Abstract
Image-guided percutaneous cryoablation is an established minimally invasive oncologic treatment. We hypothesized that cryoablation may modify the immune microenvironment through direct modulation of the tumor, thereby generating an anti-tumor response in tumors refractory to immune checkpoint inhibition (ICI). In this non-randomized phase II single-center study (NCT03290677), subjects with unresectable melanoma progressing on ICI underwent cryoablation of an enlarging metastasis, and ICI was continued for a minimum of two additional cycles. The primary endpoints were safety, feasibility and tumor response in non-ablated lesions. From May 2018 through July 2020, 17 patients were treated on study. The study met its primary endpoints with the combination strategy found to be safe and feasible with an objective response rate of 23.5% and disease control rate of 41% (4 partial response, 3 stable disease). Our data support further study of this synergistic therapeutic approach.
Similar content being viewed by others
Introduction
Immune checkpoint inhibition (ICI) has fundamentally altered the treatment paradigm for patients with metastatic melanoma, with long-term survival data demonstrating durable disease control in 30% of patients receiving single-agent PD-1 inhibition1. Despite these benefits, most patients either will not respond to front-line therapy or after an initial response, will develop acquired resistance and though important to acknowledge unique ICI response patterns, such as pseuodoprogression and delayed response, data demonstrates radiographic progression, most commonly reflect true ICI resistance2,3. The mechanisms of primary, as well as secondary resistance, are incompletely understood but include inadequate T cell infiltration into the tumor4, immunosuppressive factors within the tumor microenvironment (TME)5,6, loss of T cell function through the expression of alternative immune checkpoints6, disruption of interferon-gamma signaling via JAK2 mutations7,8 and impaired antigen presentation8,9. Efforts to overcome resistance are paramount, and novel combination strategies are developing, including combination ICI with intralesional therapy (NCT04695977) and local ablative techniques (NCT03958383, NCT05779423).
Cryoablation is a well-established treatment for primary and metastatic tumors10,11,12,13,14,15. It is an approved, percutaneous treatment that results in cellular necrosis via serial freezing and thawing16. Depending on the organ, the ablation zone can be monitored in real-time using computed tomography (CT), magnetic resonance imaging (MRI), or ultrasound (US). While percutaneous cryoablation is routinely used to treat tumors that require local control with the goal of eradication or pain palliation, pre-clinical and early clinical data illustrate immune modulatory effects of cryoablation17. In situ destruction of malignant cells via cryoablation can result in release of tumor-specific antigens and pro-inflammatory cytokines (IFNγ, TGFβ), thereby stimulating a robust tumor-specific immune response18,19. Although there have been reports of abscopal effects after cryoablation20, the magnitude and durability of an immune response from cryoablation alone does not appear to be an adequate systemic anti-tumor strategy21. Concurrent use of ICI has the potential to enhance the immunogenicity of cryoablation, resulting in a systemic anti-tumor response21,22,23,24 and overcoming the limitations of each treatment alone. Based on the hypothesis that this combined approach may improve anti-tumor outcomes we opened a phase II study evaluating cryoablation and immune checkpoint inhibition in PD-1-resistant metastatic melanoma. Here, we present the safety and efficacy data of this approach.
Results
From May 2018 through July 2020, 20 patients with unresectable or metastatic cutaneous melanoma with evidence of progressive disease (PD) in more than one site on imaging were screened for trial inclusion, with 18 patients ultimately enrolled (Table 1, Supplementary Tables 1 and 2; Supplementary Fig. 1). One patient was excluded after pulmonary lesions, suspected metastases, spontaneously regressed prior to cryoablation. A second patient was excluded due to lack of evaluable disease per RECIST criteria aside from the cryoablation target, specifically additional pulmonary metastases were <1 cm. Of 18 patients who underwent cryoablation, 17 received post-cryoablation ICI and were evaluable for efficacy assessment (Fig. 1A). All patients received single-agent PD-1 inhibition at the time of cryoablation, including one on maintenance nivolumab after ipilimumab/nivolumab. The median age at time of enrollment was 63.5 years (range 47–90), and 56% were male. Most patients (78%) had an Eastern Cooperative Oncology Group (ECOG) performance status score of 0 or 1, with 22% having an ECOG score of 2. Median LDH prior to cryoablation was 236 U/L (range 137–410; [lab range 110–210]). Four patients (22%) had brain metastases at baseline. At the time of cryoablation, seven patients (39%) were receiving first-line ICI, five were receiving second-line therapy (28%), and six (33%) were on ≥3 L of therapy. The median duration of ICI prior to cryoablation was 105 days (range 41–1250), with ten patients (58%) experiencing disease control on prior ICI (stable disease [SD] or partial response [PR]). Seven patients (41%) had progressive disease as the best response to ICI prior to cryoablation (Fig. 1B–D). At the time of cryoablation the majority of patients (78%) had progression in multiple sites (≥3 sites) with visceral progression in 78% (Supplementary Table 2).
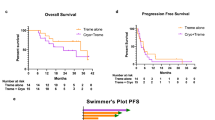
A Study schema. Visual production credits “iStock.com/DennyFachrulRozzy” and iStock.com/MaskaRad”. B Waterfall plot of best response/change in tumor burden from baseline post-cryoablation (n = 17). Source data are provided as a Source Data file. C Computed tomography of one patient with a durable response on trial (n = 1). Axial maximum intensity projection chest computed tomography (CT) of a 64-year-old male with progression of metastatic melanoma on pembrolizumab monotherapy. Baseline CT performed three months prior to cryoablation shows a 28 mm metastasis in the right lower lobe (RLL, panel 1, arrow), an 11 mm metastasis in the left upper lobe (LUL, panel 2, arrow), and right hilar lymphadenopathy (panel 3, arrow). CT images obtained to guide cryoablation of the dominant RLL metastasis demonstrate interval enlargement of the target (40 mm, panel 4, arrow), LUL metastasis (13 mm, panel 5, arrow), and right hilar lymphadenopathy (panel 6, arrow), and a new subpleural 8 mm RLL metastasis (panel 5, circle). Three-, six-, and nine-month follow-up CT shows resolution of the subpleural RLL non-ablated metastasis (panels 8, 11, 14), shrinkage of the LUL non-ablated metastasis to 7 mm, 4 mm, and 3 mm (panels 8, 11, 14, arrow), and resolution of right hilar lymphadenopathy (panels 9, 12, 15, arrow). The ablated RLL metastasis also decreased in size (panels 7, 10, 13, arrow), respectively. CT, computed tomography; LUL, left upper lobe; RLL, right lower lobe. D Duration of ICI pre- and post-cryoablation (n = 17). Cryoablation occurred on day 0. Source data are provided as a Source Data file.
Of the 17 patients who received post-ablation ICI, all successfully underwent percutaneous cryoablation by interventional radiologists. Ablation sites included enlarged lymph nodes (n = 5) as well as metastatic tumors in the lung or pleura (n = 4), soft tissue or bone (n = 4), adrenal gland (n = 3), kidney (n = 1) and liver (n = 1). Median ablative coverage was 39% of the total targeted tumor volume (range 7–100). Post-ablation, 16 patients went on to receive two additional doses of their intended post-progression ICI. A 63-year-old man who had been receiving pembrolizumab transitioned to ipilimumab/nivolumab prior to receiving the second dose of post-progression pembrolizumab due to continued symptomatic progression and was deemed a non-responder. A 90-year-old man opted to transition to hospice post-ablation without additional treatment or scans and was not included in the efficacy assessment. Both patients were included in the safety evaluation.
Safety
Eighteen patients were evaluable for peri-procedural safety (Table 2 and Supplementary Table 1). Most treatment-related and procedural-related adverse events (AEs) were mild and manageable. Significant procedure-related AEs (all grade 3) included post-procedural pain and osteomyelitis in one patient, and two patients experienced a transient lab abnormality. A 56-year-old woman had significant post-procedural pain (grade 3) at the cryoablation site (clavicle) and subsequently developed grade 3 osteomyelitis, requiring admission, intravenous antibiotics, and surgical debridement. Post-procedural lab abnormalities occurred in two patients, including one patient with transient hepatitis following cryoablation of a liver metastasis and one patient acute kidney injury following cryoablation of a renal metastasis. In both cases these lab abnormalities improved without intervention.
