Abstract
Non-Hodgkin lymphoma (NHL) is a common malignancy in the hematologic system, and traditional therapy has limited efficacy for people with recurrent/refractory NHL (R/R NHL), especially for patients with diffuse large B cell lymphoma (DLBCL). Chimeric antigen receptor (CAR) T-cell therapy is a novel and effective immunotherapy strategy for R/R hematopoietic malignancies, but relapses can occur due to the loss of CAR-T cells in vivo or the loss of antigen. One strategy to avoid antigen loss after CAR-T cell therapy is to target one more antigen simultaneously. Tandem CAR targeting CD19 and CD22 has demonstrated the reliability of tandem CAR-T cell therapy for R/R B-ALL. This study explores the therapeutic potential of tandem CD19/20 CAR-T in the treatment of R/R B cell NHL. The efficacy and safety of autologous CD19/20 CAR-T cells in eleven R/R B cell NHL adult patients were evaluated in an open-label, single-arm trial. Most patients achieved complete response, exhibiting the efficacy and safety of tandem CD19/20 CAR-T cells. The TCR repertoire diversity of CAR-T cells decreased after infusion. The expanded TCR clones in vivo were mainly derived from TCR clones that had increased expression of genes associated with immune-related signaling pathways from the infusion product (IP). The kinetics of CAR-T cells in vivo were linked to an increase in the expression of genes related to immune response and cytolysis/cytotoxicity.
Similar content being viewed by others
Introduction
Non-Hodgkin B-cell Lymphoma (NHL) encompasses a diverse range of conditions, spanning from highly aggressive and aggressive (ex. Diffuse Large B-cell Lymphoma) to indolent types of disease [1]. Approximately two-thirds of DLBCL patients can achieve complete remission (CR) by R-CHOP induction [2]. The overall response rate (ORR) to second-line therapy is reported to be 20%–30% with a median overall survival (OS) of 6 months in patients who are unsuitable for transplantation or relapse after autologous transplantation [3,4,5]. The advent of anti-CD19 Chimeric Antigen Receptor (CAR) T-cell therapy has significantly revolutionized the approach to treating B-cell malignancies, reshaping the therapeutic landscape [6,7,8,9].
Chimeric antigen receptor (CAR) T cell therapy serves as a novel and effective immunotherapy strategy for R/R hematopoietic malignancies that contribute to advantageous clinical prognosis [10]. T cells constructed with anti-CD19 CAR manifest remarkable therapeutic efficacy in patients with hematological malignancies such as B cell lymphoma [6] and B cell acute lymphocytic leukemia (B-ALL) [11]. Reported objective response rates in R/R B-cell NHL range from 50% to 93% [52%-83% for DLBCL and 71%-93% for follicular lymphoma (FL) or mantle cell lymphoma (MCL)], with CR rates ranging from 38%-88% (38%-64% for DLBCL and 53%-88% for FL or MCL) [12,13,14,15,16]. Despite the impressive therapeutic effect in hematopoietic malignancies, relapses usually occurs in some patients following CAR-T cell infusion, with a relapse rate of 14%-75% for B-ALL and 50%-60% for B cell NHL [17]. This is usually due to the loss of CAR-T cells or the loss of antigen in vivo [6, 18]. In B-ALL, escape mechanisms of antigen loss in CD19 CAR-T cell therapy include frameshift or missense mutations, and alternative splicing of CD19 mRNA [19]. In NHL, emerging evidence has proved the relapse is tightly associated with antigen loss or mutations [6, 20]. Thus, finding a way to overcome antigen loss and reduce the recurrence rate has become a critical need for CAR-T cell therapy for NHL.
One strategy to avoid antigen loss after CAR-T cell therapy is to target one more antigen simultaneously. Multiple studies reveal that T cells with two chimeric antigen receptors exhibit the reduced possibility of antigen escape by tumor cells and increased potential of cytolytic efficiency [21,22,23,24]. It has been reported a single-chain bispecific CAR targeting HER2 and CD19 augments the specificity of effector cells and counteracts antigen escape of malignant cells [25]. This bispecific CAR, also called tandem CAR, can activate T cells efficiently by combining HER2 or CD19. Tandem CAR targeting CD19 and CD22 has been used in patients with B-ALL and demonstrated the reliability of tandem CAR-T cell therapy for R/R B-ALL [26]. These exciting results attracted us to explore the therapeutic potential of tandem CD19/20 CAR in the treatment of R/R B cell NHL.
