Abstract
Artificial intelligence (AI) readers of mammograms compare favourably to individual radiologists in detecting breast cancer. However, AI readers cannot perform at the level of multi-reader systems used by screening programs in countries such as Australia, Sweden, and the UK. Therefore, implementation demands human-AI collaboration. Here, we use a large, high-quality retrospective mammography dataset from Victoria, Australia to conduct detailed simulations of five potential AI-integrated screening pathways, and examine human-AI interaction effects to explore automation bias. Operating an AI reader as a second reader or as a high confidence filter improves current screening outcomes by 1.9–2.5% in sensitivity and up to 0.6% in specificity, achieving 4.6–10.9% reduction in assessments and 48–80.7% reduction in human reads. Automation bias degrades performance in multi-reader settings but improves it for single-readers. This study provides insight into feasible approaches for AI-integrated screening pathways and prospective studies necessary prior to clinical adoption.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Breast cancer is the world’s most common cancer and a leading cause of cancer death in women1. BreastScreen Australia offers free mammographic screening targeted to women aged 50–74 years, with those over 40 years of age eligible to attend. Approximately 1 million women are screened annually, and the programme has achieved a reduction in mortality of 41–52% for screening participants and a 21% reduction in population-level breast cancer mortality2,3. However, there are challenges in accuracy, service experience, and efficiency.
In 2020, ~59 per 10,000 participants were diagnosed with breast cancer and 16 per 10,000 participants were diagnosed with ductal carcinoma in situ (DCIS)2. Despite a process of independent double reading of all mammograms by radiologists, and a third arbitration read when there is discordance (henceforth called two readers with arbitration system), in 2020 ~368 per 10,000 participants were recalled for assessment and later determined not to have breast cancer (false positive). Also, ~18.6 per 10,000 participants aged 50–74 years (2015–2017) subsequently discovered they had an interval breast cancer before their next scheduled screen after receiving an all-clear result (false negative)2.
Using artificial intelligence (AI) to help read mammograms has the potential to transform breast cancer screening by addressing the three key challenges of accuracy, service experience, and efficiency2. The evidence base for AI readers in breast cancer screening has been growing rapidly in recent years, with studies demonstrating the potential of AI to detect breast cancer on mammographic images with similar accuracy to radiologists4,5,6,7,8,9,10,11,12 and addressing key limitations of earlier concern13. Many of these studies evaluated the integration of AI into screening pathways via simulation, and they varied in the way human readers interact with the AI, where the AI was positioned in the screening pathway, and the specific screening pathway being simulated. This included the complete replacement of current diagnosis pathways by an AI system9,14, using the AI as a decision referral system to diagnose low-risk non-cancer cases without human intervention, while referring mid- and high-risk cases to human readers15,16,17, or using a recall threshold to divide the cases into human review (mid-risk) and direct recall of (high-risk) suspicious cases18. Another option was to use AI in multiple positions in the pathway: first to rule out a percentage of the non-cancer cases without human intervention, then to rule in cases to supplemental imaging after being cleared by a double reading and consensus pathway19. Finally, in a double reading and consensus pathway, one of the human readers could be replaced by the AI to study the performance of the system before and after replacement17,20.
Previous studies have primarily focused on evaluating the feasibility and effectiveness of incorporating AI into existing screening pathways6,17,18,20,21,22. These studies have shown promising results, often in terms of improved diagnostic accuracy and reduced workload, but they have been limited in the number of scenarios they evaluate6,18,21 and typically avoid (statistically) simulating the arbitration read20,22 or any direct human–AI interaction17. Testing cohorts were not always representative or didn’t have interval cancer follow-up, and operating points were not always set on a separate dataset13. They were also primarily using commercial algorithms17,18,20,21,22, which varied between studies and were assessed on different datasets, thereby precluding direct comparisons of the AI readers’ effectiveness as well as the viability of various AI-integrated scenarios. There is a need for a more in-depth analysis of where and how AI is best positioned within the screening pathway to maximise its benefits. This includes examining whether AI should be used as a primary screening tool, to assist radiologists in decision-making, or in a triage capacity to prioritise cases. An ongoing randomised controlled trial in Sweden21, which has reported a positive interim safety milestone, has limited retrospective analysis of the reading pathway under test, in part due to its reliance on human–AI interaction. As human readers are likely to remain central to the decision-making process, with concerns over the impacts of automation on radiologist performance over the short and long term23, it is crucial to evaluate the potential impacts of human–AI interaction.
In this work, we conduct detailed simulation studies incorporating human–AI interaction effects along with reader-level analysis of AI-integrated screening scenarios. We base our simulations on our retrospective testing cohort comprising data from over 90,000 screening clients in Victoria, Australia and over 600,000 mammogram images. As the AI reader, we use our in-house BRAIx AI Reader (v3.0.7), a mammography classification model based on an ensemble of open-source, image-based deep neural networks. We analyse and compare five AI-integrated screening pathways with the current standard of care. The scenarios range from AI as a standalone reader to advanced collaborative integration scenarios blending AI support with human expertise, aiming to increase screening accuracy and reduce the workload of human readers. Our simulation studies investigate possibilities for the positive, neutral and negative influence of AI integration on human reader performance for each of the five AI scenarios and our results highlight how the effectiveness of AI integration varies across different screening pathways and roles. Taken together, our work provides actionable information relevant to current directions for AI implementation21, improves on previous simulation efforts15,16,17,19,20,22, and offers insights into the optimal use of AI in enhancing screening outcomes.
Results
Study design
We evaluated the AI reader on a representative, population screening dataset collected from women who attended the BreastScreen Victoria programme from 2016–2019 in Victoria, Australia. The screening programme targets women aged 50–74 and typically collects four 2D mammograms for each client (left and right mediolateral oblique, MLO, and craniocaudal, CC) every two years. Each mammogram is read independently by two breast imaging radiologists and a third, if there is disagreement (two readers with arbitration) who has access to the outputs of the first two readers. Readers flag clients for recall for further assessment if they detect indications of breast cancer or return a no-recall decision (all-clear) if not (Fig. 1). The comparison between human readers and the AI reader was performed at the screening episode level with the positive class defined as screen-detected cancers (biopsy-confirmed cancer at assessment within 6 months) and interval cancers (clients who develop breast cancer between 6 months after a screen and the date of their next screen), and the negative class defined as any client who does not develop cancer within the screening interval (12 or 24 months). We summarised reader and system performance on the test set with area under the receiver operating characteristic (ROC) curve (AUC), and sensitivity and specificity of cancer detection decisions at specific operating points.
A Standard of care scenario: Readers 1 and 2 see the same episode and opt to recall or not-recall, if they disagree Reader 3 arbitrates. B AI standalone scenario: all decisions are taken by the AI Reader without human intervention. C AI single-reader scenario: Reader 1 takes the final decision using AI Reader input. D AI reader-replacement: same as (A) but with AI Reader replacing Reader 2. E AI band-pass scenario: AI Reader screens out episodes before Readers 1 and 2. Episodes with high scores trigger the recall decision directly, and episodes with low scores trigger the no-recall decision directly. The other episodes continue to the usual reader system. F AI triage scenario: AI reader triages the episodes before Readers 1 and 2. Episodes with high scores continue to the usual system, and episodes with low scores go through the path with only 1 reader.