In patients evaluable for post-ablation treatment-related AEs (n = 17), there were no de novo grade 3≥ immune-related adverse events (irAEs). A 67-year-old female with underlying ICI-induced hypophysitis experienced an adrenal crisis post-procedure with symptomatic hyponatremia, which was rapidly corrected with the administration of stress-dose steroids. There were no grade 4 or 5 treatment or procedural-related AEs.
Efficacy
The determination of efficacy in the study was based on the best radiologic response in non-ablated lesions to combination of cryoablation and post-progression ICI therapy. Per protocol, ablated tumors were not incorporated into RECIST 1.1 assessment. The objective response rate (ORR) and disease control rate (DCR) of the evaluable cohort (n = 17) was 23.5% (95% confidence interval [CI] 6.81–49.9) and 41.2% (95% CI 18.44–67.08), respectively, with four patients experiencing PR and three patients with SD (Fig. 1B–D). All responders had evidence of tumor regression in non-ablated sites, significantly improved than that previously reported with post-progression ICI alone (p-value: 0.076)3,25. The median duration of response was 771.5 days (range 179–1364) in responders and 356 days (range 44–1755) in patients who experienced disease control. Durable disease control, defined as continuous tumor control [SD, PR] lasting ≥6 months, was seen in patients with both PR and SD. One patient with SD by RECIST 1.1 went on to complete approximately 2 years of ICI and maintains disease control after drug cessation. Three of the four patients who experienced a PR remain alive with no alternative systemic therapy required. Though the majority (80%) of patients with evidence of disease control post-cryoablation had a normal LDH, both cryoablation site and sites of progressive disease were variable (Supplementary Table 2). Ten patients had progressive disease as their best response. Notably, one patient with a best response of PD (PD based on interval increase in lymphadenopathy with other sites disease stable or improved) continued ICI for an additional 337 days post-cryoablation. Median progression-free survival (PFS) was 59 days (95% CI 42, 179) with a 6-month PFS rate of 23.5% (95% CI 7.3%, 44.9%). Median overall survival (OS) was 387 days (95% CI 106, 608) with a 1-year OS rate of 53% (95% CI (27.6%, 73%; Supplementary Fig. 2).
Correlative analysis
Serial blood samples and pre-cryoablation tissue were collected on all patients and in select patients repeat tissue biopsy was performed after receipt of subsequent ICI. Correlative analyses were not predefined in the study protocol and were performed post-hoc.
Single-cell RNA-sequencing reveals tumor and immune correlates of response to cryoablation
To better understand the factors determining response to cryoablation therapy in the setting of ICI-resistant melanoma, we performed single-cell RNA-sequencing using both 10x Genomics and Smart-seq2 technologies on biopsies obtained from metastatic sites of 15 patients. We obtained pre-treatment samples from each patient as well as post-treatment samples from 5 patients, for a total of 20 samples across six anatomic sites (Fig. 2A, B, Method Details). Following quality control, exclusion of cells specific to anatomic site or low-frequency cell types (e.g., alveolar type II, neurons), and batch correction, we recovered 49,071 single cells across the melanocyte, endothelial, stromal, T, myeloid, and B/plasma cell lineages (Fig. 2C, D, Supplementary Fig. 3A, B, Method Details).
A Overview of the scRNA-seq cohort. Visual production credits “iStock.com/MaskaRad”. B Summary of available samples by patient and response status, organized by anatomical site and labeled by timing obtained (i.e pre-tx: pre-ablation, pre+post tx: pre-ablation and post-ablation). C Uniform manifold approximation and projection (UMAP) plot of all single cells passing quality control filtration, colored by cell lineage. D UMAP expression density plots of the following lineage-defining marker genes (left to right, top to bottom): PMEL, VWF, CD3E, LYZ, CD79A, COL1A1. E Box plots displaying (top) the percentage of T, B/plasma, and myeloid cells out of all CD45+ cells, and (bottom) the percentage of melanocyte, endothelial, and stromal cells out of CD45- cells for pre-treatment samples only. Box plots are grouped by ultimate response to cryoablation, comparing patients with progressive disease (n = 10) to those without (n = 3). Box plots indicate interquartile range, with horizontal line indicating median, and whiskers extending to maxima and minima excluding outliers (>1.5 * IQR). Indicated p values are for the Kruskal–Wallis test, uncorrected. PD progressive disease, SD stable disease, PR partial response. Source data for Fig. 2 are provided as a Source Data file.
With the available samples, we evaluated if emerging features in the tumor microenvironment could correlate with response or resistance to cryoablation. Thus, we compared cellular composition between pre-treatment tumors to see whether responders had shared features. Though there were no clear trends of cell type composition to predict the best response to cryoablation (Supplementary Fig. 3C), we found a significant increase in B/plasma cells (p = 0.014) among the three patients that did not ultimately develop progressive disease as compared to those with ultimately progressive disease (Fig. 2E), suggesting these cells may play a role in mounting a durable anti-tumor response after cryoablation.
We next performed subclustering of T cells, identified 16 clusters (Fig. 3A, Supplementary Fig. 4A), and began to identify associations between best response and T cell subtypes. Though tumors with a best response of SD appeared to have a distinct T cell profile, we did not observe pronounced differences in T cell subtype composition between response categories overall (Fig. 3B); future studies in larger cohorts may be revealing. We next performed differential gene expression analysis between T cells from lesions that showed clinical benefit from cryoablation (SD, PR) versus those that did not (PD) (Supplementary Fig. 4B, Method Details) and identified several candidate genes which could be implicated in response (Fig. 3C). For example, TXK, a gene that acts as a Th1 transcription factor and regulates IFNγ expression, and the gene CMKLR1 (chemerin-like-receptor-1), which was shown to trigger the intratumoral migration of a CD8 T-cell subset with natural killer cell properties26 were upregulated in T cells with best response of disease control, particularly among patients with SD, whereas MTHFD2 and GGH, two genes involved in one-carbon metabolism and the folate cycle, were upregulated in T cells in PD. A previous study has shown MTHFD2 to be a metabolic checkpoint particularly important for enhanced Th17 effector functions27. To understand which pathways were involved in response, we performed gene set enrichment analysis (GSEA)28 (Fig. 3D, Method Details). We found that T cells from patients with PD had enrichment of oxidative phosphorylation relative to SD and PR potentially indicating that these T cells have been exposed to chronic antigen stimulation and have failed to undergo a glycolytic shift, impairing IFNγ production and contributing to T cell exhaustion/dysfunction.
A UMAP of T cell subclustering with cluster annotations. B Box plots displaying the frequency of T cells belonging to a specific subcluster per patient comparing PD (n = 9), SD (n = 2), and PR (n = 2). Horizontal line indicates median. C Plots of DESeq2-normalized counts for highlighted genes differentially expressed by T cells between categories of best response post-cryoablation for pre-treatment samples (n = 13). FDR-adjusted p-values are for two-sided DESeq2 Wald test differential expression results; pbenefit denotes grouping SD + PR. D Gene set enrichment analysis (GSEA) for the differentially expressed T cell genes from C). Bar lengths correspond to normalized enrichment score (NES). Positive NES values indicate enrichment in SD + PR and negative values indicate enrichment in PD. Asterisks denote significance as defined in the figure. E UMAP of myeloid cell subclustering with cluster annotations. F Box plots displaying the frequency of myeloid cells belonging to a specific subcluster per patient comparing PD (n = 9), SD (n = 2), and PR (n = 2). Horizontal line indicates median. G Plots of DESeq2-normalized counts for highlighted genes differentially expressed by myeloid cells between categories of best response post-cryoablation (n = 13). FDR-adjusted p-values are for two-sided DESeq2 Wald test differential expression results; pbenefit denotes grouping SD + PR. H Timeline summary of patient course for patients with available pre- and post-cryoablation biopsies. I Box plots displaying the percent of immune cells in each lineage per sample, with lines connecting pre- and post-treatment samples from the same patient (n = 4). Box plots indicate interquartile range, with horizontal line indicating median, and whiskers extending to maxima and minima excluding outliers (>1.5 * IQR). PD progressive disease, SD stable disease, PR partial response. Source data for Fig are provided as a Source Data file.
