Abstract
We previously reported that normothermic ex vivo kidney perfusion (NEVKP) is superior in terms of organ protection compared to static cold storage (SCS), which is still the standard method of organ preservation, but the mechanisms are incompletely understood. We used a large animal kidney autotransplant model to evaluate mitochondrial function during organ preservation and after kidney transplantation, utilizing live cells extracted from fresh kidney tissue. Male porcine kidneys stored under normothermic perfusion showed preserved mitochondrial function and higher ATP levels compared to kidneys stored at 4 °C (SCS). Mitochondrial respiration and ATP levels were further enhanced when AP39, a mitochondria-targeted hydrogen sulfide donor, was administered during warm perfusion. Correspondingly, the combination of NEVKP and AP39 was associated with decreased oxidative stress and inflammation, and with improved graft function after transplantation. In conclusion, our findings suggest that the organ-protective effects of normothermic perfusion are mediated by maintenance of mitochondrial function and enhanced by AP39 administration. Activation of mitochondrial function through the combination of AP39 and normothermic perfusion could represent a new therapeutic strategy for long-term renal preservation.
Similar content being viewed by others
Introduction
The optimal renal replacement therapy for patients with end-stage kidney disease is kidney transplantation1. Expanding the use of marginal grafts and increasing the donor pool are essential strategies to shorten the waiting period for deceased donor kidney transplantation2. Although static cold storage (SCS) is widely used worldwide because of its simplicity, this preservation method has been reported to decrease the metabolic activity of the organ and aggravate ischemia-reperfusion injury3.
Ischemia-reperfusion injury is an unavoidable insult that occurs during organ preservation and is caused by a decrease in oxygen supply during ischemia, which causes cells to switch to anaerobic metabolism and impairs mitochondrial function4. Accumulation of succinate and reversal of electron transport in the mitochondrial complex during reperfusion leads to reactive oxygen species release and mitochondrial injury, which is reflected in reduced ATP production, triggering inflammation and organ damage5,6,7,8. These observations highlight the importance of maintaining cellular mitochondrial function during organ preservation.
We have studied NEVKP as a method to maintain kidneys in a physiological environment ex vivo9. We have reported that NEVKP is superior in organ protection compared to SCS3. More recently, through proteomic and genomic approaches, we found that NEVKP maintains the levels of key proteins involved in mitochondrial biosynthesis in porcine kidneys in a model of donation after cardiac death (DCD)10. We also reported that NEVKP promotes the expression of genes related to mitochondrial metabolism11. To our knowledge, there have been no previous reports evaluating mitochondrial function using live cells from preserved organs. In this study, using a porcine kidney autotransplantation model we showed that NEVKP has a superior mitochondrial protective effect as compared to SCS and that NEVKP prevents subsequent oxidative stress and inflammation.
In recent years, NEVKP has been recognized as an emerging platform for therapeutic interventions12. The ability to offer a near-physiological environment ex vivo provides the opportunity to modify a single organ prior to transplantation through cell therapy or drug administration13,14. AP39 is a compound used to supply hydrogen sulfide (H2S) within mitochondria15. Hydrogen sulfide released from AP39, which has a mitochondrial target domain, has been shown to function as an electron donor at the mitochondrial membrane, supporting mitochondrial electron transport and ATP production16,17. While there have been reports of organ protection using AP3918,19, to our knowledge, there are no reports of AP39 administered during NEVKP in large animals followed by transplantation. In this study, we aimed to enhance graft protection from ischemia by administering AP39 during perfusion after prolonged warm ischemia to stimulate the mitochondrial metabolic capacity of the cells of the renal cortex, thus further improving graft survival.
Results
5 h of NEVKP improves ischemia-reperfusion injury
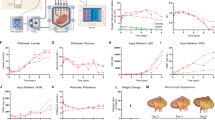
We first examined how 5 h of NEVKP affects the graft function after 60 min of warm ischemia and kidney autotransplantation(Fig. 1A). Plasma creatinine and urea nitrogen continued to increase in both the SCS and NEVKP groups after transplantation but were significantly lower in the NEVKP group from postoperative day (POD) 2 to POD 3 compared to the SCS group (Fig. 1B). Creatinine clearance and fractional excretion of sodium (FeNa), an indicator of ischemia-reperfusion injury, were measured using urine 24-h collections on the second post-transplant day (Fig. 1C). The NEVKP group showed a trend toward higher urine output compared to the no WI and SCS experimental groups. This could be explained by the diuretic effect of sodium excretion arising from ischemia-reperfusion injury and by the fact that some pigs in the SCS group had markedly low urine output. There was no significant difference in creatinine clearance between the NEVKP and SCS groups, although a trend toward higher creatinine clearance was observed in the NEVKP group (Fig. 1C). FeNa was significantly lower with machine perfusion preservation compared to SCS (Fig. 1C). Since our previous study showed that ischemia-reperfusion injury in the kidney peaked on postoperative days 2–3, we sacrificed the pigs on day 3 and evaluated the kidney tissue20. The tubular injury and inflammation scores were significantly elevated in the SCS and NEVKP groups compared to the group transplanted with healthy kidneys without ischemia (Fig. 1D).
A Schema of the study design: kidneys retrieved from 30-kg pigs were subjected to 60 min of warm ischemia, randomized to 5 h of ex vivo warm perfusion or static cold storage, and followed for 3 days after autotransplantation and contralateral nephrectomy. The no warm ischemia group that underwent transplantation after minimal storage was used as sham control (n = 5 pigs/No WI, 6 pigs/SCS, and 6 pigs/NEVKP). B Plasma creatinine levels and urea nitrogen from preoperative to day 3 (n = 5 pigs No WI, 6 pigs SCS, and 6 pigs NEVKP). C 24-h urine storage test from day 2 to day 3. 24-hour urine output, creatinine clearance, and fractional excretion of sodium (n = 5 pigs No WI, 6 pigs SCS, and 5 pigs NEVKP). In one animal in the NEVKP group, the urine sample could not be collected postoperatively due to technical error and was excluded from the analysis. D PAS stained image at the border between renal cortex and medulla obtained on day 3, comparing tubular injury score and inflammation score (n = 5 pigs No WI, 6 pigs SCS, and 6 pigs NEVKP). Scale bar, 100 μm. Results are presented as means ± SD. Comparisons between two groups were made using the two-tailed unpaired t test, and comparisons between three groups were made using ANOVA and the Tukey post hoc test. Panel (A), created with BioRender.com, was released under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International license. WI, ischemia; SCS, static cold storage; NEVKP, normothermic ex vivo kidney perfusion.
