Abstract
Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae) an invasive mealybug on cotton is primarily controlled by conventional insecticides. An endoparasitoid Aenasius arizonenesis (Girault) (Hymenoptera: Encyrtidae) is a potential biocontrol agent of this pest. We assessed the susceptibility in field populations of P. solenopsis and A. arizonensis to commonly used insecticides: profenofos, imidacloprid and thiodicarb. Reproductive traits of the parasitoid and Environmental Risk Assessment (ERA) parameters viz., Reduction coefficient, Descriptive analysis, Risk Index (RI), Selectivity ratio and Hazard quotient were measured to assess the direct and indirect effects of these insecticides on the parasitoid. Probit analysis revealed heterogeneity in the insecticide resistance development for both the cotton mealybug and its parasitoid. The field populations of P. solenopsis exhibited resistance to profenofos (18.87–59.86 folds) and thiodicarb (20.07 folds) and susceptibility to imidacloprid. Development of resistance to profenofos was observed in field populations of A. arizonensis. Exposure to lethal doses of imidacloprid and profenofos caused a reduction in parasitization (19–23%) and adult emergence (62–69%) of the parasitoid. Profenofos, thiodicarb and imidacloprid were found to be hazardous, non-selective and harmful to the endoparasitoid, A. arizonensis. There is an urgent need for optimizing insecticide applications for sustainable management of this invasive mealybug in cotton.
Similar content being viewed by others
Introduction
The introduction of alien species and their establishment outside their native range dramatically concern agricultural ecosystems worldwide1. Mealybugs are invasive insect pests which can rapidly spread to new areas due to their cryptic behaviour, hostplant plasticity2 and high reproduction rate3. Cotton mealybug Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae) is one such pernicious pest that had emerged as a pest of cotton during the 1990s in the USA4. Over the last three decades, this mealybug pest invaded and established in > 43 countries in different parts of the world5. Its invasion has caused a severe economic loss in Ecuador, Chile, Argentina, Brazil, Pakistan, Nigeria, India, China, Egypt6 and Moracco7. Management of this mealybug pest has become difficult to manage owing to its invasive spread and impervious mealy coating to insecticides8. The immediate threat to cotton production posed by P. solenopsis in Asian countries has led to the intensive and irrational use of conventional insecticides for its management9. As a consequence, field populations of P. solenopsis have developed resistance to traditional and novel insecticides9,10,11.
Biological control represents a promising and sustainable approach for the management of P. solenopsis and it has to be prioritized3. Among the fortuitous natural enemies, the solitary endoparasitoid Aenasius arizonensis (Girault) (Hymenoptera: Encyrtidae) was considered a successful biocontrol agent of P. solenopsis12, because of its high parasitism rates recorded in the field13. This parasitoid, A. arizonensis has been used for implementing nationwide biological control programs to manage P. solenopsis in India, Pakistan and China14,15,16.
Around 600 arthropod species have been reported showing resistance to at least one pesticide17. An intriguing aspect of Arthropod Pesticide Resistance Database (APRD) 2022 is the growing number of cases of resistance in non-target arthropods, with 45 reported cases of pesticide resistance in parasitoids, predators and pollinators. Parasitoids seem to exhibit a higher susceptibility to pesticides compared to predators, as they are directly exposed to selection pressure. Parasitism may enhance the detoxification system in the host18. Therefore, insecticide resistance is more likely to evolve in parasitoids whose hosts have already developed considerable resistance to insecticides.
Besides insecticide resistance, pesticides pose a negative impact on non-target beneficial arthropods, which play a vital role in the ecosystem19,20. The risk assessment of insecticides is basically required in the integrated pest management (IPM) context because their irrational use can cause serious consequences on the ecological services offered by non-target beneficial arthropods21,22. The destruction of natural enemies can exacerbate pest problems as they play an important role in regulating pest population levels. Annihilation of natural enemies in cropping systems would lead to an adverse scenario of the use of a higher dose of toxicants leaving the enhanced residue of hazardous toxicants in the environment23. Additionally, pesticides can also affect life-history parameters including growth rate, development time, reproductive functions and the preying/parasitization potential of natural enemies24.
Regulations are in place to assess the non-target effects of insecticides and safety standards have been enacted as per Document on Terrestrial Ecotoxicology (SANCO/10329/2002, 2002), SETAC/ESCORT Guidance Document25,26 and IOBC guidelines on classification of insecticides based on their non-target effects on natural enemies in agricultural eco system27. The safety standards imposed by regulatory agencies are often challenged by the abuse of insecticides when invasive pests are spread in epidemic proportions as in the case of cotton mealybug P. solenopsis. Limited literature is available on the susceptibility levels of field populations of the cotton mealybug and its parasitoids to insecticides in India9. Similarly, eco-toxicological risk assessment of insecticides in field populations of A. arizonensis has scarcely been documented28.
The field populations of the pest and parasitoid were collected from four major cotton-growing regions across India. The choice of insecticides was done based on the inputs from a Knowledge Attitude Practice survey29,30 conducted in the field locations. Detailed log dose probit analyses were done to ascertain the susceptibility levels of the pest and parasitoid to the contemporarily used insecticides in cotton. The indirect effect of insecticides on the parasitization potential of A. arizonensis was assessed through estimation of Environmental Risk Assessment (ERA) parameters such as Reduction Coefficient (Ex), Descriptive analysis (E), Risk Index, Selectivity ratio and Hazard quotient20,27,31. Understanding susceptibility levels of the field populations of the pest and assessing the target and non-target impacts of insecticides on its potential biocontrol agent would help to optimize the strategies for sustainable management of this invasive cotton mealybug.
Results
Insecticide usage history and cropping details
Details of the Knowledge-Attitude-Practice surveys are presented in Table 1. The surveys revealed that the commercial Bt cotton hybrid seeds available to the farmers had been pre-treated with imidacloprid 70WS. The mealybugs were the predominant sucking pests not only on cotton but also on other vegetable crops in the survey areas, while, whitefly, Bemisia tabaci (Genn.) (Hemiptera: Aleyrodidae), and the leafhopper, Amrasca biguttula biguttula Ishida (Hemiptera: Cicadellidae) were other sucking pests noticed on cotton. The OPs, carbamates, pyrethroids, and neonicotinoids are the predominant group of insecticides being used by the farmers to control mealybugs and other sucking pests on cotton. The number of spray applications was 10–12 in the Ludhiana and Saoner; 8–10 sprays in Junagadh and Chhindwara locations of India.
