Abstract
Polypharmacy, the concurrent use of multiple medicines, could increase the risk of kidney dysfunction among older adults because it likely burdens the aging kidneys to excrete multiple pharmaceutical ingredients and their metabolites. This study aimed to examine the relation between polypharmacy and kidney dysfunction among older patients. A nested case-control study was conducted using the National Health Insurance Service – Senior Cohort (NHIS-SC, 2009–2013), representative of the Korean senior population. It consisted of all health insurance claims linked to records of mandatory health examination. Kidney dysfunction was defined as having an eGFR lower than 60, with a decline rate of 10% or more compared to the baseline eGFR. Polypharmacy was defined based on daily counts of pharmaceutical ingredients during one year prior to the case’s event date. It was classified into polypharmacy (five to 10 ingredients) and excessive polypharmacy (10 or more ingredients). After matching case and control groups based on a range of potential confounders, conditional logistic regression was performed incorporating adjustments on disease-specific, medication-specific, and lifestyle-related risk factors. The matching resulted in 14,577 pairs of cases and controls. Exposure to polypharmacy was significantly associated with increase in the risk of kidney dysfunction; i.e., crude model (polypharmacy: OR = 1.572, 95% CI = 1.492–1.656; excessive polypharmacy: OR = 2.069, 95% CI = 1.876–2.283) and risk adjustment model (polypharmacy: OR = 1.213, 95% CI = 1.139–1.292; excessive polypharmacy: OR = 1.461, 95% CI = 1.303–1.639). The significant associations were robust across different definitions of kidney dysfunction. These findings inform healthcare providers and policy makers of the importance of polypharmacy prevention to protect older adults from kidney dysfunction.
Similar content being viewed by others
Introduction
The issue of polypharmacy, the concomitant use of multiple medicines, has been around for quite some time. While a consistent definition has yet to evolve1,2,3, its prevalence has been increasing rapidly over time4,5,6. Especially among older adults, it is quite prevalent not only in Korea7,8,9 but also in other countries5,6,10, as aging increases disease morbidity, which makes polypharmacy more prevalent among older adults11,12,13. Older adults, thus, would be at greater risk of adverse drug reactions, not only from polypharmacy but also from their weakening physiological functions, such as those of the kidneys1,14,15,16,17,18.
Polypharmacy could seriously damage the kidney because the former likely burdens the latter to excrete a wide range of drugs and their metabolites18,19. While many studies have examined the negative effect of polypharmacy on various health outcomes, such as falls20,21,22,23, fractures24, delirium25, dementia8,26, and Parkinsonism27 among older adults, only a few have investigated the association between polypharmacy and kidney function, out of which two examined the risk of acute renal failure28,29 and two examined chronic kidney diseases (CKD)30,31. However, the two studies on CKD reported inconsistent results since they used different approaches to risk adjustment for different operational definitions of CKD. Furthermore, the study results were susceptible to bias from cross-sectional data. Therefore, there is a critical need to generate more scientific evidence on the temporal relation between exposure to polypharmacy and risk of kidney dysfunction.
This study aimed to document the temporal relationship between polypharmacy and kidney dysfunction among old patients in Korea, the population of which is aging at an unprecedentedly rapid rate. Furthermore, the study further aimed to determine the significant association after risk adjustments on disease-specific, medication-specific, and lifestyle-related risk factors.
Methods
Study population
We used the population-based cohort of National Health Insurance Service–Senior Cohort (NHIS-SC) data established by the National Health Insurance Service (NHIS), the single payer of South Korea. Accordingly, the NHIS collects information on eligibility, health insurance claims, and lab values from mandatory periodic health check-ups for selected populations. During the health check-ups, information on lifestyles is collected through interviews. Moreover, the NHIS-SC was constructed based on a 10% random sample (n = 558,147) of the population aged 60 or over as of December 2002. The sample cohort was tracked for 11 years until 2013. Consequently, this study was approved by the Seoul National University Institutional Review Board (IRB No. E1801/001-001). Additionally, obtaining informed consent from the study population was waived because this study involves an analysis of existing data. All methods were performed in accordance with the approved guideline and regulation. Furthermore, our study used the NHIS-SC data from 2009 to 2013 since the key variable of serum creatinine (SCr), an indicator of kidney function, became available in 2009.
In this study, patients aged 65–84 who had a normal range of serum creatinine (SCr of 0.5–1.5) and a normal value of estimated glomerular filtration rate (eGFR of 60 or higher) at a baseline health check-up were included. In Korea, older adults are typically defined as those with ages 65 years or older to qualify for a range of senior-specific benefits. As the NHIS cohort got older, the age group of 60–64 no longer existed during the study period. Thus, the youngest age group in the study was between the ages of 65–69. The oldest age group (85 or older) was excluded because they are known to be quite different from the younger groups. Furthermore, patients who had not had their next health check-up within three years from the baseline date (n = 87,147) were excluded. Out of the aforementioned, patients with outlier values of SCr at the last check-up as well as patients with a history of renal disease prior to the case’s event date were excluded.
Study design
A nested case-control study was designed, with cases of patients who had developed kidney dysfunction during the follow-up health check-up some time in 2013. Kidney dysfunction was defined as a follow-up eGFR lower than 60 with a decline rate of 10% or more from the initial eGFR. Controls were those patients without renal disease diagnoses who had normal eGFR at initial and follow-up check-ups. After the exclusion of patients without health check-up information, cases (n = 14,657) and controls (n = 67,278) were matched 1:1 based on a wide range of covariates, excluding risk factors. The matching was exact in the year of baseline examination, gender, age, chronic kidney disease stage at baseline, and follow-up duration, but the nearest neighbor in resident area, medical insurance coverage, and income level. After matching, the numbers of the final sample were 14,577 each for case and control group (Refer to Fig. 1).