With detailed simulation studies, we compare the standard of care (two human readers with arbitration; Fig. 1A) to five AI-integrated screening pathways. We examine the AI reader as a standalone reader (AI standalone; Fig. 1B), as a reader aid for a single reader (AI single-reader; Fig. 1C), as a replacement for the second reader in a two reader with arbitration pathway (AI reader-replacement; Fig. 1D), as a filter for high confidence recall and no-recall decisions (AI band-pass; Fig. 1E), and as making a triage decision between a single reader and a two reader with arbitration pathway (AI triage, Fig. 1F). In the simulations our baseline reference point for system performance is the observed standard of care results from the original reads, with arbitration reads simulated from historical reader performance when needed. For each AI-integrated scenario, we vary the AI reader operating points to identify settings for optimal performance. We then select candidate operating points for each scenario and vary the human reader performance by modelling an interaction effect of the AI reader on human readers. We simulate these interaction effects by changing human reader decisions to agree with the AI reader (varying the proportion between 0% and 50%) when the AI reader is correct (positive interaction), incorrect (negative interaction), and irrespective of correctness (neutral interaction, typically referred to as automation bias). More details on the datasets, screening scenarios, and the simulation design can be found in the Methods.
AI as a standalone reader
We first evaluate the AI reader in the standalone scenario, assessing its performance when it replaces all human readers in the standard of care. The AI standalone scenario achieved an AUC of 0.932 (95% CI 0.923, 0.940) when evaluated on the retrospective test set (Fig. 2A). The AI standalone operating point achieved higher performance than the mean of individual radiologists (weighted by number of reads), in both sensitivity (75.0 vs. 66.3%; P < 4.11 × 10−8) and specificity (96.0 vs. 95.6%; P < 1.11 × 10−9). However, operating as a sole reader, the AI reader had lower sensitivity (75.0 vs. 79.8%; P < 1.98 × 10−5) at non-inferior specificity (96.0 vs. 96.0%: P = 0.56) than the current standard of care (two readers with arbitration; Supplementary Tables 1, 2).
A The AI reader ROC curve compared with the weighted mean individual reader and reader consensus. The AI reader achieved an AUC of 0.932 (95% CI 0.923, 0.940, n = 149,105 screening episodes) above the weighted mean individual reader performance (95.6% specificity, 66.7% sensitivity) but below the reader consensus performance (96.1% specificity, 79.8% sensitivity; standard of care). The weighted mean individual reader (black circle; n = 125 readers) is the mean sensitivity and specificity of all the individual readers (grey circles) weighted by their respective total number of reads. B, C AI reader compared against 81 individual readers (min. 1000 reads). An optimal point from each AI reader ROC curve is shown for each comparison. We show separately human readers for which both sensitivity and specificity of the AI reader point was greater than or equal to the reader (B; 74 readers, 91.3% of readers; 253,328 reads, 88.3% of reads) and readers for which the AI reader is less than or equal to the human reader in either sensitivity or specificity (C; 7 readers, 8.6%; 33,525 reads, 11.7%). Source data are provided as a Source Data file.
To better understand how the AI reader ranked amongst the reading cohort, we compared the AI reader against all individual readers in the first or second reader position with at least 1000 reads and found that with well-chosen operating points, the AI reader could achieve higher sensitivity and/or specificity than 74 out of 81 human readers (see Fig. 2B, C). When compared with the third readers on the third read cohort, the AI reader performance was found to be lower than that of the mean third reader (Supplementary Fig. 1). This result supports the use of the AI reader only in the first or second position, as we will explore in the other scenarios.
A key observation on AI reader performance was that the AI standalone scenario ROC curve was above that of the current standard of care for first-round episodes (that is, the first time a client attended the screening service) and above the weighted mean reader for second and subsequent rounds (Supplementary Fig. 2). Further comparisons were performed across other breakdowns including age, manufacturer, and radiologist morphology labels (Supplementary Figs. 3, 4). We also investigated the AI reader scores by outcome class label and found the top 10% of scores contained 92.7% of screen-detected cancers and 42.2% of interval cancers (81.8% of all cancers; Supplementary Table 3). Additionally, we benchmarked our AI reader on external datasets (some publicly available), achieving state-of-the-art performance (Supplementary Table 4).
Overall, the AI reader was a strong individual reader, outperforming individual readers on average. The first-round screening results highlight the strength of the AI readers when limited additional information is available (specifically, prior screens or other reader opinions), while the third reader and second and subsequent round analysis shows how the human readers are able to make use of the additional information to improve performance. The AI standalone scenario did not outperform the standard of care but could offer improvement in single-reader settings.
AI-integrated scenarios without human–AI interaction effects
Integrating the AI reader within a multi-reader pathway offers a more practical and clinically viable option than AI as a standalone reader, with multiple prospective trials underway globally21,24,25. We conduct detailed simulation studies of the AI reader-replacement, AI band-pass, and AI triage scenarios compared against the current standard of care in breast cancer screening in Australia, two independent readers with arbitration (Fig. 1; Methods). In this section, we vary only the AI reader operating points and simulate arbitration reads as required based on historical performance (Methods). For the AI band-pass and AI triage scenarios, we assume the human readers are blinded to AI reader outputs, and in the AI reader-replacement scenario, the first reader is also assumed to be blinded.
The AI reader-replacement scenario produced higher system sensitivity (82.3%; 95% CI 81.5–83.1, P < 0.0025) and higher specificity (96.3%; 95% CI 96.2–96.3, P < 4.3 × 10−6) than the current standard of care system (Fig. 3A and Supplementary Table 2). Across 149,105 screening episodes in our retrospective testing set, the AI reader-replacement scenario had 354 fewer unnecessary recalls (−6%, false positives) and detected 33 more cancers (+3.1%, true positives) with a reduction of 147,959 human reads (−48%), but required 11.6% more third (arbitration) reads (Table 1 and Supplementary Fig. 5). The modelled reading and assessment cost reduction was 15%.
A Human reader consensus performance compared with AI standalone, AI reader-replacement, AI band-pass and AI triage on the retrospective cohort (n = 149,105 screening episodes) without interaction effects. Representative points are shown for AI standalone (96.0% specificity, 75.0% sensitivity), AI single reader (95.6% specificity, 67.3% sensitivity), AI reader-replacement (96.3% specificity, 82.3% sensitivity), AI band-pass (96.6%, 81.7%) and AI triage (95.7% specificity, 78.0% sensitivity). Other potential operating points are shown as a continuous line. Both AI reader-replacement and AI band-pass improved performance over the human reader consensus (96.1% specificity, 79.8% sensitivity). B AI-integrated scenarios when reader performance is varied with an interaction effect when the human reader disagrees with the AI reader. From 0% to 50% of discordant decisions are reversed when the AI reader was correct (triangle, positive effect), uniformly (circle, neutral effect) and incorrect (diamond, negative effect). For AI triage to match human reader consensus performance, a 15% positive interaction effect of the AI reader on human readers is required. Source data are provided as a Source Data file.
The AI band-pass screening scenario also achieved both higher system sensitivity (81.7%, P < 0.0058) and specificity (96.6%, P < 2.2 × 10−25) than the current standard of care system (Fig. 3A and Supplementary Table 2). The AI band-pass screening scenario had 779 fewer unnecessary recalls (−13.3%) and detected 25 more cancers (+2.4%) with a reduction of 248,638 human reads (−80.7%), while also providing a 67.9% reduction in third reads (Table 1 and Supplementary Fig. 6). The modelled reading and assessment cost reduction was 28.3%.
