Abstract
The neutrophil-to-lymphocyte ratio (NLR) and lymphocyte-to-monocyte ratio (LMR) have a strong association with prognosis in patients with Stage II/III rectal cancer (RC). We attempted to explore a new system combining these two ratios, named the NLM score, and examine its prognostic value in Stage II/III RC patients undergoing neoadjuvant chemoradiotherapy (NCRT). We retrospectively analyzed data of 237 stage II/III RC patients who underwent NCRT followed by standard TME in our hospital and defined the NLM score as follows: Score 2: pre-NCRT NLR > 2.565 and pre-NCRT LMR < 2.410. Score 1: (pre-NCRT NLR > 2.565 and pre-NCRT LMR > 2.410) OR (pre-NCRT NLR < 2.565 and pre-NCRT LMR < 2.410). Score 0: pre-NCRT NLR < 2.565 and pre-NCRT LMR > 2.410. Multivariate analyses implied that lower ypTNM stage (stage 0–I vs. II–III) (hazard ratio [HR] 0.420, 95% confidence interval [CI] 0.180–0.980 for OS; HR 0.375, 95% CI 0.163–0.862 for DFS) and an NLM score ≤ 1 (HR 0.288, 95% CI 0.134–0.619 for OS; HR 0.229, 95% CI 0.107–0.494 for DFS) could independently predict better overall survival (OS) and disease-free survival (DFS). The novel scoring system, which integrated pre-NCRT NLR and pre-NCRT LMR, was an independent prognostic factor in stage II/III RC patients undergoing NRCT and had better predictive values than these ratios alone.
Similar content being viewed by others
Introduction
Neoadjuvant chemoradiotherapy followed by radical resection is the standard treatment for stage III RC patients and stage II RC patients with high-risk factors1,2. While lymph node metastasis, vessel invasion, R1 or R2 resection, and high tumor stage are identified as unfavorable prognostic factors, patients with comparable risk elements have a wide variation of oncology outcomes3,4. This possibly contributes to the differences in the patients' tumor microenvironment and immune system4,5.
Evidence has shown that the systemic inflammatory response (SIR) is associated with cancer progression, evolution, and metastasis in recent years6,7. Neutrophil-to-lymphocyte (NLR) and Lymphocyte-to-Monocyte Ratio (LMR) are markers of SIR, and they are significantly related to the clinical prognosis of patients in various tumors8,9,10. Moreover, these markers are easy to obtain and inexpensive9. Nevertheless, all previous research focused on a single index, and the results of these studies were inconsistent. Consequently, we assumed that a scoring system combining these two ratios pre-NCRT might better prognostic prediction than the single ratio.
This study defined an innovative prognostic scoring system that integrated the pre-NCRT NLR and pre-NCRT LMR named NLM score and assessed its prognostic value for RC patients undergoing NCRT.
Methods
Patients population and data collection
We retrospectively reviewed the prospective clinical database of the Department of Gastroenterology, Jiangjin DistrictCentral Hospital. Stage II/III RC patients undergoing NCRT were included. The inclusion criteria were as follows: (1) RC patients undergoing 5-FU based NCRT. (2) Confirmed diagnosis of RC by biopsy (3) RC patients undergoing radical TME. The exclusion criteria were: (1) RC patients with distant metastasis. (2) Clinical indication of inflammatory disorder or infection, such as rheumatoid arthritis or inflammatory bowel disease; (3) Recurrent tumors (4) patients with other malignancies. (5) insufficient data.
Clinicopathological data were all obtained from electronic medical records, including age, gender, ypT stage, ypN stage, ypTNM stage, pathological CRM, vascular invasion, lymphatic invasion, perineural invasion, and laboratory data. Venous blood samples were drawn close to the time of NCRT initiation. Histopathological staging (ypT and ypN) was determined according to the American Joint Committee on Cancer Staging Manual (AJCC)11.
The Ethics Committee of Jiangjin District Central Hospital approved this study. Informed consent was waived by the Ethics Committee of Jiangjin District Central Hospital since it was a retrospective study.