Kidney dysfunction
Glomerular filtration rate (GFR) is regarded as the best indicator for kidney function, and is the reference criterion for classification of kidney disease established by the National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative32,33. The cut-off point of 60 for GFR indicates chronic kidney disease32. Kidney dysfunction is operationally defined as the status of having GFR lower than 60 ml/min/1.73 m2, while having a decline rate of 10% or more from baseline GFR. The decline rate of 10% or more was to exclude patients with little difference in GFR between two health check-ups. In addition, the literature reports that annual decline rate can predict kidney disease progress34. Accordingly, annual eGFR decline rates of 3, 4, and 5 ml/min/1.73 m2/year were also used when defining kidney dysfunction in separate subgroup analyses.
It is common to use the eGFR from SCr concentration35. This study used the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation to estimate GFR. It is preferred for estimating GFR in adults because of its accuracy36,37.
Polypharmacy
Polypharmacy was computed based on the daily counts of pharmaceutical ingredients of all prescription drugs taken during 1 year prior to the case’s event date and subsequently classified into non-polypharmacy (daily use of less than five), polypharmacy (daily use of five to less than 10), and excessive polypharmacy (daily use of 10 or more). Additionally, the prescription drugs included all drugs from dental to medical care as well as inpatient and outpatient care. Furthermore, they included fixed-dose combination drugs. However, when counting the number of pharmaceutical ingredients, digestives and fillers were excluded along with OTC drugs, traditional medicines, and drugs absent in the NHIS formulary. Despite the exclusion, underestimation of polypharmacy is not much of an issue because the NHIS formulary is comprehensive; i.e., it only excludes some of the new drugs that do not meet the cost-effectiveness criteria.
where: Ki is the number of active pharmaceutical ingredients of a prescription drug i.
Risk adjustment
A wide range of well-known kidney dysfunction risk factors were identified from the literature and classified into disease-specific, medication-specific, and lifestyle-related risk factors. The disease-specific risk factors included were hypertension (HTN)38,39,40,41,42,43,44,45,46,47,48,49, diabetes mellitus (DM)38,39,40,41,42,43,46,50, congestive heart failure (CHF)42,51,52,53, ischemic heart disease (IHD)42, arrhythmia42, gout54, hypercholesterolemia (Hyper-TC)41,42,55, hypertriglyceridemia (Hyper-TG)41,42,43,55,56, lower high density lipoprotein cholesterol (Lower-HDL-C)41,42,43,55,57, higher low density lipoprotein cholesterol (Higher-LDL-C)42,55,57, and obesity38,40,50,58,59,60,61,62. The medication-specific risk factors were angiotensin-converting-enzyme inhibitors (ACEIs)63,64,65,66, angiotensin II receptor blockers (ARBs)63,64,65,66,67, metformin68, statins69, non-steroidal anti-inflammatory drugs (NSAIDs)50,63,64,65,66,70,71, proton pump inhibitors (PPIs)64,65,72,73,74, and allopurinol63,65,75. Finally, the lifestyle-related risk factors were smoking38,39,41,42,43,50,59,76,77,78,79, alcohol consumption41,43,76,80,81, and physical activity43,59,82,83,84,85.
Disease-specific risk factors were mainly determined based on the diagnosis code, the 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10), whether people had had the relevant disease code or not from the baseline to the case’s event date: HTN (I10-I15); DM (E10-E14); CHF (I50); IHD (I20-I25); arrhythmia (I49); and gout (M10). Obesity was based on Body Mass Index (BMI), and classified into underweight (less than 18.5), normal weight (18.5–22.9), overweight (23.0–24.9), and obese (more than 25), according to the Asia Pacific regional guidelines of the World Health Organization and International Obesity Task Force. All definitions related to lipid status were based on fasting lipid measure. Hyper-TC was defined as total cholesterol level more than 240 mg/dL; Lower HDL-C as HDL-C ≤ 40 mg/dL; Higher LDL-C as LDL-C ≥ 140 mg/dL; and Hyper-TG as triglycerides ≥ 150 mg/dL.
Exposure to each medication risk factor was defined depending on types of medication. First, exposures to chronic medicines (ACEIs, ARBs, Metformin, Statins) were defined based on a PDC (Proportion of Days Covered) of 50% or higher during one year prior to the case’s event date. Second, exposures to NSAIDs and PPIs were defined the same way as above using 90 days instead of one year. Third, exposure to allopurinol was defined based on a record of prescription two weeks prior to the event date.
Subsequently, smoking status was classified as smoker or non-smoker based on consecutive non-smoker responses at baseline as well as follow-up health check-ups to a question about whether a patient had smoked more than 5 boxes or 100 cigarettes in their lifespan. On the other hand, alcohol consumption status was defined based on the mean number of drinking days per week (non-drinker: 0–1 day per week) for the responses at baseline and follow-up. The exercise status was also defined based on the mean number of exercise days per week for the responses at baseline and follow-up, in which each patient performed moderate physical activity for at least 30 minutes (non-exerciser: 0–1 day per week).
Sample size and power computation
This study is a retrospective case-control study based on a 10% random sample cohort of Korean seniors. Thus, we took the approach of computing power from the number of patients who met our inclusion/exclusion criteria rather than figuring out the sample size that achieves, at least, the power level of 80% given an effect size of OR = 1.2 from a logistic regression model involving 15 covariates. Consequently, the computed power well exceeded 80% given the number of case-control pairs of 14,577.
Statistical analysis
Baseline characteristics of the cases and controls were compared using a t-test for continuous variables and chi-square test for categorical variables. Conditional logistic regression was used to calculate the odds ratio (OR) and its 95% confidence intervals (CIs). Risk factors were adjusted step by step: First, the disease and lifestyle risk factors were included in the adjusted model. Second, only exposure to medication-related factors was considered. Third, as the final model in this study, all risk factors were included. Subgroup analyses were conducted incorporating different definitions of kidney dysfunction into the final model.
Results
Description of study sample
From the cases (n = 14,657) and controls (n = 67,278) that met the inclusion/exclusion criteria in the cohort, the matching resulted in 14,577 pairs of cases and controls. Before matching, cases and controls were all different in each matching variable, except for insurance type. However, after matching, cases and controls were well-balanced with no practical differences. In fact, cases and controls were exactly the same, except for income and residential areas (Refer to details in Supplementary Table S1).