The AI triage screening scenario, without modelled AI-human interaction, produced lower sensitivity (77.2%) and specificity (95.7%) than the current reader system (Fig. 3A). Using an operating point to shift 90% of reads to a single-reader pathway, the AI triage screening scenario had 425 more unnecessary recalls (+7.3%) and detected 35 fewer cancers (−3.3%) with a reduction of 141,799 human reads (−46%), including a 77.0% reduction in third reads (Table 1 and Supplementary Fig. 6). The modelled reading and assessment cost reduction was 7.5%. This result is expected as AI triage allocates a proportion of reads to a single-reader pathway, which will always result in reduced performance in the absence of human–AI interaction.
Both the AI reader-replacement and AI band-pass scenarios offer opportunities for improved performance relative to the current standard of care across different operating points, with AI reader-replacement achieving the highest reduction in missed cancers (−12.4%) and the AI band-pass having the highest reduction in unnecessary recalls (−13.3%). The AI triage scenario offers good performance with a large workload reduction while maintaining a human decision-maker for all episodes (which AI band-pass lacks). These retrospective simulations do not take into account the change in cancer prevalence26 that both AI band-pass and AI triage would have on the episodes diverted to different pathways, nor do they consider the potential positive and negative impacts of human readers having access to AI reader outputs. Human–AI interaction effects are explored in the next section.
AI-integrated scenarios with human–AI interaction
We present three types of human–AI interaction by considering neutral, positive, and negative interactions in the AI-integrated scenarios (Fig. 3B and Supplementary Figs. 7–10). In the AI reader-replacement scenario the interaction affects the arbitration reader only. Conversely, in the AI band-pass and AI triage scenarios, the interaction affects all readers. In the AI single-reader scenario, human–AI interaction affects the single reader operating with decision support.
In all cases, we are modelling the effect of human readers revising their decision when in disagreement with the AI reader. We vary the degree of how often the human reader revises their decision upon disagreement, ranging from 0 to 1, where 0 refers to the human reader never revising their decision, and 1 refers to the human reader always revising their decision to agree with the AI (and 0.5 would refer to the human reader revising the decision 50% of the time). In the special case where the human reader changes their decisions to agree with AI fully, the collaboration between the human reader and AI reader would collapse to the AI standalone scenario. For all scenarios, we use the AI standalone operating point when considering disagreement.
The neutral human–AI interaction case, in which a reader will be inclined to agree with the AI model irrespective of the correctness of its outcome, models a general form of automation bias. Automation bias is the tendency of humans to overly agree or rely on an automated system (the AI reader in our case)27. The positive human–AI interaction case refers to the human reader changing their decision to agree with the AI reader only when the AI reader is correct, simulating human readers accurately discriminating between useful and spurious AI reader outputs. The negative case refers to the human reader changing their decision only when the AI reader is incorrect.
AI reader-replacement
In the AI reader-replacement scenario, assuming positive human–AI interaction, AI improves the system’s specificity but has minimal impact on sensitivity (the green triangles in Fig. 3B). This is because the AI reader operates with higher specificity (96.0%) and lower sensitivity (75.0%) compared to the third reader (56.3 and 97.5%). At this operating point, AI only correctly identifies a few cases of cancer missed by human readers, and human readers do not benefit much from it. Conversely, in the negative scenario, as Reader 3 is highly sensitive and AI is highly specific, agreeing with AI leads to a substantial loss of sensitivity but not specificity. With negative interaction, Reader 3 is more prone to overlooking cancer cases rather than misidentifying normal cases when learning from the AI reader.
In the neutral case, agreeing with the AI would gradually reduce the multi-reader system to a one-reader system, where the decision is solely driven by the AI reader. As a result, the performance would tend towards the AI standalone scenario at (75.0 and 96.0%). Nevertheless, in the AI reader-replacement scenario, roughly 30% or more of discordant decisions would need to be reversed in the neutral and negative interaction cases for system sensitivity to drop below that of the current standard of care (reader consensus). In other words, the AI reader-replacement scenario, without interaction effects, is so effective that human readers could accept up to 30% of the AI’s mistakes before its performance falls below the standard of care, making it substantially robust to the downside risks of human–AI interaction.
AI band-pass
For the band-pass scenario with a positive human–AI interaction effect, AI improves on specificity and sensitivity, more so than in the AI reader-replacement scenario because all three readers may benefit from AI outputs. In the negative case, the opposite is true if human reader performance suffers due to the mistakes made by AI. The sensitivity decreases significantly, with most of the contribution coming from Reader 3. The specificity also decreases slightly but remains at ~96%. In general, the AI reader tends to return more false negatives than false positives due to its high specificity.
In the neutral case, agreeing with AI would gradually lead to the collapse of the mid-band two-reader-and-consensus system into the AI standalone scenario. As the interaction effect increases, the sensitivity and specificity of the band-pass scenario will approach that of the AI standalone scenario. But unlike the AI reader-replacement scenario, the transition may not be complete, as the high-band and low-band paths continue to function, even if they only contain a limited number of episodes.
AI triage
The AI triage scenario shows the widest range in performance of the multi-reader scenarios, depending on the nature of the human–AI interaction. When there is a positive interaction, AI triage would achieve the baseline consensus (standard of care) performance when humans benefit from the AI’s ability to correct 15% of the decisions in cases of disagreement. The potential benefits are substantial: if all human readers adopt 50% of the AI’s corrections, the sensitivity could increase by 5.3 percentage points compared to the baseline where there is no interaction between humans and AI. This would result in a performance that exceeds the baseline standard of care in terms of sensitivity by 3.4 percentage points, while also operating at 97.7% specificity. In the negative interaction case, however, the potential downside is significant. The sensitivity can quickly drop by 5.6 percentage points to about 73.1% (6.7 percentage points lower than the baseline standard of care) and the specificity can drop to 93.5% when human readers follow the mistakes made by the AI reader. In the neutral case, agreeing with the AI reader does not change the performance significantly, and so performance remains a little below the standard of care in both sensitivity and sensitivity.
The performance of the AI triage scenario presents large variations when subject to positive or negative interactions, because human readers are responsible for all the recall decisions, while the AI merely supports them. When the AI offers beneficial assistance, the entire scenario improves as all readers gain from it. Conversely, if the assistance is negative, the scenario worsens in a similar way. In the neutral case, the scenario does not improve because we know from the AI standalone case that the AI reader is tuned to match the reader at about the same specificity but with much-improved sensitivity. However, the one-reader pathway contains all the low-scoring and mostly normal episodes, so improved sensitivity does not have an effect. And since the three-reader pathway has high sensitivity, the AI assistance does not detect more cancers. In this case, triage is not as susceptible to automation bias, as agreeing with AI would not harm the system’s performance.
The AI triage scenario provides a distinct contrast to the AI reader-replacement scenario. The positive and negative interactions have less impact in the AI reader-replacement scenario than in the triage scenario, because the interaction applies to fewer human readers. Also, the AI reader serves two roles in the reader-replacement scenario: as a screening tool, where it improves the system beyond the consensus performance, and as a diagnosis-assistive tool. Since it has already delivered most benefits as a screening tool, the additional improvements from its role as an assistive tool are relatively minor. The triage scenario, on the other hand, receives the impact of human–AI interaction for all human readers, and the structure of the pathway means that the AI has strong effects both as a screening tool and as an assistive tool.