NEVKP restores mitochondrial function to levels similar to those in normal kidney tissue
We next hypothesized that normothermic machine perfusion would maintain mitochondrial function ex vivo. Cell suspensions dissociated from fresh kidney biopsies, as previously shown, were plated, allowed to attach overnight, and then subjected to assessment of metabolic function in a Seahorse instrument, as previously performed21,22. Mitochondrial respiration, assessed by measuring oxygen consumption rate (OCR) was significantly lower in the SCS preservation group compared to normal, healthy tissue taken from the contralateral kidney 5-h after organ preservation (Fig. 2A). However, in the NEVKP group, OCR levels were maintained to the same degree as in healthy kidneys. The NEVKP group had significantly higher basal OCR, ATP-linked respiration, maximal respiration capacity, and reserve capacity compared to SCS-preserved kidneys during cellular metabolic stress (Fig. 2A, B). Early after transplantation, differences in mitochondrial respiratory capacity between groups were less noticeable, while ATP-linked respiration was significantly lower in the SCS group than in the healthy kidney group (Supplementary Fig. 1). The increased aerobic metabolism in NEVKP-preserved kidneys was accompanied by a significant increase in intracellular levels of ATP compared to SCS-preserved kidneys after the end of organ preservation and early (30 min) after kidney transplantation (Fig. 2C). We then examined mitochondrial morphology in the renal cortex and found that most mitochondria in the no WI group—corresponding to healthy kidneys—were elongated before transplantation, whereas in the 5-h NEVKP group, most mitochondria were fragmented and rounded (Fig. 2D). In the SCS group, mitochondrial morphology was not as much rounded, showing a pattern between the no WI and the warm perfusion group (Fig. 2D). Based on semiquantitative scoring of mitochondrial fragmentation, there was a significant difference between the no WI and NEVKP groups before transplantation, and between the SCS and NEVKP groups at 30 min after implantation (Fig. 2D). In agreement, the SCS group showed significantly increased plasma circulating cell-free mtDNA (ccf-mtDNA) levels, which are known to be released extracellularly when mitochondria are under stress, compared to the no WI group (Fig. 2E). A trend towards higher levels of plasma ccf-mtDNA was seen in the SCS group compared to NEVKP-preserved kidneys. Taken together, these data suggest that NEVKP preserves aerobic metabolism and increases ATP levels in the renal cortex, which may contribute to the ability of NEVKP to ameliorate renal ischemia-reperfusion injury after transplantation.
A Oxygen consumption rate in cells extracted from renal cortex tissue for analysis of mitochondrial respiration after organ preservation (n = 3 pigs/No WI, 3 pigs/SCS, and 3 pigs/NEVKP; n = 17 replicates/No WI, n = 17 replicates/SCS, and 18 replicates/NEVKP). Changes from baseline to after metabolic stress. B Differences in baseline oxygen consumption rates, calculated ATP-linked respiration, maximal respiration capacity, and reserve capacity (n = 3 pigs Healthy Kidney, 3 pigs SCS, and 3 pigs NEVKP; n = 17 replicates Healthy Kidney, n = 17 replicates SCS, and 18 replicates NEVKP). C ATP concentration per 100000 cells obtained from renal cortical tissue after organ storage and before implantation and at 30 min after implantation (n = 5 pigs No WI, 5 pigs SCS, and 5 pigs NEVKP). D Immunofluorescence co-staining of Hsp60 (green) and DAPI (blue) within porcine kidneys after organ storage and before implantation and at 30 min after implantation. Scale bar, 10 μm (n = 5 pigs No WI, 6 pigs SCS, and 6 pigs NEVKP). E Circulating cell-free miDNA at 3 h and 3 days after reperfusion (n = 5 pigs No WI, 6 pigs SCS, and 6 pigs NEVKP). Results are presented as means ± SD. Comparisons between two groups were made using the two-tailed unpaired t test, and comparisons between three groups were made using ANOVA and the Tukey post hoc test. WI, ischemia; SCS, static cold storage; NEVKP, normothermic ex vivo kidney perfusion; OCR, oxygen consumption rate; ATP, adenosine triphosphate; mtDNA, mitochondrial DNA.
NEVKP prevents oxidative stress in transplanted kidneys subjected to WI
Ischemia-reperfusion injury in the kidney leads to metabolic damage, mainly in the form of mitochondrial dysfunction, as well as inflammation marked by the release of reactive oxygen species and activation of neutrophils (Fig. 3A). To assess whether NEVKP affords protection, we measured TBARS, a marker of oxidative stress induced by ischemia-reperfusion injury, before organ removal and daily up to day 3 after renal transplantation (Fig. 3B). There was no significant difference between the groups at baseline and POD 1. In turn, the NEVKP group demonstrated a significant decrease in TBARS compared to the SCS group from POD 2 onward (Fig. 3B). To assess the effects of NEVKP on neutrophil activation, we measured plasma levels of myeloperoxidase (MPO). On POD3, the SCS group showed a significant increase in MPO compared to the no WI group (Fig. 3C). Lower MPO values were found in the NEVKP group compared to the SCS group, but the difference was not statistically significant (Fig. 3C). Taken together, these data suggest that the beneficial metabolic effects of NEVKP are associated with reduced oxidative stress.
A Graphical representation of the biological response triggered by mitochondrial injury in ischemia-reperfusion injury. Mitochondrial dysfunction releases reactive oxygen species and mtDNA, which in turn activate inflammatory pathways. B Comparison of plasma levels of TBARS, a marker of oxidative stress, from preoperative to day 3 post-implantation (n = 5 pigs/No WI, 6 pigs/SCS, and 6 pigs/NEVKP). C Plasma concentration of myeloperoxidase, a marker of inflammation, at 1–3 days post-implantation (n = 5 pigs No WI, 6 pigs SCS, and 6 pigs NEVKP). Results are presented as means ± SD. Comparisons between two groups were made using the two-tailed unpaired t test, and comparisons between three groups were made using ANOVA and the Tukey post hoc test. Panel (A), created with BioRender.com, was released under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International license. WI, ischemia; SCS, static cold storage; NEVKP, normothermic ex vivo kidney perfusion; ATP, adenosine triphosphate; mtDNA, mitochondrial DNA; TBARS, 2-thiobarbituric acid reactive substances; MPO, myeloperoxidase.