Here, we reported the efficacy and safety of autologous CD19/20 CAR-T cells in eleven R/R B cell NHL adult patients in an open-label, single-arm trial. Most patients went into the status of complete response and tolerated CAR-T therapy well, exhibiting the efficacy and safety of tandem CD19/20 CAR-T cells.
Material and methods
Patients and study design
This phase I-II open-label, single-arm clinical trial was conducted at the Department of Hematology and Oncology, Shenzhen University General Hospital from May 2021 to November 2022 to investigate the toxicity and efficacy of bispecific CD19/20 CAR-T cell immunotherapy in patients with R/R B cell NHL. This study was approved by the ethics committee of Shenzhen University General Hospital and was registered with ClinicalTrials.gov (NCT04723914). Informed consents were obtained from all patients. All patients were diagnosed with B cell NHL based on 2008 World Health Organization guidelines, including DLBCL, MCL, and FL. The criteria of inclusion in this study were as follows: (1) age between 14 and 75 years old, (2) histological evidence of CD19 and CD20 expression, (3) refractory or relapsed after previous therapy, and (4) Eastern Cooperative Oncology Group (ECOG) score of 2 or lower. Informed consent was obtained from all patients and their families following the Declaration of Helsinki.
CAR-T cell manufacturing and lymphodepleting chemotherapy
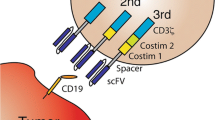
The anti-CD19/20 CAR was constructed via integrating the CD20 and CD19 single-chain variable fragment (scFv) in frame with the hinge and transmembrane domains of CD8 and the cytoplasmic domains of 4-1BB and CD3 zeta signaling domain (Fig. 1A). The heavy and light chains of Leu16 and FMC63 were linked by a (GGGGS)3 sequence and 218 s linker respectively. These two scFvs are derived from the mouse hybridoma Leu16 and FMC63 monoclonal antibody (mAb) respectively [27]. The preparation and quality control of CAR-T cells were described in a supplementary file.
A The anti-CD19/20 CAR was constructed via integrating the CD20 single-chain variable fragment (scFv) from Leu16 and CD19 scFv from FMC63 monoclonal antibody, along with the hinge and transmembrane domains of CD8 and the cytoplasmic domains of 4-1BB and CD3 zeta signaling domain. CD20 derived from Leu16 and CD19 derived from FMC63 monoclonal antibody. B Percentage-specific cleavage of CAR-T cells to target tumor cells determined by an 18-h culture LDH assay at indicated E: T ratio. C Cytokine concentrations secreted by classified anti-19 or anti-20 or anti-19&20 CAR-T cells in response to K562 tumor cells expressing K562-CD19 or K562-CD20 or K562-CD19&CD20. Specific cytokine production was determined by enzyme-linked immunosorbent assay (ELISA) (n = 3 donors). Three independent experiments were performed, and representative results are shown. NT nontransduced, TNF-a tumor necrosis factor-a, IFN-γ interferon-γ, IL-2 Interleukin-2.
Before the infusion of CAR-T cells, all patients received a conditioning chemotherapy regimen to deplete endogenous leukocytes which inhibited the anti-malignant effects of transferred CAR-T cells [26, 28]. The conditioning regimen included fludarabine (30 mg/m2 × 3 days) and cyclophosphamide (300 mg/m2 × 3 days). The doses ranged from 0.31 × 106 to 2.62 × 106 CAR-T cells per kilogram of body weight.
Toxicity and efficacy assessment
The primary endpoint was safety. Immune effector cell-associated neurological syndrome (ICANS) and cytokine release syndrome (CRS) were evaluated according to the American Society for Transplantation and Cellular Therapy (ASTCT) Consensus Criteria [29]. Interventions such as tocilizumab, or dexamethasone, were given instantly according to the patient’s tolerance and the severity of ICANS/CRS. Secondary endpoints included ORR, duration of response, progression-free survival (PFS), death, CAR-T cell persistence and B cell levels in peripheral blood, and serum cytokine levels. ORR was calculated as the sum of the CR rate and partial response (PR) rate. The response was defined according to response criteria for NHL [30]. PFS was defined as the period from the infusion to progression, death, or the last follow-up time, and OS was presented as the time from the infusion to death or the last visit. The alterations of CD19/20 CAR-T cell proportion in peripheral blood were observed by multiparameter flow cytometry.