AI single-reader
In the AI single-reader scenario, with positive interaction correcting up to 50% of the original decisions, the system can achieve a sensitivity of 74.6%, on par with the AI standalone, but with an additional 1.6% improvement in specificity (Fig. 3B). For the negative interaction case, with up to 50% of the discordant decisions turning into errors, the sensitivity and specificity decrease to ~62.6 and 93.9%, respectively. There are more gains than losses in this scenario, because disagreements occur more frequently when AI is correct than when it is wrong. In the neutral case, as the human reader increasingly agrees with the AI reader, the performance tends towards the AI standalone scenario. The automation bias turns out to be favourable here, because the AI reader outperforms the average reader by a sizable margin, and in a one-reader pathway, this directly translates to an improved system performance. Across all scenarios, a single-reader AI system is unable to match the performance of a multi-reader system when it comes to screening outcomes. However, using AI significantly narrows the gap between the single-reader system and the two-reader-and-consensus system, making a compelling case for consideration in settings limited to single-reader screening pathways.
Discussion
In this study we used detailed simulations to evaluate how an AI reader for breast cancer detection could perform in different single- and multi-reader settings in population mammographic screening. We explored positive, neutral, and negative human–AI interaction effects and identified the major upside and downside possibilities for four AI-integration scenarios depending on the nature and strength of the interaction effects. The AI reader used was a strong individual reader trained on the ADMANI training datasets28, with its performance assessed on a carefully selected and unseen ADMANI testing dataset. This AI reader achieved significantly higher sensitivity (+8.3%) and specificity than the weighted mean individual human reader, and better performance than 91% of individual human readers in our retrospective testing dataset.
The AI standalone reader’s high performance and minimal running cost (it is fully automated, so it eliminates costs associated with human readers) make a compelling case for its use in settings that follow single-reader screening practices. Many countries currently implement single-reader screening, whether for reasons of historical choices to prioritise cost and operational efficiency, as in the United States, or because the resources have not been available to establish a multi-reader screening pathway integrated into healthcare systems, as in many other countries. In such settings, AI readers could play an important role in improving screening system performance while minimising costs. However, despite the AI reader’s impressive performance as a single reader, as a standalone system, the AI reader cannot match the performance of the current standard of care (two readers with arbitration) used in Australia and many other countries including Sweden and the UK.
This performance gap necessitates some human–AI collaboration to improve screening outcomes29 and, furthermore, practical, social and legal considerations encourage retaining the human central to the decision-making process23. We observe that the AI reader can outperform human readers in the first/second position, but not in the third reader role. This observation suggests that there is a preferred position for the AI reader to maximise its advantages relative to human readers: it should serve in a more junior role where its excellent specificity optimises the performance of the whole screening pathway. Relatedly, the AI reader works notably well for first-round screening, outperforming the standard of care in this study dataset.
We studied four collaborative AI-integrated scenarios for the screening pathway: AI single-reader, AI reader-replacement, AI band-pass, and AI triage. Without any human–AI interaction effects, both AI reader-replacement and AI band-pass demonstrated significantly superior sensitivity and specificity compared to the standard of care two readers with arbitration system. Interestingly, these two approaches achieve this superior performance with different characteristics. The AI reader-replacement system gains the most from reducing missed cancers (false negatives), while the AI band-pass system gains the most from limiting unnecessary recalls to assessment (false positives). Both the AI single-reader and AI triage systems demonstrate lower performance than the standard of care and other multi-reader AI-integrated systems. However, if we assume that positive human–AI interaction yields a 15% improvement in human reader decision-making, then AI triage can match the current standard of care. Furthermore, the AI triage system has the highest possible upside if there are strong positive human–AI interaction effects. Interim results from the ongoing MASAI trial provide evidence that positive human–AI interaction is plausible21. For the AI single-reader, the superior performance of the AI reader over human readers provides a safety net, leading to improved performance even with neutral reliance on the AI reader. The relative gains from the use of AI are greater in the single-reader pathway than in the multi-reader pathways thanks to the strong performance of the AI reader as a single reader relative to single human readers. The gap between the single-reader pathway and multi-reader pathways is almost halved when AI is used in both.
The four AI-integrated scenarios present differing considerations when it comes to implementation and clinical application. AI reader-replacement is conceptually straight-forward and is the least disruptive to the reader system as it retains a human decision-maker for all episodes. As the overall reader structure is unchanged, every episode must have at least one human reader, the cancer prevalence for the first reader remains the same, and only the third reader can view AI outputs. The limited exposure of readers to the AI reader outputs mitigates the risks of negative human–AI interaction, but also limits the upside of positive human–AI interaction. The AI band-pass scenario offers perhaps the greatest potential for tuning the pathway, allowing for improved performance while minimising the radiologist's workload. However, having an AI reader make the final reading decision, without human involvement, faces challenges before clinical adoption. Concerns relating to bias, quality assurance, and medico-legal responsibility would need to be addressed23. With strong performance, the AI reader-replacement and AI triage systems seem most promising for clinical implementation as their human-in-the-loop, human-in-charge architectures minimise barriers to their adoption.
Our study features limitations based on constraints on what is feasible in complex simulations using a retrospective cohort, and other factors and considerations beyond the scope of this study. Here, we did not account for the potential effects of a change in cancer prevalence that readers in both AI triage and AI band-pass will experience. We also consider positive, neutral, and negative human–AI interaction effects in isolation, and only for a single AI reader operating point. Human readers are also likely to modulate their decisions based on scores or other supporting information, which we did not consider. Alongside prospective studies and further development of the datasets, translation into screening programmes will require, or be aided by, other programmes of work not covered in this study, including (1) Development of approaches to algorithm quality assurance to assess bias and drift on an ongoing basis; (2) Deeper examination of AI explainability for client and clinician, and their acceptance of AI reader false negatives and positives; (3) Further development of algorithms to focus on interval cancers (false negatives); (4) Utilising AI to predict short-, medium- and long-term breast cancer risk and to support personalised screening pathways. AI reader performance may be improved by integrating a longitudinal view of episodes for a given client over multiple screening rounds, taking advantage of information from prior screens as human readers do.
Taken together, we have shown, on a large retrospective dataset under different scenarios and conditions, how an AI reader can be integrated into breast cancer screening programmes to improve cancer detection, minimise unnecessary recall to assessment, and lower human reader workload and cost. Our work extends previous research by simulating arbitration reads and human–AI interaction, as well as conducting thorough analysis and comparison across various AI-integrated screening pathways using a common large retrospective dataset and AI system. Our results provide insights into optimising breast cancer screening outcomes through AI positioning and pathway design. Overall, our simulation results provide evidence that supports the prospective evaluation of the AI-integration pathways studied here and offers plausible approaches to the clinical implementation of AI readers in breast cancer screening in the near future.
Methods
Inclusion and ethics
The conceptualisation, design and implementation of this study was conducted with close collaboration between clinical staff working in the organised population breast screening service in Victoria, Australia and local academic researchers. This study was conducted using the ADMANI datasets created from the state screening programme, BreastScreen Victoria’s retrospective image and non-image database which was accessed and governed under the executed license agreement with BreastScreen Victoria and the BRAIx Project Multi-Institutional Agreement. The study’s conduct was approved by the St Vincent’s Hospital, Melbourne Human Research Ethics Committee (SVHM HREC) approval numbers; LNR/18/SVHM/162 and LNR/19/SVHM/123. All BreastScreen participants sign a consent form at screening registration that provides for the use of the de-identified data for research purposes. A unique identifier is used for the purposes of the ADMANI datasets, with all image and non-image data de-identified.
