Abstract
Anal high-risk human papillomavirus (hr-HPV) infection is widely considered a cause of anal cancer. However, epidemiological data are quite limited in Japan. This study investigated anal HPV infections and cytological abnormalities among MSM with or without HIV infection. Anal swabs were obtained, and cytological results were examined. Hybrid capture-based methodology was used for hr-HPV genotyping. The exclusion criterion was a history of vaccination against HPV. 644 subjects participated, and the overall prevalence of hr-HPV was 59.7% (95% CI 54.7–62.3), HIV-infected had higher prevalence than HIV-uninfected (68.9% vs 40.6%) p < .001. Among hr-HPV-infected participants, 82.8% (312/377) were infected with at least one of 9 valent vaccine-covered hr-HPV genotypes. From regression analysis, detection of abnormal cytology correlated positively with HIV infection (OR 2.17 [95% CI 1.51–3.13]), number of hr-HPV genotypes infected (OR 1.83 [1.59–2.10]), history of STI (OR 1.58 [1.14–2.22]) and No. of lifetime sexual partners (OR 1.56 [1.10–2.21]), albeit multivariate analysis identified the number of detected hr-HPV genotypes (adjusted OR 1.78 [1.54–2.06]) as the independent risk factor for abnormal cytology. High rates of anal hr-HPV infection, especially 9-valent HPV vaccine-preventable hr-HPV were detected among our MSM participants in Japan. HPV vaccination should also be encouraged for MSM in Japan.
Similar content being viewed by others
Introduction
Anal chronic infection by high-risk types of human papillomavirus (hr-HPV) and HPV-related malignancies are a growing concern of public health among men who have sex with men (MSM). Indeed, the prevalence of hr-HPV is extremely high not only among MSM with anal cancer but also among asymptomatic MSM1,2, and hr-HPV positivity and the risk of anal cancer are even greater in HIV-infected individuals3,4,5. Anal cancer screening using anal cytology has recently been performed in a few developed countries6, and in such countries, HPV vaccination is recommended for all male and female adolescents (from 9 to 26 years). Moreover, the Advisory Committee on Immunization Practices (ACIPs) in 2019 recommended shared clinical decision-making regarding HPV vaccination for some adults aged 27 through 45 years who are not adequately vaccinated7,8, even though preventive effects of vaccination against anal cancer or new acquisition of hr-HPV in sexually active adult populations have not been clearly demonstrated.
In Japan, the HPV vaccine for male individuals was approved only very recently, whereas genotyping of anal hr-HPV and anal-canal cancer screening are not currently approved. Furthermore, epidemiological data for MSM, including prevalent hr-HPV genotypes and related histopathological abnormalities, are quite limited, despite preliminary data suggesting high prevalence9.
In the present study, we assessed the epidemiology of anal colonization by hr-HPV and HPV-related abnormal cytology among HIV-infected and uninfected MSM and sought to discuss future strategies to prevent HPV-related anal cancer in Japan.
Results
Characteristics of the study participants
We enrolled a total of 644 MSM, including 437 HIV-infected and 207 HIV-uninfected eligible consenting participants. The demographic characteristics according to HIV-infected and uninfected subjects are presented in Table 1. The median age among HIV-infected and -uninfected participants was 46 (Interquartile range (IQR) 40–53) and 36 (IQR 29–43) years, respectively (p < 0.001).
Compared to HIV-uninfected subjects, infected subjects had a significantly larger number of sexual partners in their lifetime but had fewer sexual partners in the past 6 months. There was no significant difference in the percentage use of condoms during anal sex in either group, but a history of STIs was significantly higher in HIV-infected than -uninfected subjects. All HIV-infected individuals were being treated with anti-retroviral therapy (ART), and plasma HIV RNA was undetectable (< 20 copies/mL) in 97.7% (427/437). For viremic patients, viral load was in the range of 21–119 copies/mL. CD4 counts were high, with a median of 666 × 109/L (IQR 512–821).