Acute toxicity of insecticides on P. solenopsis
According to the probit model, there were no significant differences between the observed and the expected data, validating thus the estimated lethal concentrations for the tested chemicals. The variation in susceptibility of P. solenopsis to imidacloprid, profenofos and thiodicarb was noticed between the four field populations (Table 2). The mealybug field populations were the least susceptible to profenofos with the LC50 values being in the range of 27.74 mg L−1 (χ2 = 0.782, (df) = 5, P = 0.941) (Ludhiana) to 88.00 mg L−1 (χ2 = 0.429, (df) = 5, P = 0.980) (Saoner). When compared to laboratory susceptible check, P. solenopsis field populations were found to be 18.87–59.86 folds resistant to profenofos. Significant differences in susceptibility to thiodicarb were observed with the LC50values ranging from 5.643 mg L−1 (Saoner) to 52.88 mg L−1 (Ludhiana) and the field populations of P. solenopsis were showing up to 20.07 folds resistance to thiodicarb. Comparatively, imidacloprid was found to be relatively more toxic to P. solenopsis; the field populations were showing just 1.67–8.79 folds resistance to the neonicotinoid compound in comparison to the susceptible check.
Residual toxicity of insecticides on A. arizonensis
The Probit dose-response mortality assays revealed that profenofos was relatively more toxic to all the field populations of mealybug endoparasitoid, A. arizonensis. The LC50 values were ranging from 0.0009 mg L−1 (χ2 = 4.432, (df) = 5, P = 0.490) in Chhindwara to 0.0060 mg ai L−1 (χ2 = 10.32, (df) = 5, P = 0.066) in Junagadh population (Table 3). Imidacloprid was found to show the least residual toxicity against A. arizonensis with the LC50 values being significantly lower: 0.0010 mg L−1 (Chhindwara) to 0.0045 mg L−1 (Ludhiana). Next to profenofos, thiodicarb also had high residual toxicity to all the field populations of A. arizonensis as shown by the LC50 values in the range of 0.0018–0.0043 mg L−1. The field populations of A. arizonensis were showing 9–60 folds resistance to profenofos; 5–22.5 folds resistance to imidacloprid compared to the lab susceptible check. Relatively less resistance to thiodicarb (RR in the range of 4.28–10.24) was observed in the field populations of A. arizonensis populations.
Indirect effect of insecticides on parasitoids
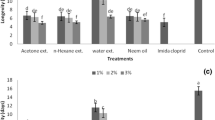
While the direct effect of insecticides on the parasitoid was revealed by the dose-response assays, the indirect effects of insecticides were assessed through estimation of parasitization potential and adult emergence of A. arizonensis. Laboratory assays have shown that the insecticidal residues (tested @ LC50 concentrations of the respective insecticides for the respective field population) significantly affected the parasitism and the emergence rates of A. arizonensis (Fig. 1). The statistical analysis revealed a significant effect of the factors insecticide, populations and their interaction on both the parasitism rate (F3, 80 = 362.44; P < 0.0001; F3, 80 = 50.13; P < 0.0001; F3, 80 = 8.08; P < 0.0001) and the emergence rate (F3, 80 = 410.91; P < 0.0001; F3, 80 = 97.12; P < 0.0001; F3, 80 = 59.09; P < 0.0001). All the tested insecticides negatively affected the parasitism and the emergence rates of A. arizonensis in comparison to the Lab population. In particular, imidacloprid significantly decreased the parasitism rate to the tune of to 19.4 ± 1.1% in A. arizonensis collected from Chhindwara location.
Effect of insecticides on the parasitism (%) emergence rate in field populations of mealybug parasitoid, A.arizonensis. Mean ± SE parasitism rate (a) and progeny emergence rate (b) of Aenasius arizonensis females exposed by contact residue of LC50 of imidacloprid, profenofos and thiodicarb. Significant effect of the factors insecticides, populations and their interactions on both the parasitism rate and the emergence rate. All the tested insecticides negatively affected the parasitism and the emergence. Columns bearing the same letter (upper case letters: within the same population; lower case letters: within the same tested insecticide) are not significantly different (LSD post hoc test for multiple comparisons at P ≥ 0.05).
Environmental risk assessment
The environmental risk assessment parameters such as Descriptive analysis (E), Reduction Coefficient (Ex), Risk Index (RI), Selectivity Ratio (SR) and Hazard quotient (HQ) were computed for categorizing the relative safety of the insecticides to the mealybug parasitoid A. arizonensis (Tables 4 and 5). Descriptive analysis (E) revealed that all the three insecticides tested were slightly toxic to A. arizonensis populations except for thiodicarb in Ludhiana region (E = 27.68). The Reduction Coefficient (Ex) values (60.84–82.77%) revealed that all the test insecticides were slightly harmful to most of the field populations of A. arizonensis (Table 5). The Risk indices (RI) were ranging from 0.04 to 0.75 in the field populations of A. arizonensis and based on the RI values, imidacloprid and thiodicarb could be categorized as low risk compounds (RI < 0.5). Conversely, profenofos posed a high risk to the parasitoid with the RI > 0.5 for all the locations. A Perusal of the selectivity ratio revealed that all the three insecticides were non selective to A. arizonensis with the selectivity ratio being less than 1 for the field populations of A. arizonensis tested. The HQ values suggest that the imidacloprid was relatively safe to the parasitoid collected from Junagadh, Ludhiana and Saoner locations.
Discussion
The cotton mealybug P. solenopsis which has entered India in 2006 as an invasive pest32 continues to be a regular pest on cotton and horticultural crops33 owing to its pronounced polyphagia. The rapid outbreak of this invasive pest and inadequate control offered by chemical insecticides necessitated the exploration of biological control options in Asian countries. Large-scale release of solitary endoparasitoid A. arizonensis was explored in India, Pakistan and China14,15,16. The crops like cotton and vegetables (wherein P. solenopsis is a serious pest) receive frequent applications of similar insecticides for the control of sucking pests. The abuse of insecticides might have compromised the efficacy of both the chemicals and biological control strategies in managing the invasive mealybug P. solenopsis in India and Pakistan34. Studies has shown widespread development of resistance to different insecticides in P. solenopsis in India9 and Pakistan35,36.
