Abstract
Background
This study aimed to investigate whether patients with stage IB NSCLC could benefit from adjuvant chemotherapy.
Methods
In the years 2010 to 2015, 1,829 NSCLC patients with stage IB disease were chosen from the SEER database. To equalize the baseline characteristics between the surgery plus adjuvant chemotherapy group (intervention) and the surgery alone group (control), propensity score matching (PSM) was used. The log-rank test plotted Kaplan–Meier survival curves to compare the overall survival (OS) and disease-specific survival (DSS). Cox proportional hazard regression was used to perform univariate and multivariate analysis on overall survival.
Results
One hundred ninety-seven patients in each group with a mean follow-up period of 65.4 months were enrolled after PSM. A significant benefit in overall survival ([intervention vs. control] HR = 0.72; 95% CI: 0.54 to 0.94; P = 0.026) was detected in the intervention group before PSM. And there were significantly improved OS (HR = 0.63; 95% CI: 0.42 to 0.92; P = 0.036) and DSS (HR = 0.73; 95% CI: 0.52 to 0.95; P = 0.044) for the patients with visceral pleural invasion (VPI) in the intervention group compared with the control group. After PSM, the patients with VPI in the intervention group had better overall survival (HR = 0.69; 95% CI: 0.40 to 0.98; P = 0.048) than those in the control group. The Cox proportional hazard regression analysis showed that VPI (HR = 1.29; 95% CI: 1.11 to 1.54; P < 0.001) was also an independent prognostic factor.
Conclusion
Stage IB NSCLC with VPI could benefit from adjuvant chemotherapy after R0 resection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With a high incidence worldwide, lung cancer has been noted as the main cause of mortality for decades [1]. Approximately 85% of lung cancer patients are diagnosed with non-small cell lung cancer (NSCLC) [2]. It is a consensus that radical resection is generally the preferred treatment for early-stage NSCLC. However, approximately 40% of early-stage NSCLC would develop recurrence in the postoperative five years, especially those with risk factors [3]. Visceral pleural invasion (VPI) was determined as a negative prognosticator for NSCLC [4]. According to the recent National Comprehensive Cancer Network (NCCN) guideline, NSCLC with VPI was classified as stage IB and had a higher recurrence compared to stage IA NSCLC, suggesting that additional therapy might be needed for stage IB NSCLC.
In recent years, adjuvant chemotherapy has been confirmed effective for locally advanced NSCLC [5]. However, it was controversial whether stage IB NSCLC would benefit from postoperative chemotherapy [6, 7]. Several retrospective studies indicated that adjuvant chemotherapy was significantly associated with better recurrence-free survival and lower mortality in stage IB NSCLC. However, bias might exist due to the small sample size and lacked up-to-date evidence in these studies. Therefore, there is an urgent need for thoracic surgeons to investigate the effect of adjuvant chemotherapy on improving survival in the stage IB NSCLC patients.
To address this issue, we conducted a retrospective study with 1:1 PSM analysis to evaluate the efficacy of surgery followed by postoperative chemotherapy for stage IB NSCLC by enrolling a homogenous subset of stage IB NSCLC patients according to the eighth edition of the TNM classification for lung cancer.
Methods
Study population
We reviewed and enrolled the stage IB NSCLC patients from the National Cancer Institute SEER database between January 2010 and December 2015. The inclusion criteria for the enrolled patients were: (I) histologically confirmed primary NSCLC according to the World Health Organization (WHO) classification of tumors; (II) stage IB was pathologically classified according to the eighth Edition of the American Joint Committee on Cancer Staging Manual; (III) between 18 and 80 years; (IV) underwent radical resection and lymph node sampling or dissection; (V) without other malignancies; and (VI) without preoperative therapy. The exclusion criteria were: (I) underwent postoperative radiotherapy or chemo-radiotherapy; (II) unavailable clinicopathological features and survival data; or (III) postoperative survival of fewer than three months.
Definition of VPI in NSCLC
Pleural invasion is divided into the following types: (I) PL0 was defined that a tumor that does not invade the visceral pleura beyond its elastic layer; (II) PL1 was defined that a tumor that invades beyond the elastic layer of the visceral pleura but is not exposed on the pleural surface; (III) PL2 was defined that a tumor that invades to the pleural surface; and (IV) PL3 was defined that a tumor that invades to the parietal pleura. PL1 and PL2 are both interpreted as VPI in the TNM staging system [8].
Definition of procedure
Radical surgical procedures included lobectomy, segmentectomy, and wedge resection, followed by lymph node sample or dissection. Systematic therapy after surgery (RX Summ–Systemic/Sur Seq = "Systemic therapy after surgery") was considered as surgery followed by adjuvant chemotherapy, and systematic therapy after surgery (RX Summ–Systemic/Sur Seq = "No/Unknown") was considered as surgery alone.