Administration of AP39 during NEVKP is feasible and safe
Having determined that NEVKP-mediated protection is associated with the preservation of mitochondrial function in the graft, we next sought to determine whether additional organ-protective effects might be achieved by further enhancing mitochondrial function with AP39 (Fig. 4A). As in the first protocol, 60 min of warm ischemia was followed by 5 h of NEVKP with subsequent autotransplantation and contralateral nephrectomy. AP39 was administered in the perfusate immediately after the start of perfusion in the intervention group (NEVKP + AP39). As we have previously reported, renal blood flow increased over time with normothermic machine perfusion (Fig. 4B), and the NEVKP + AP39 group showed a similar significant increase in renal blood flow (Fig. 4B). Perfusion was maintained under pressure fixation, and intravascular resistance decreased to a similar extent in both NEVKP- and NEVKP + AP39-treated groups as blood flow increased (Fig. 4B). Administration of AP39 significantly increased the total urine output during 5 h of perfusion compared to NEVKP alone. Urinary glucose levels were not significantly different between the two groups, suggesting that osmotic diuresis had little effect (Fig. 4B). Renal blood flow rate, intrarenal vascular resistance at 5 h, and total urine output were significantly correlated with graft function after implantation (Supplementary Fig. 2). In both NEVKP and NEVKP + AP39 groups, lactate levels decreased to a similar extent with perfusion (Fig. 4B). Glucose levels increased over time due to the administration of dextrose during perfusion, and the observed increase was similar in both groups (Fig. 4B). These results support that administration of AP39 together with NEVKP is feasible, safe, and confers physiologic improvement of kidneys subjected to WI.
A Schema of the study design: kidneys retrieved from 30-kg pigs were subjected to 60 min of warm inhibition, randomized to 5 h of ex vivo warm perfusion with or without AP39 mitochondria-targeted drug, and followed for 3 days after autotransplantation and contralateral nephrectomy (n = 6 pigs/NEVKP and 5 pigs/NEVKP + AP39). B Renal blood flow, intravascular resistance, urine volume and total urine output, urine glucose, lactate, and glucose levels during 5 h of warm perfusion (n = 5–6 pigs NEVKP and 5 pigs NEVKP + AP39). In one animal in the NEVKP group, the urine sample could not be collected postoperatively due to technical error and was excluded from the analysis. Results are presented as means ± SD. Comparisons between the two groups were made using the two-tailed unpaired t test. Panel (A), created with BioRender.com, was released under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International license. NEVKP, normothermic ex vivo kidney perfusion.
AP39 treatment improves graft function after WI
We next evaluated graft function and the extent of ischemia-reperfusion injury after kidney autotransplantation. Tubular injury scores and inflammation scores from kidney sections on POD 3 were numerically lower in the NEVKP + AP39 group compared to the NEVKP group, although the difference was not significant (Fig. 5A). Plasma creatinine and blood urea nitrogen levels started to decrease in the NEVKP + AP39 group on POD2 and were significantly lower on POD 3 compared to the NEVKP group (Fig. 5B). There was no significant difference in 24-h urine output between the two groups on the second day after transplantation (Fig. 5C). The FeNa was significantly lower on POD 3 when AP39 was administered during perfusion compared to perfusion alone (Fig. 5C). We also administered AP39 to the SCS group when flushing blood with UW solution before preservation (SCS + AP39 group). Although the SCS + AP39 group had significantly lower plasma creatinine and urea nitrogen on postoperative day 3 compared to the SCS group, they were significantly higher than in the NEVKP + AP39 group (Supplementary Fig. 3). These results indicate that administration of AP39 together with NEVKP improves graft function beyond the beneficial effects conferred by NEVKP alone.
A PAS stained image at the border between the renal cortex and medulla obtained on day 3, comparing tubular injury score and inflammation score (n = 6 pigs/NEVKP and 5 pigs/NEVKP + AP39). Scale bar, 100 μm. B Results of renal function trends. Plasma creatinine levels and urea nitrogen from preoperative to day 3 (n = 6 pigs NEVKP, 5 pigs NEVKP + AP39, and 3 pigs SCS + AP39). C 24-h urine storage test from day 2 to day 3. 24-hour urine output, creatinine clearance, and fractional excretion of sodium (n = 5 pigs NEVKP and 5 pigs NEVKP + AP39). In one animal in the NEVKP group, the urine sample could not be collected postoperatively due to technical error and was excluded from the analysis. Results are presented as means ± SD. Comparisons between the two groups were made using the two-tailed unpaired t test. NEVKP, normothermic ex vivo kidney perfusion.
AP39 administration amplifies the protective effects of NEVKP on renal mitochondrial function
We next evaluated whether AP39 treatment together with NEVKP enhances mitochondrial function compared to NEVKP alone. Intracellular ATP levels in the AP39 experimental group were significantly higher after 5 h of organ preservation, at the time of pre-implantation, but no significant differences were observed after implantation (Fig. 6A). The Seahorse analyzer was used to measure mitochondrial respiration (Fig. 6B). With the addition of AP39 during perfusion, basal OCR, ATP-linked respiration, maximal respiration capacity, and reserve capacity were all significantly increased compared to perfusion alone (Fig. 6C). Significant differences in maximal respiration capacity were also observed at the early post-transplant time (Supplementary Fig. 4). Assessment of mitochondrial morphology by Hsp60 staining showed that mitochondria in the renal cortex were more fragmented and rounded when AP39 was administered during 5 h of NEVKP (Fig. 6D). There was no significant difference between the two groups in the early post-transplant phase (Fig. 6D). There was no significant difference in ccf mtDNA levels after organ preservation or transplantation, suggesting that this mechanism of injury may not be heavily involved, although it is possible that we were underpowered to detect a significant difference in the early post-reperfusion period (Fig. 6E).
A ATP concentration per 100000 cells was obtained from renal cortical tissue at time points after organ storage and before implantation, and at 30 min after implantation (n = 5 pigs/NEVKP and 5 pigs/NEVKP + AP39). B Oxygen consumption rate in cells extracted from renal cortex tissue for analysis of mitochondrial respiration after organ preservation. Changes from baseline to after metabolic stress (n = 3 pigs NEVKP and 3 pigs NEVKP + AP39; n = 18 replicates NEVKP and 15 replicates NEVKP + AP39). C Differences in baseline oxygen consumption rates, calculated ATP-linked respiration, maximal respiration capacity, and reserve capacity (n = 3 pigs NEVKP and 3 pigs NEVKP + AP39; n = 18 replicates NEVKP and 15 replicates NEVKP + AP39). D Immunofluorescence co-staining of Hsp60 (green) and DAPI (blue) within porcine kidneys at time points after organ storage and before implantation and at 30 min after implantation (n = 6 pigs NEVKP and 5 pigs NEVKP + AP39). Scale bar, 10 μm. E Circulating cell-free miDNA at 3 h and 3 days after reperfusion (n = 6 pigs NEVKP and 5 pigs NEVKP + AP39). Results are presented as means ± SD. Comparisons between the two groups were made using the two-tailed unpaired t test. NEVKP, normothermic ex vivo kidney perfusion; OCR, oxygen consumption rate; ATP, adenosine triphosphate; mtDNA, mitochondrial DNA.