Screening programme
The BreastScreen Victoria screening programme is a population screening programme targeted at women aged 40+ with those between the ages 50–74 actively recruited. A typical BreastScreen Victoria client has a mammogram taken with a minimum of four standard mammographic views (left and right mediolateral oblique, MLO, and craniocaudal, CC) every 2 years. Annual screening is offered to a small proportion of high-risk clients (< 2%).
Every client undergoing screening through BreastScreen Victoria experiences a standardised screening pathway and data generation process (Supplementary Fig. 11). Each mammogram is read independently by two breast imaging radiologists who indicate suspicion of cancer, all-clear, or technical rescreen. If there is disagreement, a third reader, with visibility of the original two readers’ decisions, determines the final reading outcome. Clients with a suspicion of cancer are recalled for assessment. At assessment, further clinical workup and imaging is performed. Any client who has a biopsy-confirmed cancer at assessment (within six months of screening) is classified as a screen-detected cancer (true positive). Any clients who are recalled but confirmed with no cancer after follow-up assessments are classified as either benign or no significant abnormality (false positive). Clients who were not recalled at reading and do not develop breast cancer within the next screening interval are classified as normal (true negative). Clients who develop breast cancer between 6 months after a screen and the date of their next screen (12 or 24 months) are classified as interval cancers (false negative). The datasets we use are structured around individual screening episodes of clients attending BreastScreen Victoria. A screening episode is defined as a single screening round that includes mammography, reading, assessment, and the subsequent screening interval.
Study datasets
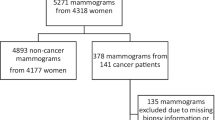
The datasets used in this study were derived from the ADMANI datasets28. The ADMANI datasets comprise 2D screening mammograms with associated clinical data collected from 46 permanent screening clinics and two mobile services across the state of Victoria, Australia. The entire datasets span 2013–2019, 2013–2015 were cancer enriched samples and not used for testing, 2016–2019 were complete screening years containing all episodes. Screening episodes that were missing any of the standard mammographic views (left and right MLO and CC), had incomplete image or clinical data, were were excluded (Fig. 4). If a screening episode had multiple screening attempts only the final attempt was used. Clients with breast implants or other medical devices were included. After exclusions, a random number generator was used to allocate 20% of all screening clients randomly to the study cohort, only the complete screening years (2016–2019) were included to ensure a representative sample. All screening episodes associated with clients were then included in the study dataset. The remaining 80% of clients and associated screening episodes were used in model training and development. The study dataset was further split into testing (75% of clients in the study) and a development dataset (25% of clients in the study) on which operating points were set. The testing dataset comprised 149,105 screening episodes from 92,839 clients, and the development dataset 49,796 screening episodes from 30,946 clients (Table 2). The mammograms were processed using the Python programming language version 3.6 using packages gdcm version 2.8.9 and pydicom 2.1.2. The non-image datasets were processed using Python programming language version 3.11 using packages numpy version 1.25.1 and pandas version 2.0.3.
Flow diagram of study exclusion criteria for screening episodes from the standardised screening pathway at BreastScreen Victoria. Missing data could be clinical data without mammograms or mammograms without clinical data, clinical data could also be incomplete missing assessment, reader or screening records. Earlier screening attempt refers to a client returning for imaging as part of the same screening round, only the last attempt was used. Failed outcome determination and failed outcome reduction refer to being unable to confirm the final screening outcome for the episode. Missing reader records refer to missing reader data. Inconsistent recall status refers to conflicting data sources on whether episodes was recalled. Incomplete screening years refers to years in which we did not have the full year of data to sample from (2013–2015), these years were excluded from testing and development datasets as they are not representative.
The study dataset has strong ground truths for all cancers (screen-detected and interval) and non-cancer (normal, benign, or no significant abnormality (NSA) with no interval cancer). Cancer was confirmed by histopathology for screen-detected cancers or obtained from cancer registries for interval cancers. The histopathological proof was predominantly from an assessment biopsy confirmed with subsequent surgery. The ground truth for clients without cancer was a non-cancer outcome after reading and no interval cancer (normal) or non-cancer outcome after assessment and no interval cancer (benign or NSA). Information on country of birth, whether or not the client identifies as Aboriginal and/or Torres Strait Islander, and age was collected at the time of screening. Responses for country of birth and Aboriginal and/or Torres Strait Islander identification were aggregated into categories of First Nations Australians and regions. No analysis on sex or gender was performed as it was not available in the dataset.
Separately to the retrospective analysis a prospective dataset was collected from December 2021 to May 2022. Data were collected in real-time (daily) from a single reading and assessment unit (St Vincent’s Breastscreen, Melbourne, Australia) using two mammography machine manufacturers from December 2021 to May 2022. The prospective dataset contains the same ground truth and demographic information with the exception of interval cancer data as it was not yet available at the time of publication. The prospective dataset consisted of a total of 25,848 episodes and 108,654 images from 25,848 clients with a total of 195 screen-detected cancers (Supplementary Table 5).
AI reader system
For this study, we used the BRAIx AI Reader (v3.0.7), a mammography classification model developed by the BRAIx research programme. The model is based on an ensemble of modern deep-learning neural networks and trained on millions of screening mammograms. We studied and created an ensemble from ResNet30, DenseNet31, ECA-Net32, EfficientNet33, Inception34, Xception35, ConvNext36 and four model architectures developed specifically for our problem, including two multi-view models that use two mammographic views of the same breast concurrently37, and two single-image interpretable models that provide improved prediction localisation and interpretability38. Each model from the ensemble was implemented in PyTorch39 and trained on data splits from the training set. The models were trained for 10–20 epochs using the Adam optimiser40 with an initial learning rate of 10−5, with weight decay of 10−6 and with the AMSGrad variant enabled41. The training set was selected to have about a 10:1 ratio for non-cancers (benign, no significant abnormality and normal) and screen-detected cancers, respectively. To enforce a specific ratio, not necessarily all the available non-cancer images in the dataset are used during the training of the models. Images were pre-processed to remove text and background annotations outside the breast region, then cropped and padded to keep the same height-to-width ratio of 2:1. Data augmentation consisted of random affine transformations42.
The AI reader is image-based and produces a score associated with the probability of malignancy for each image. Image scores are combined to produce a score for each breast, and the maximum breast score is the episode score. Decision thresholds convert each episode (or breast) score to a recall or no-recall decision. There are no minimum number of images required. Elliptical region-of-interest annotations are produced from the pixels that contribute most to the classification score, and multiple regions are ranked by importance (Supplementary Fig. 12). The reader has been evaluated on publicly available international datasets and achieved state-of-the-art performance (Supplementary Table 4). The distribution of episode scores from the study dataset, useful for inter-study comparisons, are also available (Supplementary Table 3).
Simulation design, operating points and evaluation metrics
To provide insights into the AI reader and its potential in clinical application, we performed retrospective simulation studies, where we evaluated the AI reader performance as a standalone reader and in three AI-integrated screening scenarios. The simulation studies are conducted using various packages in the R programming language version 4.2.2, including dplyr version 1.1.0, tidyr version 1.3.0 and renv version 0.15.543.
AI-integrated screening scenarios
Five scenarios were considered to evaluate the AI reader integrated in the screening pathway, AI standalone, AI single-reader, AI reader-replacement, AI band-pass, and AI triage. In the one-reader pathway, the reader makes a decision on all episodes, and the decision is final. This includes the AI standalone and the AI single-reader scenarios. In the two-reader-and-consensus pathway, the first two readers individually make a decision on whether or not to recall the client for further assessment. If the two readers agree, that is the final reading outcome. If they disagree, a third reader, who has access to the first two readers’ decisions and image annotations, arbitrates the decision. This pathway includes AI reader-replacement, AI band-pass and AI triage scenarios.