Results of our present study have demonstrated varying levels of resistance to imidacloprid, profenofos and thiodicarb in four P. solenopsis populations in India. Interestingly, we recorded for the first time, the development of insecticide resistance in four different field populations of A. arizonensis, one of the most effective parasitoids recorded against the cotton mealybug.
The insecticide exposure–response relationship revealed significant variations in the susceptibility of P. solenopsis field populations to three insecticides belonging to organophosphates, carbamates and neonicotinoids. Preliminary surveys indicated that these insecticides were widely and repeatedly applied by farmers in the surveyed locations. The high range of LC50 values recorded in P. solenopsis field populations against profenofos reveals that this OP compound can no longer effectively control P. solenopsis under field conditions. Profenofos has been one of the widely used insecticides by Indian farmers for decades for the control of bollworms, whiteflies and mealybugs in the cotton system37. Worldwide, newer classes of insecticides have replaced organophosphates and carbamates for the control of sucking pests. However, these conventional insecticides are still under use in India, because they are less expensive38. The results of our survey in major cotton-growing regions in India have also proved this point. High resistance development to profenofos in P. solenopsis had been documented earlier in the Punjab province of Pakistan35. Similarly, low to moderate resistance to profenofos in field populations of P. solenopsis was reported in cotton-growing districts of Maharashtra in India9. Also, thiodicarb and imidacloprid showed reduced toxicity to P. solenopsis in two field populations of mealybug, P. solenopsis (Table 2).
The Bt cotton varieties were introduced in India during the early 2000s and presently about 96% of cotton cropped area in this country grows transgenic Bt cotton varieties. To manage the surge in the attack of sucking pests on Bt cotton, there has been persistent use of imidacloprid (as all Bt cotton seeds are mandatorily treated with imidacloprid). The continuous use of imidacloprid has predisposed the resistance development against imidacloprid in several cotton pests in India30. Similar to the results of our study, loss in toxicity to imidacloprid against P. solenopsis has earlier been reported in Pakistan and India9,35.
Insecticides often cause a deleterious impact on insect natural enemies in agricultural systems, although they are applied for controlling target pests. Adult parasitoids are more susceptible than their preimaginal stages to encounter insecticide by contact on spray drift or by ingestion of contaminated food (e.g., nectar and/or pollen) after insecticide application on the plant surfaces. The toxic residues on plant surface directly affect the survival of released parasitoids intended for controlling the target pests. A study by Nidheesh et al.39 has shown that profenofos and a neonicotinoid, thiamethoxam were found to be highly toxic to mealybug parasitoid, A. arizonensis.
The residual toxicity assays in the present study revealed that profenofos was highly toxic to A. arizonensis field populations. The parasitoid collected from Junagadh region was 60 times (P = 0.066) more resistant to profenofos. Field populations of A. arizonensis were found to be 7–10 folds more tolerant to thiodicarb as compared to the laboratory population. However, imidacloprid was found to show 5–22 folds reduced toxicity to the field populations of A. arizonensis when compared with susceptible check. The variability of insecticide susceptibility in field populations of P. solenopsis and A. arizonensis could be attributed to the differential levels of insecticidal pressure experienced by the pest and its parasitoid.
The slopes of the regression lines of probit analysis can provide clues on the efficacy of insecticides on the target insect population. In this study, the slopes of dose–response probit curves were extremely low (< 2.0) for mealybug, and its parasitoid, suggesting the heterogeneity in resistance development against the tested insecticides in both the mealybug pest and its parasitoid A. arizonensis. Results of our study indicate that the cotton mealybug is in an early stage of developing field-evolved resistance to insecticides and there is a concomitant increase in tolerance in field populations of A. arizonensis to insecticides such as profenofos.
Resistance development in cotton mealybug to insecticides is not surprising, considering the over-reliance on conventional chemical insecticides to contain the epidemic outbreak of cotton mealybug, the P. solenopsis in India and its neighbouring countries during the 2000s the and persistent use of chemicals for controlling other sucking pests on cotton30. The first instance of P. solenopsis population showing resistance to acetamiprid was documented in Pakistan40. Presently, this pest has developed resistance against 24 insecticides and there are 196 reported cases of insecticide resistance41. Under these circumstances, there is a definite likelihood of control failures of applied insecticides against the invasive mealybug, P. solenopsis.
Studies have shown that the application of insecticides such as profenofos significantly impairs the activities of parasitoids and predators like A. arizonensis (= bambawalei), Brumus suturalis Fabricius (Coleoptera: Coccinellidae) and Scymnus coccivora Ayyar. (Coleoptera: Coccinellidae)42. Nalini and Manickavasagam43 reported the toxicity of profenofos and imidacloprid on A. arizonensis. Meenu and Ram44 had also observed that profenofos and thiodicarb resulted in maximum mortality of A. arizonensis. Our results suggest that thiodicarb is more toxic to the mealybug P. solenopsis but is relatively safer to its parasitoid A. arizonensis.
Besides the direct impact on parasitoids, the application of these insecticides significantly impacted their parasitization efficiency. Residual toxicity assays in this study showed that exposure to a lethal dose (LC50) of imidacloprid and profenofos caused a 19–23% reduction in parasitization and a 62–69% reduction in adult emergence. Earlier studies have shown that exposure of mummified mealybugs to insecticides like profenofos caused a deleterious effect on adult emergence, while, imidacloprid affected the fitness traits of A. arizonensis28.
Beneficial insects including natural enemies and pollinators constituted less than 3% of the total recorded cases of insecticide resistance in the 1980s. However, by 2015, the reported cases of insecticide resistance in natural enemies have risen to 6.4%45. According to the Arthropod Pesticide Resistance Database (APRD)17, there are about 45 cases of insecticide resistance reported in 18 species of hymenopterans against different groups of insecticides41. Ingestion of toxicants from the host mainly contributed to the development of insecticide resistance in endoparasitoids46. Insecticide resistance development in the host insect influenced the selection pressure in the hosted parasitoid18. Parasitoids associated with the resistant population of diamondback moth Plutella xylostella Linnaeus) (Lepidoptera: Plutellidae) also showed resistance to the same insecticides47. Thus, parasitoids too develop resistance to the insecticides in the long run, when the host harbouring it, is continuously exposed to the selection pressure from insecticides.
