Abstract
Interstitial lung disease (ILD) is the most common lung manifestation in patients with Sjögren syndrome (SJS) and is associated with poor outcomes. This study aimed to investigate the long-term clinical course and prognostic factors in patients with SJS-ILD. Clinical data and high-resolution computed tomography (HRCT) images of 62 patients with primary SJS-ILD were retrospectively analyzed (biopsy-proven cases, n = 16). The mean patient age was 59.8 years; 83.9% of the patients were females, and 38.7% showed a usual interstitial pneumonia (UIP) pattern on HRCT. The median follow-up period was 61.5 months. During follow-up, 15 patients (24.2%) died, 7 (11.3%) experienced acute exacerbation (AE), and 27 (43.5%) progressed. The 1-, 3- and 5-year survival rates were 93.5%, 85.8%, and 81.1%, respectively. Age (hazard ratio [HR]: 1.158, P = 0.003), C-reactive protein (CRP) level (HR: 1.212, P = 0.045), FVC (HR: 0.902, P = 0.005), and a UIP pattern on HRCT (HR: 4.580, P = 0.029) were significant prognostic factors in multivariable Cox analysis. In conclusion, death, AE, and ILD progression occurred in 25%, 10%, and 50% of the patients with SJS-ILD, respectively. Older age, higher CRP level, lower FVC, and a UIP pattern on HRCT indicated poor prognosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Primary Sjögren syndrome (SJS) is a chronic systemic inflammatory disorder characterized by impaired functioning of the lacrimal and salivary glands1,2,3. Extraglandular organ involvement including that of the skin, lung, heart, kidney, and hematopoietic system has also been reported in SJS4,5,6,7,8,9. Lung involvement is one of the most common extraglandular complications with a recently reported prevalence of 16%, and also reported to be associated with higher mortality in patients with SJS10,11. Interstitial lung disease (ILD) is one of the most frequent lung complications7. However, the long-term clinical course and prognostic factors in patients with SJS-ILD are not well-defined.
The clinical course and outcomes in patients with SJS-ILD have been reported previously12,13. Parambil et al. evaluated 18 patients with SJS-ILD (all biopsy-proven cases) and reported that over a median follow-up period of 67 months, 7 (39%) patients died and 5 (28%) showed progression (decline in forced vital capacity [FVC] ≥ 10% or diffusing capacity of carbon monoxide [DLCO] ≥ 15% from baseline)12. Similarly, F Roca et al. assessed 21 patients with SJS-ILD and showed that over a median follow-up period of 24 months, 3 (14.3%) patients died and among 19 patients excluding 2 who were lost to follow up, 7 (36.8%) patients showed progression13. Some other studies have suggested prognostic factors in patients with SJS-ILD14,15. Ito et al. assessed 33 patients with primary SJS showing lung involvement and reported that a lower baseline arterial oxygen pressure (PaO2) and presence of microscopic honeycombing on surgical lung biopsy were significant prognostic factors for mortality15. Enomoto et al. assessed 33 patients with SJS-ILD (all biopsy-proven cases) and reported that a higher arterial dioxide pressure (PaCO2), the extent of reticular abnormality on high-resolution computed tomography (HRCT), and the severity of fibroblastic foci on surgical lung biopsy were risk factors for mortality14.
However, previous studies were conducted on a small number of patients, and the long-term clinical course and prognostic factors are still not well-defined in patients with SJS-ILD. Therefore, our study aimed to evaluate the long-term clinical course and prognostic factors in a large number of patients with SJS-ILD.
Methods
Study population
Between January 2000 and December 2016, 62 patients with SJS-ILD (biopsy-proven cases: 16) were diagnosed at Asan Medical Center, Seoul, Republic of Korea, and included in this study. All patients were identified through electronic medical record search, and met the criteria of American College of Rheumatology/European League Against Rheumatism16. The presence of ILD was confirmed on HRCT images. The study was approved by the Institutional Review Board of Asan Medical Center (2018-1115), and the requirement for informed consent was waived due to the retrospective nature of this study by the Institutional Review Board of Asan Medical Center. All methods were performed in accordance with the relevant guidelines and regulations.
