promising but not yet ready for prime time. Nephrol Dial Transplant. 2012;27(4):1305–13.
Neuhaus J, Jacobs DR Jr, Baker JV, Calmy A, Duprez D, La Rosa A, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201(12):1788–95.
Kinai E, Hanabusa H. Progressive renal tubular dysfunction associated with long-term use of tenofovir DF. AIDS Res Hum Retroviruses. 2009;25(4):387–94.
Casado JL, Del Rey JM, Bañón S, Santiuste C, Rodriguez M, Moreno A, et al. Changes in kidney function and in the rate of tubular dysfunction after tenofovir withdrawal or continuation in HIV-infected patients. JAIDS J Acquired Immune Defic Syndromes. 2016;72(4):416–22.
Yombi JC, Jones R, Pozniak A, Hougardy J, Post FA. Monitoring of kidney function in HIV-positive patients. HIV Med. 2015;16(8):457–67.
Crum-Cianflone N, Ganesan A, Teneza-Mora N, Riddle M, Medina S, Barahona I, et al. Prevalence and factors associated with renal dysfunction among HIV-infected patients. AIDS Patient Care STDS. 2010;24(6):353–60.
Msango L, Downs JA, Kalluvya SE, Kidenya BR, Kabangila R, Johnson WD Jr, et al. Renal dysfunction among HIV-infected patients starting antiretroviral therapy. AIDS. 2011;25(11):1421–5.
Acknowledgements
Joel Choshi was partially supported by funding from the SAMRC through its division of Research Capacity Development under the Internship Scholarship Programme. S.E. Mabhida was also partially supported by funding from the SAMRC through its division of Research Capacity Development under the Intra-Mural Doctoral Fellowship Programme from funding received from the South African Treasury. The content hereof is the sole responsibility of the authors and does not necessarily represent the official views of the SAMRC or the funders.
Funding
Phiwayinkosi V. Dludla is supported in part by the National Research Foundation (NRF) (Grant numbers: 117829 and 141929). Sidney Hanser is also funded by the NRF Thuthuka Programme (Grant No. 107249), Health and Welfare Sector Education and Training Authority (South Africa), and the University of Limpopo (UL).
Author information
Authors and Affiliations
Contributions
Authors, J.C, S.H and P.V. D conceived and contributed to drafting the original manuscript. All other authors, including J.C, B.F, S.E. M, H. M, M.D. S, B.B.N, Z. M, D.N, A.P. K, P.V. D, and S.H reviewed and edited the manuscript. All authors approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Turfloop Research and Ethics Committee (TREC) (project number TREC/315/2019: PG), as part of the initial project approved by TREC (project number TREC/119/2016: PG). The study was explained, and informed written consent was obtained from all participants. This study was conducted in accordance with the principles of declaration of Helsinki.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Choshi, J., Flepisi, B., Mabhida, S.E. et al. Assessing renal function with the use of cystatin C in a rural cohort of people living with HIV on highly active antiretroviral therapy within the Limpopo Province, South Africa. Discov Appl Sci 6, 499 (2024). https://doi.org/10.1007/s42452-024-06197-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-024-06197-2