Administration of AP39 in combination with NEVKP reduces oxidative stress and inflammation
We then examined how oxidative stress and inflammation were altered by AP39 administration, measuring TBARS and MPO levels. At both 1 h and 5 h of perfusion, with a similar trend at 3 h of perfusion, administration of AP39 resulted in significantly lower perfusate TBARS levels compared to levels seen with perfusion alone (Fig. 7A). On POD3 after transplantation, kidneys subjected to AP39 together with NEVKP were associated with significantly lower plasma TBARS levels compared to grafts subjected to NEVKP alone (Fig. 7A). Plasma levels of MPO were significantly reduced on POD 2 in the NEVKP + AP39 group compared to in the NEVKP alone group (Fig. 7B). These results indicate that AP39 in combination with NEVKP confers further protection from ischemia-induced oxidative stress and inflammation beyond the effect conferred by NEVKP alone.
A Comparison of plasma and perfusate levels of TBARS, a marker of oxidative stress, from preoperative to day 3 post-implantation (n = 6 pigs/NEVKP and 5 pigs/NEVKP + AP39). B Plasma concentration of myeloperoxidase, a marker of inflammation, at 1–3 days post-implantation (n = 6 pigs NEVKP and 5 pigs NEVKP + AP39). Results are presented as means ± SD. Comparisons between the two groups were made using the two-tailed unpaired t test. NEVKP, normothermic ex vivo kidney perfusion; TBARS, 2-thiobarbituric acid reactive substances; MPO, myeloperoxidase.
Discussion
The molecular aspects behind the beneficial effects of normothermic ex vivo machine perfusion relative to static cold storage (the standard conventional method of organ preservation) remain incompletely understood23. It has been assumed that metabolism is maintained during normothermic perfusion, but this has not been adequately assessed until now. Here, we evaluated the mitochondrial function of biopsy-derived kidney cells after normothermic ex vivo machine perfusion. In line with our previous reports, our results indicated that 5 h of normothermic machine perfusion may reduce ischemia-reperfusion injury and protect organs in the short term20 and that oxygen consumption of living cells in the renal cortex was predominantly increased, suggesting a maintenance of the mitochondrial metabolic capacity conferred by NEVKP. In addition, ATP levels were preserved in living cells derived from ischemic kidneys subjected to warm perfusion to a similar extent to those obtained from normal kidneys. Along with the maintenance of mitochondrial function, significant differences in TBARS, a marker of oxidative stress, were observed at 3 days post-transplantation. Because the measurements were performed using plasma concentrations rather than tissue concentrations, they may reflect delayed elevation of markers of oxidative stress. These findings are consistent with reports that mitochondrial metabolic capacity during organ preservation is critical for the development of graft function and may represent a new therapeutic target in organ preservation.
Consequently, we aimed to enhance graft protection from ischemia by stimulating metabolic capacity in the renal cortex with the administration of mitochondria-targeted drugs in the perfusate. Hydrogen sulfide released from AP39, which has a mitochondrial target domain, has been shown to function as an electron donor at the mitochondrial membrane, supporting mitochondrial electron transport and ATP production16,17. AP39 has been shown to be useful in kidneys preserved by SCS and perfused under subnormothermic conditions18,24,25. Our study is the first to examine the efficacy of AP39 in the setting of perfusion under normothermic conditions and in a large animal model of transplantation. In support of these mechanisms, 5 h of NEVKP with AP39 resulted in a superior activation of mitochondrial metabolic capacity and an increase in ATP levels compared to warm perfusion without AP39. Correspondingly, oxidative stress levels in the perfusate decreased, and arterial blood flow and urine excretion, indicators of superior graft function during perfusion, increased. Observations from our prior studies did not support a strong correlation between ex vivo creatinine clearance and postoperative renal function (data not shown). Thus, we did not measure ex vivo creatinine clearance in the present study, but this could certainly be considered for future studies. This intervention also conferred a benefit with respect to graft function after transplantation. What can be drawn from these findings is the fact that maintaining mitochondrial function in ischemic kidneys is essential for good graft function after transplantation. The combination of warm perfusion, which attempts to preserve the organ as close as possible to its physiological environment outside the body, and mitochondria-activating agent may be of potential therapeutic utility. It has been reported that exposure of cultured cells to AP39 increased the concentration of hydrogen sulfide in the culture supernatant over a 7-day period17. We thus inferred that the effects of AP39 would persist within a 5-h circuit. As AP39 is a mitochondria-targeted H2S donor, we did not assess direct binding to renal tubular/cortex cells in our study. However, recent research work demonstrated that exposure of kidneys to AP39 in the context of ischemia-reperfusion injury reduced apoptotic injury of renal tubular cells19. In addition, exposure to AP39 of a number of cultured cell types, including microvascular endothelial cells, cardiac myoblasts, pancreatic islet cells, human trophoblasts, and neuroblastoma cells, has been shown to result in mitochondrial protection, suggesting direct uptake of AP39 by these cells15,17,26,27,28. While the addition of AP39 during SCS provided some degree of organ protection, the combination of warm perfusion and AP39 provided a benefit that exceeded it. Indeed, there is a growing recognition of the numerous advantages offered by treating organs alone, outside the body, such as minimizing side effects29. In this study, no apparent adverse events were observed in the group treated with the drug. Nonetheless, the practical introduction of normothermic perfusion equipment in the clinical setting may prove costly or technically challenging. If such is the case, a short cold flush with AP39 and subsequent SCS preservation can be considered.
There are several limitations to this study. One is the small number of large animals used, due to ethical and economic considerations. Second, the experimental animals did not have any of the common donor/recipient issues that can impact transplant outcomes (e.g., advanced age, multiple comorbidities, infection, resuscitation, embolization, multiple vessels, size mismatch, etc.). By eliminating these factors, we were able to directly assess the effectiveness of our strategy in organ preservation without the influence of confounding factors. Third, we used a novel method for assessing mitochondrial function in tissues. Unlike previous reports, which often used frozen serum or plasma and evaluated by mass spectrometry, we analyzed mitochondrial respiratory capacity and ATP production using living cells extracted before and after transplantation from the renal cortex, which is a highly metabolically active region, mostly due to the high energy demands required for tubular transport and reabsorption30,31. We believe this method is more appropriate because it allows us to assess mitochondrial activity in real-time and in the graft tissue itself32. ccf-mtDNA is widely used as a method to assess mitochondrial injury, however, the results in this experiment were not consistent with the other mitochondrial assessment method33,34. This assay was performed on frozen plasma and analyzed simultaneously when all samples were collected, which may have resulted in variations in the cryopreservation period among samples. In addition, mitochondrial morphology in kidney tissue is typically evaluated by electron microscopy, but because the tissue could not be preserved in an appropriate manner, it was alternatively stained with Hsp60 and evaluated using confocal microscopy. Finally, the histopathological evaluation methods we used, tubular injury score and inflammation score, are semi-quantitative methods. The accuracy and sensitivity of the tests are limited. In particular, the small number of animals used in this experimental system may have made it difficult to detect differences. Creatinine clearance measurements also have limited utility because of the difficulty of accurately measuring urine output in pig cages. We believe that serum creatinine, BUN, and fractional excretion of sodium can provide a complementary assessment of the ischemia-reperfusion injury model. These findings are similar to previous observations in which we observed superior function of kidneys transplanted after NEVKP compared to hypothermic machine perfusion, despite similar histology between the two groups3.