Variables and statistical analysis
Patients with adjuvant chemotherapy were grouped in the intervention group while those without adjuvant chemotherapy were grouped in the control group. The data were obtained for each patient as follows: gender, age, marital status, race, tumor histology, differentiation, laterality, tumor diameter, VPI, surgery, dissected LN number and survival. Disease-specific survival (DSS) and overall survival (OS) were considered evaluation indexes to assess the prognosis. OS was defined as the duration from the date of surgery to the death. DSS was defined as the duration from the date of surgery to the death of lung cancer.
The continuous variables were represented as the mean and standard deviation and were analyzed using a two-sample Students t-test. The categorical variables were shown as the number and percentage of patients and were compared with the Fisher's exact test. To balance the bias of fundamental traits across the two groups, a 1:1 propensity score matching (PSM) with a caliper of 0.1 was carried out. Age, gender, race, marital status, laterality, histology, grade, differentiation, tumor diameter, VPI status, surgery, and the number of LNs dissected were among the balanced variables. To evaluate OS between the two groups, Kaplan–Meier curves with a log-rank test were utilized, and competing risk analysis was used to assess DSS. The prognostic factors were evaluated using the Cox regression model. Data analysis was carried out using the R software (Version 4.1.2, The R Foundation, Vienna, Austria) and GraphPad Prism (Version 5.0, GraphPad Software, Inc., CA). A p-value with two tails less than 0.05 was regarded as significant.
Results
Patient characteristics
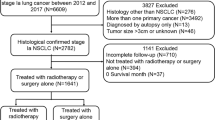
The clinical characteristics of patients were provided in Table 1. Among the 1,823 patients enrolled, 1,632 were in the control group, and 197 were in the intervention group. After matching, there were 197 patients in each group. The screening flowchart was described in Fig. 1. The propensity score distributions after matching and standardized differences of the included covariates were shown in Supplemental Fig. 1 and Supplemental Table 1, respectively, indicating a good balance of basic characteristics between the two groups. The basic characteristics of matched patients were shown in Table 1.
Overall survival
Similar follow-up was observed in the two groups. Before PSM, a significantly improved OS ([intervention group vs. control group] HR = 0.72; 95% CI: 0.54 to 0.94; P = 0.026) was detected in the intervention group compared with the control group. However, there was no remarkable difference in OS in the cohort after PSM (HR = 0.91; 95% CI: 0.63 to 1.31; P = 0.600). Among the patients with VPI, the intervention group achieved a better OS before and after PSM ([before PSM]: HR = 0.63; 95% CI: 0.42 to 0.92; P = 0.036; [after PSM]: HR = 0.69; 95% CI: 0.40 to 0.98; P = 0.048) with statistical significance compared with the control group. However, there was no significant difference in OS ([before PSM]: HR = 0.87; 95% CI: 0.62 to 1.21; P = 0.180; [after PSM]: HR = 1.07; 95% CI: 0.67 to 1.72; P = 0.770) between the two subgroups without VPI (Fig. 2A-C; Fig. 3A-C).
Overall survival and disease-specific survival in the patient population with stage IB between the two groups before PSM. (A) overall survival of the total patient population; (B) overall survival of the patient population without VPI; (C) overall survival of the patient population with VPI; (D) disease-specific survival of the total patient population; (E) disease-specific survival of the patient population without VPI; (F) disease-specific survival of the patient population with VPI. OS, overall survival; CI, credibility interval; NA, not available
Overall survival and disease-specific survival in the patient population with stage IB between the two groups after PSM. (A) overall survival of the total patient population; (B) overall survival of the patient population without VPI; (C) overall survival of the patient population with VPI; (D) disease-specific survival of the total patient population; (E) disease-specific survival of the patient population without VPI; (F) disease-specific survival of the patient population with VPI. OS, overall survival; CI, credibility interval; NA, not available
Disease-specific survival
Before PSM, no significantly improved DSS (HR = 0.82; 95% CI: 0.64 to 1.04; P = 0.360) was detected in the intervention group compared with the control group. And similar results (HR = 1.12; 95% CI: 0.72 to 1.77; P = 0.612) were shown between the two groups after PSM. Among the patients with VPI, a significantly better DSS was detected in the intervention group before PSM (HR = 0.73; 95% CI: 0.52 to 0.95; P = 0.044) compared with the control group; however, there was no difference in disease-specific survival (HR = 1.04; 95% CI: 0.52 to 2.09; P = 0.913) in the two groups after PSM. However, there was no significant difference in OS ([before PSM]: HR = 0.87; 95% CI: 0.62 to 1.21; P = 0.890; [after PSM]: HR = 1.17; 95% CI: 0.64 to 2.14; P = 0.598) between the two subgroups without VPI (Fig. 2D-F; Fig. 3D-F).