Clinical data
Clinical and survival data, and pathologic reports for surgical lung biopsy were retrospectively collected (Y.J.K) from medical records, telephone interviews, and/or the records of the National Health Insurance of Korea. Spirometric parameters, total lung capacity (TLC) by plethysmography, and DLCO were measured according to the American Thoracic Society (ATS)/European Respiratory Society (ERS) recommendations17,18,19, and the results were expressed as percentages of normal predicted values. A six-minute walk test (6MWT) was performed according to the ERS/ATS recommendations20. Bronchoalveolar lavage (BAL) was performed according to the ATS guidelines21.
Data from follow-up assessments at 3–6-month intervals or from hospitalization events were reviewed to determine the development of complications such as pneumonia, acute exacerbation (AE), pulmonary hypertension, or malignant tumors. AE was defined according to the criteria suggested by Collard et al.22, as a worsening of dyspnea within 30 days, with new bilateral lung infiltration and no evidence of infection or other alternative causes of dyspnea (e.g., pulmonary embolism or left heart failure). Pulmonary hypertension was measured by transthoracic echocardiography, and a high probability of pulmonary hypertension was defined as a tricuspid regurgitation Vmax of > 3.4 m/s according to the 2015 European Society of Cardiology/ERS guidelines23. ILD progression was determined as an absolute decline of at least 10% in the predicted FVC or at least 15% in the DLco24.
HRCT evaluation
HRCT scans were obtained in accordance with standard protocols at full inspiration without contrast enhancement. HRCT scan images were reviewed by a radiologist (J.C.) and a pulmonologist (J.W.S.) blinded to the clinical and pathologic information. Overall, the HRCT pattern was categorized as usual interstitial pattern (definite UIP), probable UIP, indeterminate for UIP, or an alternative diagnosis, based on the idiopathic pulmonary fibrosis (IPF) diagnostic criteria3. Alternative diagnosis pattern on HRCT were further categorized according to the 2013 international multidisciplinary classification of idiopathic interstitial pneumonias25. Discrepancies were resolved by a consensus. A UIP pattern was defined by a subpleural and basal predominance of reticular abnormalities, honeycombing with or without traction bronchiectasis, and absence of inconsistent findings with a UIP pattern such as extensive ground-glass opacities, micro-nodules, discrete cysts, or segmental/lobar consolidations.
Statistical analysis
All values are expressed as mean ± standard deviation for continuous variables and as percentages for categorical variables. The Student’s t-test or Mann–Whitney U test was used for analyzing continuous data, and Pearson’s chi-squared or Fisher’s exact test was used for analyzing categorical data. The linear mixed-effects model was used to estimate the pulmonary function changes over time in each patient. The intercept and slope were both fitted as random effects to address the inter-patient differences at baseline and the different rates of pulmonary function changes during the follow-up. We calculated the slope of FVC or DLco to time from the baseline (in years) using FVC or DLco as the dependent variables, and measurement time from the baseline as the independent variable. We used the slope as the annual rate of change in lung function (the absolute change of % predicted value). Changes in lung function were evaluated using a Student’s t-test or analysis of variance (ANOVA) for intergroup comparisons. Kaplan–Meier survival analysis and the log-rank test were performed to evaluate survival. Survival time was calculated as the number of months from the date of diagnosis until death or time of censoring. All patients were followed up during study period, and patients were censored if they were alive on December 31, 2016. The unadjusted or multivariable Cox proportional hazard model was used to evaluate risk factors for mortality. Variables with a P value of < 0.1 in the unadjusted analysis were entered into the multivariable models (backward log-likelihood ratio statistics method). All P-values were two-tailed, with statistical significance set at a P value of < 0.05. All statistical analyses were performed using IBM SPSS Statistics for Windows, Version 24.0. (IBM Corp., Armonk, NY, USA).