As antigen presentation is expected to play a major role in dictating response following cryoablation, we performed subclustering of myeloid cells and identified 11 clusters (Fig. 3E, Supplementary Fig. 4C). We identified emerging associations between three subclusters with best response to cryoablation; in particular, there was a higher proportion of IL7R+ myeloid cells in three of the tumors with clinical benefit, indicating that these cells have been activated29, and a higher proportion of MAF + tumor-associated macrophages in those with PD than those with PR (Fig. 3F). We then performed differential gene expression analysis between myeloid cells from lesions with best response of SD/PR versus PD (Fig. 3G, Method Details). A candidate gene implicated in failure to respond to cryoablation was MARCKS, a gene that has been shown to reduce expression of proinflammatory cytokines in macrophages (Fig. 3G)30,31. Differential expression between categories of ultimate response to cryoablation revealed additional potential associations; IL10, an anti-inflammatory cytokine that inhibits macrophage activation32, and ANKRD22, which has been shown to increase the suppressive function of MDSCs33, were significantly higher in patients with PD (Fig. 3G, Supplementary Fig. 4D). Then, we performed GSEA on the differentially expressed genes and found that IFNγ response, TNFα signaling via NF-κB, and monocyte chemotaxis pathways were associated with PD (Supplementary Fig. 4E). Based on previous studies34,35,36,37, we hypothesize that chronic IFNγ and TNFα response of myeloid cells in this group of patients who have been previously treated with ICI induced a stronger suppressive function in these cells (e.g., MDSCs) that could lead to a counter-regulatory mechanism leading to T cell exhaustion/dysfunction and therapy resistance. Finally, we performed subclustering of melanoma cells to define the landscape of ICB-resistant tumors treated with cryoablation, but we were unable to draw associations with response due to insufficient samples from patients with SD (Supplementary Fig. 4F, G).
Finally, we sought to compare the immune cell landscape from matched tumors pre- and post-cryoablation to see whether the therapy-induced characteristic changes in the tumor microenvironment (Fig. 3H). Despite the limited number of samples, there may be an emerging trend that B cells are increased in the tumor post-cryoablation (Fig. 3I).
Inflammatory state of the tumor microenvironment prior to cryoablation
Nine patients had tumor tissue evaluable for PD-L1 tumor proportion score (TPS), CD8+ tumor-infiltrating lymphocytes (TIL), and stromal CD8+ lymphocytes by immunohistochemistry (IHC). Pre-ablation tumors were uniformly associated with a “cold” TME, as all pre-ablation tumor samples were PD-L1 negative (TPS of 0%) and the majority had low levels of CD8 + TIL, defined as ˂5% of tumor cells associated with CD8+ TILs. CD8+ staining of the stroma was heterogeneous, ranging from 1% to 50% of nucleated stromal cells in evaluable samples (in two patients, stromal scoring could not be performed). Two patients had tumor tissue evaluable post-ablation; one continued to demonstrate negative PD-L1 staining, and the other had a mild increase in PD-L1 TPS to 5%.
Circulating tumor DNA
Out of fifteen patients with serial blood samples, three had adequate cfDNA (>3% tumor fraction) for pan-cancer analysis. The change in cfDNA levels was consistent with the observed tumor response in these patients according to RECISTv1.1 for the time points at which blood samples were collected with most common mutations and tumor mutational burden of available samples highlighted (Supplementary Fig. 5), suggesting that cfDNA could be used to monitor response to combined cryoablation and ICI therapy.
Discussion
Here, we present the safety and efficacy data from a phase II study of cryoablation and immune checkpoint inhibition in PD-1-resistant metastatic melanoma. Several important observations are presented. First, this combination was found to be both feasible and safe. All enrolled patients, even those with an ECOG performance status of 2, safely underwent cryoablation. Peri-procedural events occurred at anticipated rates38,39,40,41,42, and there was no increased rate of irAEs post-ablation. Second, though limited by cross-trial comparisons, the ORR of 23.5% and progression-free and overall survival rates at one year are comparable to or better than the existing data in the post-anti-PD-1 setting of combination anti-PD-1 plus tyrosine kinase inhibitors, toll like receptor agonists, and epigenetic modifiers43,44,45. Furthermore, of the 17 patients who had been progressing on ICI prior to cryoablation, 7 (41%) experienced disease control (SD or PR) after cryoablation. This is particularly notable in a population of patients who had relatively cold tumors (100% PD-L1 negative; 90% CD8+ TIL 0 or 1+ staining in evaluable patients). We hypothesize that cryoablation stimulates effective immune activation in a subset of patients treated with ICI leading to clinical benefit. Of the 17 evaluable patients, four (23.5%) experienced a partial response in non-ablated lesions with a disease control rate of 41% and at the time of publication four patient continue to experience disease control off therapy. This data highlights the therapeutic potential of this well-tolerated strategy in PD-1 resistant melanoma.
These findings are supported by the preclinical and early clinical data that suggest that cryoablation is more effective at breaking down barriers to immune cell infiltration and stimulating immune responses compared to heat-based ablation46. Additionally, whereas heat-based thermal ablation results in protein denaturation and coagulative necrosis, possibly reducing the availability of intact neoantigens, cryoablation has been shown to leave many native antigen structures preserved24. Furthermore, cryoablation has been shown to induce polyclonality and intra-tumoral T cell repertoire remodeling47, trigger a tumor-specific T cell response18 as well as a release of both tumor neoantigens and pro-inflammatory intracellular contents17,22. We hypothesize that this inflammatory reaction post-cryoablation stimulates the innate immune system to take up the released tumor neoantigens and activate the adaptive immune system to attack tumor sites elsewhere in the body. Correlative data from our study illustrates that evaluable pre-ablation tumor samples were “cold”, meaning that they lacked PD-L1 expression and CD8 + TIL presence. Only two patients had post-ablation tumors evaluable for histological analysis, limiting the ability to discern if cryoablation resulted in an increased inflammatory TME. Furthermore, though there were no clear trends of cell type composition to predict best response to cryoablation, an increase in B/plasma cells was seen in patients with disease control, suggesting these cells may play a role in mounting a durable anti-tumor response after cryoablation. Indeed, prior work has demonstrated the association of tumor-associated B cells and response to ICI in malignant melanoma, with tumor growth in B16 melanoma models48 and improved OS in a B cell-rich environment49. Though data vary on the exact role of B cells in generating an anti-tumor response, perhaps cryoablation results in pro-tumorigenic B cell activation and improved disease control. With respect to clinical predictors of response, due to small sample size and number of responders, we lack the statistical power to make conclusive inferences, however the majority (80%) of patients who experienced sustained response and/or control had baseline (pre-cryoablation) LDH levels within normal limits and M1a or M1b disease. There was no clear association of cryoablation site and response, though with correlative data highlighting the possible role of B cells, future work will examine if there is an optimal target (i.e. lymph nodes metastasis). Based on these data, we conclude that combination of cryoablation and immune checkpoint inhibition warrants additional study in larger patient cohorts.
Limitations of this study include a lack of randomization, the small sample size, and a finite number of specimens for robust correlative analysis, as well as a lack of post-cryoablation tissue samples for all patients. Additionally it is hard to control for novel patterns of ICI response, however the ORR and DCR seen in our cohort exceeds anticipated rates of pseuodoprogression and delayed response from ICI alone50,51. Although the trial population is small, the demographic characteristics of included patients mirrors clinical practice with inclusion of high-risk patients and those with poor prognostic factors including EGOG performance status of 2, primary resistance to ICI, multiple prior lines of therapy, CNS disease, and elevated LDH. The ability to demonstrate safety and efficacy in this population – one generally less fit than in most clinical studies – is significant. Additionally, PD-1 refractory melanoma represents an area of unmet need, as treatment options are limited to salvage ipilimumab/nivolumab, clinical trial enrollment, or targeted therapies in tumors with actionable alterations. Notably, the efficacy of ipilimumab/nivolumab after treatment failure to anti-PD-1 therapy is modest with ORR ranging from 20 to 25%, DCR 33 to 44% with rates of grade 3–4 AEs approximately 30%52, and likely worse in the post-nivolumab-relatlimab setting53. Thus, effective and safe alternative therapeutic strategies are needed, especially for patients ineligible for clinical trials.