Univariate and multivariate analysis
In the univariate analysis, females (HR = 0.66; 95% CI: 0.56 to 0.76; P < 0.001), older than 60 years (HR = 2.04; 95% CI: 1.67 to 2.49; P < 0.001), squamous cell carcinoma (HR = 1.61; 95% CI: 1.38 to 1.88; P < 0.001), poorly differentiated (HR = 1.38; 95% CI: 1.19 to 1.61; P < 0.001), sublobectomy (HR = 0.66; 95% CI: 1.95 to 2.40; P < 0.001) and with VPI (HR = 1.29; 95% CI: 1.11 to 1.54; P < 0.001) were shown as risk factors of OS for NSCLC with stage IB. All of the above were detected as independent risk factors after the multivariate analysis (Fig. 4).
Discussion
It was debatable whether adjuvant chemotherapy would be beneficial for stage IB NSCLC following radical resection. According to this study, there was no discernible difference in OS between stage IB NSCLC patients who received adjuvant chemotherapy after surgery and those who did not. However, stage IB NSCLC patients with VPI may benefit from adjuvant treatment in terms of enhanced DFS and OS.
VPI is a part of the tumor node metastasis (TNM) staging system and is known to have a poor predictive impact on NSCLC patients [9]. For NSCLC tumors sized 3 cm or less, the presence of VPI increases the T stage from T1 to T2. Compared with those without VPI, a worse prognosis was shown in NSCLC with VPI. It might be because of the direct invasion of the pleura followed by diffuse dissemination of cancer cells throughout the pleural cavity by pleural fluid. Postoperative chemotherapy was theoretically effective because it was considered a systemic therapy and could improve regional invasion and dissemination control. Our work showed improved DFS and OS in the NSCLC with VPI receiving adjuvant chemotherapy than those receiving radical surgery alone. Similar results were reported in the previous studies, confirming the benefit of postoperative chemotherapy for stage IB NSCLC [10, 11].
Stage IB NSCLC is a distinct entity in lung cancer due to its remarkable heterogeneity in prognosis. Making prognostic stratifications and predicting the oncological fate of stage IB NSCLC is still a problem that needs to be solved for thoracic surgeons [12, 13]. In the univariate and multivariate analysis, females, older than 60 years, poorly differentiated, squamous cell carcinoma, sublobectomy and VPI were shown as risk factors of OS for NSCLC with stage IB, which was consistent with the results in the previous literature. It was reported inthe previous studies that stage I NSCLC patients with poorly differentiated histology had worse outcomes and that stage IB NSCLC patients with tumors bigger than 4 cm had a higher risk of mortality [14]. According to the study by Horiana et al. [15], stage I NSCLC patients with lung squamous cell carcinoma have a higher risk of mortality than those with lung adenocarcinoma. Even though sublobectomy has demonstrated non-inferiority for OS compared to lobectomy, lobectomy and systematic mediastinal lymphadenectomy are still thought to be the gold standard procedure for treating early-stage lung cancer.
According to international guidelines, stage IB NSCLC patients may not require adjuvant therapy after radical surgery; however, for high-risk patients, adjuvant therapy was suggested. Our study showed that postoperative chemotherapy was preferred in the patients with low age or adenocarcinoma. The reason might be the unsafety of adjuvant chemotherapy for old patients and good prognosis for squamous cell lung cancer. However, in our work, adjuvant chemotherapy did not provide better overall survival in the whole population of NSCLC with stage IB. The results of PSM analysis also showed that adjuvant chemotherapy was not beneficial for the whole population of stage IB NSCLC. Therefore, screening patients suitable for postoperative chemotherapy will be the focus of further research.
There still were some limitations. First, this was a retrospective study. Second, efficacy of different chemotherapy regimens might be different. Still, a comparison of the effectiveness of different chemotherapy regimens was not performed in our study due to the lack of comparable data. Third, there might be a difference in prognosis between PL1 and PL2, as reported in previous studies. However, we did not conduct the subgroup analysis according to the degree of visceral pleura metastasis. In addition, this study focused on adjuvant chemotherapy's efficacy for stage IB NSCLC. However, adjuvant chemotherapy might not be preferred for some NSCLCs, such as those with EGFR mutation after surgery. Due to insufficient data, we did not perform subgroup analysis for EGFR-mutation population.