This study did not match cases and controls on risk factors, but instead included those risk factors later for risk adjustment models. As seen in the study sample description, exposures to polypharmacy and excessive polypharmacy were higher among cases than among controls: 33.15% vs. 25.23% respectively for polypharmacy and 8.49% vs. 4.98% for excessive polypharmacy. Exposures to all other risk factors were also significantly higher among cases than controls except for Hyper-TC, Higher-LDL-C, smoking, and physical activity (Table 1).
Risk of kidney dysfunction from polypharmacy
Compared to controls without kidney dysfunction, cases with kidney dysfunction were significantly associated with higher odds of exposure to polypharmacy, not only in the crude conditional logistic regression model (polypharmacy: Crude OR = 1.572, 95% CI = 1.492–1.656; excessive polypharmacy: Crude OR = 2.069, 95% CI = 1.876–2.283) but also in each risk-adjusted model. In Model 1, adjusted for the disease-specific and lifestyle-related risk factors, Adj. OR was 1.287 (95% CI = 1.212–1.366) for polypharmacy and 1.603 (95% CI = 1.439–1.787) for excessive polypharmacy. The significant associations were also present for the other two risk adjustment models (Table 2).
Significantly positive associations between exposure to each risk factor and kidney dysfunction were observed for all disease-related risk factors except for IHD and arrhythmia; in Model 3 that was adjusted for all risk factors, IHD and arrhythmia respectively had Adj. OR of 1.066 (95% CI = 0.984–1.154) and of 1.110 (95% CI = 0.910–1.354). As for the medication-specific risk factors, only two medications had significant associations with kidney dysfunction; Adj. OR for ACEI was 1.348 (95% CI: 1.183–1.536), for ARB 1.449 (95% CI: 1.361–1.543). Metformin and allopurinol had a significant association in Model 2, but no longer when all other risk factors were adjusted.
Finally, for the lifestyle-related risk factors, overweight people were more likely to have kidney dysfunction (normal weight: Adj. OR = 1.216, 95% CI: 1.060–1.396; overweight: Adj. OR = 1.281, 95% CI: 1.112–1.475; obese: Adj. OR = 1.377, 95% CI: 1.197–1.584, ref = underweight). For lipid measures, Hyper-TG and Lower-HDL-C were significantly associated with kidney dysfunction (Hyper-TG: Adj. OR = 1.171, 95% CI: 1.111–1.235; Lower-HDL-C: Adj. OR = 1.171, 95% CI: 1.091–1.257; Higher-LDL-C: Adj. OR = 1.000, 95% CI: 1.000–1.001). As for smoking, drinking, and physical activity, only smoking was significantly associated with kidney dysfunction (Adj. OR: 1.075, 95% CI: 1.001–1.154).
Subgroup analyses for different definitions of kidney dysfunction
Study findings would be robust when they were consistent across different definitions of kidney dysfunction. We generated several subgroups of cases and controls using different definitions. In the original model, we defined kidney dysfunction using eGFR lower than 60 with a decline of 10% or more compared to baseline eGFR. We constructed subgroups using annual rather than total decline rate. Sub-case A was defined as patients with next eGFR less than 60 with an annual decline rate of 3 ml/min/1.73 m2 or more. The matched controls were sub-control A (each N = 14,329). Sub-case/control B (each N = 12,950) and sub-case/control C (each N = 11,746) were defined using the annual decline rates of 4 ml/min/1.73 m2 or more and 5 ml/min/1.73 m2 or more respectively.
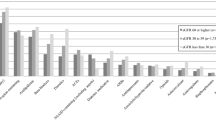
Across different subgroups, polypharmacy and excessive polypharmacy were significantly associated with kidney dysfunction: respective crude ORs were 1.572 and 2.069 for subgroup A; 1.574 and 2.095 for subgroup B; and 1.624 and 2.216 for subgroup C (Fig. 2 and Supplementary Table S2). In addition, all the adjusted ORs of polypharmacy and excessive polypharmacy were significant: 1.220 and 1.468 for subgroup A; 1.232 and 1.525 for subgroup B; and 1.240 and 1.551 for subgroup C, respectively. All other risk factors had the same significant association with kidney dysfunction to the original analysis, except that the use of PPI was also significantly associated with kidney dysfunction, with Adj. OR of 1.212 (95% CI: 1.004–1.251) for subgroup A, 1.140 (95% CI: 1.016–1.280) for subgroup B, and 1.160 (95% CI: 1.027–1.311) for subgroup C. (Fig. 2. Refer to details in Supplementary Table S2)
Odd ratios of exposures to each risk factor and kidney dysfunction for different Operationalisations of kidney dysfunction. (Left: Results from the main analysis; Right: Results from the subgroup analyses: red dot for subgroup A; black diamond for subgroup B; and blue dot for subgroup C). PP: Polypharmacy, use of five to 10 drugs; E– PP: Excessive polypharmacy, use of 10 or more drugs; HTN: hypertension; DM: diabetes mellitus; CHF: congestive heart failure; IHD: ischemic heart disease; Underweight: BMI < 18.5; Normal: BMI < 23; Overweight: BMI < 25; Obese: BMI ≥ 25; ACEIs: Angiotensin-Converting-Enzyme Inhibitors; ARBs: Angiotensin II Receptor Blockers; NSAIDs: Non-Steroidal Anti-Inflammatory Drugs; PPIs: Proton Pump Inhibitors.