Single-cell RNA sequencing and TCR sequencing
Cell preparation and library preparation
Peripheral blood (PB) samples were obtained from a single patient on day 21 after CAR-T cell infusion. The CAR-T cells were isolated from both the infused product (IP) and PB on day 21 using the EasySep PE Positive Selection Kit II (Stemcell) following the protocol. The isolated cells were then suspended in PBS as single-cell suspensions. These suspensions were loaded into microfluidic devices using the Singleron Matrix® Single Cell Processing System (Singleron). scRNA-seq libraries were generated using the GEXSCOPE® Single Cell RNA Library Kits (Singleron) following the manufacturer’ protocol. Additionally, scTCR-seq libraries were constructed using the GEXSCOPE Single Cell Immuno-TCR Kit (Singleron Biotechnologies) protocol.
Sequencing data analysis and pathway analysis
All libraries were sequenced using Illumina NovaSeq 6000 with 150 bp paired-end reads. Details regarding the analysis of sequencing data are described in the supplementary file.
To identify differentially expressed genes (DEGs), we used the Seurat FindMarkers function based on the Wilcox likelihood-ratio test with default parameters. The criteria for distinguishing DEGs can be found in the supplementary document.
Data from the previous Seurat FindMarkers function analysis were used for gene set enrichment analysis (GSEA). Additionally, Gene Ontology (GO) annotation and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis were applied to investigate the potential functions of differentially expressed genes.
Statistical analysis
All statistical analyses were performed using Graphpad Prism 8 and R package (v4.2.3). Continuous variables are presented as medians and ranges, and categorical variables are presented as frequencies and percentages. Kaplan-Meier method was used to analyze the probability ratio of OS and PFS.
Results
Analysis of the function of CD19/CD20 CAR-T cells compared with single-targeted CAR-T cells
We extensively evaluated the CD19/CD20 CAR-T cells in immunological assays in vitro. Our results demonstrated that CD19/CD20 CAR-T cells exhibited comparable cytotoxicity to CD20 or CD19 monovalent CAR-T cells (Fig. 1B). Furthermore, the CD19/CD20 CAR-T cells exhibited a strong induction of cytokines in the presence of target tumor cells, which was similar to that of monovalent CAR-T cells (Fig. 1C). These results suggested that CD19/CD20 CAR-T cells had the potential to be an effective treatment for CD19/CD20-expressing tumors.
Patient characteristics
Between May 2021 and November 2022, 14 patients were screened in our hospital, 13 patients underwent leukapheresis and a total of 11 NHL patients who met the inclusion criteria were enrolled in our study to receive anti-CD19/20 CAR-T cell therapy (Fig. 2). The median age of the patients was 59 years (range: 43–68 years), including seven females (64%) and four males (36%). Table 1 shows the demographic and clinical characteristics of patients with three major lymphoma types including DLBCL, MCL, and FL. 9 patients had DLBCL of GCB or nonGCB. Case 5 was diagnosed with FL, while case 6 had MCL. All patients (100%) were CAR naive and had stage III or IV disease. 4 were double-expressed (expression of c-MYC and BCL2) DLBCL. In half of the patients, biopsies showed a Ki67 positive rate of over 70%. 3 patients had refractory disease, and the other 8 NHL patients experienced relapse after antineoplastic therapy.
10 patients had positive expression of both CD19 and CD20 as confirmed by immunohistochemistry.
Safety and adverse events
Eleven patients received CD19/20 CAR-T cell products. Five patients (45%) developed CRS with a median onset of 4 days after infusion (range 1–16) and a median duration of 2 days (range 1–3) (Table 2). Cases 1, 2, 7, and 8 had CRS grades of 1 or 2, while case 3 had CRS grades of 3 with symptoms such as fever, hypotension, and severe hypoxemia. Interestingly, due to relapse and pulmonary infection, case 2 developed late-onset CRS (grade 3) 161 days after CAR-T cell infusion, characterized by fever, hypoxia, and hypotension, with IL6 levels up to 3441 pg/ml. Two patients (18%) developed ICANS and were assessed as grade 3. The median time to onset of ICANS was 11 days (range 6–16) post-infusion, and the median duration was 1 day. Both CRS and ICANS were treated according to institutional guidelines, with 2 patients (18%) receiving 2 doses of tocilizumab and 1 patient (9%) receiving dexamethasone. No obvious residual neurological impairments were observed in the enrolled patients. Grade 3 or above adverse events included edema (n = 1, 9%), hyponoia (n = 1, 9%), anorexia (n = 2, 18%), nausea (n = 1, 9%), URI (n = 2, 18%), lung infection (n = 3, 27%), skin infection (n = 1, 9%), sinus tachycardia (n = 1, 9%), heart failure (1, 9%), anemia (n = 2, 18%), hypokalemia (n = 1, 9%), neutropenia (n = 5, 45%), thrombocytopenia (n = 2, 18%) and musculoskeletal disorders (n = 2, 18%).