A comprehensive ecotoxicological risk assessment is needed to better understand the adverse impact of insecticides on non-target organisms. Regulations are in place to assess the non-target effects of insecticides and safety standards have been evolved like Document on Terrestrial Ecotoxicology26 and IOBC guidelines on the classification of insecticides based on their non-target effects on natural enemies in an agricultural ecosystem27.
Insecticide exposures severely affect the fitness traits of natural enemies48,49. There are limited studies on mortality assessment of insecticides on field populations of P. solenopsis together with its parasitoid35,50,51. The present study assessed the mortality in field populations of the cotton mealybug and its parasitoid and the insecticide effects on the reproductive traits of the endoparasitoid, A. arizonensis. Quantitative estimates such as Reduction Coefficient (Ex) and Descriptive analysis (E), selectivity ratio, Risk index and Hazard quotient have considered both the mortality of parasitoids and their parasitization efficiency to estimate the hazardous effect of pesticides on natural enemies27,31. Comparative assessment of Reduction Coefficient (Ex) revealed that the three commonly used insecticides (profenofos, imidacloprid and thiodicarb) for controlling P. solenopsis in the major cotton-growing regions could be considered slightly toxic to A. arizonensis according to the IOBC classification. The deleterious effect of these three insecticides has earlier been documented by Badshah et al.50. Imidacloprid was found to be slight to moderately toxic to the adults of A. arizonensis based on the assessment of Reduction Coefficient (Ex) as per an earlier report28. The harmfulness of profenofos and thiodicarb against A. arizonensis has well been documented earlier44,51. The results of the descriptive analysis revealed that, except for thiodicarb application in the Ludhiana population, all three insecticides were found to be slightly toxic to A. arizonensis in the study locations. Pazini, et al.49 classified imidacloprid as slightly harmful to Telenomus podisi Ashmead (Hymenoptera: Scelionidae) based on the assessment of Descriptive analysis.
The risk indices were found to be ranging from 0.04 to 0.73 for different field populations of the pest and parasitoid. Comparatively lower Risk Index suggests that application of thiodicarb and imidacloprid pose a relatively low risk to parasitization by the endoparasitoid. The OP compound, profenofos poses the highest risk to the parasitization by the endoparasitoid.
The selectivity ratio reveals that all the three insecticides were non-selective to the mealybug pest. Further studies are needed to identify insecticidal molecules or formulations selective to the cotton mealybug with the least non-target effect on its fortuitous parasitoid A. arizonensis.
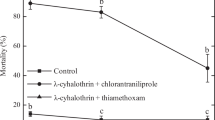
A hazard ratio HQ < 50 for a pesticide is considered safe for natural enemies of a pest. The HQ values recorded in this study suggest that the imidacloprid is relatively safe to the field populations of A. arizonensis. Even though HQ values suggest profenofos and thiodicarb are slight to moderately toxic to the parasitoid, their application at recommended label rates would likely to cause 90% mortality of A. arizonensis (Fig. 2). The vulnerability of this endoparasitoid the field recommended dose of profenofos and thiodicarb has earlier been documented28.
Comparison of contact LC90 values of insecticides to mealybug and parasitoid with their field recommended concentrations. The expected mortality of field populations of P. solenopsis as achieved by label rates is represented graphically. The toxic impact of these insecticides on A. arizonensis field populations could be deduced from this graph. Susceptibility of the four field populations of P. solenopsis as given by the estimates of LC50 and 95% confidence limits for the tested insecticides was compared with the maximum recommended field dose of these insecticides by Central Insecticides Board and Registration Committee (CIBRC), Government of India. As per CIBRC, the recommended doses for the tested insecticides against sucking pests were: imidacloprid 0.00625 mg L−1; profenofos: 0.125 mg L−1; thiodicarb: 0.185 mg L−1.
Currently, as many as 36 insecticides have been approved for use against sucking pest complex in cotton and other crops by the Central Insecticide Board of Registration Committee in India52. Our study has provided new knowledge on the direct and indirect impact of currently used insecticides on contemporary field populations of P. solenopsis and its main parasitoid in India. Both the cotton mealybug and its parasitoid are at the early stage of development of field resistance to insecticides. By deploying elaborate ERA parameters, our study has demonstrated that profenofos, thiodicarb and imidacloprid being widely used by cotton farmers are hazardous, non-selective and harmful to the potential biocontrol agent of mealybugs in the cotton ecosystem.
Detailed studies regarding the side effect of pesticides should be addressed towards the behavior of beneficial arthropods53 as well as the combination of pesticides with other stressors (e.g., temperature)20. There is a need for advocating the replacement of widely used insecticides belonging to OP and carbamates with newer chemistries for integrated management of the invasive mealybug, P. solenopsis in India and Pakistan.
Our studies reiterate the need for optimizing the insecticide usage for mitigating the insecticide resistance development in mealybug and conserving the fortuitous parasitoid, A. arizonensis in the cotton ecosystem. Adoption of biorational approaches involving botanical insecticides such as Neem pesticides, use of entomopathogens like Metarhizium anisopliae, Beauveria bassiana, Lecanicillium lecanii, and identifying safer and selective insecticidal molecules with the least non-target effect to natural enemies would ensure the sustainable management of P. solenopsis and other sap-sucking pests in the cotton crop.
Methods
Guidelines
All methods were performed following the relevant guidelines and regulations approved by the institution and funding agency. All experimental protocols were as per the technical programme of the research project as approved by the Institute Research Council ethics committee of the ICAR- Indian Agricultural Research Institute, New Delhi. Since the survey was interview-based with humans, before conducting the survey, we informed the farmers about the purpose and the utilization of the survey; an informed consent was obtained from each of the participants.
Collection of insects, maintenance and rearing
No specific permissions were required for these locations/activities as the plant/insect species covered in this study are not endangered or protected species. The pest infestation was seen in natural conditions at different locations and their collection does not require any permission from any regulatory authority under the prevalent laws.