All methods were performed in accordance with the relevant guidelines and regulations.
Definition of pre-NCRT NLR, pre-NCRT LMR, and NLM score
We defined pre-NCRT NLR as a ratio of absolute neutrophil to lymphocyte counts., while pre-NCRT LMR as a ratio of absolute lymphocyte to monocyte counts.
The operating curve (ROC) analysis was adopted to determine the optimal pre-NCRT NLR and pre-NCRT LMR cut-off values for predicting death. The cut-off values were 2.565 and 2.410 for NLR and LMR, respectively. Same methods were performed for the optimal cut-off points for pre-NCRT CEA and CA19-9. The cut-off points for them were 3.55 and 19.0, respectively.
The NLM score was defined as: Score 2: NLR > 2.565 and LMR < 2.410. Score 1: NLR > 2.565 and LMR > 2.410 OR NLR < 2.565 and LMR < 2.410. Score 0: NLR < 2.565 and LMR > 2.410.
Treatment, follow-up and endpoints
The multidisciplinary team meeting (MDT) conducted the treatment plan after considering recommendations of NCCN12, ESMO13, and JSCCR14 colorectal cancer guidelines and the patient's physical condition. All included patients received 5-fluorouracil-based chemoradiotherapy, followed by the standard total mesorectum excision (TME) procedure and pathological assessment of specimen12,13,14.
Patients were followed up every three months in the first three years after surgery and every six months after that. Blood tests were accomplished at each follow-up. Chest and abdominal CT scans were conducted every six months, with total colonoscopy one year after the operation and every two years afterward12,13,14. Clinical, radiological, or histological findings were used to monitor tumor recurrence13.
Follow-up data was gained by telephone or directly from outpatient clinic records15. Disease-free survival (DFS) and overall survival (OS) were primary endpoints15. OS was the time interval from operation date to death date or last visit, DFS is defined as the time interval from operation date to recurrence, metastasis, or last visit date15,16. Patients were censored if alive at the last follow-up16.
Statistical analysis
Statistical analysis was performed by SPSS 22.0. Continuous and categorical variables were expressed as median ± interquarteral range and patients' numbers (%), respectively. Association between NLR or LMR and CEA and CA19-9 were assessed by Kaplan–Meier analysis with log-rank test was used for OS and DFS evaluation17.
Cox proportional hazard regression modeling was conducted for univariate (UV) and multivariate (MV) analyses18. UV analysis was performed to evaluate potential risk factors that could be associated with prognosis based on previous research and clinical knowledge19. Variables with a p value < 0.10 were included in MV regression analyses20. To avoid NLR and LMR's influence on the NLM score in the MV analysis, two models excluding and including the NLM score were established.
All statistical tests were bilateral, and 5% was set as the level of statistical significance18,19,20.
Results
Patients characteristics
We incorporated 237 patients according to the inclusion and exclusion criteria. The mean follow-up was 37 (range: 2–122) months. Thirty-six (15.2%) patients died during the follow-up. Among the 237 patients, 150 (63.3%) were male, and 87 (36.7%) were female. During pathological assessment after NCRT and operation, 45 (19.0%) patients presented ypTNM stage 0 (complete response), while 57 (24.1%) in ypTNM stage I, 72 (30.4%) in ypTNM stage II, and 63 (26.6%) in ypTNM stage III. Sixty (25.3%) patients presented lymph node metastasis, thirteen (5.5%) with vascular invasion, thirteen (5.5%) with lymphatic invasion, and forty-one (17.3%) with perineural invasion. The median (IQR) were 4.15 (2.18–10.07) for pre-NCRT CEA, 13.56 (7.80–25.40) for, 2.27 (1.77–2.98) and Detailed information was shown in Table 1.
