Abstract
It is essential to identify high risk transient ischemic attack (TIA) patients. The previous study reported that the CSR (comprehensive stroke recurrence) model, a neuroimaging model, had a high predictive ability of recurrent stroke. The aims of this study were to validate the predictive value of CSR model in TIA patients and compare the predictive ability with ABCD3-I score. Data were analyzed from the prospective hospital-based database of patients with TIA which defined by the World Health Organization time-based criteria. The predictive outcome was stroke occurrence at 90 days. The receiver-operating characteristic (ROC) curves were plotted and the C statistics were calculated as a measure of predictive ability. Among 1186 eligible patients, the mean age was 57.28 ± 12.17 years, and 474 (40.0%) patients had positive diffusion-weighted imaging (DWI). There were 118 (9.9%) patients who had stroke within 90 days. In 1186 TIA patients, The C statistic of CSR model (0.754; 95% confidence interval [CI] 0.729–0.778) was similar with that of ABCD3-I score (0.717; 95% CI 0.691–0.743; Z = 1.400; P = 0.1616). In 474 TIA patients with positive DWI, C statistic of CSR model (0.725; 95% CI 0.683–0.765) was statistically higher than that of ABCD3-I score (0.626; 95% CI 0.581–0.670; Z = 2.294; P = 0.0245). The CSR model had good predictive value for assessing stroke risk after TIA, and it had a higher predictive value than ABCD3-I score for assessing stroke risk for TIA patients with positive DWI.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Transient ischemic attack (TIA) is one of the most common acute cerebrovascular disease and is associated with high risk of early stroke1,2. Therefore, it is essential to identify TIA patients at high risk and give effective treatment to prevent stroke occurrence.
The use of brain magnetic resonance imaging (MRI) has shown that some patients with a clinical definition of TIA have neuroimaging evidence of brain injury on diffusion-weighted imaging (DWI). Moreover, recent studies demonstrated that adding imaging information, e.g. ABCD3-I score, showed improved risk predictive ability in TIA patients3,4,5. However, without more detailed imaging parameters, such as DWI pattern, the ABCD3-I score would have no good predictive ability of stroke in TIA patients with positive DWI.
DWI patterns may suggest differences in pathogenesis, which are predictive for stroke recurrence6. For example, multiple acute infarctions are inclined to be caused by a plaque rupture with multiple distal emboli or a cardiac source of embolism7. The TIAregistry.org project showed that acute infarctions, especially for multiple infarctions, portended an increased risk of stroke in TIA and minor stroke patients8. Recently, the comprehensive stroke recurrence (CSR) model, a neuroimaging-based model including DWI patterns, has been proved that had a high predictive value of recurrent stroke for stroke patients9. As a promising risk prediction tool for TIA, CSR model still needs to be validated in different patient population before it can be widely utilized.
Therefore, the aims of our study are: (1) validating the predictive value of the CSR model in TIA patients; (2) comparing the predictive ability of CSR model with ABCD3-I score.
Method
Study population
The data of our study was from the hospital-based TIA database of the First Affiliated Hospital of Zhengzhou University. The background and methods of the database were described in other articles3,10. This prospective cohort study consecutively recruited 1652 TIA patients whose onset time is less than 7 days from October 2010 to August 2017. Patients were diagnosed based on the traditional TIA criteria, an acute loss of focal cerebral or ocular dysfunction lasting less than 24 h attributed to embolic or thrombotic vascular diseases11. Patients were excluded if they met the following criteria: (1) unavailable CSR score or ABCD3-I score. (2) Failure to complete imaging assessment before stroke occurrence. (3) Lost to follow-up. The research was approved by the ethics committee of the First Affiliated Hospital of Zhengzhou University. The informed consent forms were obtained from all of the patients or their legal proxies.
Data collection
Trained physicians recorded all detailed baseline data of enrolled TIA patients, including demographics such as age, sex, vascular risk factors such as current smoking, hypertension, diabetes mellitus, dyslipidemia, previous stroke, atrial fibrillation or coronary heart disease, imaging features, and treatment for secondary prevention (antiplatelet agents, anticoagulant, lipid-lowing agents, antihypertension agents and hypoglycemic agents).