SCS has long been the standard method of preserving kidneys35. It is widely used throughout the world because of its simplicity, low cost, and ease of technique. Currently, hypothermic ex vivo perfusion (HMP) with or without oxygen is becoming the new standard method of organ preservation for kidneys from DCD and donation after brain death (DBD) with expanded criteria in European countries36,37,38. HMP has been shown to reduce delayed graft function and improve graft survival after transplantation compared to SCS. HMP has relatively simple logistics and economic advantages over NEVKP, while NEVKP better enables the potential assessment of organ function. In addition, their proximity to the physiological environment could offer the potential for future interventions to repair damaged organs or induce immune tolerance through cell therapy. Studies that directly compare NEVKP and HMP are currently limited but should be the focus of future investigation39.
In this study we also evaluated mitochondrial morphology after organ preservation and transplantation by fluorescent immunostaining. Our major finding at the morphology level was that mitochondria protected by warm perfusion were more fragmented, small, and rounded. This suggests that these morphological changes may indicate structural mitochondrial remodeling. It is generally accepted that elongated mitochondria have higher membrane potential due to increased OXPHOS activity than fragmented mitochondria40. Based on this, it is logical to think that inducing elongation may be beneficial for cell function and survival41. However, there is emerging evidence that fragmentation may be beneficial for cell health and viability under some stress conditions42,43. The purpose of mitochondrial fragmentation is not entirely clear, but it has been shown that fragmentation could be a precursor to prepare cells to degrade damaged mitochondria and/or induce death in defective cells44. Further, mitochondrial fragmentation occurs during oxygen deprivation in many systems. During hypoxia, mitochondria also alter their oxidative phosphorylation activity to decrease oxygen consumption and ROS production44. Since fragmented mitochondria show decreased mitochondria potential, it is possible that fragmentation may be another mechanism for reducing oxygen consumption or decreasing ROS production. In fact, C.elegans with a mutation in mitochondria fusion genes showed rounded fragmented mitochondria, however, they were more resistant to oxidative stress compared to the wild type or the fission mutant43, suggesting that pre-fragmented mitochondria may have some protective properties under oxidative stress conditions. Recently, localized mitochondrial fission at the site of membrane damage was shown to signal for membrane repair42. It is thus plausible that fragmentation of mitochondria is not detrimental to the cell or indicative of irreversible injury but, instead, may reflect an adaptive cellular response to stress to aid in the survival of the cell45. Nevertheless, since the significance of this finding has not yet been sufficiently investigated, further cause-effect studies using clinical and experimental specimens will be required.
In conclusion, this study shows that the organ-protective effects of normothermic perfusion are associated with the maintenance of mitochondrial function. This concept had not been previously investigated in detail and could be of therapeutic interest. This work also demonstrates that AP39 activates mitochondrial function and clearly improves renal function after transplantation. Activation of mitochondrial function through the combination of AP39 and normothermic perfusion could represent a potential new therapeutic target for long-term renal preservation. We speculate that the clinical application of this novel normothermic perfusion technique with AP39 could increase the number of viable organs for transplantation. The potential for extended preservation time could help solve the geographic problem of organ transfer, resulting in reduced economic costs. Our plan is to move forward and study the effects of AP39 in combination with warm perfusion in the clinic.
Methods
Animals
All preclinical research protocols were performed using 12-week-old male Yorkshire pigs (29–31 kg). Pigs were housed and acclimated in the pens for one week prior to the study. All pigs were raised under conditions of 18–22 °C, 30–50% humidity with a 12 h light/dark cycle and had free access to water and food. All protocols were reviewed and approved by the Animal Care Committee, Toronto General Hospital and adhered to the Canadian Council on Animal Care Certificate of Good Animal Practices Guidelines.
Experimental design
The study design involved two major protocols. First, based on the hypothesis that warm perfusion would preserve mitochondrial function during organ preservation after warm ischemia, a comparison was made between SCS and NEVKP. The kidneys were resected after 60 min of warm ischemia and stored in SCS (n = 6) or NEVKP (n = 6) for 5 h. Subsequently, we performed removal of the contralateral kidney and autotransplantation and followed the pigs for 3 days. In addition, a ‘no warm ischemia’ group (no WI, n = 5), in which transplantation is performed promptly without ischemia after kidney retrieval, was added as a sham group. Second, we tested whether supplementation with the mitochondria-targeted hydrogen sulfide donor, AP39, during 5 h of NEVKP could further contribute to functional improvement after warm ischemia (NEVKP + AP39, n = 5). The warm ischemia time and follow-up period were the same as in the first protocol. Cases with surgical complications (anastomotic stenosis, abdominal wall scar hernia) were excluded.
Kidney procurement and autotransplant
The surgical procedure was performed as previously described20. After sedation, the pigs were intubated and ventilated. Anesthesia was maintained with fentanyl, isoflurane, and propofol. A central venous catheter was placed in the internal jugular vein for blood collection and drug administration. After a central abdominal incision was placed, the right kidney was dissected, and the renal artery and vein were clamped for 60 min to mimic DCD conditions and then excised. Grafts were immediately flushed with 300 ml of University of Wisconsin (UW) solution until no visible blood outflow was noticed from the vein. For the SCS group, kidneys filled with UW solution were kept on ice. For the NEVKP group, kidneys were connected to a perfusion machine. The abdomen was subsequently closed, and after recovery from anesthesia, the pigs were returned to the pen. At the end of organ preservation, the pigs were transported back to the operating room, anesthetized, and intubated. The median incision was reopened, and the left kidney was removed. After 5 h of preservation, grafts were flushed again with 300 ml of UW solution, and the renal artery and vein were anastomosed to the aorta and vena cava, respectively. Cold ischemia time was within 2–3 min. Anastomosis time was 25–30 min. After reperfusion, we performed end-to-end anastomosis of the ureters. A 6Fr 20 cm ureteral stent was placed inside to prevent stenosis.