In the AI standalone scenario, the AI reader replaces the (only) human reader in the one-reader pathway and provides the same binary recall or no-recall outcome as the human readers on all episodes. In the AI single-reader scenario, the AI reader acts as an assistive tool to human readers. It provides the binary recall or no-recall outcome to the human reader, but it does not make any decision on its own. The human reader with access to the AI output would first make a decision and then consider whether to revise its decision should there be disagreement with the AI output.
In the AI reader-replacement scenario, the AI reader replaces one of the first two readers in the screening pathway and provides the same binary recall or no-recall outcome as the human readers. The first and second readers are replaced at random (with equal probability) for each episode. As the AI reader could trigger a third read that did not exist in the original dataset, the third reader was simulated for all episodes even if an original third read was present. This approach is to prevent unduly tying the result to the dataset and to obtain better variability estimates. Sensitivity analysis, where the third reader uses the real data when possible and where the replaced second reader is used as the third reader, was also performed (Supplementary Table 6). The third reader in our retrospective cohort operated with a sensitivity of 97.5% and a specificity of 56.3%, and we simulated the third reader with respect to this performance. Concretely, whenever an episode reaches the simulated third reader, the reader will make a recall decision by first inspecting the actual episode outcome and then using it as a prediction 97.5% of the time if the outcome is cancer (and 2.5% of the time using the opposite case as prediction), or 56.3% if the outcome is normal. This is achieved by sampling from the uniform(0,1) distribution with the corresponding probability, and it ensures that the simulated performance matches the real-world performance. Confidence intervals were generated through 1000 repetitions of each simulation.
As a remark, we emphasise that the simulation of the third reader should be performed with reference to the real-world Reader 3, rather than by reusing data of the earlier replaced readers (e.g. Reader 1 or Reader 2)20,22. Reusing data is convenient, as the replaced readers have seen all the episodes, and it avoids the simulation of the third reader. However, this overlooks the fact that while Reader 1 and 2 make independent judgements, they are conditionally dependent. In simple terms, a difficult cancer case is difficult for any reader. In such cases, the two readers would frequently miss together even when they make independent judgement, and the overall sensitivity would drop if either of them is used as an arbiter. In general, Reader 3 (the arbiter) makes decisions differently than Readers 1 and 2 because Reader 3 has access to their decisions and analyses. If Readers 1 and 2 were used in place of Reader 3 for simulation, then the result would be distorted, as we see in Supplementary Table 6.
In the AI band-pass scenario, the AI reader was used analogously to a band-pass filter. The AI reader provided one of three outcomes: recall, pass, and no-recall. All episodes with recall outcomes were automatically recalled. All episodes with the no-recall outcome were not recalled. All episodes with the pass outcome were sent to the usual human screening pathway. The AI reader made the final decision on the recall and no-recall episodes with no human reader involvement, and for all episodes that passed the human screening pathway, the original reader decisions were used.
In the AI triage scenario, the AI reader triages the episodes before the human readers. Episodes with high scores continue to the standard pathway, and episodes with low scores go through the pathway with only 1 reader. For episodes sent to the standard pathway, the original reader decisions were used, and for episodes sent to the single-reader path, the reader decision is sampled randomly (with equal probability) from the first and second readers. The AI reader made no final decision on any of the episodes.
AI operating points
Three sets of operating points were used as part of the study: the AI reader-replacement reader, the AI band-pass reader and the AI triage reader. There are three sets for five scenarios because the AI standalone reader and the AI single reader uses the same operating point as the AI reader-replacement reader. All operating points were set on the development set (Supplementary Fig. 13).
The AI reader-replacement operating point used a set of manufacturer-specific thresholds to convert the prediction scores into a binary outcome: recall or no-recall. The operating point was chosen to improve on the weighted mean individual reader’s sensitivity and specificity. The weighted mean individual reader was the weighted (by number of reads) mean of the sensitivity and specificity of the individual (first and second) radiologists when they were operating as a first or second reader. For all operating points that improved on the weighted mean individual readers sensitivity and specificity, the point with the maximum Youden’s index44 was chosen.
The AI band-pass reader used two sets of manufacturer-specific thresholds to convert the prediction scores into three outcomes: recall, pass and no-recall. The AI band-pass simulation was evaluated at different AI reader thresholds via a grid search. At each evaluation, two thresholds for each manufacturer were set to a target high specificity and high sensitivity point. All episodes with a score above the high sensitivity point were given the recall outcome, all episodes below the high specificity point the no-recall outcome, and all episodes in between points were given the pass outcome. The final AI band-pass reader thresholds were chosen from the simulation result with the maximum Youden’s index of the points with non-inferior sensitivity and specificity than that of the two readers with an arbitration system.
The AI triage reader used the 90% quantile of the prediction scores as the threshold to convert the prediction scores into the triage outcome: the standard pathway or the one-reader pathway. Episodes with prediction scores less than the threshold are assigned to the one-reader pathway; otherwise, they are assigned to the standard pathway.
When there is interaction between the AI reader and the human reader, i.e. the human may revise their decision based on the AI output, the AI reader, in all cases, uses the reader-replacement operating point (which is also the standalone operating point). To clarify, taking the AI triage scenario as an example, the AI triage reader uses the triage operating point to decide whether an episode should go to the standard pathway or the one-reader pathway. Once that is decided and the episode reaches any reader, the reader would have access to an AI-assist reading tool that operates at the reader-replacement operating point. So overall, there would be two operating points functioning at the same time.
Human–AI interaction
We simulate three interaction effects, the positive, the neutral and the negative effect. All three interactions involve an AI reader and a human reader. The AI reader first makes a decision about recall (using the assistive operating point), and then the human reader makes their decision with access to the AI output. The human may adjust their decisions if they differ from the AI’s, and this happens (100 × p)% of the time, where p is a parameter that varies between 0 and 1 across multiple simulations. For example, when p = 0.1, human readers will adjust the decision 10% of the time when their decisions differ from the AI, and when p = 1, human readers will change all their decisions to align with the AI. This models the automation effect, which we refer to as the neutral interaction.
For positive interactions, the human readers would only adjust the decision if the AI is correct. This models the situation where AI enhances human readings by reducing occasional misses and assisting in complex cases. And for negative interactions, the human readers would change the decision only if the AI is incorrect. This models the situation where human is confused by the AI reader’s output and mistakenly changes their correct decision into an incorrect one.
Evaluation metrics
The AUC, based on the receiver operating characteristic or ROC curve, is used to summarise the AI reader’s standalone performance.
Sensitivity and specificity are used to compare the AI reader with the radiologists and the AI-integrated screening scenarios with the current screening pathway. Sensitivity, or the true positive rate (TPR), is computed by dividing the number of correctly identified cancers (the true positives) by the total number of observed cancers (all positives, i.e. including both screen-detected cancers and interval cancers). It measures the success rate of the classifier in detecting the cancer. This is a key performance metric because early detection of cancer leads to more effective treatment45, due to a timely intervention, requirement for a less aggressive treatment, and improved survival rates. Specificity, or the true negative rate (TNR), is computed by dividing the number of correctly identified non-cancer episodes (true negatives) by the total number of observed non-cancer episodes (all negatives). It measures the success rate of the classifier in correctly not recalling a client when cancer is absent in the client. This is a key performance metric because unnecessarily recalling clients to the assessment centre is costly and induces significant stress on clients and their families46.