All patients without contradictions of magnetic resonance imaging (MRI) were recommended to undergo an etiologic work-up according to a standard protocol at baseline within 7 days of the index TIA. The index TIA was defined as the most recently preceding assessment by a stroke specialist. The standardized work-up included brain MRI (3.0 T, diffusion-weighted imaging [DWI], apparent diffusion coefficient [ADC], T1/T2-weighted imaging, fluid-attenuated inversion recovery [FLAIR] sequences) and arterial examination (at least one assessment of intracranial arteries, and at least one assessment of extracranial arteries). Intracranial artery imaging included 3-dimensional time-of-flight magnetic resonance angiography, computed tomography angiography, or digital subtraction angiography. Extracranial artery imaging included carotid doppler, computed tomography angiography, contrast-enhanced magnetic resonance angiography, or digital subtraction angiography. Two trained readers independently reviewed the imaging data and were blinded to the patients’ baseline and outcome information. Any disagreement was decided by a third reader.
ABCD3-I score and CSR model
The ABCD3-I score12 is a 13-point scale consisting of six clinical variables (age, blood pressure, clinical features, duration of symptoms, and history of diabetes mellitus, dual TIA [an earlier TIA within 7 days of the index event]) and two imaging findings (ipsilateral ≥ 50% stenosis of internal carotid artery and acute DWI hyperintensity). The CSR model is a scale that totally based on neuroimaging parameters9. It is an 8-point scale including five neuroimaging parameters (multiple stage lesion, isolated cortical lesion, WMH, OLI and relevant arterial stenosis; Table 1).
Multiple stage lesion (i.e. lesions of varying age) was defined as multiple DWI lesions that met at least 2 of the following: (1) hyperacute: hypointense on ADC and isointense on FLAIR imaging; (2) early acute: hypointense on ADC and hyperintense on FLAIR; (3) late acute to subacute: isointense on ADC and hyperintense on FLAIR. Isolated cortical lesion was defined as DWI lesions that involved only cortical areas without subcortical involvement. WMH was rated on FLAIR imaging using the Fazekas scale in peri-ventricular and subcortical areas, respectively, then summed up the score in two areas and dichotomized into mild (0–2) and severe (3–6) WMH. OLI was initially rated as numbers according to well-defined lesions on MRI (maximum diameter in the axial plane ≥ 3 mm), and then divided them into three categories (OLI, 0; OLI, 1–2 and OLI ≥ 3). Relevant arterial stenosis was defined as ≥ 50% narrowing in the lumen of the intracranial or extracranial artery responsible for the neurological symptoms. The rate of arterial stenosis is calculated based on the North American symptomatic carotid endarterectomy study (NASCET)13:
D referred to diameter of normal lumen, and d referred to diameter of the narrowest lumen.
Follow-up and outcome
All registered patients were followed up over the telephone by a neurologist blinded to the clinical information and prognostic scores. All patients who were suspected of having a stroke were followed up via face-to-face interview. The predictive outcome was stroke occurrence within 90 days. Stroke was defined as a rapidly developed clinical symptom of focal (or occasionally global) disturbance of cerebral function, lasting more than 24 h or until death, with no apparent non-vascular cause14.
Statistics analysis
Participants were assigned to the group of positive DWI or negative DWI according to the presence or absence of DWI-identified lesions and analyses were performed in all TIA patients and subgroup of DWI positive or DWI negative patients. We used chi-square test for categorical variables; t-test for normally distributed continuous variables and Mann–Whitney U test for non-normally distributed continuous variables. Cochran-Armitage trend test was performed for patients for each CSR model score. Cumulative event curves were constructed for the end point by the Kaplan–Meier method, and the risk of stroke (stratified according to the score) was compared by the log-rank test. Univariate cox proportional hazards regression modeling was conducted to determine the risk factors associated with stroke occurrence. If there were statistical differences by univariate analysis at baseline, confounding factors were selected to examine the relationship between the risk predictive scores and stroke occurrence using multivariable Cox proportional hazards regression. Associations were presented as hazard ratio (HR) with corresponding 95% confidence interval (CI). To investigate the extent to which CSR model improved the risk assessment of incident stroke in comparison with the ABCD3-I score, the C statistics, integrated discrimination improvement (IDI), and net reclassification index (NRI) was calculated.