Ex vivo kidney perfusion
NEVKP was performed as previously described3,9. The renal artery and vein were cannulated, the retrieved organ was placed in a chamber with a clean bag over it, and the renal artery and vein were connected to the circuit. A feeding tube was inserted into the ureter to allow the collection of urine. Perfusate was mainly based on leukocyte filtered erythrocytes collected from third-party pigs and 5% human albumin solution (Supplementary Table 1). The temperature of the perfusate was set at 38 °C to ensure that the temperature at the kidney surface was 36–37 °C using a heat exchanger. 95% oxygen and 5% carbon dioxide were supplied at a total rate of 2 L/min. Nonpulsatile perfusate was circulated at a constant rate by a centrifugal pump. Perfusion was pressure-controlled and initiated at 75 mmHg, reduced to 70 mmHg after 1 h, and maintained at 65 mmHg after 2 h. Physiological parameters were recorded every hour during the 5 h of perfusion. Ringer’s Lactate Solution was supplemented to compensate for urine produced during perfusion. Using a syringe pump, glucose (0.25 g/h), essential amino acids (0.1 g/h), and insulin (5 IU/h) were continuously administered into the venous reservoir, and verapamil (0.25 mg/h) was administered into the arterial line.
Administration of AP39
AP39, a mitochondria-targeted hydrogen sulfide donor, was administered to the NEVKP + AP group aiming to enhance mitochondrial function18. The compound was purchased from Cayman Chemical (Ann Arbor, MI, USA). AP39 was administered at a final concentration of 200 nM dissolved in dimethyl sulfoxide for UW solution and perfusate with reference to previous reports15,16. Immediately after the start of perfusion, AP39 was administered as a bolus in the perfusate.
Sample collection
Tissues were collected at baseline, before implantation after the 5-h storage period, 30-min post-reperfusion, and on day 3. After transplantation, renal biopsies were taken to include both the cortex and medulla for the histological evaluation or only the cortex for the other analyses. Blood was collected at baseline, pre-implantation, 1–3 h, and 1–3 day(s) post-transplant for blood gas analysis using RAPIDPoint 500 Systems (Siemens, Munich, Germany) and plasma biochemistry tests using Piccolo Xpress (Abaxis, CA, USA). The remaining plasma was stored. Perfusate was collected hourly from baseline. Urine during perfusion was obtained and stored every 2 h. Transplanted pigs were kept in a cage, and 24-hour urine was collected on the second to third postoperative day. Urine creatinine and sodium concentrations were measured using an Abbott Architect Chemistry Analyzer (Abbott Laboratories, IL, USA), and creatinine clearance (CCr) and fractional excretion of sodium (FeNa) were calculated. In one animal in the NEVKP group, the urine sample could not be collected postoperatively due to technical error and was excluded from the analysis.
Histology
Graft tissue at day 3, when the degree of ischemia-reperfusion injury reaches its maximum, was used for evaluation. Paraffin-embedded blocks were prepared from formalin-fixed tissue, and periodic acid-Schiff (PAS) staining was performed on 3-micron sections. Tubular injury and inflammation scores were determined in a blinded fashion by a nephropathologist (RJ), as previously described46. The tubular injury score is a 0–3 decision semiquantitative rating based on the following indices: loss of tubular brush border, tubular dilation, cast formation, and vacuolation of tubular epithelial cells. The inflammation score is a 0–3 grading scale based on the degree of inflammatory cell infiltration into the interstitium lesion47,48.
Assessment of metabolic function in the graft
Mitochondrial respiration and glycolysis were evaluated by measuring the oxygen consumption rate (OCR) and extracellular acidification rate (ECAR), respectively, in live cells extracted from the renal cortex using a Seahorse XFe96 analyzer (Agilent, CA, USA)49. Live cell suspensions were first obtained from the biopsies collected from the renal cortex. To maximize cell viability, this process was performed immediately after tissue collection. The tissue was mechanically dissected using a scalpel and placed in RPMI 1640 medium containing 0.1 mg/ml DNAase, 10 mg/mL collagenase, and 2990 units/mL neutral protease and agitated regularly at 37 °C for 30 min to obtain a single cell suspension21. Biopsy-derived cells were plated in growth medium at a density of 100,000 cells/well, in an XFe96 Cell Culture. Four to six replicates of each condition were studied21. After letting the cells adhere overnight at 37 °C, growth medium was replaced by Seahorse assay medium (Agilent) containing glutamine (2 mM), pyruvate (1 mM), and glucose (5.55 mM). Cells were then placed in a CO2-free incubator at 37 °C for 1 h. To induce metabolic stress, a series of agents were injected into Seahorse microplate wells: oligomycin (1 μM), FCCP (0.3 μM), 2-DG (50 mM), and Rot + AA (1 μM). The basal level, ATP-linked Respiration, Maximal Respiration Capacity, and Reserve Capacity were calculated from the average OCR curve of each condition.
Measurement of intracellular ATP levels
Intracellular ATP levels in biopsy-derived kidney cells were measured using the CellTiter-Glo® 2.0 Assay kit and rATP standard (Promega, WI, USA). Six replicates of 10,000 live cells/well were seeded in white 96-well plates and incubated overnight at 37 °C 5% CO2. Cells were washed with PBS, and Cell-Titer Glo reagent was added. The plates were placed in the incubator for 10 min to allow the reagent to react with the cells. Luminescence was measured using a microplate reader.
Immunofluorescence microscopy
Porcine renal biopsy samples were fixed in 4% paraformaldehyde and embedded in paraffin. 5 µm sections were obtained, de-paraffinized in 2 washes of xylene (10 min each), and rehydrated in 100%, 95%, and 80% ethanol (5 min each). Antigen retrieval was conducted by incubating the sections in sodium citrate buffer (pH 6.0) for 20 min in a pressure cooker. Samples were then exposed to a blocking solution containing 10% normal goat serum (Wisent) and 5% BSA (w/v) diluted in TBST (0.1% Tween 20 v/v) for 1 h at RT. Next, samples were probed with primary antibody against Hsp60 (Abcam, ab46798, 1:500) and subsequently with anti-rabbit Alexa 488 secondary antibody (Thermofisher, A-11008, 1:1000), both diluted in blocking solution. Nuclei were stained and visualized using DAPI. Fluorescent Mounting Medium (Agilent, S302380-2) was used to mount coverslips onto slides. Images were taken on a Zeiss LSM980 laser-scanning confocal microscope equipped with an Airyscan 2 module with a 63x oil immersion objective lens. Zen 3.3 (Zeiss) software was used for image acquisition.
Manual quantification of mitochondrial morphology
The immunofluorescence images of renal tissues stained for the mitochondrial protein HSP60 were used to score for mitochondrial morphology within the tubular epithelial cells. Tubules were graded from 1 to 5 based on the mitochondrial morphology of their epithelial cells, where a score of 1 was given to tubules that possessed epithelial cells that all had circular (fragmented) mitochondria and a 5 to those that only had elongated mitochondria (see Fig. 2). A tubule was rated a 3 if it contained medium-length mitochondria. A score of 2 was given to tubules that had a mixture of cells with fragmented or medium-length mitochondria and a score of 4 to tubules with medium to fully elongated mitochondria. No tubules contained a combination of cells with elongated mitochondria and cells with circular mitochondria. At least 25 tubules were scored over 3 different sections (images) for each kidney sample, and the average score was calculated. For each condition, 5 to 6 kidneys were analyzed.