Results
Baseline characteristics
The baseline clinical characteristics of the patients are summarized in Table 1. Among a total of 62 patients, the mean age was 59.8 years and 83.9% were females. The median follow-up period was 61.5 months (interquartile range, 24.0–99.5 months), and 15 patients (24.2%) died. Major causes of death were underlying ILD progression (66.7%) and pneumonia (13.3%) (see Supplementary Table S1). Total of 53 patients (85.5%) received steroid and/or immunosuppressants (median treatment duration: 15 months [interquartile range: 5–28 months], the initial dose of steroid [mean, prednisolone equivalent dose]: 31.3 mg) (Supplementary Table S2). Non-survivors were older and had less frequent anti-SSA/Ro positivity lower lung function (FVC, DLCO, and TLC), poorer exercise capacity (distance and the lowest oxygen saturation during 6MWT), and lower lymphocyte counts in the BAL fluid than survivors (Table 1). Non-survivors also showed a UIP pattern on HRCT more frequently than survivors. However, there were no differences in the treatment during follow-up between non-survivors and survivors.
Clinical course
The clinical course of patients with SJS-ILD is summarized in Table 2. Of the 62 patients, AE, pneumonia, pulmonary hypertension, and ILD progression occurred in 7 (11.3%), 9 (14.5%), 4 (6.5%), and 27 (43.5%) patients during follow-up, respectively. Lung cancer and lymphoma were not found in any of the patients. Non-survivors experienced more frequent pneumonia, pulmonary hypertension, and ILD progression than survivors; however, there were no differences in the development of AE between the 2 groups (Table 2). Non-survivors also showed higher decline rates in FVC and DLCO than survivors.
Clinical course according to HRCT pattern
Of the 62 patients, 24 (38.7%) were classified as having a UIP pattern on HRCT, 26 (41.9%) as having probable UIP, and 12 (19.4%) as having alternative diagnosis (7 nonspecific interstitial pneumonia [NSIP], 4 lymphocytic interstitial pneumonia [LIP], and 1 organizing pneumonia [OP]). Histopathology was available for 16 patients with SJS-ILD, and radiopathologic correlation are summarized in Fig. 1. All patients (n = 2) with a UIP pattern on HRCT had UIP on biopsy. In patients (n = 9) with a probable UIP pattern on HRCT, NSIP on biopsy was the most common (66.7%), followed by UIP (11.1%) and OP (22.2%). In patients (n = 5) with an alternative diagnosis pattern on HRCT, NSIP on biopsy was also the most common (60.0%), followed by LIP (40%). Baseline characteristics of patients according to the HRCT pattern are summarized in Supplementary Table S3. Patients with a UIP pattern were older and had a shorter walking distance during 6MWT than those in the other groups; however, there was no difference in the prevalence of complications among the 3 groups (Table 3).
Flowchart of histopathology and HRCT pattern in patients with SJS-ILD. HRCT high-resolution computed tomography, ILD interstitial lung disease, LIP lymphocytic interstitial pneumonia, NSIP nonspecific interstitial pneumonia, OP organizing pneumonia, SJS Sjögren syndrome, UIP usual interstitial pneumonia.
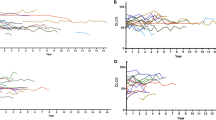
The Kaplan–Meier survival curve of patients with SJS-ILD according to the HRCT pattern is shown in Fig. 2. Patients with a UIP pattern on HRCT showed lower survival (median survival, 91 months; 5-year survival, 58.8%) than those with probable UIP (5-year survival, 90.3%, P < 0.001) or alternative diagnosis (5-year survival, 80.2%, P = 0.008); however, there was no difference in survival between the probable and alternative diagnosis groups (P = 0.294). The UIP group also showed a higher decline rate in DLCO and a tendency for a faster decline in FVC and TLC than other groups, but without statistical significance (Table 3).
Kaplan–Meier curves of patients with SJS-ILD according to HRCT patterns. Cross marks, open circles, and open squares show censored cases in the alternative diagnosis, UIP, and probable UIP groups, respectively. The prognosis of UIP was significantly different from that of the others (vs. probable UIP; P < 0.001, vs. alternative diagnosis; P = 0.008, Tukey–Kramer test). The prognosis was not significantly different between probable UIP and alternative diagnosis (P = 0.294, Tukey–Kramer test). HRCT high-resolution computed tomography, ILD interstitial lung disease, SJS Sjögren syndrome, UIP usual interstitial pneumonia.