All statistical analyses were performed using SPSS software for Windows (version 25.0; SPSS). A two-tailed value of P < 0.05 was considered statistically significant.
Results
Of all 1652 consecutive patients with a diagnosis of TIA, 457 patients with unavailable scores (286 with unavailable CSR score, 60 with unavailable ABCD3-I score, and 111 with unavailable CSR score and ABCD3-I score) and 9 patients lost to 90-day follow-up were excluded. Finally, there were 1186 eligible patients in this study. Comparison of baseline characteristics of the included and excluded groups showed no significant differences (Table S1). Among the 1186 patients, the mean age was 57.28 ± 12.17 years, 719 (60.6%) of patients were men, and 474 (40.0%) patients had positive DWI on MRI. Table 2 showed baseline characteristics in TIA patients. Compared to TIA patients with negative DWI, those with positive DWI were more likely to be men with smoking, have higher ABCD3-I and CSR scores, have a history of hypertension and anticoagulant agents takers.
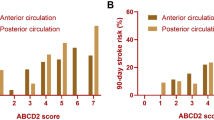
We analyzed the incidence of stroke at different follow-up periods. In TIA patients, the stroke rate at day 2, 7 and 90 was 3.5%, 5.2% and 9.9%, respectively. In those with positive DWI, the stroke rate at day 2, 7, and 90 was 7.0%, 10.1% and 18.4%. In those with negative DWI, the stroke rate at day 2, 7 and 90 was 1.1%, 2.0% and 4.4%. Figure 1 showed the stroke risk according to different CSR score in TIA patients and TIA patients with positive DWI, which illustrated an overall increase in the rate of stroke with increasing CSR score. A linear trend for occurrence rates was observed with the Cochran-Armitage trend test in TIA patients (Z = − 11.627; P < 0.0001) and TIA patients with positive DWI (Z = − 6.9986; P < 0.0001). TIA patients were categorized into low CSR score group (0–3) and high CSR score group (4–8) based on optimal cut-off point which was calculated by the maximum Youden index. Survival-free-of stroke curves categorized by CSR model were shown in Fig. 2. In TIA patients, survival curve was significantly different between the low-risk and high-risk categories (log-rank test = 121.70; P < 0.001). Similar result was found in TIA patients with positive DWI (log-rank test = 33.75; P < 0.001). But the trend was not found in those with negative DWI.
(A) Kaplan–Meier curves of TIA patients stratified according to the CSR model at 90 days (log-rank test = 121.70, P < 0.001); (B) Kaplan–Meier curves of DWI-positive subjects stratified according to the CSR model at 90 days (log-rank test = 33.75, P < 0.001); (C) Kaplan–Meier curves of DWI-negative subjects stratified according to the CSR model at 90 days (log-rank test = 6.89, P = 0.142).
Table 3 showed the clinical characteristics and risk factors associated with stroke in TIA patients. Those who undergone stroke were more likely to have history of hypertension, higher ABCD3-I score and CSR score in TIA patients. In those with positive DWI, those who undergone stroke were more likely to be women, have history of hypertension and stroke, higher ABCD3-I score and CSR score. Final multivariate analysis revealed that history of hypertension (HR 1.547; 95% CI 1.031–2.32), high ABCD3-I score (HR 1.216; 95% CI 1.116–1.326) and CSR score ≥ 4 (HR 3.708; 95% CI 2.487–5.53) were independent risk factors of stroke occurrence in TIA patients. In TIA patients with positive DWI, the final multivariate analysis showed that history of hypertension (HR 1.795; 95% CI 1.094–2.945), history of stroke (HR 1.641; 95% CI 1.040–2.590), high ABCD3-I score (HR 1.181; 95% CI 1.058–1.319), and CSR score ≥ 4 (HR 3.075; 95% CI 1.942–4.868) were independent risk factors of stroke occurrence.