Discussion
Compared to controls who had kept their normal kidney function since the baseline examination, the matched cases with kidney dysfunction had a higher exposure to polypharmacy as well as to excessive polypharmacy (polypharmacy: 33.15% vs. 25.23: excessive polypharmacy: 8.49% vs. 4.98%). The higher exposure to polypharmacy among cases of kidney dysfunction than among controls with normal kidney function was also observed in the crude conditional logistic regression (polypharmacy: OR = 1.572, 95%CI = 1.492–1.656; excessive polypharmacy: OR = 2.069, 95% CI = 1.876–2.283), as well as in the risk adjusted model (polypharmacy: Adj. OR = 1.213, 95% CI = 1.139–1.292; excessive polypharmacy: Adj.OR = 1.461, 95% CI = 1.303–1.639). These results are consistent with the findings of three previous studies28,29,30. Konig et al.30 reported a crude OR of 2.07 (95% CI: 1.54–2.74) and an adjusted OR of 1.54 (95% CI: 1.14–2.08) based on the Berlin Aging Study II (BASE –II) cohort. The other two studies also reported a significant association between polypharmacy and kidney dysfunction, while using different concepts of polypharmacy (duration of polypharmacy, polypharmacy of cardiovascular medicines) and focusing on kidney dysfunction of acute renal failure and injury. However, our study results were not consistent with Sutaria et al.’s study31. Their study found a negative effect of polypharmacy on CKD based on an unadjusted model, but a protective, though not statistically significant, effect of polypharmacy on CKD when adjusting for age, cardiovascular disease, and diabetes mellitus. There are some differences between the studies. Firstly, their study is a cross-sectional, while ours is a nested case control. Their study did not control for the other important risk factors such as those lifestyle-related and medication-specific. Furthermore, the effect of polypharmacy might be masked by the large variations of age effect, which was matched between cases and controls in our study. In other words, our study focused on risk factors for kidney dysfunction after exactly matching other covariates between cases and controls.
Moreover, this study confirmed that most of the risk factors known to impair kidney function are associated with kidney dysfunction (Table 2). However, interestingly, ACEIs/ARBs50,63,64,65,66,67,70 were found to be significantly associated with kidney dysfunction despite the fact that they are recommended as first-line hypertension therapy for patients with a compelling condition of CKD86,87,88,89 due to their reno-protective effect. Although it is initially tempting to claim that the adverse associations just reflect the fact that patients who take ACEI/ARB are likely to have had kidney dysfunction, the associations from our case-control study are from a temporal sequence where exposure to ACEI/ARB precedes the outcome occurrence of kidney dysfunction. On the other hand, it is plausible that patients who take ACEI/ARB are likely to have had conditions of HTN/DM and are thus more likely to develop kidney dysfunction, not from the medications but from the diseases. Recognizing the potential confounding effects of HTN/DM, our study did control for presence of the diseases in the risk adjustment model to separate the disease- and medication-specific effects. As the result, our study found that risk of kidney dysfunction was associated not only with presence of HTN/DM but also with exposure to ACEI/ARB. Our study is not the first to report that use of ACEI/ARB may not always be reno-protective, especially in real world settings.90,91,92
The other risk factors known to damage kidneys but not found significant in this study are LDL, drinking, Proton Pump Inhibitors (PPIs), Metformin, Statin, and NSAIDs. However, plenty of evidence supports our study. Moreover, LDL is the least likely risk factor for kidney dysfunction among cholesterol types50,57. In addition, drinking, especially moderate alcohol consumption, has no adverse effect on kidney function93. Accordingly, PPIs have the weakest level of evidence for being risk factors for kidney dysfunction74. In our study, PPIs were not significantly associated with kidney dysfunction in the main analysis but were significantly associated in the subgroup analysis. Furthermore, some evidence in the literature suggests that Metformin94,95 and statin96 are not associated with kidney function. Despite the widely-known adverse association of NSAIDs with kidney dysfunction50,63,64,65,66,70, our study reports no such association. Therefore, perhaps the contradictory findings may have resulted from two different study populations. While our study findings come from patients with normal kidney functioning, the findings in the literature are based on patients with kidney dysfunction. Alternatively, it is likely that the duration of NSAID exposure could affect the occurrence of reno-toxicity. Our study did not stratify NSAID exposure into long-term vs. short-term. Instead, we dichotomized NSAID exposure based on a proportion of days covered (PDC) of 50% or higher for 90 days prior to the kidney dysfunction. While our study is not the first to report the contradictory finding97,98 future studies need to examine whether the risk of reno-toxicity depends on long-term vs. short-term exposure to NSAID. Additionally, allopurinol is known to be reno-protective for hyperuricemia75, but can also be reno-toxic for interstitial nephritis50,63,65,66,70. In our study, allopurinol had an adverse effect on the kidneys when not adjusted for gout but lacked an adverse effect when adjusted for gout. As a result, more studies are required in order to understand this phenomenon as reflected in the comments of Stamp et al.99.
A key strength of this study is the use of linked data of health check-up information with prescription claims, which was constructed as cohort data under the universal health coverage system in Korea. Although various studies with slightly different operational definitions have examined the association between polypharmacy and kidney disease, they all had limitations, in that they didn’t consider obesity and smoking, which are important risk factors for kidney function28,29,31, or were biased due to using a self-reported questionnaire30. Consequently, considering the information available from our data, the risk factors for kidney disease considered and reflected in the study model are more comprehensive than in other studies. Moreover, despite adjusting for various covariates and/or risk factors, we have identified the associative risk of polypharmacy for kidney dysfunction. In addition, factors identified associated with kidney dysfunction, apart from exposure to polypharmacy, are hypertension, diabetes, congestive heart failure, gout, obesity, hyper-TG, lower-HDL-C, smoking, and use of ACEIs/ARBs (as well as the use of PPIs in a more rigorous sub-group). Despite past studies having identified associative risks for kidney disease, medicines taken by patients, which are one of the factors that burden kidney function, were not considered in those studies. These medicines not only include polypharmacy-related exposure, but also other medicines known for being associated with the kidneys, such as ACEIs/ARBs, Metformin, Statins, NSAIDs, PPIs, and Allopurinol.
However, there are limitations in our study. Firstly, other medicines with potential nephrotoxicity were not considered in the statistical model due to the limited number of their takers and little difference between case and control groups resulting from basic statistical analysis. Such drugs include osmotic agents, contrast, methotrexate, calcineurin inhibitors, and certain antibiotics (refer to Supplementary Table S3). Secondly, only prescribed medicines in the NHIS formulary are included while accounting for polypharmacy. Consequently, not considering the use of non-formulary drugs, drug samples, over-the-counter (OTC) drugs, supplements, and vitamins leads to underestimating polypharmacy and a restricted interpretation of the association between polypharmacy and kidney dysfunction. Thirdly, confounders might be associated with kidney function despite adjusting more covariates than before. For example, parenteral medicines and other diseases were not accounted for.