To compare the sensitivity and specificity of the AI-integrated screening scenarios with the current screening pathway, McNemar’s test (with continuity correction to improve the approximation of binomial distribution by the chi-squared distribution) is used to test for differences47, and the binomial exact test (one-sided) is used to test for superiority. Both tests adhere to the correct design for McNemar’s test, in which a 2-by-2 contingency table is constructed based on the paired samples from the two comparison scenarios. The samples are paired by the episode ID, and the tests are conducted once for each of the sensitivity and specificity48,49 at a significance level of 5%. For the effect size calculation, Cramér’s V (also known as Φ) is used for McNemar’s test. It is calculated as \(\sqrt{\frac{{\chi }^{2}}{N}}\), where χ2 is the test statistics and N is the number of samples. For the binomial test, Cohen’s h is used, calculated as \(\left\vert 2\,\,{\mbox{arcsin}}\,\sqrt{{p}_{1}}-2\,\,{\mbox{arcsin}}\,\sqrt{{p}_{2}}\right\vert \), where p1 is the test statistics, and p2 is the expected proportion under the null hypothesis.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Source data files are provided with this paper for all figures and tables. The non-transformed image and non-image data that established the ADMANI datasets were accessed under license agreement with BreastScreen Victoria. Further details about the ADMANI datasets are available in the data descriptor paper28. The three datasets used as external validation are publicly available or available via request. The Chinese Mammography Dataset (CMMD) is publicly available from the following website: https://wiki.cancerimagingarchive.net/pages/viewpage.action?pageId=70230508. The Cohort of Screen-age Women - Case-control (CSAW-CC) dataset is available via request from the following website: https://snd.gu.se/en/catalogue/study/2021-204. The BreastScreen Reader Assessment Strategy Australia (BREAST Australia) is available via request from the following website: https://breast-australia.sydney.edu.au/research/. Source data are provided with this paper.
Code availability
The code used for training the BRAIx AI reader is based on open-source algorithms and training techniques but is not able to be shared. We are required to protect potentially commercially valuable project intellectual property, which the source code constitutes, as part of our multi-institution agreement and grant obligations. The description of the model training procedure and models used are provided in the Methods section and can be implemented with open-source frameworks. The main conclusions drawn in our work relate to our AI reader simulation experiments. The code used to simulate the AI reader operating within the screening programme and validate the external results is available publicly43.
References
World Cancer Research Fund. Breast cancer. https://www.wcrf.org/dietandcancer/breast-cancer/ (2021).
Australian Institute of Health and Welfare. BreastScreen Australia Monitoring Report 2022 (Australian Institute of Health and Welfare, 2022).
Morrell, S., Taylor, R., Roder, D. & Dobson, A. Mammography screening and breast cancer mortality in australia: an aggregate cohort study. J. Med. Screen. 19, 26–34 (2012).
Rodríguez-Ruiz, A. et al. Stand-alone artificial intelligence for breast cancer detection in mammography: comparison with 101 radiologistsdembrower. J. Natl. Cancer Inst. 111, 916–922 (2019).
Rodríguez-Ruiz, A. et al. Detection of breast cancer with mammography: effect of an artificial intelligence support system. Radiology 290, 305–314 (2019).
McKinney, ScottMayer et al. International evaluation of an ai system for breast cancer screening. Nature 577, 89–94 (2020).
Kim, Hyo-Eun et al. Changes in cancer detection and false-positive recall in mammography using artificial intelligence: a retrospective, multireader study. Lancet Digit. Health 2, e138–e148 (2020).
Ribli, Dezső., Horváth, A., Unger, Z., Pollner, P. éter & Csabai, István Detecting and classifying lesions in mammograms with deep learning. Sci. Rep. 8, 1–7 (2018).
Salim, M. et al. External evaluation of 3 commercial artificial intelligence algorithms for independent assessment of screening mammograms. JAMA Oncol. 6, 1581–1588 (2020).
Schaffter, T. et al. Evaluation of combined artificial intelligence and radiologist assessment to interpret screening mammograms. JAMA Netw. Open 3, e200265–e200265 (2020).
Wu, N. et al. Deep neural networks improve radiologists’ performance in breast cancer screening. IEEE Trans. Med. Imag. 39, 1184–1194 (2020).
Frazer, HelenM. L., Qin, A. K., Pan, H. & Brotchie, P. Evaluation of deep learning-based artificial intelligence techniques for breast cancer detection on mammograms: results from a retrospective study using a breastscreen victoria dataset. J. Med. Imag. Rad. Oncol. 65, 529–537 (2021).
Freeman, K. et al. Use of artificial intelligence for image analysis in breast cancer screening programmes: systematic review of test accuracy. BMJ 374, n1872 (2021).
Larsen, M. et al. Artificial intelligence evaluation of 122,969 mammography examinations from a population-based screening program. Radiology 303, 502–511 (2022).
Yala, A., Schuster, T., Miles, R., Barzilay, R. & Lehman, C. A deep learning model to triage screening mammograms: a simulation study. Radiology 293, 38–46 (2019).
Shoshan, Y. et al. Artificial intelligence for reducing workload in breast cancer screening with digital breast tomosynthesis. Radiology 303, 69–77 (2022).
Leibig, C. et al. Combining the strengths of radiologists and ai for breast cancer screening: a retrospective analysis. Lancet Digit. Health 4, e507–e519 (2022).
Lauritzen, A. D. et al. An artificial intelligence–based mammography screening protocol for breast cancer: Outcome and radiologist workload. Radiology 304, 41–49 (2022).
Dembrower, K. et al. Effect of artificial intelligence-based triaging of breast cancer screening mammograms on cancer detection and radiologist workload: a retrospective simulation study. Lancet Digit. Health 2, e468–e474 (2020).
Sharma, N. et al. Multi-vendor evaluation of artificial intelligence as an independent reader for double reading in breast cancer screening on 275,900 mammograms. BMC Cancer 23, 460 (2023).
Lång, K. et al. Artificial intelligence-supported screen reading versus standard double reading in the mammography screening with artificial intelligence trial (masai): a clinical safety analysis of a randomised, controlled, non-inferiority, single-blinded, screening accuracy study. Lancet Oncol. 24, 936–944 (2023).
Marinovich, M. L. et al. Artificial intelligence (AI) for breast cancer screening: BreastScreen population-based cohort study of cancer detection. EBioMedicine 90, 104498 (2023).
Carter, S. M. et al. The ethical, legal and social implications of using artificial intelligence systems in breast cancer care. Breast 49, 25–32 (2020).
Byng, D. et al. Abstract ot3-18-03: the praim study: a prospective multicenter observational study of an integrated artificial intelligence system with live monitoring. Cancer Res. 83, OT3–18 (2023).
Strand, F. Artificial intelligence in large-scale breast cancer screening (screentrustcad). ClinicalTrials.gov identifier: NCT04778670. Updated: 2023-03-14. Accessed: 2024-04-08. https://clinicaltrials.gov/study/NCT04778670.
Al-Bazzaz, H., Janicijevic, M. & Strand, F. Reader bias in breast cancer screening related to cancer prevalence and artificial intelligence decision support—a reader study. Eur. Radiol. 34, 5415–5424 (2024).