In conclusion, the anti-tumor efficacy of combining cryoablation and post-progression ICI was superior to what would be expected with the use of post-progression ICI alone with an acceptable safety profile. Additional prospective research is needed in this burgeoning space of combination intra-tumoral ablation and ICI. An ongoing study (NCT05779423) is evaluating the combination of cryoablation and ipilimumab/nivolumab in patients with metastatic melanoma with progression on PD-1 inhibition. The primary objective is to investigate the clinical benefit of cryoablation and dual ICI with translational aims to further evaluate the immunomodulatory effect of cryoablation and cell types associated with response. Critical unanswered questions include the determination of the optimal site of ablation, optimal volume of tumor-tissue ablated, ideal ICI regimen (anti-PD1/L1, anti-CTLA-4, anti-LAG3, or combination), timing of ICI administration in relation to ablation as well as biomarkers to identify patients most appropriate for this strategy.
Methods
Trial design
The study was performed at the Massachusetts General Hospital (Boston, MA). The protocol was written by the investigators and funded by the William M. Wood Foundation and the Society of Interventional Oncology. The protocol was reviewed and approved by the Dana Farber Cancer Institute / Harvard Cancer Center (DFCI/HCC) institutional review board. Written consent included agreement to publish clinical information potentially identifying individuals was obtained. The study design and conduct complied with all relevant regulations regarding the use of human study participants and was conducted in accordance with the criteria set by the Declaration of Helsinki.
Based on the hypothesis that cryoablation may stimulate an immune response in PD-1-resistant tumors we conducted a non-randomized single-center Phase II trial. The aim was to examine the safety and efficacy of combination cryoablation and post-progression ICI in patients with cutaneous metastatic melanoma (NCT03290677) and non-small cell lung cancer. Enrollment is closed for both cohorts. Notably, the data pertaining to the melanoma cohort only is included in this analysis. The NSCLC cohort will be published in a separate publication.
The primary endpoints were safety and feasibility of this combination approach as well as tumor response in non-ablated lesions. Efficacy was measured by radiologic response rate using RECIST 1.1. Patients were followed 90 days post-ablation to monitor for treatment-related toxicities including procedural adverse events (AEs) and immune-related adverse events (irAEs).
Patients and eligibility
Patients 18 years of age or older with histologically confirmed, unresectable or metastatic cutaneous melanoma who were receiving commercial ICI were eligible for inclusion. Eligible patients had progressive disease for which an additional two cycles (4–6 weeks) of post-progression immunotherapy was deemed an acceptable therapy by their treating oncologist. Demographic data including sex/gender (self-reported per patient) was collected though was not considered in trial design and study treatment. Patients must have had a growing tumor in the chest, abdomen, or pelvis amenable to percutaneous cryoablation. Amenable lesions were present if an interventional radiologist was able to define a path free of bones, major vessels, and other critical structures such as nerves or the ureter between the skin and the target. Patients were required to have measurable disease by RECIST 1.1 criteria54 independent of the lesion to be ablated. Uncontrollable coagulopathy led to exclusion.
The trial was officially registered on Clinicaltrials.gov (NCT03290677) on 9/25/2017 with the first patient enrolled on 1/18/2019 with the last patient enrolled on 7/22/2021. The study was officially closed for further accrual on 9/22/2021.
Treatment
Cryoablation
Patients underwent cryoablation with the Visual ICE system (Boston Scientific, Marlborough, Massachusetts) with 14-gauge (IceFORCE 2.1 CX, IcePEARL 2.1 CX) or 17-gauge (IceROD 1.5 CX, IceSPHERE 1.5 CX) probes. Depending on the patient preference and comorbidities, conscious sedation, monitored anesthesia care or general anesthesia was administered. Patients were placed on the table of a LightSpeed (GE Healthcare, Chicago, Illinois) computed tomography (CT) scanner and the cryoprobe(s) were guided into the target using CT with or without the addition of US. One or more probes were used depending on the size and location of the target with the goal of ablating at least 1 cm3 of the targeted tumor. After confirming appropriate placement of the probe(s), cryoablation was performed using a three-cycle freeze–thaw phase protocol for lung lesions and a dual-freeze protocol for extra-thoracic lesions. Non-contrast CT images were obtained to visualize the evolving ablation zone. Technical success was defined as lethal ice defined as the isotherm at −40 degrees Celsius covering part of the tumor. Subsequently, probe(s) were removed, and patients were monitored according to institutional protocol. When a lesion in the lung was targeted, the patient was positioned puncture side down and remained in this position during the 3-h recovery period. Chest radiographs were obtained at 1 and 3 h to evaluate for delayed pneumothorax or hemothorax, and patients were electively admitted for overnight observation under the care of a thoracic surgeon. When an extra-thoracic lesion was targeted, serial post procedure chest radiographs and admission for overnight observation were not required and only standard post-procedure monitoring per anesthesia guidelines were followed.
Post-progression immunotherapy
Following cryoablation, patients were planned to receive, at minimum, two additional cycles of post-progression ICI therapy with the same ICI agent they had been receiving prior to progression. Mandatory CT scans of chest, abdomen and pelvis to assess for safety and response was performed at two pre-specified time points: 1) 6–8 weeks after cryoablation, and 2) 8–12 weeks after cryoablation.
Continued use of post-progression ICI (after the initial two cycles) was at the discretion of the treating provider and further drug delivery was not specified by the protocol. Patients were able to transition to alternative drug therapy at any time, as part of routine care. However, patients initiated on an alternative drug therapy prior to completing the protocol-specified two cycles of treatment and/or response assessments were classified as “non-responders”.
Correlative studies
Tissue biopsies were performed in all patients directly prior to cryoablation, and after receipt of subsequent ICI, when feasible. Serial blood samples were collected on all patients. Correlative analyses were not predefined. They were performed post-hoc. For all correlative studies, patients provided written informed consent for research and genomic profiling, as approved by the Dana-Farber/Harvard Cancer Center Institutional Review Board (DF/HCC Protocol 11-181).
Immunohistochemistry
To investigate the inflammatory state of the TME prior to cryoablation, IHC for PD-L1 and CD8 were performed using a monoclonal anti-PD-L1 antibody (Clone E1L3N, 1:100, Cell Signaling Technologies; Danvers, MA) and a CD8 monoclonal antibody (4B11, RTU, Leica Biosystems, Buffalo Grove, IL), respectively, with an automated stainer (Bond III, Leica Microsystems, Buffalo Grove, IL). PD-L1 tumor proportion score (TSP) was measured on a continuous basis (0–100%) based on the proportion of tumor cells with positive membranous stating (complete or incomplete circumferential staining) of any intensity. CD8+ tumor-infiltrating T cells was scored as 0–3 and each grade defined based on the fraction of tumor cells on top of which positive T cells were present: 0, none or rare; 1, <5%; 2, ≥5% and <25%; 3, ≥25%. CD8+ staining of the stromal was scored on a continuous basis (0–100%) and scored as 0% vs. 1–39% vs. 40–100%, based on the proportion of stromal nucleated cells with positive staining.
Single cell RNA sequencing
Exploratory single-cell RNA sequencing (ScRNAseq) analysis was performed on pre-ablation tissue biopsy (n = 15) and on post-ICI tissue biopsy (n = 4) in evaluable patients. Fresh tumor samples were rapidly dissociated using methods that have been optimized for small tumor core needle biopsies, resulting in high cellular viability and complexity, as we have shown in melanoma55. After sequencing single cells using droplet-based (5′ V1, 10x Genomics platform) and plate-based (SmartSeq2) technologies, we identified major lineages (within immune, tumor and stromal cells) and major cell states within each lineage.