Comparing the predictive ability of ABCD3-I score and CSR model in TIA patients using the receiver-operating characteristic curve analysis (Fig. 3). The area under the curve (AUC) was 0.717 (95% CI 0.691–0.743) by the ABCD3-I score and 0.754 (95% CI 0.729–0.778) by the CSR score for the prediction of 90-day stroke occurrence. The comparison of the AUC was performed by Z test, which revealed that the C statistic for CSR model was higher than that for the ABCD3-I score (Z = 1.400; P = 0.1616) but the result was not statistically significant. Similar results were found in those with negative DWI. However, the comparison of the two scores was statistically significant in TIA patients with positive DWI. The analysis suggested that the AUC was 0.626 (95% CI 0.581–0.670) by the ABCD3-I score and 0.725 (95% CI 0.683–0.765) by the CSR score for the prediction of 90-day stroke occurrence. The Z test revealed that the C statistic for CSR model was higher than that for the ABCD3-I score (Z = 2.294; P = 0.0245). The discriminatory power and risk reclassification also appeared to be substantially better (IDI, 7.9%, P < 0.001; category NRI, 55.1%, P < 0.001).
Ninety-day receiver-operating characteristic curves as a predictive value of the CSR score and ABCD3-I score. (A) The C statistic of CSR model (0.754; 95% CI 0.729–0.778) was similar with that of ABCD3-I score (0.717; 95% CI 0.691–0.743, Z = 1.400; P = 0.1616) in TIA patients; (B) CSR score showed better discrimination (Z = 2.294, P = 0.0245) with the C statistic of 0.725 (95% CI 0.683–0.765) than ABCD3-I score (0.626; 95% CI 0.581–0.670) in DWI-positive subjects. (C) The C statistic of CSR model (0.585; 0.548–0.622) was similar with that of ABCD3-I score (0.588; 0.551–0.624, Z = 0.0362; P = 0.9711) in TIA patients with negative DWI.
Discussion
The major findings of this study were the following: (1) usefulness of CSR model to predict the 90-day risk of stroke after TIA was validated; (2) the CSR model were superior to the ABCD3-I score in predicting the risk of 90-day stroke in TIA patients with positive DWI. In this study, the 90-day stroke rate was 9.9% in TIA patients, which was identical to the pooled risk (9.2%) based on a random effects model in a systematic review and meta-analysis15. However, the TIAregistry.org project reported that the risk of stroke occurrence after TIA was 3.7% at 90 days8 and another meta-analysis of recent intervention studies reported that the pooled stroke risk was 3.42% at 90 days16. One of possible reasons for the discrepancy may be that only hospitalized patients were enrolled in our study, which had high incidence of stroke compared with patients from clinic or emergency department. Moreover, the definition of TIA was distinguishing in different studies. In our study, the definition of TIA was time-based, however, the TIAregistry.org project8 defined TIA as new symptomatic neurologic deterioration lasting less than 24 h with no new infarction on neuroimaging, which was tissue-based definition. This definition did not take TIA patients with positive DWI into account, which was another potential reason of low rate of stroke occurrence. Correspondingly, the stroke risk for TIA with negative DWI was 4.4% in our study, which are consistent with these studies.
Recent studies demonstrated that the combination of neuroimaging and vascular information could further improve the predictive value of stroke after TIA17,18,19. ABCD3-I score provided risk estimates based on neuroimaging in addition to clinical predictors, which was widely used to stratify the risk factors of stroke in many studies20,21. In our study, the ABCD3-I score showed a good predictive value of stroke in TIA patients, which was similar to previous studies. And CSR model, based on more detailed neuroimaging, provided good discrimination for risk prediction of early stroke among TIA patients in our study.