In conclusion, this study found that exposure to polypharmacy was significantly associated with increases in the risk of kidney dysfunction among older patients. There was a temporal association for different risk adjustments as well as for different subgroup analyses. These findings inform healthcare providers and policy makers of the importance of polypharmacy prevention to protect older adults from kidney dysfunction.
Data Availability
The dataset generated and analysed in this study are available from the corresponding author on reasonable requests.
References
Gnjidic, D. et al. Polypharmacy cutoff and outcomes: five or more medicines were used to identify community-dwelling older men at risk of different adverse outcomes. J Clin Epidemiol 65, 989–995, https://doi.org/10.1016/j.jclinepi.2012.02.018 (2012).
Mortazavi, S. S. et al. Defining polypharmacy in the elderly: a systematic review protocol. BMJ Open 6, e010989, https://doi.org/10.1136/bmjopen-2015-010989 (2016).
Masnoon, N., Shakib, S., Kalisch-Ellett, L. & Caughey, G. E. What is polypharmacy? A systematic review of definitions. BMC Geriatr 17, 230, https://doi.org/10.1186/s12877-017-0621-2 (2017).
Guthrie, B., Makubate, B., Hernandez-Santiago, V. & Dreischulte, T. The rising tide of polypharmacy and drug-drug interactions: population database analysis 1995-2010. BMC Med 13, 74, https://doi.org/10.1186/s12916-015-0322-7 (2015).
Moriarty, F., Hardy, C., Bennett, K., Smith, S. M. & Fahey, T. Trends and interaction of polypharmacy and potentially inappropriate prescribing in primary care over 15 years in Ireland: a repeated cross-sectional study. BMJ Open 5, e008656, https://doi.org/10.1136/bmjopen-2015-008656 (2015).
Charlesworth, C. J., Smit, E., Lee, D. S., Alramadhan, F. & Odden, M. C. Polypharmacy among adults aged 65 years and older in the United States: 1988-2010. J Gerontol A Biol Sci Med Sci 70, 989–995, https://doi.org/10.1093/gerona/glv013 (2015).
Park, S. et al. Association between screening and the thyroid cancer “epidemic” in South Korea: evidence from a nationwide study. BMJ 355, i5745, https://doi.org/10.1136/bmj.i5745 (2016).
Park, H. Y., Park, J. W., Song, H. J., Sohn, H. S. & Kwon, J. W. The association between polypharmacy and dementia: a nested case-control study based on a 12-year longitudinal cohort database in South Korea. PLoS One 12, e0169463, https://doi.org/10.1371/journal.pone.0169463 (2017).
Kim, H. A., Shin, J. Y., Kim, M. H. & BJ, P. Prevalence and predictors of polypharmacy among Korean elderly. PLoS One 9, 10.1371/ (2014).
Castioni, J., Marques-Vidal, P., Abolhassani, N., Vollenweider, P. & Waeber, G. Prevalence and determinants of polypharmacy in Switzerland: data from the CoLaus study. BMC Health Serv Res 17, 840, https://doi.org/10.1186/s12913-017-2793-z (2017).
Veehof, L., Stewart, R., Haaijer-Ruskamp, F. & BM, J. The development of polypharmacy. A longitudinal study. Fam Pract 17, 261–267 (2000).
Molokhia, M. & Majeed, A. Current and future perspectives on the management of polypharmacy. BMC Fam Pract 18, 70, https://doi.org/10.1186/s12875-017-0642-0 (2017).
Hilmer, S. N., McLachlan, A. J. & Le Couteur, D. G. Clinical pharmacology in the geriatric patient. Fundam Clin Pharmacol 21, 217–230, https://doi.org/10.1111/j.1472-8206.2007.00473.x (2007).
McLean, A. J. & Le Couteur, D. G. Aging biology and geriatric clinical pharmacology. Pharmacol Rev 56, 163–184, https://doi.org/10.1124/pr.56.2.4 (2004).
Le Couteur, D. G., McLachlan, A. J. & de Cabo, R. Aging, drugs, and drug metabolism. J Gerontol A Biol Sci Med Sci 67, 137–139, https://doi.org/10.1093/gerona/glr084 (2012).
Bushardt, R. L., Massey, E. B., Simpson, T. W., Ariail, J. C. & Simpson, K. N. Polypharmacy: Misleading, but manageable. Clin Interv Aging 3, 383–389 (2008).
Musso, C. G., Belloso, W. H., Scibona, P., Bellizzi, V. & Macias Nunez, J. F. Impact of renal aging on drug therapy. Postgrad Med 127, 623–629, https://doi.org/10.1080/00325481.2015.1063957 (2015).
Modig, S., Lannering, C., Ostgren, C. J., Molstad, S. & Midlov, P. The assessment of renal function in relation to the use of drugs in elderly in nursing homes; a cohort study. BMC Geriatr 11, 1, https://doi.org/10.1186/1471-2318-11-1 (2011).
Turnheim, K. Drug therapy in the elderly. Exp Gerontol 39, 1731–1738, https://doi.org/10.1016/j.exger.2004.05.011 (2004).
Baranzini, F. et al. Fall-related injuries in a nursing home setting: is polypharmacy a risk factor? BMC Health Serv Res 9, 228, https://doi.org/10.1186/1472-6963-9-228 (2009).
Kojima, T. et al. Association of polypharmacy with fall risk among geriatric outpatients. Geriatr Gerontol Int 11, 438–444, https://doi.org/10.1111/j.1447-0594.2011.00703.x (2011).
Pan, H. H., Li, C. Y., Chen, T. J., Su, T. P. & Wang, K. Y. Association of polypharmacy with fall-related fractures in older Taiwanese people: age- and gender-specific analyses. BMJ Open 4, e004428, https://doi.org/10.1136/bmjopen-2013-004428 (2014).
Dhalwani, N. N. et al. Association between polypharmacy and falls in older adults: a longitudinal study from England. BMJ Open. 7, 1–8, https://doi.org/10.1136/bmjopen-2017-016358 (2017).