Goddard, K., Roudsari, A. & Wyatt, J. C. Automation bias: a systematic review of frequency, effect mediators, and mitigators. J. Am. Med. Inform. Assoc. 19, 121–127 (2012).
Frazer, HelenM. L. et al. Admani: annotated digital mammograms and associated non-image datasets. Radiol. Artif. Intell. 5, e220072 (2022).
Wilder, B., Horvitz, E. & Kamar, E. Learning to complement humans. In IJCAI (ed. Bessiere, C.) 1526–1533 (ijcai.org, 2020).
He, K., Zhang, X., Ren, S. & Sun, J. Deep residual learning for image recognition. In Proc. IEEE Conference on Computer Vision and Pattern Recognition 770–778 (IEEE, 2016).
Huang, G., Liu, Z., van der Maaten, L. & Weinberger, K. Q. Densely connected convolutional networks. In Proc. IEEE Conference on Computer Vision and Pattern Recognition (CVPR) 7 (IEEE, 2017).
Wang, Q. et al. ECA-Net: efficient channel attention for deep convolutional neural networks. In CVPR 11531–11539 (Computer Vision Foundation / IEEE, 2020).
Tan, M. & Le, Q. Efficientnet: rethinking model scaling for convolutional neural networks. In Proc. 36th International Conference on Machine Learning 6105–6114 (PMLR, 2019).
Szegedy, C., Ioffe, S., Vanhoucke, V. & Alemi, A. A. Inception-v4, inception-resnet and the impact of residual connections on learning. In Thirty-first AAAI Conference on Artificial Intelligence (AAAI Press, 2017).
Chollet, F. Xception: deep learning with depthwise separable convolutions. In Proc. IEEE Conference on Computer Vision and Pattern Recognition (CVPR) 7 (IEEE, 2017).
Liu, Z. et al. A convnet for the 2020s. In Proc. IEEE/CVF Conference on Computer Vision and Pattern Recognition 11976–11986 (IEEE, 2022).
Chen, Y. et al. Multi-view local co-occurrence and global consistency learning improve mammogram classification generalisation. In Medical Image Computing and Computer Assisted Intervention – MICCAI 2022 3–13 (Cham, Springer, 2022).
Wang, C. et al. Knowledge distillation to ensemble global and interpretable prototype-based mammogram classification models. In Medical Image Computing and Computer Assisted Intervention – MICCAI 2022 14–24 (Springer, 2022).
Paszke, A. et al. PyTorch: an imperative style, high-performance deep learning library. In 33rd Conference on Neural Information Processing Systems 8024–8035 (Curran Associates, Inc., 2019).
Kingma, D. P. & Ba, J. Adam: a method for stochastic optimization. arXiv preprint arXiv:1412.6980 (2014).
Reddi, S. J., Kale, S. & Kumar, S. On the convergence of adam and beyond. arXiv preprint arXiv:1904.09237 (2019).
Oyelade, O. N. & Ezugwu, A. E. A deep learning model using data augmentation for detection of architectural distortion in whole and patches of images. Biomed. Signal Process. Control 65, 102366 (2021).
Kwok, C. F. & Elliott, M. S. Braix-project/retrospective-cohort-study: v3.0.0. Zenodo https://doi.org/10.5281/zenodo.12633016 (2024).
Youden, W. J. Index for rating diagnostic tests. Cancer 3, 32–35 (1950).
Cancer Australia. Guidance for the management of early breast cancer: recommendations and practice points. Cancer Australia (2020).
Lee, J. M. et al. Breast cancer risk, worry, and anxiety: effect on patient perceptions of false-positive screening results. Breast 50, 104–112 (2020).
McNemar, Q. Note on the sampling error of the difference between correlated proportions or percentages. Psychometrika 12, 153–157 (1947).
Trajman, A. & Luiz, R. R. Mcnemar χ2 test revisited: comparing sensitivity and specificity of diagnostic examinations. Scand. J. Clin. Lab. Invest. 68, 77–80 (2008).
Kim, S. & Lee, W. Does mcnemar’s test compare the sensitivities and specificities of two diagnostic tests? Stat. Methods Med. Res. 26, 142–154 (2017).
Acknowledgements
The authors would like to acknowledge Rita Butera, Luke Neill, and Georgina Marr from BreastScreen Victoria and the leadership and staff of St Vincent’s Hospital Melbourne for their support of the project. The authors would like to acknowledge Katrina Kunicki, Anne Johnston, Colleen Elso, Elizabeth Campbell and Tom Kay of St Vincents Institute of Medical Research (SVI) for their extensive help and support with many aspects of this project. The authors thank Wayne Crismani for thoughtful comments on the manuscript. This work is supported by funding from the Australian Government under the Medical Research Future Fund Grant (MRFAI000090) for the Transforming Breast Cancer Screening with Artificial Intelligence (BRAIx) Project awarded to H.M.L.F., D.J.M., P.B., J.F.L., J.L.H., G.C. and a National Health and Medical Research Council Investigator Grant (GNT1195595) awarded to D.J.M. This work is also supported by a Ramaciotti Health Investment Grant awarded to D.J.M. and funding from a Royal Australian and New Zealand College of Radiologists Clinical Research Grant and the St Vincent’s Hospital Melbourne Research Endowment Fund awarded to HMLF. The funders had no role in the work or decision to publish. SVI provided significant in-kind support comprising IT support, infrastructure, and GPUs used for numerical calculations in this paper enabled via funding from the Victorian State Government Operational Infrastructure Support Programme to St Vincent’s Institute of Medical Research. HMLF acknowledges the generous support of the Medical Device Partnering Programme for enabling the prior foundational research to be undertaken. D.J.M. acknowledges generous support from Paul Holyoake and Marg Downey.
Author information
Authors and Affiliations
Consortia
Contributions
Conceptualisation: H.M.L.F., D.J.M., J.F.L., J.L.H. and G.C.; Methodology: H.M.L.F., C.A.P.-S., C.F.K., M.S.E., Y.C., C.W., P.B., G.C., D.J.M.; AI model and code development: C.A.P.-S., C.F.K., M.S.E., Y.C. and C.W.; Dataset development: H.M.L.F., M.S.E., C.A.P.-S. and C.F.K.; Data analysis: C.A.P.-S., C.F.K. and M.S.E.; Resources: D.J.M., H.M.L.F., P.B., J.F.L., J.L.H. and G.C.; Data preparation: M.S.E., B.H. and R.K.; Data interpretation: all authors; Results interpretation: all authors; Writing—original draft, review and editing: C.A.P.-S., C.F.K., M.S.E., H.M.L.F., D.J.M. and G.C.; Supervision: H.M.L.F., D.J.M. and G.C.; Project administration: H.M.L.F., D.J.M. and G.C.; Funding acquisition: H.M.L.F., D.J.M., P.B., J.F.L., J.L.H. and G.C.
Corresponding author
Ethics declarations
Competing interests
P.B. is an employee of annalise.ai. C.W., Y.C., D.J.M., M.S.E., H.M.L.F. and G.C. are inventors on a patent, 'WO2024044815—Improved classification methods for machine learning', a model used in versions of the BRAIx AI reader. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Frazer, H.M.L., Peña-Solorzano, C.A., Kwok, C.F. et al. Comparison of AI-integrated pathways with human-AI interaction in population mammographic screening for breast cancer. Nat Commun 15, 7525 (2024). https://doi.org/10.1038/s41467-024-51725-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-51725-8
- Springer Nature Limited