Field populations of cotton mealybug P. solenopsis and its parasitoid A. arizonensis were collected from cotton fields located in Ludhiana, Junagadh, Saoner and Chhindwara of India by random sampling method. Uniform infestation of mealybug and its parasitoid in cotton fields of major cotton-growing districts encouraged us to choose these sites for collection of the pest and the parasitoid. Details of the sampling locations are given in Table 1. Between 2000 and 2500 mealybugs were collected from cotton fields over a radius of 5 km in each location. The cotton mealybug P. solenopsis adult females bearing well-formed ovisacs were collected and brought to the laboratory. The mealybugs were then transferred to insecticide-free sprouted potato tubers Solanum tuberosum (L.) (Solanaceae). Mummified mealybug containing parasitoid A. arizonensis collected from these locations were brought to the laboratory and were reared on 3rd instar nymphs of P. solenopsis from the respective population. Laboratory populations of mealybug, P. solenopsis and its parasitoid A. arizonensis (unexposed to insecticides for at least 20 generations) were maintained as susceptible check for toxicity comparisons.
Matured adults of P. solenopsis and A. arizonensis were observed under the stereomicroscope and identified through morphological keys54,55. The insect populations were maintained in plastic cages (30 × 30 × 30 (L × B × H) containing sprouted potatoes for three generations before being used in the bioassays. Insect rearing was maintained at standardized environmental conditions, as follows: 25 ± 2 °C, 75 ± 5% R.H., 12L:12D photoperiod, according to the methodology developed by Nagrare et al.56. Non-Bt cotton plants, Gossypium hirsutum L, var. “LRA 5166” (Malvaceae) were grown from seeds under greenhouse conditions (20 ± 5 °C, 70 ± 10% R.H.) avoiding any pesticide application. The seeds of cotton variety “LR5166” were obtained from the ICAR Central Institute for Cotton Research, Regional Station, Coimbatore, Tamil Nadu, India. Clean cotton leaves used for the bioassays were collected from 60-day-old cotton plants that reached the phenological stage identified as BBCH Code 5157.
Insecticide usage pattern in the study area
Knowledge Attitude Practice (KAP) surveys were conducted before the start of the experiment by following the protocol used by Yadouleton et al.29 and Naveen et al.30 to understand the insecticide usage pattern in the study area. Minimum of ten farmers in each locality were informally interviewed by using a semi-structured questionnaire focusing on insecticide usage patterns in each farm. Further, data were collected on cropping patterns and, control strategies through direct observations and group discussions. The details of the survey are presented in Table 1.
Insecticides
Technical grades of imidacloprid (ai. 93%), thiodicarb (ai. 89%) (Sigma Aldrich, USA) and profenofos (ai. 89%) (Pesticide Industries Ltd., India) were used for the bioassays. Technical grade insecticides were dissolved in acetone, and serial concentrations were prepared using deionized water containing Triton X-100 (0.1 g L−1) as a non-ionic surfactant30. Based on the usage pattern of insecticides, these insecticides were selected, as they represented the OPs, pyrethroids and neonicotinoids concurrently used for control of mealybugs and other sucking pests in the respective regions where the mealybug populations were collected.
Bioassays
Susceptibility levels of P. solenopsis populations
The level of susceptibility in P. solenopsis field populations to imidacloprid, profenofos and thiodicarb was assessed through the IRAC method 001 for toxicological bioassays with a slight modification from Nauen and Elbert58. Briefly, for each active ingredient, between 6 and 7 concentrations were prepared. Fresh cotton leaves previously infested with ten coetaneous 3rd instar nymphs (having the same age or date of origin) were dipped into the chosen insecticide concentration for 5 s and allowed to dry for 1 h under a fume hood in laboratory conditions. Once dried, each insecticide-sprayed cotton leaf was placed between two superposed ventilated Petri dishes (10 cm in diameter). The distal portion of the leaf petiole was immersed under a 2 ml Eppendorf® tube filled with distilled water and sealed with Parafilm®. Mortality was recorded 24 h after exposure to insecticides. Mealybugs showing no coordinated movement or not responding when gently touched with a soft paintbrush were considered dead. Each insecticide-concentration combination and the control were replicated five times. This bioassay was conducted separately for each population of P. solenopsis under the above-mentioned experimental conditions.
Residual contact toxicity of insecticides on A. arizonensis
The residual contact toxicity of imidacloprid, profenofos and thiodicarb on each field population of A. arizonensis was evaluated through the method described by Desneux et al.21. Briefly, glass vials (12 × 5 cm) were filled with 2 ml of insecticidal solution, flipped horizontally and poured out for allowing them to dry. Thus, five couples (i.e., 5 females and 5 males) of newly emerged adult parasitoids (0–24 h-old) from the rearing were released into ventilated glass vials covered by a fine mesh net. Each insecticide for each concentration and the control were replicated five times. Mortality was assessed after 24 h. The parasitoids were considered dead if they did not respond when touched with a soft paintbrush. The bioassay for each population was conducted separately keeping the same laboratory conditions. Residual toxicity of these insecticides was compared with that of laboratory susceptible check for assessing relative tolerance of A. arizonensis field populations to insecticides.
Effect of insecticides on parasitization potential of A. arizonensis
The effect of imidacloprid, profenofos and thiodicarb on A. arizonensis was assessed by evaluating the reproductive traits (i.e., parasitism and emergence rates) of the survived adult females exposed to insecticide dry residues on glass. According to the methodology described above, 50 A. arizonensis mated females (24 h old) of each population were transferred from the rearing system into glass vials treated with the previously calculated median lethal concentration (LC50) of each insecticide. Glass vials treated only with a solution of acetone and water were included as control. Six hours after the exposure to insecticide residues on glass, 20 survived females were randomly selected and transferred in a glass jar containing a cotton leaf preliminary infested with a hundred 3rd instar nymphs of P. solenopsis from the respective population. Females of A. arizonensis were allowed to parasitize the mealybug nymphs for 24 h and then removed. The parasitism rate was recorded 7 days after the exposure by counting the number of mummified mealybugs that showed light brown color, while the parasitoid emergence rate was recorded after 12 days.