Associations between the pre-NCRT NLR, pre-NCRT LMR, and NLM score and prognosis
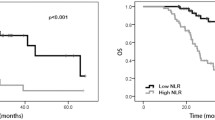
The pre-NCRT NLR > 2.565 had significantly worse OS and DFS than the pre-NCRT NLR < 2.565 (OS, p = 0.002, Fig. 1A; DFS, p = 0.002, Fig. 1B). The pre-NCRT LMR < 2.410 had significantly worse OS and DFS than pre-NCRT LMR > 2.410 (OS, p = 0.001, Fig. 2A; DFS, p = 0.001, Fig. 2B). The higher NLM score predicted the worse OS and DFS (OS, Fig. 3A; DFS, Fig. 3B).
Univariate and multivariate analyses for DFS and OS
On univariate analysis, ypTNM stage, pre-NCRT CEA, pre-NCRT CA19-9, pre-NCRT NLR, pre-NCRT LMR and NLM score were significantly associated with OS (Table 2) and DFS (Table 3). MV analysis model 1 suggested that only ypTNM stage was an independent predictor for OS (HR 0.405, 95% CI 0.174–0.945, p = 0.037), while pre-NCRT NLR (HR 0.525, 95% CI 0.244–1.131, p = 0.100) and pre-NCRT LMR (HR 2.239, 95% CI 0.967–5.182, p = 0.060) were not. ypTNM (HR 0.353, 95% CI 0.154–0.811, p = 0.014) and pre-NCRT LMR (HR 2.739, 95% CI 1.190–6.304, p = 0.018) were significant predictors for DFS, while pre-NCRT NLR (HR 0.510, 95% CI 0.240–1.083, p = 0.510) was not. In MV analysis model 2, ypTNM (HR 0.420, 95% CI 0.180–0.980 for OS; HR 0.375 95% CI 0.163–0.862 for DFS) and NLM score (HR 0.288, 95% CI 0.134–0.619 for OS; HR 0.229, 95% CI 0.107–0.494 for DFS) were significant predictors for both OS and DFS.
Relationship between NLR, LMR and CEA CA19-9 or other clinicopathological factors
Relationship between NLR, LMR and other clinicopathological factors were presented in Tables 4 and 5. Pre-NCRT NLR or LMR had no significant association between other factors.
Discussion
Our research focused on the relationship between pre-NCRT NLR, pre-NCRT LMR, NLM score and OS and DFS in RC patients receiving NCRT. The main finding was that the NLM score was an independent prognostic factor in RC patients and was superior to pre-NCRT NLR and pre-NCRT LMR alone for predicting prognosis.
Previous studies explored the prognostic value of blood cell ratios for RC patients21,22,23. Shen et al.24 investigated 199 patients with locally advanced RC treated with NCRT followed by surgery. They presented a high NLR (≥ 2.8) independently related to poor OS in MV analysis but not to DFS24. The relationship between NLR and prognosis and the cut-off value was similar to ours, but their median follow-up period was relatively short (31 months). Carruthers et al.25 assessed 115 patients, showing that high NLR (≥ 5.0) was an independent prognostic factor for worse OS, decreased time to local recurrence, and shorter DFS25. Their results were consistent with ours in UV analysis. However, they only focused on the prognostic value of the single blood index, not comparing it to others nor combing it with others for better prognosis prediction.
Zhang et al.26 analyzed 472 LARC patients undergoing NCRT and radical surgery. They indicated a high NLR (> 2.3) was an independent predictor for OS and DFS in MV analysis, while LMR was only in UV analysis26. We found that NLR was an independent predictor for only DFS, while LMR was neither. Although we had similar results, they did not combine NLR and LMR for a more powerful prognostic factor. Our study presented that the NLM score had a better prognostic value than both NLR and LMR for both OS and DFS.
Although the mechanism of inflammation index affecting the prognosis of RC is not clear, some meaningful progress has been made. Tumor-associated neutrophils (TANs) origin from peripheral neutrophils27,28. They play an essential role in tumor progression since they could promote tumor growth, cause genetic instability and stimulate angiogenesis27,28. Tumor-associated macrophages (TAMs) are derived from circulating monocytic precursors and are essential in tumor progression's inflammatory microenvironment29. TAMs can produce angiogenesis and growth factors and protease enzymes, which promote extracellular matrix degradation, angiogenesis, tumor cell proliferation, and metastasis30. Different from monocytes and neutrophils, lymphocytes is essential in host cell-mediated immune regulation, which helps destroy residual malignant cells and related micrometastases29. Temporarily, tumor-infiltrating lymphocytes are related to improving the clinical prognosis of cancer27,28,29,30. All of the mechanisms we examined may explain why patients with a high NLR or a low LMR have poor prognosis.