Conclusion
There was no significant difference in OS in the population with stage IB NSCLC who received adjuvant chemotherapy than those who underwent radical surgery alone. However, adjuvant chemotherapy could improve DFS and OS for stage IB NSCLC with VPI. The results will be further confirmed in the randomized control trials.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available in the SEER repository (https://seer.cancer.gov/), and available from the corresponding author on reasonable request.
References
Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Duma N, Santana-Davila R, Molina JR. Non-small cell lung cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc. 2019;94(8):1623–40.
Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303(11):1070–6.
De Giglio A, Di Federico A, Gelsomino F, Ardizzoni A. Prognostic relevance of pleural invasion for resected NSCLC patients undergoing adjuvant treatments: a propensity score-matched analysis of SEER database. Lung Cancer. 2021;161:18–25.
McElnay P, Lim E. Adjuvant or neoadjuvant chemotherapy for NSCLC. J Thorac Dis. 2014;6 Suppl 2(Suppl 2):S224-227.
Zuo Z, Zhang G, Song P, et al. Survival nomogram for stage IB non-small-cell lung cancer patients, based on the SEER database and an external validation cohort. Ann Surg Oncol. 2021;28(7):3941–50.
Nagasaka M, Gadgeel SM. Role of chemotherapy and targeted therapy in early-stage non-small cell lung cancer. Expert Rev Anticancer Ther. 2018;18(1):63–70.
Rami-Porta R, Bolejack V, Crowley J, et al. The IASLC lung cancer staging project: proposals for the revisions of the T descriptors in the forthcoming eighth edition of the TNM classification for lung cancer. J Thorac Oncol. 2015;10(7):990–1003.
Tu Z, Li C, Tian T, Chen Q. A risk classification system predicting the cancer-specific survival for postoperative stage IB non-small-cell lung cancer patients without lymphovascular and visceral pleural invasion. Lung Cancer. 2021;161:114–21.
Zhang Z, Xie S, Cai W, et al. A nomogram to predict the recurrence-free survival and analyze the utility of chemotherapy in stage IB non-small cell lung cancer. Transl Lung Cancer Res. 2022;11(1):75–86.
Li X, Zhang C, Sun Z, et al. Propensity-matched analysis of adjuvant chemotherapy for completely resected stage IB non-small-cell lung cancer patients. Lung Cancer. 2019;133:75–82.
Wu LL, Liu X, Jiang WM, et al. Stratification of patients with stage IB NSCLC based on the 8th edition of the American Joint Committee on Cancer (AJCC) staging manual. Front Oncol. 2020;10:571.
Zhai W, Duan F, Li D, et al. Risk stratification and adjuvant chemotherapy after radical resection based on the clinical risk scores of patients with stage IB-IIA non-small cell lung cancer. Eur J Surg Oncol. 2022;48(4):752–60.
Tu Z, Tian T, Chen Q, Li C. Overall survival analyses following adjuvant chemotherapy or nonadjuvant chemotherapy in patients with stage IB non-small-cell lung cancer. J Oncol. 2021;2021:8052752.
Grosu HB, Manzanera A, Shivakumar S, Sun S, Noguras Gonzalez G, Ost DE. Survival disparities following surgery among patients with different histological types of non-small cell lung cancer. Lung Cancer. 2020;140:55–8.
Acknowledgements
None.
Funding
This study was supported by the National Postdoctoral Innovation Talents Support Program of China (No. BX20230083), National Natural Science Foundation of China (No. 82303564), and Youth Foundation of Zhongshan Hospital, Fudan University (No. 2022–020).
Author information
Authors and Affiliations
Contributions
Xinyu Yang, Guang Han, Mingxiang Feng, Ming Li: Conceptualization, Methodology, Software; Yunfan Hu, Mengnan Zhao, Tian Jiang, Changhao Ren: Data curation; Xinyu Yang, Guang Han, Ming Li: Writing- Original draft preparation; Xinyu Yang, Guang Han, Ming Li: Visualization, Investigation. Mingxiang Feng, Ming Li: Supervision; Xinyu Yang: Software, Validation; Xinyu Yang, Ming Li: Writing- Reviewing and Editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Informed consent was waived by our Institutional Review Board because of the retrospective nature of our study.
Competing interests
The authors have no financial or other interests regarding the submitted manuscript.
Disclosure Statement: There is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplemental Figure 1.
Jitter plot for the propensity score distributions of patients before and after PSM.
Additional file 2: Supplemental Table 1.
Standardized differences of the included covariates after matching.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, X., Han, G., Hu, Y. et al. Adjuvant chemotherapy on survival of patients with stage IB non‑small cell lung cancer: a comparison study with propensity score matching. CCB 3, 1 (2024). https://doi.org/10.1007/s44272-023-00006-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44272-023-00006-4