Prevalence of human papilloma virus
Among the participants, sufficient anal samples for hr-HPV genotyping were available for 632/644 (98.1%) (425 HIV infected and 207 HIV uninfected). Figure 1 displays the distribution of infection with different hr-HPV genotypes in both HIV-infected and -uninfected patients. The overall rate of hr-HPV positivity was 59.7% [95% confidence interval (CI) 55.8–63.4], with HIV-infected MSM (68.9% [95% CI 64.4–73.2]) having a significantly higher rate than HIV-uninfected MSM (40.6% [95% CI 34.1–47.4]). The most significant difference between HIV-infected and -uninfected individuals was found for HPV52 and -58 (p < 0.001); HPV16, -52 and -58 were the most common HPV genotypes. The number of hr-HPV genotypes found in each participant stratified by HIV serostatus is illustrated in Fig. 2. Although there were more participants without hr-HPV infection among HIV-uninfected individuals than HIV-infected individuals (123/207 (59.4%) vs 132/425 (31.1%) (p < 0.001)), subjects with multiple infections were more prevalent among the latter than the former (182/425 (42.8%) vs 29/207 (14.0%) (p < 0.001).
Additionally, we observed that 312 of 377 h-HPV-infected subjects (82.8%), including 246 HIV-infected (84.0%) and 66 HIV-uninfected (78.6%) participants (p = 0.249), carried at least one of 9 valent vaccine-covered hr-HPV genotypes (genotypes 16, 18, 31, 33, 45, 52, and 58) (see Supplementary Table S1 online). When assessing according to age group in all subjects, most genotypes were equally prevalent, however, HPV52 and HPV56 prevalence rates tend to increase significantly with age (p = 0.0081 and p = 0.0058 respectively), (see Supplementary Figure S1 online).
Cytology results
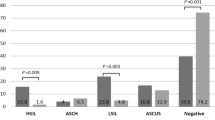
Cytology results were available for all participants, with sufficient anal samples for 644 (437 HIV infected and 207 HIV uninfected) subjects. Findings among participants with NILM (negative for intraepithelial lesion or malignancy), termed as normal cytology, or ASCUS + (atypical squamous cell of undetermined significance), termed as abnormal cytology, are shown in Table 2. Despite no age difference between participants with normal or abnormal anal cytology, HIV-infected MSM more often had abnormal anal cytology. Overall, we found no difference in the number of sexual partners in the past 6 months or in the percentage use of condoms during receptive anal sex in either group. Participants with abnormal anal cytology showed a prevalence of 25.8% and 12.9% for HPV16 and HPV18 infection, respectively, compared to 11.6% and 4.7% for those with normal anal cytology (p < 0.001). The prevalence of all hr-HPV types was 45.0% (182/404) for normal anal cytology, 79.2% (152/192) for ASC-US, 94.4% (17/18) for LSIL, 94.4% (17/18) for ASC-H, and 75.0% (9/12) for HSIL. Anal abnormal cytology results were observed in 37.3% (240/644) of all participants, with more cases among HIV-infected individuals (187/437; 42.8%) than HIV-uninfected individuals (53/207; 25.6%) (p < 0.001). Next, we assessed the impact of hr-HPV genotype on abnormal anal cytology between HIV-infected and -uninfected participants. Figure 3a depicts the frequency of anal abnormal cytology results based on each hr-HPV genotype. Compared to HIV-uninfected individuals, HIV-infected individuals had a significantly higher prevalence of anal abnormal cytology overall (86.6% versus 62.3%, p < 0.001), but with a significant difference only detected for HPV31, -51, -56 and -68. Correlation between the frequency of anal abnormal cytology and the number of hr-HPV genotypes detected in a subject is shown in Fig. 3b, and there was a significant chi-square trend for all subjects with abnormal cytology (p < 0.001) (Supplementary Table S2 shows stratification of the result by HIV serostatus).
Frequency of abnormal anal cytology via anal swabs in HIV-infected and -uninfected patients. (a) The frequency of abnormal anal cytology results (ASC-US, LSIL, ASC-H, or HSIL) is presented in accordance with each hr-HPV genotype in both subjects (644). (b) The frequency of abnormal cytology results was calculated in accordance with the number of hr-HPV genotypes found in each subject (see Supplementary Table S2 for stratification by HIV serostatus). HPV human papillomavirus, hr-HPV high-risk types of human papillomavirus.