The raw sequencing data (single-cell RNA-seq) from this study has been deposited in the dbGAP database [https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs003579.v1.p1].
The codes generated during this study are available at Github repository: https://github.com/lasalletj/CryoPD1_Melanoma.
Method details
Tissue dissociation
In this study, all samples underwent rapid dissociation post-surgery using Miltenyi Biotec’s Human Tumor Dissociation kit (cat#130-095-929), as recently described56. The tissue was first fragmented into small pieces in a 1.5 ml Eppendorf tube filled with 932 µl DMEM, 42 µl of enzyme H, 21 µl of enzyme R, and 5 µl of enzyme A. The tube containing the tissue was then vortexed briefly for 2 s and set in a thermomixer (Eppendorf; F1.5) at 37 °C, 350 rpm for 15 min. Subsequently, the tissue was placed on a 50 µm filter (Sysmex; Cat# 04-004-2327), minced further, and rinsed with 10 ml of DMEM containing 10% FCS. The supernatant was discarded after centrifugation at 1500 rpm and 4 °C for 5 min. ACK buffer (Gibco, cat#A1049201) was added to eliminate red blood cells when needed. The sample was then suspended in DMEM with 10% FCS and manually counted using a hemocytometer (Bright-line; Cat# 1492) to ascertain cell count and viability. For SmarSeq2 protocol, samples were first stained with live/dead Zombie Violet Dye (Biolegend, 423114) for 15 min at RT. The cells were then washed and resuspended with 1X PBS containing 1.5% FCS for cell surface labeling using standard protocols with the following antibodies: Human TrueStain FcX (Biolegend; Cat# 422302), PE anti-human CD45 (Biolegend; Cat# 304008), and APC/Cyanine7 anti-human CD235a (Biolegend; Cat# 349116). Sorting of single live ZombielowCD235a−CD45+ or ZombielowCD235a−CD45− into 96 well PCR plates containing 10 µl TCL Buffer (QIAGEN, Cat# 1031576) with 1% 2-Mercaptoethanolwas was performed using a Sony MA900 cell sorter. Plates were stored at −80 °C until processed for cDNA and library generation. For 10x Genomics microfluidic protocol, the EasySep Dead Cell Removal (Annexin V) kit (STEMCELL; cat#17899) was used prior to the encapsulation step if the cell viability fell below 70%56
10x data generation
Single-cell gene expression (GEX) libraries were produced using the 10x Genomics Chromium Single Cell 5’ Library and Gel Bead Kit v1 chemistry (10x Genomics; Cat#1000006), following the manufacturer’s guidelines. After creating cDNA, quality control assessment was undertaken using the Qubit dsDNA High Sensitivity kit (Invitrogen; Cat#Q32854) for concentration evaluation and the Agilent High Sensitivity BioA DNA kit (Agilent; Cat#5067-4626) for measuring size distribution. Samples were then sequenced on the NextSeq 500 sequencer using the 75 cycles flow-cell/kit (Illumina; Cat#20024906) with paired-end reads: 26 reads for the first read and 55 for the second.
Smart-Seq2 cDNA preparation, library construction, and sequencing
cDNA for CD45+ or CD45− single cells was generated using the Smart-Seq2 as previously described55. First, RNA was extracted using Agencourt RNAClean XP SPRI beads (Beckman Coulter, Cat# A63987). The samples were then suspended in 4 µL of Mix-1 [1 µl (10 µM) RT primer (DNA oligo) 5′–AAGCAGTGGTATCAACGCAGAGTACT30VN-3′; 1 µl (10 µM) dNTPs; 1 µl (10%, 4 U/µl) recombinant RNase inhibitor; 1 µl nuclease-free water] and underwent a denaturing process at 72 °C for 3 min, followed by rapid cooling on ice for 1 min. Second, 7 µL of Mix-2 [0.75 µl nuclease-free water; 2 µl 5X RT buffer (Thermo Fisher Scientific, Cat# EP0753); 2 µl (5 M) betaine; 0.9 µl (100 mM) MgCl2; 1 µl (10 µM) TSO primer (RNA oligo with LNA) 5′-AAGCAGTGGTATCAACGCAGAGTACATrGrG+G-3′; 0.25 µl (40 U/µl) recombinant RNase inhibitor; 0.1 µl (200 U/µl) Maxima H Minus Reverse Transcriptase] was added and the reverse transcription reaction ran at 50 °C for 90 min, and then at 85 °C for 5 min. Third, we added 14 µL of Mix-3 [1 µl nuclease-free water; 0.5 µl (10 µM) ISPCR primer (DNA oligo) 5′-AAGCAGTGGTATCAACGCAGAGT-3′; 12.5 µl 2X KAPA HiFi HotStart ReadyMix] and the cDNA amplification step was performed at 98 °C for 3 min, 21 cycles of [98 °C for 15 s, 67 °C for 20 s, and 72 °C for 6 min], and a final extension at 72 °C for 5 min. Finally, two cDNA cleanup cycles were done using Agencourt AMPureXP SPRI beads (Beckman Coulter, Cat# A63881) to discard any small-size fragments (e.g., primer dimers). Next, we generated libraries using the Nextera XT Library Prep kit (Illumina, Cat# FC-131-1024) as previously described55. This step was followed by a cleanup process to eliminate any remaining primer dimers. Each library containing 384 CD45+ or CD45− single cells was sequenced on a NextSeq 500 sequencer (Illumina) using the 75 cycles flow-cell/kit (Illumina; Cat#20024906), with paired-end, reads of 38 bases (for read1 and read2). Prior to and after library preparation, we checked each sample’s quality by measuring their concentrations with the Qubit dsDNA high-sensitivity assay kit (Invitrogen, Cat# Q32854) and cDNA size distribution with the High-Sensitivity DNA Bioanalyzer Kit (Agilent, Cat# 5067-4626)
Quantification and statistical analysis
scRNA-seq alignment
We built a custom FASTA generated from the Homo Sapiens (human) genome assembly GRCh38 (hg38) following exclusion of ALT, HLA, and Decoy contigs according to the Broad Institute GTEx-TOPMed RNA-seq pipeline (https://github.com/broadinstitute/gtex-pipeline/). GENCODE v35 was used for annotation. For SS2 samples, raw FASTQ files were aligned to the custom genome FASTA in the Terra platform with the Broad Institute GTEx pipeline using STAR v2.7.5a, and expression quantification based on a collapsed annotation was performed using RSEM v1.3.0. For 10x samples, CellRanger v7.0.1 was used to align reads to the GRCh38-2020-A transcriptome, with the option of including introns set to true.
scRNA-seq quality control filtration
We filtered out cells with more than 25% mitochondrial reads. For the SS2 data, we used RNA-SeQC 257 (https://github.com/getzlab/rnaseqc) to calculate additional quality control metrics for each cell. We discarded cells with less than 2000 genes expressed, greater than 1,300,000 reads, median 3’ bias outside the range of 0.3 to 0.75, median coefficient of variation (CV) of exon coverage >1, median absolute deviation over all exon CVs >0.8, and exonic rate less than 35%. For the 10x data, we discarded cells with less than 200 genes expressed or less than 1000 reads.
Batch correction, clustering, and dimensionality reduction
Following separate quality control processing for 10x and SS2, separate Seurat objects were generated for each patient sample and SCTransform was applied to each individual sample for normalization and variance stabilization. We removed cells which reflected the tumor site and would have no comparison with other samples (i.e. alveolar type II cells, lung epithelium, neurons, keratinocytes, lymphatic vessel endothelium). SelectIntegrationFeatures was run on the list of objects, and then the Seurat objects were merged into one using the merge function. The selected integration features were set as the merged object’s VariableFeatures. PCA was run on the object using RunPCA on the SCT assay. We then used RunHarmony, using the SCT assay, PCA reduction with the first 50 dimensions, and regressing the effects of batch and sequencing technology. The UMAP was generated with the RunUMAP function, and clustering was performed using FindNeighbors and FindClusters. To check for successful integration, we looked at the distribution of cells from each sample across the clusters. To identify other potential doublets, we performed subclustering of each lineage using the NormalizeData and ScaleData Seurat functions and found clusters with high expression of other lineage markers. We then performed additional rounds of subclustering, adding the cells previously grouped in the wrong lineage to subclustering the appropriate group. Redistributed cells that were found to again form their own cluster were excluded as likely doublets. We retained a total of 49,071 high quality single cells for downstream analysis. The integrated UMAP was generated using SCT values, and the lineage subcluster UMAPs were generated with log-normalized values.