Although similar studies focused on the prognostic value of immune indexes, they only focused on single factors and did not integrate these biomarkers nor compare their prognostic values8,21,22,25,26,29. This study established a novel scoring system combining pre-NCRT NLR and pre-NCRT LMR, named NLM score. The NLM score was superior to single ratios for predicting RC prognosis. Our research found that pre-NCRT NLR has a better prognosis prediction effect than pre-NCRT LMR, indicating neutrophils and monocyte could have different effects on tumor micrometastasis. More profound research in this area may be valuable.
This study had several limitations. Firstly, it was a retrospective study conducted at a single center. Secondly, in this analysis, the optimal cut-off points for pre-NCRT NLR and pre-NCRT LMR were 2.565 and 2.410, respectively. Nevertheless, the cut-off point in this study may only apply to our center's population. If doctors from other medical centers try to apply this prognostic scoring system, we recommend conducting their analysis to determine the optimal cut-off point for a particular patient group. Thirdly, we only enrolled the pre-NCRT NLR and pre-NCRT LMR into our prognostic scoring system. Other systemic inflammatory biomarkers still have high prognostic significance, such as the prognostic nutritional index.22,33 Regrettably, they were not available regularly in our department. Future research on the prognostic scoring system should include as many systemic inflammation indices as possible.
Conclusion
In conclusion, we established a novel prognostic system combing pre-NCRT NLR and pre-NCRT LMR for RC patients. We demonstrated an NLM score ≤ 1 could independently predict better survival. Further studies are necessary to verify its prognostic value.
References
Markovina, S. et al. Improved metastasis- and disease-free survival with preoperative sequential short-course radiation therapy and FOLFOX chemotherapy for rectal cancer compared with neoadjuvant long-course chemoradiotherapy: Results of a matched pair analysis. Int. J. Radiat. Oncol. Biol. Phys. 99(2), 417–426 (2017).
Ruppert, R. et al. Avoidance of overtreatment of rectal cancer by selective chemoradiotherapy: Results of the optimized surgery and MRI-based multimodal therapy trial. J. Am. Coll. Surg. 231(4), 413–25.e2 (2020).
Sun, Q. et al. Perineural and lymphovascular invasion predicts for poor prognosis in locally advanced rectal cancer after neoadjuvant chemoradiotherapy and surgery. J. Cancer 10(10), 2243–2249 (2019).
Tan, Y. et al. Predictors and risk factors of pathologic complete response following neoadjuvant chemoradiotherapy for rectal cancer: A population-based analysis. Front. Oncol. 9, 497 (2019).
Zhang, H. et al. Rectal cancer patients with downstaging after neoadjuvant chemoradiotherapy and radical resection do not benefit from adjuvant chemotherapy. Ann. Transl. Med. 8(12), 743 (2020).
Diakos, C. I., Charles, K. A., McMillan, D. C. & Clarke, S. J. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 15(11), e493-503 (2014).
Roxburgh, C. S. & McMillan, D. C. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 6(1), 149–163 (2010).
Li, K. J. et al. Predictive value of lymphocyte-to-monocyte ratio (LMR) and neutrophil-to-lymphocyte ratio (NLR) in patients with oesophageal cancer undergoing concurrent chemoradiotherapy. BMC Cancer 19(1), 1004 (2019).
Trinh, H. et al. Prognostic value of changes in neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) and lymphocyte-to-monocyte ratio (LMR) for patients with cervical cancer undergoing definitive chemoradiotherapy (dCRT). Clin. Chim. Acta 510, 711–716 (2020).
Yalon, M. et al. Elevated NLR may be a feature of pediatric brain cancer patients. Front. Oncol. 9, 327 (2019).