Risk factors for mortality
The hazard ratio (HR), 95% confidence interval (CI), and P value in the unadjusted analysis for each variable using Cox’s proportional hazard model for the risk of death are shown in Table 4. Statistically significant parameters in the unadjusted analysis were older age, smoking history, higher C-reactive protein (CRP) level, lower FVC, DLCO, and TLC, and a UIP pattern on HRCT. In the multivariable analysis, age (HR 1.158; 95% CI 1.050–1.278; P = 0.003), CRP level (HR 1.212; 95% CI 1.004–1.462; P = 0.045), FVC (HR 0.902; 95% CI 0.839–0.969; P = 0.005), and a UIP pattern on HRCT (UIP to non-UIP, HR 4.580; 95% CI 1.167–17.975; P = 0.029) were independently associated with survival.
Discussion
In our study of over 5 years of follow-up, death, AE, and ILD progression occurred in approximately 25%, 10%, and 50% of patients with SJS-ILD, respectively. Older age, higher CRP level, lower FVC, and a UIP pattern on HRCT were significant prognostic factors for mortality in patients with SJS-ILD.
Patients with connective tissue disease-associated interstitial lung disease (CTD-ILD) are known to be at risk of an AE26,27. In our study, 6 patients (9.7% of total subjects) experienced an AE. In the study by Suda et al., which retrospectively reviewed 83 CTD-ILD patients with mean observation periods of 6 years, 6 (7.2%) patients developed AE, and 5 of them (83.3%) died despite aggressive anti-inflammatory and immunosuppressive therapy, indicating a poor prognosis of AE in CTD-ILD, which is compatible with that of IPF27. In their study, among 17 patients with SJS-ILD, 1 (5.9%) patient developed AE during a mean follow-up period of 6 years27. Manfredi et al. assessed 78 patients with CTD-ILD over a mean follow-up period of 23.9 months and reported that 9 (11.5%) patients experienced AE and showed poorer survival than those without AE (overall survival, 31.1% vs. 86.5%; P < 0.001)26. In their study, among 16 patients with SJS-ILD, 1 (6.3%) patient developed AE26.
Our results showed that approximately half of the patients experienced ILD progression during follow-up. Only a few studies have investigated the clinical course of SJS-ILD. Parambil et al. assessed 18 patients with SJS-ILD and reported that 5 (28%) patients showed ILD progression (decline in FVC ≥ 10% or DLco ≥ 15%) over a median follow-up period of 38 months12. F. Roca et al. evaluated 19 patients with SJS-ILD and also showed that 7 (36.8%) patients experienced ILD progression (decline in FVC ≥ 10% or DLco ≥ 15%) over a median follow-up period of 24 months13. However, our data showed a numerically higher rate of progression than previous reports. This finding might be due to the longer follow-up period in our study than in previous studies, indicating the chronically deteriorating nature of the disease. Moreover, ILD progression may have been overestimated by the calculation method of our study; ILD progression was evaluated based on the lung function value at the time of one follow-up observation. Patients with CTD-ILDs often show some degree of fluctuation in lung function measurements28.
Among all participants in this study, a UIP pattern on HRCT was present in 38.7%, which is consistent with the findings of previous reports13,14. Roca et al. assessed 21 patients with SJS-ILD and reported that 5 (23.8%) showed a UIP pattern on HRCT13. Enomoto et al. evaluated 33 patients with SJS-ILD (all biopsy-proven cases), also demonstrating that 11 (33.3%) patients showed a UIP pattern on HRCT14. However, Kamiya et al. evaluated 99 radiologically diagnosed cases of SJS-ILD and reported that when HRCT scans were categorized according to the 2018 Fleischner society guideline, a UIP pattern on HRCT was present in only 9 (9%) cases29. Our study also showed that patients with a UIP pattern on HRCT had a poorer clinical course and survival than those without it, and a UIP pattern was an independent prognostic factor in the multivariable analysis. A previous report supports our findings13. Roca et al. reported that a UIP pattern on HRCT appeared more frequently in patients with ILD deterioration than in those without (42.9% vs. 16.7%)13. However, Kamiya et al. demonstrated that HRCT patterns were not related to poor outcomes in unadjusted (HR 0.41; 95% CI 0.06–3.09; P = 0.39) and multivariable (HR 0.40; 95% CI 0.05–3.10; P = 0.38) Cox analyses adjusted by age and sex29. These different results might be attributable to differences in the study population (prevalence of a UIP pattern, 9% [Kamiya et al.] vs. 38.7% [our study]).