Lai, S. W. et al. Polypharmacy correlates with increased risk for hip fracture in the elderly: a population-based study. Medicine (Baltimore) 89, 295–299, https://doi.org/10.1097/MD.0b013e3181f15efc (2010).
Hein, C. et al. Impact of polypharmacy on occurrence of delirium in elderly emergency patients. J Am Med Dir Assoc 15, 850 e811–855, https://doi.org/10.1016/j.jamda.2014.08.012 (2014).
Lai, S. W. et al. Association between polypharmacy and dementia in older people: a population-based case-control study in Taiwan. Geriatr Gerontol Int 12, 491–498, https://doi.org/10.1111/j.1447-0594.2011.00800.x (2012).
Park, H. Y., Park, J. W., Sohn, H. S. & Kwon, J. W. Association of parkinsonism or parkinson disease with polypharmacy in the year preceding diagnosis: a nested case–control study in South Korea. Drug Saf 40, 1109–1118, https://doi.org/10.1007/s40264-017-0559-5) (2017).
Chang, Y. P., Huang, S. K., Tao, P. & CW, C. A population-based study on the association between acute renal failure (ARF) and the duration of polypharmacy. BMC Nephrol 13 (2012).
Chao, C. T. et al. Cumulative cardiovascular polypharmacy is associated with the risk of acute kidney injury in elderly patients. Medicine (Baltimore) 94, e1251, https://doi.org/10.1097/MD.0000000000001251 (2015).
König, M., Gollasch, M., Demuth, I. & Steinhagen-Thiessen, E. Prevalence of impaired kidney function in the German elderly: results from the Berlin Aging Study II (BASE-II). Gerontology 63, 201–209, https://doi.org/10.1159/000454831) (2017).
Sutaria A, Liu L & Z., A. Multiple medication (polypharmacy) and chronic kidney disease in patients aged 60 and older: a pharmacoepidemiologic perspective. Ther Adv Cardiovasc Dis 10, 242–250, 10.1177/ (2016).
Levey, A. S. et al. National Kidney Foudation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med 139, 137–147 (2003).
Webster, A. C., Nagler, E. V., Morton, R. L. & Masson, P. Chronic Kidney Disease. The Lancet 389, 1238–1252, https://doi.org/10.1016/s0140-6736(16)32064-5 (2017).
Tsai, C. W., Ting, I. W., Yeh, H. C. & Kuo, C. C. Longitudinal change in estimated GFR among CKD patients: A 10-year follow-up study of an integrated kidney disease care program in Taiwan. PLoS One 12, e0173843, https://doi.org/10.1371/journal.pone.0173843 (2017).
Pottel, H. et al. An estimated glomerular filtration rate equation for the full age spectrum. Nephrol Dial Transplant 31, 798–806, https://doi.org/10.1093/ndt/gfv454 (2016).
Matsushita, K. et al. Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA 307, 1941–1951 (2012).
Levey, A. S., Becker, C. & Inker, L. A. Glomerular filtration rate and albuminuria for detection and staging of acute and chronic kidney disease in adults: a systematic review. JAMA 313, 837–846, https://doi.org/10.1001/jama.2015.0602 (2015).
Fox, C. S. et al. Predictors of new-onset kidney disease in a community-based population. JAMA 291, 844–850 (2004).
Haroun, M. K. Risk factors for chronic kidney disease: a prospective study of 23,534 men and women in Washington county, Maryland. J Am Soc Nephrol 14, 2934–2941, https://doi.org/10.1097/01.asn.0000095249.99803.85 (2003).
Jha, V. et al. Chronic kidney disease: global dimension and perspectives. The Lancet 382, 260–272, https://doi.org/10.1016/s0140-6736(13)60687-x (2013).
Yamagata, K. et al. Risk factors for chronic kidney disease in a community-based population: a 10-year follow-up study. Kidney Int 71, 159–166, https://doi.org/10.1038/sj.ki.5002017 (2007).
Chen, S. C. et al. Association of dyslipidemia with renal outcomes in chronic kidney disease. PLoS One 8, e55643, https://doi.org/10.1371/journal.pone.0055643 (2013).
Kang, H. T. et al. Association between the ratio of triglycerides to high-density lipoprotein cholesterol and chronic kidney disease in Korean adults: the 2005 Korean National Health and Nutrition Examination Survey. Kidney Blood Press Res 34, 173–179, https://doi.org/10.1159/000323895 (2011).
Barri, Y. M. Hypertension and kidney disease: a deadly connection. Curr Cardiol Rep 8, 411–417 (2006).
Hanratty, R. et al. Relationship between blood pressure and incident chronic kidney disease in hypertensive patients. Clin J Am Soc Nephrol 6, 2605–2611, https://doi.org/10.2215/CJN.02240311 (2011).
Lea, J. P., &, S. B. & Diabetes, N. mellitus and hypertension: key risk factors for kidney disease. J Natl Med Assoc 94, 7S–15S (2002).
Mwasongwe, S. et al. Masked hypertension and kidney function decline: the Jackson Heart Study. J Hypertens 36, 1524–1532, https://doi.org/10.1097/HJH.0000000000001727 (2018).
Vaes, B. et al. The correlation between blood pressure and kidney function decline in older people: a registry-based cohort study. BMJ Open 5, e007571, https://doi.org/10.1136/bmjopen-2015-007571 (2015).
Yano, Y. et al. Association between prehypertension and chronic kidney disease in the Japanese general population. Kidney Int 81, 293–299, https://doi.org/10.1038/ki.2011.346 (2012).
Russo, G. T. et al. Plasma triglycerides and HDL-C levels predict the development of diabetic kidney disease in subjects with type 2 diabetes: the AMD Annals initiative. Diabetes Care 39, 2278–2287, https://doi.org/10.2337/dc16-1246/-/DC1 (2016).
Shiba, N. & Shimokawa, H. Chronic kidney disease and heart failure–Bidirectional close link and common therapeutic goal. J Cardiol 57, 8–17, https://doi.org/10.1016/j.jjcc.2010.09.004 (2011).