Data analysis
The Levene and Shapiro–Wilk tests were used to check the homogeneity and normality of variance of the dependent variables and the dataset was log-transformed whenever needed. Mortality data from concentration–response bioassay were subjected to probit analyses59 using Polo Plus 2.0 software (LeOra Software, USA). The LC50 and LC90 values with Fiducial limits, slopes of the regression lines standard errors, and χ2 significance tests, were thus estimated. Values were considered significantly different whether their 95% fiducial limits did not overlap. The observed mortality was corrected for control mortality through Abbott’s formula. The parasitism rate was calculated as the per cent of parasitized mummies on the total offered mealybug hosts. The emergence rate was calculated as the per cent of emerged parasitoids on developed mummies. For assessing toxic effect of insecticide on the parasitoid, we tested the effect of insecticide, population and the potential interaction of these two factors (insecticide × population) on the proportion of developed mummies (i.e., parasitoid pupae) and the proportion of the newly emerged parasitoids by carrying out a one-way ANOVA followed by Least Significant Difference (LSD) post hoc test (P < 0.05) for multiple mean comparisons among the treatments. This analysis was performed in IBM® SPSS® Statistics for Macintosh, Version 23.0.0.0 (IBM Corp. Released 2015. Armonk, NY: IBM Corp).
Likelihood of control failure of insecticides on field populations of P. solenopsis
The likelihood of control failure of insecticides was estimated based on Silva60 and Naveen et al.30. The current level of susceptibility of the field populations of P. solenopsis as given by the estimates of LC50 and 95% confidence limits for the tested insecticides was compared with the maximum recommended field dose of these insecticides by the Central Insecticides Board and Registration Committee (CIBRC), Government of India. As per CIBRC, the recommended doses for the tested insecticides against sucking pests were: imidacloprid 0.00625 mg L−1; profenofos: 0.125 mg L−1; thiodicarb: 0.185 mg L−1; The expected mortality of field populations of P. solenopsis as achieved by label rates in comparison with the estimated LC50 of the tested insecticides is represented graphically. The toxic impact of these insecticides on A. arizonensis field populations could be deduced from this graph.
Environmental risk assessment (ERA)
The indirect effect of imidacloprid, profenofos and thiodicarb on parasitization potential of A. arizonensis was assessed by calculating the risk assessment parameters as described below.
Reduction coefficient (Ex)
The Ex, that summarizes the potential insecticide deleterious effects, was calculated as described by Urbaneja et al.61 using the formula:
where Emx represents the corrected mortality calculated as per Abbott62 of the parasitoid when exposed to a given insecticide, while, Efx denotes parasitization capacity determined as follows:
where Fx and Fc represent the mean percent parasitization recorded for insecticide x and the untreated control, respectively. The Reduction coefficients (Ex) were used for classifying the insecticides according to the International Organization for Biological Control (IOBC) standards into four categories: (1) Ex < 30%—harmless; (2) Ex: 30–80%—slightly harmful; (3) Ex: 80–99%—moderately harmful; (4): Ex > 99%—harmful.
Descriptive analysis
Descriptive statistics E was calculated as described by Hassan et al.27
wherein E refers to the percent reduction in parasitism or emergence; T and C denote the mean percent reduction in parasitism or emergence in the treatment and control groups, respectively. Based on the E values range, the insecticides were grouped into four classes following the IOBC guidelines: (1) E < 30%: harmless (Class 1); (2) 30% ≤ E ≤ 79% as slightly harmful (Class 2); (3) 80% ≤ E ≤ 99% as moderately harmful (class 3); (4) E > 99% as harmful (class 4).
Risk index (RI)
The indirect toxic effect of insecticides on parasitoid A. arizonensis was expressed as Risk Index (RI) which refers to the reduction in natural potential parasitization due to insecticide application. Risk Index was calculated following Vercruysse and Steurbaut63.
Selectivity ratio
Selectivity ratio was estimated as described by Şengonca and Liu64 using the formula given below:
Selectivity ratio < 1 indicates that the chemical is more toxic to the parasitoid than to the P. solenopsis (non-selective); The ratio > 1 indicates that the chemical is less toxic to the parasitoid.
Hazard quotient
The Hazard quotient65, was calculated to estimate the ecological risk of pesticides as follows:
An hazard quotient < 50 indicates that the compound is non-hazardous to parasitoids for a given exposure rate.
Ethics declarations
This study does not involve any human subjects.
Data availability
All the data were provided in the manuscripts.
References
Hulme, P. E. et al. Grasping at the routes of biological invasions: A framework for integrating pathways into policy. J. Appl. Ecol. 45, 403–414 (2008).
ScaleNet. Phenacoccus solenopsis Tinsley 1898 (Pseudococcidae: Phenacoccus). http://scalenet.info/catalogue/Phenacoccus%20solenopsis/ (2022). Accessed 1 June 2022.
Tong, H., Yan, A., Li, Z., Ying, W. & Jiang, M. Invasion biology of the cotton mealybug, Phenacoccus solenopsis Tinsley: Current knowledge and future directions. J. Integr. Agric. 18, 758–770 (2019).
Fuchs, T., Stewart, J., Minzenmayer, R. & Rose, M. First record of Phenacoccus solenopsis Tinsley in cultivated cotton in the United States. Southwestern Entomol. 16, 215–221 (1991).
Waqas, M. S. et al. Biology, ecology, and management of cotton mealybug Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae). Pest Manag. Sci. 77, 5321–5333 (2021).
El-Zahi, E.-Z. S., Aref, S. A. E.-S. & Korish, S. K. M. The cotton mealybug, Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae) as a new menace to cotton in Egypt and its chemical control. J. Plant Protect. Res. 56 (2016).
El Aalaoui, M. & Sbaghi, M. First record of the mealybug Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae) and its seven parasitoids and five predators in Morocco. EPPO Bull. 51, 299–304 (2021).
Sequeira, R. V., Khan, M. & Reid, D. J. Chemical control of the mealybug Phenacoccus solenopsis (Hemiptera: Pseudococcidae) in Australian cotton: Glasshouse assessments of insecticide efficacy. Aust. Entomol. 59, 375–385 (2020).
Nagrare, V. et al. Resistance development in Cotton mealybug, Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae) to insecticides from Organophosphate, Thiadiazines and Thiourea derivatives. Int. J. Trop. Insect Sci. 40, 181–188 (2020).