Cell type abundance comparisons
We compared the cell type abundance (either lineage or subcluster) using the Wilcoxon rank-sum test for two-group comparisons and the Kruskal–Wallis test for comparisons of three groups. P values are unadjusted for cell type abundance comparisons.
Differential expression analysis
We performed differential gene expression analysis of T cells and myeloid cells. To generate pseudo-bulk RNA profiles for each sample, we summed all the counts of all cells of a given lineage (T or myeloid) for each. Differential expression analyses were performed between the pseudo-bulk samples using the DESeq2 package in R58 without additional covariates. We performed analyses between both best response categories of SD + PR vs. PD and ultimate PD categories of PD vs. no PD.
Gene set enrichment analysis
We performed gene set enrichment analysis using the fgsea package in R59 using both the Hallmark and C5 GO BP pathway databases from MSigDB Release v7.2. Gene ranks were assigned according to the −1*sign(average_log2FC)*log10(p-value) metric.
Circulating tumor DNA
Ultra-low pass – whole genome sequence
cfDNA Extraction from Whole Blood: Whole blood was collected in EDTA, CellSave, or Streck tubes and processed for plasma extraction utilizing two spins. Blood tubes were centrifuged at 1900 × g for 10 min and plasma was transferred to a second tube before further centrifugation at 15,000 × g for 10 min. Supernatant plasma was stored at −80C until cfDNA extraction. cfDNA was extracted using the QIAsymphony DSP Circulating DNA Kit according to the manufacturer’s instructions. This is a magnetic-particle technology-based chemistry used in conjunction with the QIAsymphony SP instrument manufactured by Qiagen. The cfDNA is bound to magnetic particles. The particle-bound cfDNA is separated from the solution using a covered magnetic rod head. Several wash steps follow to eliminate debris and protein residue from the sample. The machine finishes with a 60 uL cfDNA elution (Qiagen, 2017). Library Construction: Initial DNA input was normalized to be within the range of 25–52.5 ng in 50 uL of TE buffer (10 mM Tris HCl 1 mM EDTA, pH 8.0) according to picogreen quantification. Library preparation was performed using a commercially available kit provided by KAPA Biosystems (KAPA HyperPrep Kit with Library Amplification product KK8504) and IDT’s duplex UMI adapters. Unique 8-base dual index sequences embedded within the p5 and p7 primers (purchased from IDT) are added during PCR. Enzymatic clean-ups are performed using Beckman Coultier AMPure XP beads with elution volumes reduced to 30 µL to maximize library concentration.
Post Library Construction Quantification and Normalization: Library quantification was performed using the Invitrogen Quant-It broad range dsDNA quantification assay kit (Thermo Scientific Catalog: Q33130) with a 1:200 PicoGreen dilution. Following quantification, each library was normalized to a concentration of 35 ng/µL, using Tris-HCl, 10 mM, pH 8.0. Library Pool Creation for Ultra-low Pass Sequencing: In preparation for the sequencing of the ultra-low pass libraries (ULP), approximately, 4 µL of the normalized library is transferred into a new receptacle and further normalized to a concentration of 2 ng/µL using Tris-HCl, 10 mM, pH 8.0. Following normalization, up to 95 ultra-low pass WGS samples are pooled together using equivolume pooling. The pool is quantified via qPCR and normalized to the appropriate concentration to proceed to sequencing. Cluster amplification and sequencing: Cluster amplification of library pools was performed according to the manufacturer’s protocol (Illumina) using Exclusion Amplification cluster chemistry and HiSeqX flowcells. Flowcells were sequenced on v2 Sequencing-by-Synthesis chemistry for HiSeqX flowcells. The flowcells are then analyzed using RTA v.2.7.3 or later. Each pool of ultra-low pass whole genome libraries is run on one lane using paired 151 bp runs.
Pan-Cancer analysis (TWIST Custom Hybrid Selection) was performed on samples with adequate ULP signals; defined as > 3% of detectable tumor fraction.
Pan-cancer analysis (TWIST Custom Hybrid Selection)
Post Library Construction Quantification and Normalization: Library quantification was performed using the Invitrogen Quant-It broad range dsDNA quantification assay kit (Thermo Scientific Catalog: Q33130) with a 1:200 PicoGreen dilution. Following quantification, each library is normalized to a concentration of 35 ng/µL, using Tris-HCl, 10 mM, pH 8.0. In-solution hybrid selection for exome or custom panels: After library construction, hybridization and capture are performed using the relevant components of IDT’s XGen hybridization and wash kit and following the manufacturer’s suggested protocol, with several exceptions. A set of 12-plex pre-hybridization pools are created. These pre-hybridization pools are created by equivolume pooling of the normalized libraries, Human Cot-1 and IDT XGen blocking oligos. The pre-hybridization pools undergo lyophilization using the Biotage SPE-DRY. Post lyophilization, custom exome bait (TWIST Biosciences) along with hybridization mastermix is added to the lyophilized pool prior to resuspension. Samples are incubated overnight. Library normalization and hybridization setup are performed on a Hamilton Starlet liquid handling platform, while target capture is performed on the Agilent Bravo automated platform. Post capture, a PCR is performed to amplify the capture material. After post-capture enrichment, library pools were quantified using qPCR (automated assay on the Agilent Bravo). Based on qPCR quantification, pools were normalized using a Hamilton Starlet to 2 nM and sequenced using Illumina sequencing technology. Cluster amplification and sequencing: Cluster amplification of library pools was performed according to the manufacturer’s protocol (Illumina) using Exclusion Amplification cluster chemistry and HiSeq X flowcells. Flowcells were sequenced on v2 Sequencing-by-Synthesis chemistry for HiSeq X flowcells. The flowcells are then analyzed using RTA v.2.7.3 or later. Each pool of libraries was run on paired 151 bp runs, reading the dual-indexed sequences to identify molecular indices and sequenced across the number of lanes needed to meet coverage for all libraries in the pool.
Statistical considerations
This was a phase II study with the primary objectives of determining the safety, feasibility and radiologic response rate of cryoablation in patients receiving post-progression immune checkpoint inhibitor therapy. The safety and feasibility of the procedure was determined based on observed adverse events with combination cryoablation/ICI deemed safe and feasible if the rate of serious adverse events related to cryoablation was no more than 15%. The study was conducted in two stages with pre-outlined criteria to halt enrollment after 10 patients if 1) more than 2 patients experienced SAEs related to cryo and 2) if there were zero responses. At the end of the study, the procedure was deemed to be safe and consequently feasible as no more than 4 patients experienced SAE related to cryoablation in each arm, and deemed worthy of further investigation as at least 3 patients are observed as having radiologic response in a study arm. The safety stopping criteria for the first stage guaranteed with 95% CI that the upper bound for the true rate of SAE related to cryoablation is no higher than 40%. By the end of the study, this upper bound was no higher than 40%.
Demographic and baseline characteristics, as well as activity and safety observations, were summarized as medians and ranges for continuous variables and as frequencies and percentages for categorical variables. Response rate was calculated as the proportion of patients with RECIST 1.1 partial (PR) or complete response (CR) divided by the total number of patients treated. Disease control rate (DCR) was calculated as the proportion of patients with RECIST 1.1 PR, CR and stable disease (SD) divided by the total number of patients treated. Confidence intervals (CI) were calculated using the exact binomial method. Time-to-progression was defined as the interval from beginning of cryoablation to RECIST 1.1 progression or death.