Sarnak, M. J. A patient with heart failure and worsening kidney function. Clin J Am Soc Nephrol 9, 1790–1798, https://doi.org/10.2215/CJN.11601113 (2014).
Silverberg, D., Wexler, D., Blum, M., Schwartz, D. & Iaina, A. The association between congestive heart failure and chronic renal disease. Curr Opin Nephrol Hypertens 13, 163–170, https://doi.org/10.1097/01.mnh.0000119526.79618.5a (2004).
Roughley, M. J., Belcher, J., Mallen, C. D. & Roddy, E. Gout and risk of chronic kidney disease and nephrolithiasis: meta-analysis of observational studies. Arthritis Res Ther 17, 90, https://doi.org/10.1186/s13075-015-0610-9 (2015).
Tsimihodimos, V., Mitrogianni, Z. & Elisaf, M. Dyslipidemia associated with chronic kidney disease. Open Cardiovasc Med J 5, 41–48 (2011).
Zubovic, S. V., Kristic, S., Prevljak, S. & Pasic, I. S. Chronic kidney disease and lipid disorders. Med Arch 70, 191–192, https://doi.org/10.5455/medarh.2016.70.191-192 (2016).
Lanktree, M. B., Theriault, S., Walsh, M. & Pare, G. HDL cholesterol, LDL cholesterol, and triglycerides as risk factors for CKD: a mendelian randomization study. Am J Kidney Dis 71, 166–172, https://doi.org/10.1053/j.ajkd.2017.06.011 (2018).
Hall, M. E. et al. Obesity, hypertension, and chronic kidney disease. Int J Nephrol Renovasc Dis 7, 75–88, https://doi.org/10.2147/IJNRD.S39739 (2014).
Stengel, B., Tarver-Carr, M. E., Powe, N. R., Eberhardt, M. S. & Brancati, F. L. Lifestyle factors, obesity and the risk of chronic kidney disease. Epidemiology 14, 479–487, https://doi.org/10.1097/01.EDE.0000071413.55296.c4 (2003).
Grubbs, V. et al. Body mass index and early kidney function decline in young adults: a longitudinal analysis of the CARDIA (Coronary Artery Risk Development in Young Adults) study. Am J Kidney Dis 63, 590–597, https://doi.org/10.1053/j.ajkd.2013.10.055 (2014).
Xie, L. et al. BMI is associated with the development of chronic kidney diseases in hypertensive patients with normal renal function. J Hypertens, https://doi.org/10.1097/HJH.0000000000001817 (2018).
Evangelista, L. S., Cho, W. K. & Kim, Y. Obesity and chronic kidney disease: a population-based study among South Koreans. PLoS One 13, e0193559, https://doi.org/10.1371/journal.pone.0193559 (2018).
Singh, N. P., Ganguli, A., &, A. & Drug-induced, P. kidney diseases. J Assoc Physicians India 51, 970–979 (2003).
Taber, S. S. & Pasko, D. A. The epidemiology of drug-induced disorders: the kidney. Expert Opin Drug Saf 7, 679–690, https://doi.org/10.1517/14740330802410462 (2008).
Pazhayattil, G. S. & Shirali, A. C. Drug-induced impairment of renal function. Int J Nephrol Renovasc Dis 7, 457–468, https://doi.org/10.2147/IJNRD.S39747 (2014).
Mansfield, K. E., Nitsch, D., Smeeth, L., Bhaskaran, K. & Tomlinson, L. A. Prescription of renin-angiotensin system blockers and risk of acute kidney injury: a population-based cohort study. BMJ Open 6, e012690, https://doi.org/10.1136/bmjopen-2016-012690 (2016).
Kobori, H., Mori, H., Masaki, T. & Nishiyama, A. Angiotensin II blockade and renal protection. Curr Pharm Des 19, 3033–3042 (2013).
Inzucchi, S. E., Lipska, K. J., Mayo, H., Bailey, C. J. & McGuire, D. K. Metformin in patients with type 2 diabetes and kidney disease: a systematic review. JAMA 312, 2668–2675, https://doi.org/10.1001/jama.2014.15298 (2014).
Sanguankeo, A., Upala, S., Cheungpasitporn, W., Ungprasert, P. & Knight, E. L. Effects of statins on renal outcome in chronic kidney disease patients: a systematic review and meta-analysis. PLoS One 10, e0132970, https://doi.org/10.1371/journal.pone.0132970 (2015).
Onuigbo, M. A. & Can, A. C. E. inhibitors and angiotensin receptor blockers be detrimental in CKD patients? Nephron Clin Pract 118, c407–419, https://doi.org/10.1159/000324164 (2011).
Gooch, K. et al. NSAID use and progression of chronic kidney disease. Am J Med 120, 280 e281–287, https://doi.org/10.1016/j.amjmed.2006.02.015 (2007).
Lazarus, B. et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med 176, 238–246, https://doi.org/10.1001/jamainternmed.2015.7193 (2016).
Xie, Y. et al. Long-term kidney outcomes among users of proton pump inhibitors without intervening acute kidney injury. Kidney Int 91, 1482–1494, https://doi.org/10.1016/j.kint.2016.12.021 (2017).
Kamal, F., Khan, M. A., Molnar, M. Z. & Howden, C. W. The association between proton pump inhibitor use with acute kidney injury and chronic kidney disease. J Clin Gastroenterol 52, 468–476, https://doi.org/10.1097/MCG.0000000000001035 (2018).
Krishnamurthy, A. et al. The effect of allopurinol on renal function. J Clin Rheumatol 23, 1–5, https://doi.org/10.1097/RHU.0000000000000480 (2017).
Shankar, A., Klein, R. & Klein, B. E. The association among smoking, heavy drinking, and chronic kidney disease. Am J Epidemiol 164, 263–271, https://doi.org/10.1093/aje/kwj173 (2006).
Nagasawa, Y., Yamamoto, R., Rakugi, H. & Isaka, Y. Cigarette smoking and chronic kidney diseases. Hypertens Res 35, 261–265, https://doi.org/10.1038/hr.2011.205 (2012).