Afzal, M. B. S., Shad, S. A., Ejaz, M. & Ijaz, M. Selection, cross-resistance, and resistance risk assessment to deltamethrin in laboratory selected Phenacoccus solenopsis (Homoptera: Pseudococcidae). Crop Prot. 112, 67–73 (2018).
Nazar, M. Z. et al. Characteristics of biochemical resistance mechanism of novel insecticides in Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae). Crop Prot. 138, 105320 (2020).
Kumar, R., Kranthi, K., Monga, D. & Jat, S. Natural parasitization of Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae) on cotton by Aenasius bambawalei Hayat (Hymenoptera: Encyrtidae). J. Biol. Control. 23, 457–460 (2009).
Dhawan, A., Singh, K., Saini, S., Aneja, A. & Singh, J. Parasitizing potential of parasitoid (Aenasius bambawalei) on mealybug (Phenacoccus solenopsis) in cotton (Gossypium spp) and weed plants. Indian J. Agric. Sci. 81, 97–99 (2011).
Mahmood, R., Aslam, M., Solangi, G. & Samad, A. in 5th Meeting of ICAC’s Asian Cotton Research and Development Network. 23–25.
Vennila, S. et al. Spatio-Temporal Distribution of Host Plants of Cotton Mealybug, Phenacoccus solenopsis Tinsley in India Vol. 50 (National Centre for Integrated Pest Management, 2011).
Li, J. et al. Investigation on the occurrence of parasitic wasps of Phenacoccus solenopsis Tinsley in Guangxi. J. Southern Agric. 51, 853–861 (2020).
APRD. Arthropod Pesticide Resistance Database (APRD). https://www.pesticideresistance.org/search.php. Accessed on 21.11.2020. (2020).
Liu, S.-S., Li, Z.-M., Liu, Y.-Q., Feng, M.-G. & Tang, Z.-H. Promoting selection of resistance to spinosad in the parasitoid Cotesia plutellae by integrating resistance of hosts to the insecticide into the selection process. Biol. Control 41, 246–255 (2007).
Desneux, N., Decourtye, A. & Delpuech, J.-M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 52, 81–106 (2007).
Kim, K., Kabir, E. & Jahan, S. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 575, 525–535 (2017).
Guedes, R., Smagghe, G., Stark, J. & Desneux, N. Pesticide-induced stress in arthropod pests for optimized integrated pest management programs. Annu. Rev. Entomol. 61, 43–62 (2016).
Ricupero, M. et al. Combined thermal and insecticidal stresses on the generalist predator Macrolophus pygmaeus. Sci. Total Environ. 729, 138922 (2020).
Huang, J. et al. Uncovering the economic value of natural enemies and true costs of chemical insecticides to cotton farmers in China. Environ. Res. Lett. 13, 064027 (2018).
Pinheiro, L. A. et al. Side effects of pesticides on the olive fruit fly parasitoid Psyttalia concolor (Szépligeti): A review. Agronomy 10, 1755 (2020).
Barrett, K., Grandy, N., Harrison, E., Hassan, S. & Oomen, P.(ed ESCORT workshop (European Standard Characteristics of Non-Target Arthropod Regulatory Testing)) 53.
EFSA. Panel on Plant Protection Products and their Residues (PPR), Scientific Opinion addressing the state of the science on risk assessment of plant protection products for non-target arthropods. EFSA J. 11, 3290 (2015).
Hassan, S. et al. in Guidelines to Evaluate Side-Effects of Plant Protection Products to Non-target Arthropods. 107–119 (2000).
Karmakar, P. & Shera, P. Lethal and sublethal effects of insecticides used in cotton crop on the mealybug endoparasitoid Aenasius arizonensis. Int. J. Pest Manag. 66, 13–22 (2018).
Yadouleton, A. W. M. et al. Development of vegetable farming: A cause of the emergence of insecticide resistance in populations of Anopheles gambiae in urban areas of Benin. Malaria J. 8, 1–8 (2009).
Naveen, N. et al. Insecticide resistance status in the whitefly, Bemisia tabaci genetic groups Asia-I, Asia-II-1 and Asia-II-7 on the Indian subcontinent. Sci. Rep. 7, 1–15 (2017).
Chen, X. D., Gill, T. A., Pelz-Stelinski, K. S. & Stelinski, L. L. Risk assessment of various insecticides used for management of Asian citrus psyllid, Diaphorina citri in Florida citrus, against honey bee, Apis mellifera. Ecotoxicology 26, 351–359 (2017).
Nagrare, V. et al. Widespread infestation of the exotic mealybug species, Phenacoccus solenopsis (Tinsley) (Hemiptera: Pseudococcidae), on cotton in India. Bull. Entomol. Res. 99, 537–541 (2009).
Spodek, M. et al. The cotton mealybug, Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae) in Israel: Pest status, host plants and natural enemies. Phytoparasitica 46, 45–55 (2018).
Saddiq, B. et al. Resistance in the mealybug Phenacoccus solenopsis Tinsley (Homoptera: Pseudococcidae) in Pakistan to selected organophosphate and pyrethroid insecticides. Crop Prot. 66, 29–33 (2014).
Ahmad, M. & Akhtar, S. Development of resistance to insecticides in the invasive mealybug Phenacoccus solenopsis (Hemiptera: Pseudococcidae) in Pakistan. Crop Prot. 88, 96–102 (2016).
Ismail, M., Ejaz, M., Abbas, N., Shad, S. A. & Afzal, M. B. S. Resistance risk assessment to chlorpyrifos and cross-resistance to other insecticides in a field strain of Phenacoccus solenopsis Tinsley. Crop Prot. 94, 38–43 (2017).
Sagar, D., Balikai, R., Patil, R., Udikeri, S. & Bheemanna, M. Insecticide usage pattern in major Bt cotton growing districts of Karnataka, India. J. Exp. Zool. India 16, 461–466 (2013).
DPPQS. http://ppqs.gov.in/statistical-database. (2021).
Nidheesh, T., Shylesha, N. & Sharma, K. Effect of some insecticides on Aenasius arizonensis (Girault) parasitizing cotton mealybug Phenacoccus solenopsis Tinsley. Indian J. Entomol. 82, 731–734 (2020).