We hypothesized that combination cryoablation and post-progression ICI would be superior to spontaneous late response to post-progression ICI, which is estimated to be 5%3,25. Two-sided Exact binomial test was used for assessing the difference between observed response rate with cryoablation and post-progression ICI and an assumed null for ICI alone of 5%.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The raw sequencing data (single-cell RNA-seq) from this study has been deposited in the dbGAP database under accession number phs003579.v1.p1 [https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs003579.v1.p1]. These data are available under controlled access and use (data use must be related to the specified disease, melanoma and other skin cancer). Access can be requested through dbGAP. Source data are provided with this paper including de-identified participant data. Additional individual de-identified participant data can be shared upon request to the Corresponding author. The full study protocol, including statistical analysis plan, is available in the Supplementary Information. The remaining data are available within the Article, Supplementary Information or Source Data file. Source data are provided with this paper.
Code availability
The codes generated during this study are available at Github repository (https://github.com/lasalletj/CryoPD1_Melanoma). All code required to run the analyses in this manuscript is deposited in the associated Github repository (https://github.com/lasalletj/CryoPD1_Melanoma). Any additional information required to reanalyze the data reported in this work paper is available from the corresponding author upon request.
References
Robert, C. et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 372, 2521–2532 (2015).
Borcoman, E. et al. Novel patterns of response under immunotherapy. Ann. Oncol. 30, 385–396 (2019).
Topp, B. G. et al. Tumor dynamics in patients with solid tumors treated with pembrolizumab beyond disease progression. Cancer Cell. 41, 1680–1688.e2 (2023).
Hugo, W. et al. Genomic and transcriptomic features of response to anti-PD-1 therapy in metastatic melanoma. Cell 165, 35–44 (2016).
Spranger, S. et al. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci. Transl. Med. 5, 200ra116 (2013).
Koyama, S. et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat. Commun. 7, 10501 (2016).
Dunn, G. P., Sheehan, K. C. F., Old, L. J. & Schreiber, R. D. IFN unresponsiveness in LNCaP cells due to the lack of JAK1 gene expression. Cancer Res. 65, 3447–3453 (2005).
Zaretsky, J. M. et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N. Engl. J. Med. 375, 819–829 (2016).
Restifo, N. P., Smyth, M. J. & Snyder, A. Acquired resistance to immunotherapy and future challenges. Nat. Rev. Cancer 16, 121–126 (2016).
Bang, H. J. et al. Percutaneous cryoablation of metastatic lesions from non-small cell lung carcinoma: Initial survival, local control, and cost observations. J. Vasc. Inter. Radio. Jvir. 23, 761–769 (2012).
Fintelmann, F. J. et al. Percutaneous Cryoablation: Safety and Efficacy for Pain Palliation of Metastases to Pleura and Chest Wall. J. Vasc. Inter. Radio. Jvir. 31, 294–300 (2020).
Callstrom, M. R. et al. Multicenter study of metastatic lung tumors targeted by interventional cryoablation evaluation (SOLSTICE). J. Thorac. Oncol. Publ. Int Assoc. Study Lung Cancer 15, 1200–1209 (2020).
Callstrom, M. R. et al. Percutaneous image-guided cryoablation of painful metastases involving bone. Cancer 119, 1033–1041 (2013).
Zargar, H. et al. Cryoablation for small renal masses: selection criteria, complications, and functional and oncologic results. Eur. Urol. 69, 116–128 (2016).
Bahn, D. K. et al. Targeted cryoablation of the prostate: 7-year outcomes in the primary treatment of prostate cancer. Urology 60, 3–11 (2002).
Erinjeri, J. P. & Clark, T. W. I. Cryoablation: mechanism of action and devices. J. Vasc. Inter. Radio. Jvir. 21, S187–S191 (2010).
Kato, T. et al. Characterization of the cryoablation-induced immune response in kidney cancer patients. Oncoimmunology 6, e1326441 (2017).
Sabel, M. S. et al. Immunologic response to cryoablation of breast cancer. Breast Cancer Res. Treat. 90, 97–104 (2005).
Takahashi, Y. et al. Optimized magnitude of cryosurgery facilitating anti-tumor immunoreaction in a mouse model of Lewis lung cancer. Cancer Immunol. Immunother. Cii. 65, 973–982 (2016).
Thakur, A. et al. Induction of specific cellular and humoral responses against renal cell carcinoma after combination therapy with cryoablation and granulocyte-macrophage colony stimulating factor: a pilot study. J. Immunother. Hagerstown Md. 34, 457–467 (2011).
Waitz, R. et al. Potent induction of tumor immunity by combining tumor cryoablation with anti-CTLA-4 therapy. Cancer Res. 72, 430–439 (2012).
McArthur, H. L. et al. A pilot study of preoperative single-dose ipilimumab and/or cryoablation in women with early-stage breast cancer with comprehensive immune profiling. Clin. Cancer Res. J. Am. Assoc. Cancer Res. 22, 5729–5737 (2016).
Sabel, M. S. Cryo-immunology: a review of the literature and proposed mechanisms for stimulatory versus suppressive immune responses. Cryobiology 58, 1–11 (2009).
Yakkala, C., Chiang, C. L. L., Kandalaft, L., Denys, A. & Duran, R. Cryoablation and immunotherapy: an enthralling synergy to confront the tumors. Front Immunol. 10, 2283 (2019).
Gandara, D. R. et al. Atezolizumab treatment beyond progression in advanced NSCLC: results from the randomized, phase III OAK study. J. Thorac. Oncol. Publ. Int Assoc. Study Lung Cancer 13, 1906–1918 (2018).
Ballet, R. et al. Chemerin triggers migration of a CD8 T cell subset with natural killer cell functions. Mol. Ther. 31, 2887–2900 (2023).
Sugiura, A. et al. MTHFD2 is a metabolic checkpoint controlling effector and regulatory T cell fate and function. Immunity 55, 65–81.e9 (2022).
Bettonville, M. et al. Long-term antigen exposure irreversibly modifies metabolic requirements for T cell function. eLife. 7, e30938 (2018).
Al-Mossawi, H. et al. Context-specific regulation of surface and soluble IL7R expression by an autoimmune risk allele. Nat. Commun. 10, 4575 (2019).
Lee, S. M., Suk, K. & Lee, W. H. Myristoylated alanine-rich C kinase substrate (MARCKS) regulates the expression of proinflammatory cytokines in macrophages through activation of p38/JNK MAPK and NF-κB. Cell Immunol. 296, 115–121 (2015).
Manček-Keber, M. et al. MARCKS as a negative regulator of lipopolysaccharide signaling. J. Immunol. 188, 3893–3902 (2012).
O’Farrell, A. M., Liu, Y., Moore, K. W. & Mui, A. L. IL-10 inhibits macrophage activation and proliferation by distinct signaling mechanisms: evidence for Stat3-dependent and -independent pathways. EMBO J. 17, 1006–1018 (1998).
Chen, H. et al. ANKRD22 is a potential novel target for reversing the immunosuppressive effects of PMN-MDSCs in ovarian cancer. J. Immunother. Cancer 11, e005527 (2023).
Pai, C. C. S. et al. Clonal deletion of tumor-specific T cells by interferon-γ confers therapeutic resistance to combination immune checkpoint blockade. Immunity 50, 477–492.e8 (2019).
Mazet, J. M. et al. IFNγ signaling in cytotoxic T cells restricts anti-tumor responses by inhibiting the maintenance and diversity of intra-tumoral stem-like T cells. Nat. Commun. 14, 321 (2023).
Han, J., Wu, M. & Liu, Z. Dysregulation in IFN-γ signaling and response: the barricade to tumor immunotherapy. Front. Immunol. 14, 1190333 (2023).
Sade-Feldman, M. et al. Tumor necrosis factor-α blocks differentiation and enhances suppressive activity of immature myeloid cells during chronic inflammation. Immunity 38, 541–554 (2013).