Oxidative stress and inflammation assay
Measurement of malondialdehyde (MDA) is an established method for monitoring lipid peroxides and assessing oxidative stress50. We quantified MDA in perfusate and plasma using the thiobarbituric acid reactive substance (TBARS) assay kit (Cell Biolabs, CA, USA) according to the manufacturer’s instructions. Myeloperoxidase (MPO) is an inflammatory enzyme that causes both oxidative stress and inflammation in the pathogenesis of reperfusion injury51,52. Plasma MPO levels were measured using the Pig MPO / Myeloperoxidase ELISA Kit (LS Bio, WA, USA) kit according to the manufacturer’s instructions. Plasma was diluted 50-fold to bring its optical density measurements within the standard curve of this assay.
Circulating cell-free mitochondrial DNA
Circulating cell-free mitochondrial DNA (ccf-mtDNA) was determined in porcine plasma as previously reported33,53. DNA was extracted from plasma and purified using a QIAamp DNA Mini Kit spin column (Qiagen, Hilden, Germany). ccf-mtDNA was collected from a total of 150 μl plasma. The absolute levels of ccf-mtDNA were estimated by interpolation using a standard curve created by serial dilution of oligonucleotides of PCR products of known concentration (Integrated DNA Technologies). A quantitative polymerase chain reaction was performed using MT-ND4 primers to amplify the ND4 gene, which represents the mitochondrial genome.
Statistical analysis
Statistical analyses and data visualization were conducted with GraphPad Prism version 9. Continuous values were presented as mean and standard deviation (SD). Comparisons between two groups were made using the two-tailed unpaired t test, and comparisons between three groups were made using ANOVA and the Tukey post hoc test. P < 0.05 was considered statistically significant.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data are available in the main text or the supplementary materials. Source data are provided in this paper.
References
Wolfe, R. A. et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N. Engl. J. Med. 341, 1725–1730 (1999).
Hart, A. et al. OPTN/SRTR 2019 Annual data report: Kidney. Am. J. Transplant. 21, 21–137 (2021).
Urbanellis, P. et al. Normothermic ex vivo kidney perfusion improves early DCD graft function compared with hypothermic machine perfusion and static cold storage. Transplantation 104, 947–955 (2020).
Jassem, W. & Heaton, N. D. The role of mitochondria in ischemia/reperfusion injury in organ transplantation. Kidney Int. 66, 514–517 (2004).
Luongo, T. S. et al. The mitochondrial Na(+)/Ca(2+) exchanger is essential for Ca(2+) homeostasis and viability. Nature 545, 93–97 (2017).
Vercesi, A. E. et al. Mitochondrial calcium transport and the redox nature of the calcium-induced membrane permeability transition. Free Radic. Biol. Med. 129, 1–24 (2018).
Chouchani, E. T. et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 515, 431–435 (2014).
Chouchani, E. T. et al. A unifying mechanism for mitochondrial superoxide production during ischemia-reperfusion injury. Cell Metab. 23, 254–263 (2016).
Kaths JM, et al. Normothermic ex vivo kidney perfusion for the preservation of Kidney grafts prior to transplantation. J. Vis. Exp. 101, e52909 (2015).
Urbanellis, P. et al. Prolonged warm ischemia time leads to severe renal dysfunction of donation-after-cardiac death kidney grafts. Sci. Rep. 11, 17930 (2021).
McEvoy, C. M. et al. Normothermic ex-vivo kidney perfusion in a porcine auto-transplantation model preserves the expression of key mitochondrial proteins: An unbiased proteomics analysis. Mol. Cell. Proteomics 20, 100101 (2021).
Albert, C. et al. Monobody adapter for functional antibody display on nanoparticles for adaptable targeted delivery applications. Nat. Commun. 13, 5998 (2022).
DiRito, J. R. et al. Lysis of cold-storage-induced microvascular obstructions for ex vivo revitalization of marginal human Kidneys. Am.J. Transplant. 21, 161–173 (2021).
Lohmann, S. et al. Mesenchymal stromal cell treatment of donor Kidneys during ex vivo normothermic machine perfusion: A porcine renal autotransplantation study. Am. J. Transplant. 21, 2348–2359 (2021).
Nishime, K. et al. Preservation of pancreas in the University of Wisconsin solution supplemented with AP39 reduces reactive oxygen species production and improves islet graft function. Am. J. Transplant. 21, 2698–2708 (2021).
Szczesny, B. et al. AP39, a novel mitochondria-targeted hydrogen sulfide donor, stimulates cellular bioenergetics, exerts cytoprotective effects and protects against the loss of mitochondrial DNA integrity in oxidatively stressed endothelial cells in vitro. Nitric Oxide 41, 120–130 (2014).
Gerő, D. et al. The novel mitochondria-targeted hydrogen sulfide (H(2)S) donors AP123 and AP39 protect against hyperglycemic injury in microvascular endothelial cells in vitro. Pharmacol. Res. 113, 186–198 (2016).
Lobb, I. et al. Hydrogen sulfide protects renal grafts against prolonged cold ischemia-reperfusion injury via specific mitochondrial actions. Am. J. Transplant. 17, 341–352 (2017).
Dugbartey GJ, et al. Static cold storage with mitochondria-targeted hydrogen sulfide donor improves renal graft function in an ex vivo porcine model of controlled donation-after-cardiac-death Kidney transplantation. Int. J. Mol. Sci. 24, 14017 (2023).
Kaths, J. M. et al. Normothermic ex vivo Kidney perfusion for graft quality assessment prior to transplantation. Am. J. Transplant. 18, 580–589 (2018).
McEvoy, C. M. et al. Single-cell profiling of healthy human Kidney reveals features of sex-based transcriptional programs and tissue-specific immunity. Nat. Commun. 13, 7634 (2022).
Clotet-Freixas, S. et al. Sex differences in kidney metabolism may reflect sex-dependent outcomes in human diabetic Kidney disease. Sci. Transl. Med. 16, eabm2090 (2024).
Hosgood, S. A. et al. Normothermic machine perfusion versus static cold storage in donation after circulatory death Kidney transplantation: a randomized controlled trial. Nat. Med. 29, 1511–1519 (2023).
Juriasingani S, et al. Evaluating the effects of subnormothermic perfusion with AP39 in a novel blood-free model of ex vivo kidney preservation and reperfusion. Int. J. Mol. Sci. 22, 7180 (2021).