Van Laecke, S. & Van Biesen, W. Smoking and chronic kidney disease: seeing the signs through the smoke? Nephrol Dial Transplant 32, 403–405, https://doi.org/10.1093/ndt/gfw448 (2017).
Yacoub, R. et al. Association between smoking and chronic kidney disease: a case control study. BMC Public Health 10, 731, https://doi.org/10.1186/1471-2458-10-731 (2010).
White, S. L. et al. Alcohol consumption and 5-year onset of chronic kidney disease: the AusDiab study. Nephrol Dial Transplant 24, 2464–2472, https://doi.org/10.1093/ndt/gfp114 (2009).
Schaeffner, E. & Ritz, E. Alcohol and kidney damage: a Janus-faced relationship. Kidney Int 81, 816–818, https://doi.org/10.1038/ki.2012.14 (2012).
Robinson-Cohen, C. et al. Physical activity and rapid decline in kidney function among older adults. Arch Intern Med 169, 2116–2123 (2009).
Hawkins, M. S. et al. Association between physical activity and kidney function: National Health and Nutrition Examination Survey. Med Sci Sports Exerc 43, 1457–1464, https://doi.org/10.1249/MSS.0b013e31820c0130 (2011).
Bowlby, W. et al. Physical activity and metabolic health in chronic kidney disease: a cross-sectional study. BMC Nephrol 17, 187, https://doi.org/10.1186/s12882-016-0400-x (2016).
West, S. L., Ma, C., Chaudhry, M., Thomas, S. G. & Lok, C. E. The association of daily activity levels and estimated kidney function in men and women with predialysis chronic kidney disease. Kidney Int Rep 2, 874–880, https://doi.org/10.1016/j.ekir.2017.05.003 (2017).
James, P. A. et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 311, 507–520, https://doi.org/10.1001/jama.2013.284427 (2014).
Hsu, T. W. et al. Renoprotective effect of renin-angiotensin-aldosterone system blockade in patients with predialysis advanced chronic kidney disease, hypertension, and anemia. JAMA Intern Med 174, 347–354, https://doi.org/10.1001/jamainternmed.2013.12700 (2014).
Sarafidis, P. A., Stafylas, P. C., Kanaki, A. I. & Lasaridis, A. N. Effects of renin-angiotensin system blockers on renal outcomes and all-cause mortality in patients with diabetic nephropathy: an updated meta-analysis. Am J Hypertens 21, 922–929, https://doi.org/10.1038/ajh.2008.206 (2008).
Xie, X. et al. Renin-angiotensin system inhibitors and kdney and crdiovascular outcomes in patients with CKD: a bayesian network meta-analysis of randomized clinical trials. Am J Kidney Dis 67, 728–741, https://doi.org/10.1053/j.ajkd.2015.10.011 (2016).
Ahmed, A., Jorna, T. & Bhandari, S. Should we stop angiotensin converting enzyme inhibitors/angiotensin receptor blockers in advanced kidney disease? Nephron 133, 147–158, https://doi.org/10.1159/000447068 (2016).
Weiss, J. W., Thorp, M. L. & O’Hare, A. M. Renin-angiotensin system blockade in older adults with chronic kidney disease: a review of the literature. Curr Opin Nephrol Hypertens 19, 413–419, https://doi.org/10.1097/MNH.0b013e32833b8d6b (2010).
Schmidt, M. et al. Serum creatinine elevation after renin-angiotensin system blockade and long term cardiorenal risks: cohort study. BMJ 356, j791, https://doi.org/10.1136/bmj.j791 (2017).
Menon, V. et al. Alcohol consumption and kidney function decline in the elderly: alcohol and kidney disease. Nephrol Dial Transplant 25, 3301–3307, https://doi.org/10.1093/ndt/gfq188 (2010).
Bell, S., Soto-Pedre, E., Connelly, P., Livingstone, S. & Pearson, E. Clarifying the relationship between metformin, acute kidney injury and lactic acidosis. Nat Rev Nephrol 14, 70, https://doi.org/10.1038/nrneph.2017.172 (2017).
Heaf, J. Metformin in chronic kidney disease: time for a rethink. Perit Dial Int 34, 353–357, https://doi.org/10.3747/pdi.2013.00344 (2014).
Agarwal, R. Effects of statins on renal function. Mayo Clin Proc 82, 1381–1390, https://doi.org/10.4065/82.11.1381 (2007).
Horl, W. H. Nonsteroidal anti-inflammatory drugs and the kidney. Pharmaceuticals (Basel) 3, 2291–2321, https://doi.org/10.3390/ph3072291 (2010).
Moller, B. et al. Chronic NSAID use and long-term decline of renal function in a prospective rheumatoid arthritis cohort study. Ann Rheum Dis 74, 718–723, https://doi.org/10.1136/annrheumdis-2013-204078 (2013).
Stamp, L. K., Chapman, P. T. & Palmer, S. C. Allopurinol and kidney function: An update. Joint Bone Spine 83, 19–24, https://doi.org/10.1016/j.jbspin.2015.03.013 (2016).
Acknowledgements
We thank the Korea National Health Insurance Service for providing NHIS-Senior data.
Author information
Authors and Affiliations
Contributions
Kang H. analysed the data and wrote the main manuscript text. Hong S.H. also wrote and reviewed the main manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kang, H., Hong, S.H. Risk of Kidney Dysfunction from Polypharmacy among Older Patients: A Nested Case-Control Study of the South Korean Senior Cohort. Sci Rep 9, 10440 (2019). https://doi.org/10.1038/s41598-019-46849-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-46849-7
- Springer Nature Limited
This article is cited by
-
Association of polypharmacy with incidence of CKD: a retrospective cohort study
Clinical and Experimental Nephrology (2023)
-
Prevalence and factors associated with polypharmacy among patients with rheumatoid arthritis: a single-centre, cross-sectional study
Clinical Rheumatology (2023)
-
Effectiveness of polypharmacy reduction policy in Japan: nationwide retrospective observational study
International Journal of Clinical Pharmacy (2022)