In our study, age, FVC, and a UIP pattern on HRCT were independent prognostic factors in patients with SJS-ILD, and our findings were consistent with the findings of previous reports14,15,29. Ito et al. assessed 33 patients with lung involvement in primary SJS and reported that lower PaO2 and presence of microscopic honeycombing on surgical lung biopsy were poor prognostic factors for mortality15. Enomoto et al. assessed 33 patients with SJS-ILD and also reported that higher PaCO2, the extent of reticular abnormalities on HRCT, and the severity of fibroblastic foci on surgical lung biopsy were associated with poorer prognosis14. Moreover, Kamiya et al. evaluated 99 patients with SJS-ILD and demonstrated that older age and lower FVC were significantly associated with worse survival29. However, our study was the first to show that a UIP pattern on HRCT could be an independent prognostic factor for survival in patients with SJS-ILD.
A probable UIP pattern on HRCT in CTD-ILD is known to have similar prognostic effects as in IPF30. Yunt et al., in 158 patients with rheumatoid arthritis (RA)-ILD, reported that there was no difference in survival between patients with definite UIP pattern on HRCT and those with possible UIP (median survival: 8.27 vs. 6.14 years, p = 0.99), but those with definite or possible UIP had worse survival than those with NSIP (p = 0.03)30. However, there is also a contradicting finding showing that patients with definite UIP on HRCT have a poorer prognosis than those with probable UIP in CTD-ILD31. Jacob et al., in 157 patients with RA-ILD, reported that when CT findings are categorized in 3 groups (definite UIP, probable and inconsistent with UIP), those with a definite UIP pattern (3-year survival: 55%) had similar survival to IPF patients (n = 284, 3-year survival: 42%), but worse than those with a probable UIP (3-year survival: 56%)31. In our study, a UIP pattern on HRCT was linked to worse survival compared to probable UIP. This finding might be due to the higher frequency of pathological NSIP pattern in patients with probable UIP pattern on HRCT in SJS-ILD than that in IPF or RA-ILD; in our study, the most common histopathologic pattern was NSIP in patients with radiologic probable UIP pattern (6 out of 9, 66.7%).
This study has some limitations. First, it was a retrospective observational study conducted at a single center. However, the baseline characteristics of the subjects were similar to those reported in other studies12,13,14,15,32. Second, most of the patients were treated with steroid and/or immunosuppressants, which could have influenced their clinical course and outcomes. However, the treatment was not different between non-survivors and survivors, and thus, was not associated with the prognosis of patients in our study. Finally, the date of diagnosis of patients spans a wide range from 2000 to 2016. However, the survival rates between patients diagnosed before and after 2010 were not different (5-year survival rate: 82.1% and 76.1% respectively, P = 0.570). Despite these limitations, our study showed the long-term clinical course and outcomes in a large number of patients with SJS-ILD.
In conclusion, we identified the chronically deteriorating nature of SJS-ILD with around half of the patients experiencing ILD progression over 5 years. Our results suggest that older age, higher CRP, lower FVC and a UIP pattern on HRCT indicate a poorer prognosis in patients with SJS-ILD.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Pertovaara, M. et al. Clinical follow up study of 87 patients with sicca symptoms (dryness of eyes or mouth, or both). Ann. Rheum. Dis. 58, 423–427. https://doi.org/10.1136/ard.58.7.423 (1999).
Ramos-Casals, M., Tzioufas, A. G. & Font, J. Primary Sjogren’s syndrome: New clinical and therapeutic concepts. Ann. Rheum. Dis. 64, 347–354. https://doi.org/10.1136/ard.2004.025676 (2005).