Jennings, J. W. et al. Cryoablation for palliation of painful bone metastases: the MOTION multicenter study. Radio. Imaging Cancer 3, e200101 (2021).
de Baere, T. et al. Evaluating cryoablation of metastatic lung tumors in patients—safety and efficacy the ECLIPSE trial—interim analysis at 1 year. J. Thorac. Oncol. Publ. Int Assoc. Study Lung Cancer 10, 1468–1474 (2015).
Fintelmann, F. J. et al. Catecholamine surge during image-guided ablation of adrenal gland metastases: predictors, consequences, and recommendations for management. J. Vasc. Inter. Radio. Jvir. 27, 395–402 (2016).
Parvinian, A. et al. A single-institution experience in percutaneous image-guided cryoablation of lymph node metastases. AJR. Am. J. Roentgenol. 217, 152–156 (2021).
Junker, T. et al. Partial Nephrectomy versus Percutaneous Cryoablation of Small Renal Cell Carcinomas: A Comparison of Adverse Events in a Prospective Multicenter Cohort Study. J. Vasc. Inter. Radio. Jvir. 33, 1375–1383.e7 (2022).
Arance, A. et al. Phase II LEAP-004 Study of Lenvatinib Plus Pembrolizumab for Melanoma With Confirmed Progression on a Programmed Cell Death Protein-1 or Programmed Death Ligand 1 Inhibitor Given as Monotherapy or in Combination. J. Clin. Oncol. J. Am. Soc. Clin. Oncol. 41, 75–85 (2023).
Ribas, A. et al. KEYNOTE-022 update: phase 1 study of first-line pembrolizumab (pembro) plus dabrafenib (D) and trametinib (T) for BRAF-mutant advanced melanoma. Ann. Oncol. 28, v430 (2017).
Agarwala, S.S. et al. Efficacy and safety of entinostat (ENT) and pembrolizumab (PEMBRO) in patients with melanoma progressing on or after a PD-1/L1 blocking antibody. JCO 36, 9530 (2018).
Shao, Q. et al. Engineering T cell response to cancer antigens by choice of focal therapeutic conditions. Int J. Hyperth. J. Eur. Soc. Hyperthermic Oncol. North Am. Hyperth. Group. 36, 130–138 (2019).
Page, D. B. et al. Deep Sequencing of T-cell Receptor DNA as a Biomarker of Clonally Expanded TILs in Breast Cancer after Immunotherapy. Cancer Immunol. Res. 4, 835–844 (2016).
DiLillo, D. J., Yanaba, K. & Tedder, T. F. B cells are required for optimal CD4+ and CD8+ T cell tumor immunity: therapeutic B cell depletion enhances B16 melanoma growth in mice. J. Immunol. Balt. Md. 184, 4006–4016 (2010).
Griss, J. et al. B cells sustain inflammation and predict response to immune checkpoint blockade in human melanoma. Nat. Commun. 10, 4186 (2019).
Robert, C. et al. Nivolumab in Previously Untreated Melanoma without BRAF Mutation. N. Engl. J. Med. 372, 320–330 (2015).
Weber, J. S. et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 16, 375–384 (2015).
Pires da Silva, I. et al. Ipilimumab alone or ipilimumab plus anti-PD-1 therapy in patients with metastatic melanoma resistant to anti-PD-(L)1 monotherapy: a multicentre, retrospective, cohort study. Lancet Oncol. 22, 836–847 (2021).
Menzies, A. M. et al. CTLA-4 Blockade Resistance after Relatlimab and Nivolumab. N. Engl. J. Med. 386, 1668–1669 (2022).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer. 45, 228–247 (2009).
Sade-Feldman, M. et al. Defining T Cell States Associated with Response to Checkpoint Immunotherapy in Melanoma. Cell 175, 998–1013.e20 (2018).
Bill, R. et al. CXCL9:SPP1 macrophage polarity identifies a network of cellular programs that control human cancers. Science 381, 515–524 (2023).
Graubert, A., Aguet, F., Ravi, A., Ardlie, K. G. & Getz, G. RNA-SeQC 2: efficient RNA-seq quality control and quantification for large cohorts. Bioinforma. Oxf. Engl. 37, 3048–3050 (2021).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Korotkevich, G., et al. Fast gene set enrichment analysis. bioRxiv, https://www.biorxiv.org/content/10.1101/060012v3 (2021).
Acknowledgements
This study was supported by grants provided by the William M. Wood Foundation, the Society of Interventional Oncology, the Melanoma Research Foundation, Adelson Medical Research Foundation (N.H.), and U54CA224068 (N.H.). T.J.L. was supported by T32GM007753 from the National Institute of General Medical Sciences (NIGMS). J.S. was supported by the Rosztoczy Foundation and AG was supported by the German Academic Exchange Service. Notably funding agents were not involved in study design, data collection and analysis or manuscript writing
Author information
Authors and Affiliations
Contributions
R.S., G.B., M.S.-F., N.H. supervised the project. M.J.M., F.F., C.A. devised study concept and design. F.F. and R.U. performed the cryoablation. M.J.M., H.K., R.J. and D.L. identified and enrolled patients onto the study. T.L., A.M. performed statistical analysis. A.L. and T.S. assisted with specimen (tissue, blood) collection and data storage. T.L., J.F., E.B., A.G., I.G., M.S.-F. and N.H. performed the single-cell RNA sequencing and computational analysis. M.M.-K. performed the immunohistochemistry. S.S performed the circulating-free DNA and tumor mutational burden analysis. F.F., J.S. and A.G. provided RECIST measurements. M.J.M. wrote the manuscript. Interpretation of the data, critical revisions of the manuscript for important intellectual content, and approval of the final submitted version: all authors.
Corresponding author
Ethics declarations
Competing interests
M.J.M. has served as a consultant/received honorarium from AstraZeneca, Immunai, Bristol Myers Squib, Xilio Therapeutics, Nektar Therapeutics, Catalyst Pharmaceuticals, Regeneron Pharmaceuticals. F.J.F. reports research support from Pfizer as well as consulting and speaking for Boston Scientific. T.J.L. has served as a consultant for Chemomab Therapeutics. M.M.K. has served as a consultant/received honorarium from AstraZeneca, Janssen Oncology, Pfizer, Innate, Repare, Sanofi, and Daiichi-Sankyo, and has received royalties from Elsevier. H.L.K. is an employee of Ankyra Therapeutics and has served on advisory boards for Castle Biosciences, MidaTech Pharma, Tatum Biosciences, and Virogin Biotech. R.W.J. is on the advisory board for XSphera Biosciences and has received research support from Monopteros Therapeutics and has served as a paid consultant/received honoraria from Incyte, G1 Therapeutics, and Bioxcel Therapeutics. R.N.U.: has served as paid consultant for Boston Scientific, German Accelerator. G.M.B. has sponsored research agreements through her institution with: Olink Proteomics, Teiko Bio, InterVenn Biosciences, Palleon Pharmaceuticals. She served on advisory boards for: Iovance, Merck, Nektar Therapeutics, Novartis, and Ankyra Therapeutics. She has served as a consultation for: Merck, InterVenn Biosciences, Iovance, and Ankyra Therapeutics. She holds equity in Ankyra Therapeutics. N.H. holds equity in and advises Danger Bio/Related Sciences, is on the scientific advisory board of Repertoire Immune Medicines and CytoReason, owns equity in BioNtech, and receives research funding from Bristol Myers Squibb and Calico Life Science. R.J.S. has served as a paid consultant/member of scientific advisory board from BMS, Eisai, Iovance, Merck, Novartis, OncoSec, Pfizer; and has received research funding from Merck. M.S.F. received funding from Calico Life Sciences, Bristol-Myers Squibb, Istari Oncology and served as a consultant for Galvanize Therapeutics. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Anastasios Dimou and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mooradian, M.J., Fintelmann, F.J., LaSalle, T.J. et al. Cryoablation and post-progression immune checkpoint inhibition in metastatic melanoma: a phase II trial. Nat Commun 15, 7357 (2024). https://doi.org/10.1038/s41467-024-51722-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-51722-x
- Springer Nature Limited