The Fig. 3 displayed the dynamics of serum cytokine and C-reactive protein (CRP) levels in all patients during the first month following CAR-T infusion. Among the cytokines detected, IL-2, IL-6, IL-8, and IL-10 were elevated in 5 patients (cases 1, 2, 3, 7, and 8) with CRS. IL-6 was associated with CRS grade.
Response and survival
Before the infusion of CAR-T cells, all patients received a conditioning regimen. The median number of CD19/20 CAR-T cells infused was 1.05 (range: 0.31–2.62) × 106/kg (Supplementary Table 2). Of all enrolled patients, case 3 died of severe pneumonia and CRS prior to evaluation, and the remaining 10 patients were evaluated. Nine out of ten patients responded to CAR-T therapy (ORR = 90%). Seven of nine responders achieved CR as the best response (CR rate = 70%), and the other two responders achieved PR. Among seven patients achieving CR, case 4 and case 6 achieved CR at approximately one month, while cases 1, 5, and 7 exhibited PR at the first efficacy assessment around one month, and subsequently achieved CR in the following assessments (around 3 to 6 months). The median duration of response for the nine responders was 11.83 months (range: 4.70–18.50 months) (Fig. 4A), with the longest response time being 18.50 months after CAR-T cell infusion (Case 1). The PET-CT images for three representative patients before CAR-T therapy and at the best response after infusion were displayed in Fig. 4B.
The median follow-up of all efficacy-evaluated patients was 12.63 months (range: 6.70–18.50 months). Only case 2 died from disease progression, with a survival time of 6.70 months. The median OS and PFS after CAR-T cell infusion were not reached. The estimated 3-month survival rate was 90.91% (95% CI 62.26%-99.53%) and the 12-month survival rate was 81.82% (95% CI 52.30%-96.77%) as shown in Fig. 4C. The estimated 3-month PFS rate was 90% (95%CI 59.59%-99.49%) and 12-month PFS rate was 60% (95% CI 31.27%-83.18%) (Fig. 4D).
CAR-T cell expansion, persistence, and B cell recovery
Flow cytometry was utilized to monitor the presence of CAR-T cells in peripheral blood at various time points after CAR-T cell infusion. The CAR-T cell expansion data for all patients were sorted and plotted as dynamic curves, which varied among the patients (Fig. 5A, B). The dynamic percentage of CAR-T cells in lymphocytes was consistent with the CAR-T cell number. The first high expansion of CAR-T cells was detected within the second week (from day 7 to day 14) after infusion. Interestingly, in cases 2, 3, and 7, a second peak in CAR-T cell expansion was observed during a later period of follow-up (Supplementary Fig. 1). At peak expansion, up to 65.41% CAR-T cells were detected in peripheral blood mononuclear cells (PBMC) (median 22.12%; range, 3.41%–65.41%; Fig. 5B). The highest peak number of CAR-T cells in the peripheral blood was 1127 cells/μl at day 27 in case 3, who experienced severe CRS symptoms, and this was also the second peak for this patient. In case 1, the highest peak percentage of CAR-T cells in lymphocytes of peripheral blood was 53.89%, and CD19/20 CAR-T cells were still detectable in vivo up to 460 days post-infusion, which was the longest CAR-T cell persistence in this clinical trial before the cutoff day and maintained an ongoing CR status. As of the last available datapoint, five of the nine responders had detectable CAR-T cells and all five patients maintained a persistent CR status (Fig. 5A, B). These findings revealed the in vivo expansion and persistence of bispecific CAR-T cells in the enrolled patients.
A CAR-T cell number in peripheral blood mononuclear cells (PBMCs). Absolute counts of CAR-T cells in peripheral blood were measured using flow cytometry. B Presence of CAR-expressing T cells among lymphocytes as quantified by flow cytometry. C Percentage of CD19+ cells among lymphocytes as quantified by flow cytometry.
B cells in the peripheral blood of all patients dropped below detection after the CAR-T cell infusion, and CD19 + B cells returned to normal in 4 patients (36.4%) 100 days after CAR-T cell infusion (Fig. 5C).
TCR clonotypes of CAR-T cells, differentially expressed genes, and pathway analysis
To better understand the dynamics of the CAR-T cell repertoire following CAR-T cell infusion, we analyzed the T cell clonotype features of CAR-T cells from IP and from PB on day 21. A total of 1796 clones were identified in CAR-T infusion products. Out of these, only 168 clones remained, and some of them were found to be amplified in peripheral blood samples 21 days after the infusion. We observed a lower proportion of the top 9 clonotypes of CAR-T cells in the IP, in contrast to almost 100% on day 21 (Fig. 6A). These data demonstrated that CAR-T cells exhibited lower diversity and higher levels of clonal expansion of T cells in their blood after CAR-T cell infusion.