Lynch, D. A. et al. Diagnostic criteria for idiopathic pulmonary fibrosis: A Fleischner Society White Paper. Lancet Respir. Med. 6, 138–153. https://doi.org/10.1016/s2213-2600(17)30433-2 (2018).
Asmussen, K., Andersen, V., Bendixen, G., Schiodt, M. & Oxholm, P. A new model for classification of disease manifestations in primary Sjogren’s syndrome: Evaluation in a retrospective long-term study. J. Intern. Med. 239, 475–482. https://doi.org/10.1046/j.1365-2796.1996.418817000.x (1996).
Baldini, C. et al. Primary Sjogren’s syndrome as a multi-organ disease: Impact of the serological profile on the clinical presentation of the disease in a large cohort of Italian patients. Rheumatology (Oxford) 53, 839–844. https://doi.org/10.1093/rheumatology/ket427 (2014).
Francois, H. & Mariette, X. Renal involvement in primary Sjogren syndrome. Nat. Rev. Nephrol. 12, 82–93. https://doi.org/10.1038/nrneph.2015.174 (2016).
Kokosi, M., Riemer, E. C. & Highland, K. B. Pulmonary involvement in Sjogren syndrome. Clin. Chest Med. 31, 489–500. https://doi.org/10.1016/j.ccm.2010.05.007 (2010).
Rashtak, S. & Pittelkow, M. R. Skin involvement in systemic autoimmune diseases. Curr. Dir. Autoimmun. 10, 344–358. https://doi.org/10.1159/000131754 (2008).
Vassiliou, V. A., Moyssakis, I., Boki, K. A. & Moutsopoulos, H. M. Is the heart affected in primary Sjogren’s syndrome? An echocardiographic study. Clin. Exp. Rheumatol. 26, 109–112 (2008).
Palm, O. et al. Clinical pulmonary involvement in primary Sjogren’s syndrome: Prevalence, quality of life and mortality—A retrospective study based on registry data. Rheumatology (Oxford) 52, 173–179. https://doi.org/10.1093/rheumatology/kes311 (2013).
Ramos-Casals, M. et al. Characterization of systemic disease in primary Sjögren’s syndrome: EULAR-SS Task Force recommendations for articular, cutaneous, pulmonary and renal involvements. Rheumatology (Oxford) 54, 2230–2238. https://doi.org/10.1093/rheumatology/kev200 (2015).
Parambil, J. G., Myers, J. L., Lindell, R. M., Matteson, E. L. & Ryu, J. H. Interstitial lung disease in primary Sjogren syndrome. Chest 130, 1489–1495. https://doi.org/10.1378/chest.130.5.1489 (2006).
Roca, F. et al. Interstitial lung disease in primary Sjogren’s syndrome. Autoimmun. Rev. 16, 48–54. https://doi.org/10.1016/j.autrev.2016.09.017 (2017).
Enomoto, Y. et al. Prognostic factors in interstitial lung disease associated with primary Sjogren’s syndrome: A retrospective analysis of 33 pathologically-proven cases. PLoS ONE 8, e73774. https://doi.org/10.1371/journal.pone.0073774 (2013).
Ito, I. et al. Pulmonary manifestations of primary Sjogren’s syndrome: A clinical, radiologic, and pathologic study. Am. J. Respir. Crit. Care Med. 171, 632–638. https://doi.org/10.1164/rccm.200403-417OC (2005).
Lundberg, I. E. et al. 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann. Rheum. Dis. 76, 1955–1964. https://doi.org/10.1136/annrheumdis-2017-211468 (2017).
Macintyre, N. et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur. Respir. J. 26, 720–735. https://doi.org/10.1183/09031936.05.00034905 (2005).
Miller, M. R. et al. Standardisation of spirometry. Eur. Respir. J. 26, 319–338. https://doi.org/10.1183/09031936.05.00034805 (2005).
Wanger, J. et al. Standardisation of the measurement of lung volumes. Eur. Respir. J. 26, 511–522. https://doi.org/10.1183/09031936.05.00035005 (2005).
Holland, A. E. et al. An official European Respiratory Society/American Thoracic Society technical standard: Field walking tests in chronic respiratory disease. Eur. Respir. J. 44, 1428–1446. https://doi.org/10.1183/09031936.00150314 (2014).