Juriasingani S, et al. Subnormothermic perfusion with H(2)S donor AP39 improves DCD porcine renal graft outcomes in an ex vivo model of kidney preservation and reperfusion. Biomolecules 11, 446 (2021).
Zhu, C. et al. Supplementing preservation solution with mitochondria-targeted H(2) S donor AP39 protects cardiac grafts from prolonged cold ischemia-reperfusion injury in heart transplantation. Am. J. Transplant. 19, 3139–3148 (2019).
Covarrubias, A. E. et al. AP39, a Modulator of mitochondrial bioenergetics, reduces antiangiogenic response and oxidative stress in hypoxia-exposed trophoblasts: Relevance for preeclampsia pathogenesis. Am. J. Pathol. 189, 104–114 (2019).
Marwah, M. K. et al. Transdermal delivery of mitochondrial-targeted hydrogen sulphide donor, AP39 protects against 6-hydroxydopamine-induced mitochondrial dysfunction. Eur. J. Pharm. Biopharm. 191, 166–174 (2023).
Goldaracena, N. et al. Inducing hepatitis C virus resistance after pig liver transplantation-A proof of concept of Liver graft modification using warm ex vivo perfusion. Am. J. Transplant. 17, 970–978 (2017).
Patel, K. et al. The effects of oxygenation on ex vivo Kidneys undergoing hypothermic machine perfusion. Transplantation 103, 314–322 (2019).
Schlegel, A. et al. Hypothermic oxygenated perfusion protects from mitochondrial injury before Liver transplantation. EBioMedicine 60, 103014 (2020).
Bhargava, P. & Schnellmann, R. G. Mitochondrial energetics in the Kidney. Nat. Rev. Nephrol. 13, 629–646 (2017).
Jeong, H. et al. Peripheral biomarkers of mitochondrial dysfunction in adolescents with bipolar disorder. J. Psychiatr. Res. 123, 187–193 (2020).
Iske, J. et al. Senolytics prevent mt-DNA-induced inflammation and promote the survival of aged organs following transplantation. Nat. Commun. 11, 4289 (2020).
Bon, D. et al. New strategies to optimize kidney recovery and preservation in transplantation. Nat. Rev. Nephrol. 8, 339–347 (2012).
Moers, C. et al. Machine perfusion or cold storage in deceased-donor kidney transplantation. N. Engl. J. Med. 360, 7–19 (2009).
Jochmans, I. et al. Oxygenated versus standard cold perfusion preservation in kidney transplantation (COMPARE): a randomised, double-blind, paired, phase 3 trial. Lancet 396, 1653–1662 (2020).
Husen, P. et al. Oxygenated end-hypothermic machine perfusion in expanded criteria donor Kidney transplant: A randomized clinical trial. JAMA Surg. 156, 517–525 (2021).
Vallant, N. et al. A comparison of pulsatile hypothermic and normothermic ex vivo machine perfusion in a porcine Kidney model. Transplantation 105, 1760–1770 (2020).
Yu, T., Robotham, J. L. & Yoon, Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc. Natl. Acad. Sci. USA 103, 2653–2658 (2006).
Madan, S., Uttekar, B., Chowdhary, S. & Rikhy, R. Mitochondria lead the way: Mitochondrial dynamics and function in cellular movements in development and disease. Front. Cell Dev. Biol. 9, 781933 (2021).
Horn A., Raavicharla S., Shah S., Cox D. & Jaiswal, JK. Mitochondrial fragmentation enables localized signaling required for cell repair. J. Cell Biol. 219, e201909154 (2020).
Machiela, E. et al. Disruption of mitochondrial dynamics increases stress resistance through activation of multiple stress response pathways. FASEB J. 34, 8475–8492 (2020).
Fuhrmann, D. C. & Brüne, B. Mitochondrial composition and function under the control of hypoxia. Redox Biol. 12, 208–215 (2017).
Chi, L. et al. Loss of functional peroxisomes leads to increased mitochondrial biogenesis and reduced autophagy that preserve mitochondrial function. Cell Mol. Life Sci. 80, 183 (2023).
Kaths, J. M. et al. Eight-hour continuous normothermic ex vivo Kidney perfusion is a safe preservation technique for Kidney transplantation: A new opportunity for the storage, assessment, and repair of Kidney grafts. Transplantation 100, 1862–1870 (2016).
Goujon, J. M. et al. Histological evaluation of proximal tubule cell injury in isolated perfused pig kidneys exposed to cold ischemia. J. Surg. Res. 82, 228–233 (1999).
Jayle, C. et al. Comparison of protective effects of trimetazidine against experimental warm ischemia of different durations: early and long-term effects in a pig kidney model. Am. J. Physiol. Renal Physiol. 292, F1082–F1093 (2007).
Clotet-Freixas, S. et al. Extracellular matrix injury of Kidney allografts in antibody-mediated rejection: A proteomics study. J. Am. Soc Nephrol 31, 2705–2724 (2020).
Agarwal, R., Vasavada, N., Sachs, N. G. & Chase, S. Oxidative stress and renal injury with intravenous iron in patients with chronic kidney disease. Kidney Int. 65, 2279–2289 (2004).
Matthijsen, R. A. et al. Myeloperoxidase is critically involved in the induction of organ damage after renal ischemia reperfusion. Am. J. Pathol. 171, 1743–1752 (2007).
Bolisetty, S. & Agarwal, A. Neutrophils in acute kidney injury: not neutral any more. Kidney Int. 75, 674–676 (2009).
Ali, A. et al. Static lung storage at 10 °C maintains mitochondrial health and preserves donor organ function. Sci. Transl. Med. 13, eabf7601 (2021).
Acknowledgements
The study was funded by the Canadian Donation and Transplantation Research Program, the Canadian Institutes of Health Research (L.A.R., Grant# PJT-169167), and the Canada Research Chairs Program (L.A.R.).
Author information
Authors and Affiliations
Contributions
Conceptualization: M.K., T.R., M.S., and L.R. Methodology: M.K., L.M., S.G., R.J., S.C.F., A.K., A.A., S.L., P.K., M.S., and L.R. Investigation: M.K., C.P., S.R., L.M., E.N., Y.N., T.G., B.A., S.G., T.T., and K.L. Visualization: M.K., S.C.F., and S.L. Funding acquisition: M.S. and L.R. Supervision: M.S. and L.R. Writing – original draft: M.K. Writing – review & editing: C.P., S.C.F., A.A., S.L., T.R., M.S., and L.R.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Tom Darius and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kawamura, M., Parmentier, C., Ray, S. et al. Normothermic ex vivo kidney perfusion preserves mitochondrial and graft function after warm ischemia and is further enhanced by AP39. Nat Commun 15, 8086 (2024). https://doi.org/10.1038/s41467-024-52140-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-52140-9
- Springer Nature Limited