A Stacked bar plot represents the abundance of the first 9 top TCR clonotypes of CAR-T cells from infusion product (IP) and peripheral blood (PB) on day 21 after infusion. B The volcano plot of significantly changed genes in the top 3 TCR clonotypes vs. other TCR clonotypes of CAR-T cells from IP. The criteria for identifying differentially expressed genes (DEGs) with significant differences using a volcano plot are as follows: the absolute value of the log2 fold-change (|log2FC | ) must be equal to or greater than 1, and the adjusted p-value (based on Bonferroni correction using all features in the dataset) must be less than 10e-06. The DEGs were up-regulated or down-regulated according to the log2FC value > 1 or < − 1. C Top KEGG terms enriched in upregulated genes in 3 top clonotypes compared to other clones in CAR-T cell from IP. Fisher’s exact test. KEGG Kyoto Encyclopedia of Genes and Genomes.
The top 3 TCR clones accounted for more than 80% of the total CAR-T cells (Fig. 6A and Supplementary Table 3). We then focused on the top 3 TCR clonotypes of CAR-T cells (Supplementary Table 3) derived from both IP and PB samples on day 21. By comparing the differentially expressed genes between the top 3 TCR clones of CAR-T cells and other clones from IP, we discovered that the top 3 TCR clones exhibited a higher fold-change in genes associated with immune-related signaling pathways (Fig. 6B, C). However, on day 21, there was no difference in gene expression between the two types of CAR-T cells (data not shown).
We focused on analyzing the differentially expressed genes of CAR-T cells in IP and PB at day 21. Compared to CAR-T cells from IP, the expression of 16 genes increased in CAR-T cells on day 21 (Fig. 7A). We used GSEA to illustrate the significant properties of these differentially expressed genes. Interestingly, CAR-T cells on day 21 showed signatures associated with cell adhesion molecules (Fig. 7B). Additionally, we analyzed the biological processes (BP) based on the GO database [31] of differentially expressed genes in CAR-T cells. CAR-T cells on day 21 exhibited immune response and cytolysis/cytotoxicity compared to CAR-T cells from IP (Fig. 7C, D). Two out of the top three BPs (2/3) were directly linked to activating cell surface receptor signaling pathways (Fig. 7C).
A Differentially expressed genes (DEGs) of CAR-T cells on day 0 vs. day 21. x-axis: the difference between the percentage of cells highly expressing one certain gene in the designated cluster; y-axis: log2 fold-change (FC) of the average expression between the two groups. B Gene set enrichment analysis (GSEA) plots of differentially expressed genes in CAR-T cells on day 21 compared to those on day 0. GESA based on a Kolmogorov–Smirnov test. NES normalized ES, FDR false discovery rate, NOM p normalized p-value. The adjusted p-value < 0.05 was considered statistically significant. C Top GO terms enriched in upregulated genes in CAR-T cells on day 21 compared to those on day 0, Fisher’s exact test. GO: Gene Ontology. BP: biological processes. D Top KEGG terms enriched in upregulated genes in CAR-T cells on day 21 compared to those on day 0, Fisher’s exact test. KEGG: Kyoto Encyclopedia of Genes and Genomes.
Discussion
Multiple studies have demonstrated that CD19 is highly and specifically expressed in malignant and normal B cells, making it as a promising target for CAR-T immunotherapy in the treatment of R/R NHL [6, 20]. However, antigen loss after CD19 CAR-T cell therapy has been a significant obstacle in achieving long-term remission for patients [24]. To address this issue, several strategies have been applied to expand antigen coverage and prevent antigen-loss-induced recurrence after the infusion of single-target CAR-T cells. One effective approach has been to develop CARs that target both CD19 and CD20, as CD20 is also an effective treatment target for B-cell malignancies, expressed on the surface of B cells but not on hematopoietic stem cells [18]. Both Tandem CAR (TanCAR-T) and dual CAR constructs targeting diverse antigens have been tested in clinical trials. However, the sustained activity of TanCAR-T cells has been found to be superior to that of T cells with dual CARs due to the unanimous expression of CARs, better agglomeration of F-actin, and polarization of MTOC [21].