Meyer, K. C. et al. An official American Thoracic Society clinical practice guideline: The clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am. J. Respir. Crit. Care Med. 185, 1004–1014. https://doi.org/10.1164/rccm.201202-0320ST (2012).
Collard, H. R. et al. Acute exacerbations of idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 176, 636–643. https://doi.org/10.1164/rccm.200703-463PP (2007).
Galie, N. et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Heart J. 37, 67–119. https://doi.org/10.1093/eurheartj/ehv317 (2016).
American Thoracic Society. Idiopathic pulmonary fibrosis: Diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am. J. Respir. Crit. Care Med. 161, 646–664. https://doi.org/10.1164/ajrccm.161.2.ats3-00 (2000).
Travis, W. D. et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 188, 733–748. https://doi.org/10.1164/rccm.201308-1483ST (2013).
Manfredi, A. et al. Acute exacerbation of interstitial lung diseases secondary to systemic rheumatic diseases: A prospective study and review of the literature. J. Thorac. Dis. 11, 1621–1628. https://doi.org/10.21037/jtd.2019.03.28 (2019).
Suda, T. et al. Acute exacerbation of interstitial pneumonia associated with collagen vascular diseases. Respir. Med. 103, 846–853. https://doi.org/10.1016/j.rmed.2008.12.019 (2009).
Paschalaki, K. E., Jacob, J. & Wells, A. U. Monitoring of lung involvement in rheumatologic disease. Respiration 91, 89–98. https://doi.org/10.1159/000442890 (2016).
Kamiya, Y. et al. Prognostic factors for primary Sjögren’s syndrome-associated interstitial lung diseases. Respir. Med. 159, 105811. https://doi.org/10.1016/j.rmed.2019.105811 (2019).
Yunt, Z. X. et al. High resolution computed tomography pattern of usual interstitial pneumonia in rheumatoid arthritis-associated interstitial lung disease: Relationship to survival. Respir. Med. 126, 100–104. https://doi.org/10.1016/j.rmed.2017.03.027 (2017).
Jacob, J. et al. Predicting outcomes in rheumatoid arthritis related interstitial lung disease. Eur. Respir. J. https://doi.org/10.1183/13993003.00869-2018 (2019).
Gao, H. et al. Prevalence, risk factors, and prognosis of interstitial lung disease in a large cohort of Chinese primary Sjogren syndrome patients: A case-control study. Medicine 97, e11003. https://doi.org/10.1097/md.0000000000011003 (2018).
Acknowledgements
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and Technology (NRF-2019R1A2C2008541), and a Grant (2020IL0036) from the Asan Institute for Life Sciences, Asan Medical Center, Seoul, Republic of Korea. The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
J.W.S. is the guarantor of the paper and takes responsibility for the integrity of the work as a whole. J.W.S., Y.J.K. and H.J.K. take responsibility for the data analysis, interpretation of results and drafted the initial manuscript. J.C. performed the radiological evaluation of the study subjects. All authors discussed the results and reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, Y.J., Choe, J., Kim, H.J. et al. Long-term clinical course and outcome in patients with primary Sjögren syndrome-associated interstitial lung disease. Sci Rep 11, 12827 (2021). https://doi.org/10.1038/s41598-021-92024-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-92024-2
- Springer Nature Limited
This article is cited by
-
Malnutrition is associated with mortality in Sjögren’s syndrome-associated interstitial lung disease
Scientific Reports (2024)
-
Clinical outcomes and risk factors of progressive pulmonary fibrosis in primary Sjögren’s syndrome-associated interstitial lung disease
BMC Pulmonary Medicine (2023)
-
Clinical course and risk factors for development and progression of interstitial lung disease in primary Sjögren’s syndrome
Scientific Reports (2023)
-
Risk factors and prognosis of interstitial lung disease for primary Sjögren syndrome patients: A retrospective case‒control study
Clinical Rheumatology (2023)
-
Risk factors for progression of interstitial lung disease in Sjögren’s syndrome
Clinical Rheumatology (2022)