An abnormal DWI has been validated as increasing the early stroke risk in many studies, especially DWI patterns12,18, such as infarction numbers8,22, different infarction stages23,24, and infarction location17,18. For example, Amarenco et al.8 showed that acute multiple infarctions portended an increased risk of stroke in TIA and minor stroke patients. Sylaja et al.25 reported that patients with multiple DWI lesions of different age were at 3.6-times-higher risk of having new lesions at 30 days than those having lesions of the same age. Purroy et al.26 demonstrated that single cortical lesions were also associated with cardioembolism, whereas subcortical acute lesions were associated with recurrent stroke. CSR model, a neuroimaging-based model including DWI patterns, showed better predictive value than ABCD3-I score for TIA patients with positive DWI In our present study. The superiority in predicting the subsequent stroke of CSR model could be explained by detailed imaging features of infarction provided the prognostic information that related to the underlying stroke mechanism. For instance, multiple infarcts of different ages indicated repeated ischemic events at different time points and had prognostic significance because they signify one or more “unstable” underlying causative lesions19. Cortical lesion was usually related to cardioembolism and cryptogenic with pathogenesis of embolism22,23. OLI or severe WMH indicated small vessel disease which could cause hostile brain conditions that were more vulnerable to ischemic insults24,27. In a study of 959 patients with first-ever acute ischemic stroke (AIS), the CSR model applied within 7 days of AIS provided an AUC of 0.81 for 2-year risk of recurrent stroke, and internal and external validation analysis also showed good predictive power9. Our study provided further evidence that the CSR model could predict stroke risk in TIA patients with positive DWI. Therefore, CSR model could stratify TIA patients, especially those with positive DWI. In clinical practice, ABCD3-I score could be used for initial assessment for its easier evaluation method, and CSR model could stratify DWI-positive patients further based on detailed neuroimaging findings and determine individualized secondary prevention strategies for the purpose of reducing the risk of stroke after TIA.
The study has several limitations. First, a total of 466 excluded patients (28.2%) would cause selection bias. As post-hoc analysis of prospective research, this limitation cannot be avoided. Incomplete MRI imaging sequences or imaging examinations in other hospitals lead to unavailable CSR score. However, baseline characteristics of included and excluded patients in the study were similar. Second, we evaluated only hospitalized patients at a single center, which could lead to selective bias. These findings need further validation by multicenter prospective studies.
Conclusion
CSR model and ABCD3-I score had good predictive value for predicting the risk of 90-day stroke in patients with definite TIA. Moreover, CSR model was superior to ABCD3-I score in predicting the risk of 90-day stroke in TIA patients with positive DWI. Using CSR model, we could stratify high-risk TIA patients further.
Ethical statement
The study was performed according to the guidelines of the Helsinki Declaration. The study was undertaken with the understanding and written consent of each subject and was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University.
Data availability
Data are available to researchers on request for purposes of reproducing the results or replicating the procedure by directly contacting the corresponding author.
References
von Weitzel-Mudersbach, P., Andersen, G., Hundborg, H. H. & Johnsen, S. P. Transient ischemic attack and minor stroke are the most common manifestations of acute cerebrovascular disease: A prospective, population-based study—The Aarhus TIA study. Neuroepidemiology 40, 50–55 (2013).
Giles, M. F. & Rothwell, P. M. Risk of stroke early after transient ischaemic attack: A systematic review and meta-analysis. Lancet Neurol. 6, 1063–1072 (2007).
Song, B. et al. Validation of the ABCD3-I score to predict stroke risk after transient ischemic attack. Stroke 44, 1244–1248 (2013).
Zhao, M. et al. Comparison of stroke prediction accuracy of ABCD2 and ABCD3-I in patients with transient ischemic attack: A meta-analysis. J. Stroke Cerebrovasc. Dis. 26, 2387–2395 (2017).
Knoflach, M. et al. Predictive value of ABCD2 and ABCD3-I scores in TIA and minor stroke in the stroke unit setting. Neurology 87, 861–869 (2016).
Engelter, S. T. et al. Optimizing the risk estimation after a transient ischaemic attack—The ABCDE⊕ score. Eur. J. Neurol. 19, 55–61 (2012).
Hyafil, F. et al. Rupture of nonstenotic carotid plaque as a cause of ischemic stroke evidenced by multimodality imaging. Circulation 129, 130–131 (2014).
Amarenco, P. et al. One-year risk of stroke after transient ischemic attack or minor stroke. N. Engl. J. Med. 374, 1533–1542 (2016).