In this study, we have combined two scFvs into a single molecule with antigen specificity to both CD19 and CD20 and constructed TanCAR-T cells that display wide antigen coverage and strong antitumor activity in vitro (Fig. 1) [18]. To verify the in vitro outcomes and evaluate the response and duration of these bispecific CAR-T cells in clinical application, we conducted a phase I-II clinical trial on patients with R/R NHL. All patients were diagnosed as NHL with relapsed or refractory characteristics, and some patients suffered from high tumor burden or aggressive disease. Remarkably, the therapy achieved impressive responses, with nine out of ten patients responding to the bispecific CAR-T immunotherapy, resulting in a 90% OR rate and a 70% CR rate. Notably, five out of ten patients still survived with CR status with a median PFS of 13.47 months. Two patients achieved CR within 3 months after infusion but then relapsed with a median PFS duration of 9.62 months. The previous trials demonstrated that the median PFS after CD19 CAR-T-cell therapy for R/R NHL was approximately 6 months [6, 7, 20, 32]. In our study, the median PFS after CAR-T cell infusion was not reached at a median follow-up time of 12.63 months (range: 6.70–18.50 months). Case 1 achieved long-term disease/relapse-free survival, indicating the CD19/20 CAR-T cell infusion can exert a long-period therapeutic effect for relapsed or refractory high-risk NHL patients. Our preliminary results demonstrated that patients with R/R NHL who received bispecific CD19/20 CAR-T-cell immunotherapy achieved a high percentage of complete remission.
ICANS and CRS are the common adverse events associated with CAR-T cell immunotherapy and require close monitoring. In some studies of CAR-T cell therapy, patients with concerns about potentially lethal ICANS have been excluded [33, 34]. The ZUMA-1 trial showed that the rates of grade 3 or higher ICANS were as high as 32% [35], and in the JULIET trial, the rates of grade 3 or higher CRS were as high as 23% [36]. CRS is the most frequent and potentially fatal side effect of CAR-T therapy, with symptoms including fever, hypoxia, and hypotension [37]. In our current study, cases 1, 2, 7, and 8 had CRS grades of 1 or 2 after the infusion of CD19/20 CAR-T cells. Case 3 developed severe CRS symptoms, which were assessed as grade 3. Generally, CRS occurs within several days after the infusion of CAR-T cells [38], but there was one special case in our study. Case 2 was enrolled with refractory DLBCL and achieved PR status after the infusion of CD19/20 CAR-T cells. The clinical response of case 2 sustained for over 4.70 months. Intriguingly, the CAR-T cells of case 2 expanded rapidly again and achieved the peak (23 cells/µl blood) at day 164 after CAR-T infusion (Supplementary Fig. 1). During this period, she experienced serious CRS of grade 3 with the symptoms of fever, hypotension, and hypoxemia due to relapse and pulmonary infection. This phenomenon indicates CRS could also occur in the late term of the response stage after CAR-T therapy, and the re-expansion of CAR-T cells still possesses the potential risk for late CRS. Regarding the adverse events of ICANS in our current study, cases 1 and 3 were the only two patients who experienced grade 3 ICANS, and whose symptoms were reversible with prompt treatment. This suggests that CD19/20 CAR-T cell therapy is a safe approach to treat patients with R/R NHL.
In this study, the expansion of CAR-T cells was robust, with the most significant increase observed during the first 7–14 days after infusion. These findings align with those seen in other CAR-T cell therapies [6, 7]. IL-6 increased in parallel with initial CAR-T cell expansion and correlated with the grade of CRS (Fig. 3).
In this study, we utilized a combination of scTCR-seq and RNA-Seq analyses to assess both TCR clonal dynamics and functional gene expression in a single patient undergoing CAR-T cell therapy. We found a decrease in TCR repertoire diversity on day 21 after CAR-T cell infusion. The T cell clones of CAR-T cells that exhibited high expression of genes associated with immune-related signaling pathways from IP were likely to become the dominant TCR clones by day 21. Additionally, dynamic changes in CAR-T cells in vivo were linked to a significant increase in the expression of genes related to immune response and cytolysis/cytotoxicity. This finding suggested that CAR-T cells played a critical role in combating B lymphoma cells upon antigen encounter. Similarly, one previous study about CD19 CAR-T cells also confirmed that the clonal diversity was highest in the IP and decreased after infusion. Certain clones exhibit superior abilities to proliferate, and/or persist in the bloodstream following infusion [39].
Conclusions
In summary, this phase I/II trial demonstrates the safety and robust clinical effectiveness of autologous CAR-T therapy targeting CD19 and CD20 for treating R/R NHL, highlighting the value of dual-antigen targeting in cell-based immunotherapy. However, given the small sample size of this study, a larger cohort of patients will be necessary to further validate the long-term efficacy and safety of this approach. The proliferation of TCR clones in vivo originates primarily from clones with elevated gene expression involved in immune-related signaling pathways in infused products, while the dynamics of CAR-T cells in vivo are linked to heightened expression of genes associated with immune response and cytolysis/cytotoxicity.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request
References
Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–90.
Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, Castaigne S, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood. 2010;116:2040–5.
Elstrom RL, Martin P, Ostrow K, Barrientos J, Chadburn A, Furman R, et al. Response to second-line therapy defines the potential for cure in patients with recurrent diffuse large B-cell lymphoma: implications for the development of novel therapeutic strategies. Clin Lymphoma Myeloma Leuk. 2010;10:192–6.
Crump M, Neelapu SS, Farooq U, Van Den Neste E, Kuruvilla J, Westin J, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR−1 study. Blood. 2017;130:1800–8.
Hamadani M, Hari PN, Zhang Y, Carreras J, Akpek G, Aljurf MD, et al. Early failure of frontline rituximab-containing chemo-immunotherapy in diffuse large B cell lymphoma does not predict futility of autologous hematopoietic cell transplantation. Biol Blood Marrow Transpl. 2014;20:1729–36.
Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl J Med. 2017;377:2531–44.
Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380:45–56.
Hunter BD, Rogalski M, Jacobson CA. Chimeric antigen receptor T-cell therapy for the treatment of aggressive B-cell non-Hodgkin lymphomas: efficacy, toxicity, and comparative chimeric antigen receptor products. Expert Opin Biol Ther. 2019;19:1157–64.
Abramson, Palomba JS, Gordon LI ML, Lunning MA, Wang M, Arnason J, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396:839–52.
Sadelain M, Riviere I, Riddell S. Therapeutic T cell engineering. Nature. 2017;545:423–31.
Park JH, Riviere I, Gonen M, Wang X, Senechal B, Curran KJ, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378:449–59.
June CH, Sadelain M. Chimeric antigen receptor therapy. N Engl J Med. 2018;379:64–73.
Turtle CJ, Hanafi LA, Berger C, Hudecek M, Pender B, Robinson E, et al. Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci Transl Med. 2016;8:355ra116.
Chavez JC, Yassine F, Sandoval-Sus J, Kharfan-Dabaja MA. Anti-CD19 chimeric antigen receptor T-cell therapy in B-cell lymphomas: current status and future directions. Int J Hematol Oncol. 2021;10:IJH33.
Ying Z, Song Y, Zhu J. Effectiveness and safety of anti-CD19 chimeric antigen receptor-T cell immunotherapy in patients with relapsed/refractory large B-cell lymphoma: a systematic review and meta-analysis. Front Pharm. 2022;13:834113.
Majzner RG, Mackall CL. Clinical lessons learned from the first leg of the CAR T cell journey. Nat Med. 2019;25:1341–55.
Furqan F, Shah NN. Bispecific CAR T-cells for B-cell malignancies. Expert Opin Biol Ther. 2022;22:1005–15.
Tong C, Zhang Y, Liu Y, Ji X, Zhang W, Guo Y, et al. Optimized tandem CD19/CD20 CAR-engineered T cells in refractory/relapsed B-cell lymphoma. Blood. 2020;136:1632–44.
Sotillo E, Barrett DM, Black KL, Bagashev A, Oldridge D, Wu G, et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART−19 immunotherapy. Cancer Discov. 2015;5:1282–95.
Schuster SJ, Svoboda J, Chong EA, Nasta SD, Mato AR, Anak O, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med. 2017;377:2545–54.
Hegde M, Mukherjee M, Grada Z, Pignata A, Landi D, Navai SA, et al. Tandem CAR T cells targeting HER2 and IL13Ralpha2 mitigate tumor antigen escape. J Clin Invest. 2016;126:3036–52.
Zah E, Lin MY, Silva-Benedict A, Jensen MC, Chen YY. T cells expressing CD19/CD20 bispecific chimeric antigen receptors prevent antigen escape by malignant B cells. Cancer Immunol Res. 2016;4:498–508.
Hamieh M, Dobrin A, Cabriolu A, van der Stegen SJC, Giavridis T, Mansilla-Soto J, et al. CAR T cell trogocytosis and cooperative killing regulate tumour antigen escape. Nature. 2019;568:112–6.
Majzner RG, Mackall CL. Tumor antigen escape from CAR T-cell therapy. Cancer Discov. 2018;8:1219–26.