Risk factors for abnormal cytology
Finally, univariate and multivariate regression analyses were performed to identify factors affecting development of abnormal cytology. In univariate analysis, HIV infection (OR 2.17; 95% CI 1.51–3.13), number of detected hr-HPV genotypes (OR 1.83; 95% CI 1.59–2.10), past treatment history of STI (OR 1.58; 95% CI 1.14–2.22) and No. of lifetime sexual partners (OR 1.56; 95% CI 1.10–2.21) were significantly associated with abnormal cytology results, as indicated in Table 3. In multivariate analysis, HIV infection, history of STI and No. of lifetime sexual partners did not show independent risk factors for abnormal cytology, but the number of infected hr-HPV genotypes showed strong significance (aOR 1.78; 95% CI 1.54–2.06], p < 0.001).
Discussion
Anal HPV infection and its contribution to the risk of anal cancer is a growing concern, especially among MSM. However, epidemiological data remain limited for MSM in Japan, regardless of their HIV serostatus. Our study was performed at one of the largest healthcare centers for the HIV-infected population in Tokyo, and 90% of HIV seropositive individuals in Japan are MSM. The center also has the largest sexual health clinic for HIV-seronegative MSM in Japan as of now, where regular check-ups for HIV and STI testing, as well as HIV pre- and post-exposure prophylaxis, are carried out. Therefore, this study should generally represent a large proportion of MSM in eastern Japan. The results of the study showed that the prevalence of anal hr-HPV infection among all participants was 59.7% [95% CI 55.8–63.4] and that the most frequently detected hr-HPV genotypes were HPV-52 (18.8%), HPV-16 (17.2%), and HPV-58 (16.0%). Our previous study conducted 10 years ago in HIV-infected MSM identified a prevalence of hr-HPV infection as high as 75.9%, and the most frequent hr-HPV genotypes were HPV-58 (30.2%), HPV-16 (28.8%) and HPV-52 (22.2%)9. These 3 genotypes might be the predominant hr-HPV genotypes among Japanese MSM during the last decade. In this study, the prevalence of anal hr-HPV among HIV-infected subjects was 68.9% [95% CI 64.4–73.2], with a lower rate of 40.6% for HIV-uninfected subjects [95% CI 34.1–47.4]. The high prevalence of hr-HPV in HIV-seropositive MSM is similar to reports for other Asian countries, but the distribution of hr-HPV genotypes differs somewhat4,10,11. For example, the prevalence of hr-HPV infection is reportedly 85.2% in Taiwan, 82.7% in China and 85% in Thailand, and the most frequent hr-HPV genotypes are HPV-16, HPV-51 and HPV-52 in Taiwan and Thailand and HPV-16 and HPV-18 in China4,10,11.
In terms of anal cytology, the prevalence of hr-HPV was 45% for normal anal cytology, 79.2% for ASC-US, 94.4% for both LSIL and ASC-H, and 75.0% for HSIL. These findings are consistent with previous reports10. In addition, HIV-infected individuals were found to carry more hr-HPV genotypes than HIV-uninfected individuals and had an increased risk of abnormal anal cytology. Moreover, this study clearly documents for the first time in Japan that the prevalence of abnormal cytology correlates positively with the number of hr-HPV genotypes detected (Fig. 3b), as was discussed in a previous study10. This correlation was even stronger in HIV-infected than -uninfected, as shown in Supplementary Table S2. On the other hand, the prevalence of abnormal cytology was not greatly influenced by the difference in genotype (Fig. 3a). Furthermore, 82.8% (312/377) of subjects infected with hr-HPV (246 HIV infected and 66 HIV uninfected), harbored at least one of the 9 valent vaccine-covered hr-HPV genotypes, suggesting that HPV vaccination should be recommended, regardless of HIV serostatus and even to those who are already infected with some hr-HPV genotypes. Such measures would prevent acquisition of vaccine-preventable hr-HPV genotypes, as indicated previously12. Another associated risk factor for abnormal cytology was HIV infection, though among our participants, HIV infection was well controlled by antiretroviral therapy (ART). Nevertheless, it is not clear from the present cross-sectional study whether HIV infection preceding hr-HPV infection causes impaired clearance of hr-HPV infection or whether HIV-infected individuals re-acquire hr-HPV more readily.