Nam, K. W., Kwon, H. M., Lim, J. S., Han, M. K. & Lee, Y. S. Clinical relevance of abnormal neuroimaging findings and long-term risk of stroke recurrence. Eur. J. Neurol. 24, 1348–1354 (2017).
Song, B. et al. Efficacy of high-intensity statin use for transient ischemic attack patients with positive diffusion-weighted imaging. Sci. Rep. 9, 1173 (2019).
Hatano, S. Experience from a multicentre stroke register: A preliminary report. Bull. World Health Organ. 54, 541–553 (1976).
Merwick, A. et al. Addition of brain and carotid imaging to the ABCD2 score to identify patients at early risk of stroke after transient ischaemic attack: A multicentre observational study. Lancet Neurol. 9, 1060–1069 (2010).
Barnett, H. J. M. et al. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N. Engl. J. Med. 325, 445–453 (1991).
Whisnant, J. P. et al. Classification of cerebrovascular diseases III. Stroke 21, 637–676 (1990).
Wu, C. M. et al. Early risk of stroke after transient ischemic attack: A systematic review and meta-analysis. Arch. Intern. Med. 167, 2417–2422 (2007).
Valls, J. et al. A current estimation of the early risk of stroke after transient ischemic attack: A systematic review and meta-analysis of recent intervention studies. Cerebrovasc. Dis. 43, 90–98 (2017).
Jung, J. M. et al. Predictors of recurrent stroke in patients with symptomatic intracranial arterial stenosis. Stroke 43, 2785–2787 (2012).
Wang, G. et al. Does all single infarction have lower risk of stroke recurrence than multiple infarctions in minor stroke? BMC Neurol. 19, 7 (2019).
Wardlaw, J. M. et al. Vascular risk factors, large-artery atheroma, and brain white matter hyperintensities. Neurology 82, 1331–1338 (2014).
Purroy, F. et al. Predictive value of brain and vascular imaging including intracranial vessels in transient ischaemic attack patients: External validation of the ABCD3-I score. Eur. J. Neurol. 20, 1088–1093 (2013).
Dai, Q. et al. From clinical to tissue-based dual TIA: Validation and refinement of ABCD3-I score. Neurology 84, 1426–1432 (2015).
Lee, D. K., Kim, J. S., Kwon, S. U., Yoo, S. H. & Kang, D. W. Lesion patterns and stroke mechanism in atherosclerotic middle cerebral artery disease: Early diffusion-weighted imaging study. Stroke 36, 2583–2588 (2005).
Bang, O. Y. et al. Specific DWI lesion patterns predict prognosis after acute ischaemic stroke within the MCA territory. J. Neurol. Neurosurg. Psychiatry 76, 1222–1228 (2005).
Lau, K. K. et al. Total small vessel disease score and risk of recurrent stroke: Validation in 2 large cohorts. Neurology 88, 2260–2267 (2017).
Sylaja, P. N. et al. Acute ischemic lesions of varying ages predict risk of ischemic events in stroke/TIA patients. Neurology 68, 415–419 (2007).
Purroy, F. et al. Patterns of diffusion-weighted magnetic resonance imaging associated with etiology improve the accuracy of prognosis after transient ischaemic attack. Eur. J. Neurol. 18, 121–128 (2011).
Song, T. J. et al. Total cerebral small-vessel disease score is associated with mortality during follow-up after acute ischemic stroke. J. Clin. Neurol. 13, 187–195 (2017).
Acknowledgements
We thank all the members working for TIA database of the First Affiliated Hospital of Zhengzhou University and all the participants in the study.
Funding
This work was supported by the Henan Province Medical Science and Technology Research Plan (SBGJ2018031).
Author information
Authors and Affiliations
Contributions
L.Z. and S.C. contributed to study design and manuscript drafting; L.P. contributed to acquisition of data; H.F. and H.L. contributed to the statistics analysis; J.W., S.S., Y.G. and B.S. contributed to critical revisions of the manuscript. Y.X. contributed to the conception and design of the study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, L., Cao, S., Pei, L. et al. Validation of CSR model to predict stroke risk after transient ischemic attack. Sci Rep 12, 604 (2022). https://doi.org/10.1038/s41598-021-04405-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-04405-2
- Springer Nature Limited