Grada Z, Hegde M, Byrd T, Shaffer DR, Ghazi A, Brawley VS, et al. TanCAR: a novel bispecific chimeric antigen receptor for cancer immunotherapy. Mol Ther Nucleic Acids. 2013;2:e105.
Dai H, Wu Z, Jia H, Tong C, Guo Y, Ti D, et al. Bispecific CAR-T cells targeting both CD19 and CD22 for therapy of adults with relapsed or refractory B cell acute lymphoblastic leukemia. J Hematol Oncol. 2020;13:30.
Schneider D, Xiong Y, Wu D, Nӧlle V, Schmitz S, Haso W, et al. A tandem CD19/CD20 CAR lentiviral vector drives on-target and off-target antigen modulation in leukemia cell lines. J Immunother Cancer. 2017;5:42.
Turtle CJ, Hanafi LA, Berger C, Gooley TA, Cherian S, Hudecek M, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest. 2016;126:2123–38.
Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transpl. 2019;25:625–38.
Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–68.
Gene Ontology C. The Gene Ontology resource: enriching a gold mine. Nucleic Acids Res. 2021;49:D325–D34.
Bachy E, Le Gouill S, Di Blasi R, Sesques P, Manson G, Cartron G, et al. A real-world comparison of tisagenlecleucel and axicabtagene ciloleucel CAR T cells in relapsed or refractory diffuse large B cell lymphoma. Nat Med. 2022;28:2145–54.
Karschnia P, Jordan JT, Forst DA, Arrillaga-Romany IC, Batchelor TT, Baehring JM, et al. Clinical presentation, management, and biomarkers of neurotoxicity after adoptive immunotherapy with CAR T cells. Blood. 2019;133:2212–21.
Frigault MJ, Dietrich J, Martinez-Lage M, Leick M, Choi BD, DeFilipp Z, et al. Tisagenlecleucel CAR T-cell therapy in secondary CNS lymphoma. Blood. 2019;134:860–6.
Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20:31–42.
Schuster SJ, Tam CS, Borchmann P, Worel N, McGuirk JP, Holte H, et al. Long-term clinical outcomes of tisagenlecleucel in patients with relapsed or refractory aggressive B-cell lymphomas (JULIET): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2021;22:1403–15.
Grigor EJM, Fergusson D, Kekre N, Montroy J, Atkins H, Seftel MD, et al. Risks and benefits of chimeric antigen receptor T-cell (CAR-T) therapy in cancer: a systematic review and meta-analysis. Transfus Med Rev. 2019;33:98–110.
Lefebvre B, Kang Y, Smith AM, Frey NV, Carver JR, Scherrer-Crosbie M. Cardiovascular effects of CAR T cell therapy: a retrospective study. JACC CardioOncol 2020;2:193–203.
Sheih A, Voillet V, Hanafi LA, DeBerg HA, Yajima M, Hawkins R, et al. Clonal kinetics and single-cell transcriptional profiling of CAR-T cells in patients undergoing CD19 CAR-T immunotherapy. Nat Commun. 2020;11:219.
Funding
Chinese National Major Project for New Drug Innovation (2019ZX09201002003), National Natural Science Foundation of China (82030076, 82070161, 81970151, 81670162 and 81870134), Shenzhen Science and Technology Foundation (JCYJ20190808163601776, JCYJ20200109113810154), Shenzhen Key Laboratory Foundation (ZDSYS20200811143757022), Sanming Project of Medicine in Shenzhen (SZSM202111004), Natural Science Foundation of Shenzhen University General Hospital (SUGH2019QD012), Shenzhen Natural Science Fund (the Stable Support Plan Program, 20200830182623001), HaiYa Young Scientist Foundation of Shenzhen University General Hospital (2024-HY002), and Shenzhen Medical Research Fund (C2301003).
Author information
Authors and Affiliations
Contributions
Lixin Wang, Chuling Fang, Yisheng Li, and Li Yu contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Chuling Fang, Qingzheng Kang, Wenfa Huang, Ziren Chen, Weiqiang Zhao, Lei Wang, Yiran Wang, Kun Tan, Xiao Guo, Yuanyuan Xu, Shuhong Wang, Lijun Wang, Jingqiao Qiao, Zhixiong Tang, Chuan Yu, Yang Xu. The first draft of the manuscript was written by Chuling Fang and Qingzheng Kang, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, L., Fang, C., Kang, Q. et al. Bispecific CAR-T cells targeting CD19/20 in patients with relapsed or refractory B cell non-Hodgkin lymphoma: a phase I/II trial. Blood Cancer J. 14, 130 (2024). https://doi.org/10.1038/s41408-024-01105-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41408-024-01105-8
- Springer Nature Limited