Amin, M. B. et al. The eighth edition AJCC cancer staging manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 67(2), 93–99 (2017).
Benson, A. B. et al. Rectal cancer, version 2.2018, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 16(7), 874–901 (2018).
Glynne-Jones, R. et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 28(suppl_4), iv22–iv40 (2017).
Watanabe, T. et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int. J. Clin. Oncol. 23(1), 1–34 (2018).
Burke, H. B. Overall survival vs disease-specific survival. JAMA Oncol. 4(4), 586 (2018).
Broglio, K. R. & Berry, D. A. Detecting an overall survival benefit that is derived from progression-free survival. J. Natl. Cancer Inst. 101(23), 1642–1649 (2009).
Rich, J. T. et al. A practical guide to understanding Kaplan–Meier curves. Otolaryngol. Head Neck Surg. 143(3), 331–336 (2010).
Hsu, C. H. & Yu, M. Cox regression analysis with missing covariates via nonparametric multiple imputation. Stat. Methods Med. Res. 28(6), 1676–1688 (2019).
Kawada, T. Dietary factors and incidence of dementia: Cox regression analysis with special emphasis on the number of events. J. Am. Geriatr. Soc. 62(12), 2467 (2014).
Nicolai, P. et al. Prognostic determinants in supraglottic carcinoma: Univariate and Cox regression analysis. Head Neck 19(4), 323–334 (1997).
Braun, L. H. et al. Neutrophil-to-lymphocyte ratio in rectal cancer-novel biomarker of tumor immunogenicity during radiotherapy or confounding variable?. Int. J. Mol. Sci. 20(10), 2448 (2019).
Portale, G., Cavallin, F., Valdegamberi, A., Frigo, F. & Fiscon, V. Platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio are not prognostic biomarkers in rectal cancer patients with curative resection. J. Gastrointest. Surg. 22(9), 1611–1618 (2018).
Yoshida, D. et al. Prognostic impact of the neutrophil-to-lymphocyte ratio in stage I–II rectal cancer patients. J. Surg. Res. 245, 281–287 (2020).
Shen, L. et al. Baseline neutrophil–lymphocyte ratio (≥2.8) as a prognostic factor for patients with locally advanced rectal cancer undergoing neoadjuvant chemoradiation. Radiat. Oncol. 9, 295 (2014).
Carruthers, R. et al. Systemic inflammatory response is a predictor of outcome in patients undergoing preoperative chemoradiation for locally advanced rectal cancer. Colorectal Dis. 14(10), e701–e707 (2012).
Zhang, Y. et al. Prognostic value of pretreatment systemic inflammatory markers in patients with locally advanced rectal cancer following neoadjuvant chemoradiotherapy. Sci. Rep. 10(1), 8017 (2020).
Kim, J. & Bae, J. S. Tumor-associated macrophages and neutrophils in tumor microenvironment. Mediat. Inflamm. 2016, 6058147 (2016).
Mizuno, R. et al. The role of tumor-associated neutrophils in colorectal cancer. Int. J. Mol. Sci. 20(3), 529 (2019).
Masucci, M. T., Minopoli, M. & Carriero, M. V. Tumor associated neutrophils. Their role in tumorigenesis, metastasis, prognosis and therapy. Front. Oncol. 9, 1146 (2019).
Wu, L., Saxena, S., Awaji, M. & Singh, R. K. Tumor-associated neutrophils in cancer: Going pro. Cancers 11(4), 564 (2019).
Author information
Authors and Affiliations
Contributions
J.Z. contribute to the Conception and design. Y.H. contribute to the administrative support and provision of study materials or patients. Y.G., P.D., L.Z. contribute to the collection and assembly of data. J.Z., L.Z. contribute to the data analysis and interpretation. All authors contribute to the manuscript writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, J., Zhang, L., Gou, Y. et al. The combination of pre-neoadjuvant chemoradiotherapy inflammation biomarkers could be a prognostic marker for rectal cancer patients. Sci Rep 12, 4286 (2022). https://doi.org/10.1038/s41598-022-07726-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-07726-y
- Springer Nature Limited