There are some limitations to be considered in the present study. First, this was a single-site, clinic-based, cross-sectional study in Tokyo that does not represent the entire MSM population in Japan. Second, the study did not assess whether a case of hr-HPV infection was a transient infection, a persistent infection, or a reinfection. Third, this study only analyzed hr-HPV genotypes; therefore, not all hr-HPV genotypes were assessed. Finally, cytology results for HIV-uninfected subjects were reported only through a Pap smear and then translated to the Bethesda system using a previously reported relationship for the two systems13.
In conclusion, we found that hr-HPV, especially 9-valent HPV vaccine-preventable hr-HPV genotypes, is highly prevalent among MSM in Japan. Our study showed that an increased number of hr-HPV genotypes infected, is strongly associated with development of anal abnormal cytology; thus, HPV vaccination for Japanese MSM, those already infected or not, should be considered to prevent further acquisition of more hr-HPV genotypes, that would lead to HPV related anal malignancy. Further studies are needed to assess the health impact and cost-effectiveness of nine-valent HPV vaccination.
Methods
Ethics statement
Written informed consent for HPV and anal cytology testing was obtained from all participants. This study was approved by the ethics committee of the National Center for Global Health and Medicine (approval no. NCGM-G-002390). This study was implemented in accordance with the provisions of the Declaration of Helsinki.
Study population
There is an existing group of HIV-infected MSM being treated at AIDS Clinical Center (ACC), National Center for Global Health and Medicine (NCGM), Tokyo, as well as a non-HIV-infected MSM group at the Sexual Health Clinic (SHC) outpatient in the same center. We conducted a cross-sectional study in these two groups between January 1, 2019, and March 31, 2019, for HIV-uninfected individuals and June 1 to August 31, 2019, for HIV-infected individuals. Eligibility criteria included male sex, age ≥ 16 years from SHC and age ≥ 20 years from ACC, self-reported history of having anal receptive sex with men, willingness to undergo anal swab sampling to test for hr-HPV infection, and anal cytology. Exclusion criteria was a history of HPV vaccination.
Data collection
Each participant completed a standardized questionnaire. The information gathered included sociodemographic data (age), sexual behavior data (number of lifetime sexual partners, number of sexual partners in the last 6 months, percentage use of condoms (using measurement scales of 0 to 100, with intervals of 10), and history of sexually transmitted infection (including genital/oral/anal chlamydia, gonorrhea and herpes, syphilis, and genital/anal warts).
Anal specimen collection for HPV genotyping and cytology
Samples were collected at the enrollment visit by well-trained health-care professionals using digene® specimen collection swabs, with a 5 cm insertion into the anal canal and a slow 360-degree rotating extraction. The swab was then shaken in BD SurePath liquid-based cytology fluid, and all samples were processed at the external laboratory LSI Medience Corporation Tokyo, Japan. HPV DNA was detected by hybrid capture-based methodology, as described previously14, which included only oncogenic or hr-HPV genotypes (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68)15. Anal cytology slides were interpreted by two cytopathologists blinded to the HIV or HPV infection status. Liquid-based cytology was prepared for routine Pap staining. The cytology results were reported first using Papanicolaou Classification (Class I–V)16 and also reported using Bethesda system classification categories, as follows: NILM, ASC-US, LSIL (low-grade squamous intraepithelial lesion), ASC-H (atypical squamous cells, cannot exclude high-grade lesion) and HSIL (high-grade squamous intraepithelial lesions)16. Slides with discordant interpretations were reviewed again under a multihead microscope to reach an agreement. Cytology results for HIV-uninfected subjects were reported only using Pap smears and then translated to the Bethesda system using the previously reported relationship of the two systems13; results for HIV-infected subjects were reported using both Pap smears and the Bethesda system.
Statistical analysis
All statistical tests were evaluated at a 0.05 level of significance. Statistical analyses were performed with GraphPad Prism 7.0 and regression analysis with Statistical Package for Social Sciences (SPSS) software, version 23.0. We report categorical data as numbers (n) and percentages (%) and continuous variables as median and interquartile range (IQR). We compared differences in sociodemographics and sexual behaviors between HIV-infected and uninfected subjects and performed descriptive analysis. Mann–Whitney U tests were employed for continuous variables, and Chi-squared/Fisher's exact tests were used for categorical variables. The odds ratios (ORs) of significant factors associated with hr-HPV infection and the detection of anal abnormal cytological lesions were calculated using logistic regression analysis. Multivariate logistic regression models were then applied to the significant factors (p < 0.05) in univariate analysis.