Afzal, M. B. S., Shad, S. A., Abbas, N., Ayyaz, M. & Walker, W. B. Cross-resistance, the stability of acetamiprid resistance and its effect on the biological parameters of cotton mealybug, Phenacoccus solenopsis (Homoptera: Pseudococcidae), Pakistan. Pest Manag. Sci. 71, 151–158 (2014).
https://www.pesticideresistance.org/search.php (2022). Accessed 21 Oct 2020.
Sahito, H. A. et al. Screening of pesticides against cotton mealybug Phenacoccus solenopsis Tinsley and its natural enemies on cotton crop. Int. Res. J. Biochem. Bioinform. 19, 232–236 (2011).
Nalini, T. & Manickavasagam, S. Toxicity of selected insecticides to mealybug parasitoids, Aenasius bambawalei Hayat and Aenasius advena Compere (Hymenoptera: Encyrtidae). J. Biol. Control. 25, 14–17 (2011).
Meenu & Ram, P. Effect of insecticides on different developmental stages of Aenasius bambawalei Hayat (Hymenoptera: Encyrtidae), a parasitoid of solenopsis mealybug, Phenacoccus solenopsis Tinsley. Biol. Control. 28, 204–209 (2014).
Bielza, P. Advances in Insect Control and Resistance Management 313–329 (Springer International Publishing, 2016).
Wu, S. & Zhang, R. A new invasive pest, Phenacoccus solenopsis threatening seriously to cotton production. Chin. Bull. Entomol. 46, 159–162 (2009).
Sparks, T. C. & Nauen, R. IRAC: Mode of action classification and insecticide resistance management. Pestic. Biochem. Physiol. 121, 122–128 (2015).
EFSA, S. C. Guidance to develop specific protection goals options for environmental risk assessment at EFSA, in relation to biodiversity and ecosystem services. EFSA J. 14, e04499 (2016).
Pazini, J. D. B. et al. Differential impacts of pesticides on Euschistus heros (Hem.: Pentatomidae) and its parasitoid Telenomus podisi (Hym.: Platygastridae). Sci. Rep. 9, 1–10 (2019).
Badshah, H., Ullah, F., Calatayud, P. A., Ullah, H. & Ahmad, B. Can toxicants used against cotton mealybug Phenacoccus solenopsis be compatible with an encyrtid parasitoid Aenasius bambawalei under laboratory conditions?. Environ. Sci. Pollut. Res. 24, 5857–5867 (2017).
Nagrare, V. et al. Relative toxicity of insecticides against cotton mealybug Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae) and its fortuous parasitod Aenasius bambawalei Hayat (Hymenoptera: Encyrtidae). J. Appl. Nat. Sci. 8, 987–994 (2016).
DPPQS. http://ppqs.gov.in/sites/default/files/1._major_uses_of_pesticides_-_insecticide_as_on_30.06.2021.pdf (2021). Accessed 30 June 2022.
Dai, C. et al. Can contamination by major systemic insecticides affect the voracity of the harlequin ladybird?. Chemosphere 256, 126986 (2020).
Thomas, A. & Ramamurthy, V. Morphological and molecular studies on the intraspecific variations between populations of the cotton mealybug Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae). Entomol. News 123, 339–347 (2014).
Hayat, M. Description of a new species of Aenasius Walker (Hymenoptera: Encyrtidae), parasitoid of the mealybug, Phenacoccus solenopsis Tinsley (Homoptera: Pseudococcidae) in India. Biosystematica 3, 21–26 (2009).
Nagrare, V. et al. Compendium of Cotton Mealybugs 42 (Central Institute for Cotton Research, 2011).
Feller, C. et al. Phänologische Entwicklungsstadien von Gemüsepflanzen: II. Fruchtgemüse und Hülsenfrüchte. Nachrichtenbl. Deut. Pflanzenschutzd 47, 217–232 (1995).
Nauen, R. & Elbert, A. European monitoring of resistance to insecticides in Myzus persicae and Aphis gossypii (Hemiptera: Aphididae) with special reference to imidacloprid. Bull. Entomol. Res. 93, 47–54 (2003).
Finny, D. Probit Analysis: A Statistical Treatment of the Sigmoid Response Curve 256 (Cambridge University Press, 1947).
Silva, C. A. D. D. Occurrence of new species of mealybug on cotton fields in the states of Bahia and Paraíba, Brazil. Bragantia 71, 467–470 (2012).
Urbaneja, A. et al. Efficacy of five selected acaricides against Tetranychus urticae (Acari: Tetranychidae) and their side effects on relevant natural enemies occurring in citrus orchards. Pest Manag. Sci. Formerly Pesticide Sci. 64, 834–842 (2008).
Abbott, W. S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol 18, 265–267 (1925).
Vercruysse, F. & Steurbaut, W. POCER, the pesticide occupational and environmental risk indicator. Crop Prot. 21, 307–315 (2002).
Şengonca, Ç. & Liu, B. Influence of mixed biocide GCSC-BtA on the pupae and adult stages of Apanteles plutellae Kurd. (Hym., Braconidae) and its host. Plutella xylostella (L.) (Lep., Plutellidae). J. Pest Sci. 74, 145–149 (2001).
EPPO. Side-effects on honey bees. Efficacy evaluation of plant protection products. Vol. 40 323–331 (Bull OEPP/EPPO Bull, 2010).
Acknowledgements
The authors gratefully acknowledge the support from the DST-SERB Government of India for the research Grant and Director, ICAR-IARI, New Delhi, Director, ICAR-CICR, Nagpur and PC & Head, ICAR-CICR, Regional Station, Coimbatore for providing facilities for the conduct of this research.
Funding
This research work was supported by the grants from DST-SERB Grant no. DST No: No.SR/FT/LS-33/2012 Department of Science and Technology, Government of India.
Author information
Authors and Affiliations
Contributions
K.S. has contributed to the project administration, funding acquisition, methodology, investigation, analysis and writing of the manuscript. M.R. contributed review and editing of the manuscript. S.S. has contributed to the conceptualization, writing—review and editing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shankarganesh, K., Ricupero, M. & Sabtharishi, S. Field evolved insecticide resistance in the cotton mealybug Phenacoccus solenopsis and its direct and indirect impacts on the endoparasitoid Aenasius arizonensis. Sci Rep 12, 16764 (2022). https://doi.org/10.1038/s41598-022-20779-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-20779-3
- Springer Nature Limited