References
Vuyst, D. H., Clifford, G. M., Nascimento, M. C., Madeleine, M. M. & Franceschi, S. Prevalence and type distribution of human papillomavirus in carcinoma and intraepithelial neoplasia of the vulva, vagina and anus: A meta-analysis. Int. J. Cancer 124, 1626–1636 (2009).
Daling, J. R. et al. Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer. Cancer 101, 270–80 (2004).
D’Souza, G. et al. Incidence and epidemiology of anal cancer in the multicenter AIDS cohort study. J. Acquir. Immune Defic. Syndr. 48, 491–499 (2008).
Phanuphak, N. et al. Anal human papillomavirus infection among Thai men who have sex with men with and without HIV infection: prevalence, incidence, and persistence. J. Acquir. Immune Defic. Syndr. 63, 472–479 (2013).
Ferlay, J. et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 136, E359–E386 (2015).
Park, I. U. & Palefsky, J. M. Evaluation and management of anal intraepithelial neoplasia in HIV-negative and HIV-positive men who have sex with men. Curr. Infect. Dis. Rep. 12, 126–133 (2010).
Meites, E. et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb. Mortal. Wkly. Rep. 68, 698–702 (2019).
Food and Drug Administration. Prescribing information [package insert]. Gardasil 9 (Human Papillomavirus 9-Valent Vaccine, Recombinant). US Department of Health and Human Services, Food and Drug Administration. https://www.fda.gov/media/90064/download (2018).
Nagata, N. et al. Prevalence of anal human papillomavirus infection and risk factors among HIV-positive patients in Tokyo, Japan. PLoS ONE 10(9), e0137434 (2015).
Ping-Feng, W. et al. Anal human papillomavirus and its associations with abnormal anal cytology among men who have sex with men. Sci. Rep. 10, 3165. https://doi.org/10.1038/s41598-020-59967-4 (2020).
Li, X. et al. Anal HPV/HIV co-infection among men who have sex with men: A cross-sectional survey from three cities in China. Sci. Rep. 6, 21368 (2016).
King, E. M. et al. Human papillomavirus DNA in men who have sex with men: type-specific prevalence, risk factors and implications for vaccination strategies. Br. J. Cancer. 112, 1585–1593 (2015).
van Hamont, D., van Ham, M. A., Bakkers, J. M., Massuger, L. F. & Melchers, W. J. Evaluation of the SPF10-INNO LiPA human papillomavirus (HPV) genotyping test and the Roche Linear Array HPV genotyping test. J. Clin. Microbiol. 44, 3122–3129 (2006).
Yoshikawa, H. et al. Detection and typing of multiple genital human papillomaviruses by DNA amplification with consensus primers. Jpn. J. Cancer Res. 82, 524–531 (1991).
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans Human papillomaviruses, Vol. 90, 1–636. World Health Organization (2007).
Hugh, M., Roman, L., William, W. & Robert, A. The current status of the Papanicolaou smear. CA Cancer J. Clin. 45, 305–320 (1995).
Acknowledgements
The authors thank all staff members of AIDS Clinical Center, National Center for Global Health and Medicine, Tokyo, Japan, for their contributions in this study.
Funding
This study was supported in part by funds from the National Center for Global Health and Medicine [Grant No. 19A2011].
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: D.S., D.M., M.T. Performed the experiments: D.S., D.M., K.W., N.A., H.U., Y.Y., A.T., J.T., K.T., K.T., Y.K. Analyzed the data: D.S., D.M., K.W., H.G., S.O. Supervised the study and manuscript drafting: D.M., K.W., H.G., S.O. Wrote the paper: D.S. All of the authors critically revised this manuscript for important intellectual content and approved the final version submitted for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shiojiri, D., Mizushima, D., Takano, M. et al. Anal human papillomavirus infection and its relationship with abnormal anal cytology among MSM with or without HIV infection in Japan. Sci Rep 11, 19257 (2021). https://doi.org/10.1038/s41598-021-98720-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-98720-3
- Springer Nature Limited