Abstract
The drivers of sexual dimorphism in heart failure phenotypes are currently poorly understood. Divergent phenotypes may result from differences in heritability and genetic versus environmental influences on the interplay of cardiac structure and function. To assess sex-specific heritability and genetic versus environmental contributions to variation and inter-relations between echocardiography traits in a large community-based cohort. We studied Framingham Heart Study participants of Offspring Cohort examination 8 (2005–2008) and Third Generation Cohort examination 1 (2002–2005). Five cardiac traits and six functional traits were measured using standardized echocardiography. Sequential Oligogenic Linkage Analysis Routines (SOLAR) software was used to perform singular and bivariate quantitative trait linkage analysis. In our study of 5674 participants (age 49 ± 15 years; 54% women), heritability for all traits was significant for both men and women. There were no significant differences in traits between men and women. Within inter-trait correlations, there were two genetic, and four environmental trait pairs with sex-based differences. Within both significant genetic trait pairs, men had a positive relation, and women had no significant relation. We observed significant sex-based differences in inter-trait genetic and environmental correlations between cardiac structure and function. These findings highlight potential pathways of sex-based divergent heart failure phenotypes.
Similar content being viewed by others
Introduction
Clinical manifestations and presentations of heart failure (HF) are known to differ between women and men, with women more frequently developing HF with preserved ejection fraction (HFpEF)1. Over the lifespan, women compared to men are found to have higher degrees of left ventricular (LV) thickening and stiffening as well as higher levels of circumferential shortening and torsion with aging2,3,4. These alterations manifest as measurable differences in LV structure and function between men and women, with greater concentric remodeling and diastolic dysfunction seen in women5. The underlying determinants of these sex differences remain incompletely understood. Accumulating evidence points to the likely role of sexual dimorphism in gene expression as well as response to environmental factors6,7. To this end, quantitative linkage trait analysis can be used to assess heritability as well as relative contributions of shared genetic and environmental influences to quantitative measures of cardiac phenotypes and sex differences in this regard, assuming the availability of pedigree information8,9. Therefore, the objective of this study was to investigate sex-specific differences in heritability as well as genetic versus environmental contributions to variation in cardiac structural and functional traits measured by echocardiography. By identifying these sex differences in a large community-based cohort, we aim to further understand the potential underlying drivers of differences that can result in divergent HF phenotypes consistently seen in clinical practice.
Results
The study population included 5674 participants (mean age 49 ± 15 years; 54% women). As previously seen in sex-pooled analyses, heritability tended to be higher for cardiac structural traits than for cardiac functional traits10. For men, heritability ranged from 0.33 (for left ventricular wall thickness [LVWT]) to 0.57 (aortic root diameter [AoD]) for cardiac structural traits, and from 0.17 (mitral annular plane systolic excursion [MAPSE]) to 0.44 (log longitudinal synchrony [LSS]) for ventricular functional measures (P < 0.0001 for all) (Table 1). Heritability estimates for women appeared somewhat lower but after accounting for multiple testing were statistically similar for structural traits, ranging 0.26 (LVWT) to 0.39 (LV diastolic dimension [LVDD]), as well as for functional traits, ranging 0.22 (global longitudinal strain [GLS]) to 0.48 (log[LSS]). However, heritability of one particular structural trait, AoD, was significantly higher in men compared to women (0.57 ± 0.05 vs 0.37 ± 0.05, P = 0.01).
We also examined genetic and environmental correlations between trait pairs separately by sex. As shown in Table 2, strong but expected genetic correlations were observed for several pairs or cardiac traits. In men, genetic correlations were seen for certain pairs of cardiac function traits: − 0.89 ± 0.11 (global circumferential strain [GCS] with LV ejection fraction [LVEF]), and structural traits: 0.76 ± 0.06 (LVWT with LVMi) and 0.72 ± 0.06 (LVDD with LV mass index [LVMi]); these pairs were expected due to the inherent correlations between these measurement methods. In women, the strongest genetic correlations between cardiac traits were similar: − 0.71 ± 0.10 (GCS with LVEF), 0.67 ± 0.06 (LVWT with LVMi), and 0.81 ± 0.05 (LVDD with LVMi). For environmental correlations, significant and strongly associated findings were also noted in expected pairs: 0.64 ± 0.03 (LVWT with LVMi) and − 0.28 ± 0.05 (GCS with LVEF) for men with similarly significant correlations of 0.68 ± 0.03 (LVWT with LVMi) and − 0.37 ± 0.04 (GCS with LVEF) for women.
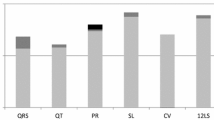
Sex differences were seen for two genetically influenced and four environmentally influenced cardiac trait pairs (Fig. 1), after accounting for multiple testing. For both of the genetic trait pairs that demonstrated sex differences, men had a positive relation, and women had no significant relation: left atrial systolic dimension [LAD] with log[LSS] (men: 0.32 ± 0.09, women: − 0.03 ± 0.09, Pdifference = 0.006), and MAPSE with LVMi (men: 0.41 ± 0.17, women: − 0.06 ± 0.12, Pdifference = 0.04). For the four environment trait pairs that demonstrated sex differences, men had an inverse relation and women had no significant relation in one: LVWT with MAPSE (men: − 0.11 ± 0.05, women: 0.05 ± 0.05, Pdifference = 0.03); men had no significant relation and women had a positive relation in two: LVDD with MAPSE (men: − 0.04 ± 0.05, women: 0.19 ± 0.05, Pdifference = 0.002) and LAD with LVEF (men: − 0.06 ± 0.06, women: 0.10 ± 0.05, Pdifference = 0.05); and, there was a significant sex-discordant relation observed for one trait pair: MAPSE with LVMi (men: − 0.10 ± 0.05, women: 0.10 ± 0.05, Pdifference = 0.006).
Discussion
The mechanistic underpinnings of sex-specific differences in HF phenotypes remains unclear. Our study, which represents a large-scale sex-specific analysis of heritability and genetic-environmental correlation of cardiac phenotypes, offers a few salient observations regarding differences in cardiac inter-trait correlations between men and women. First, men appeared to have slightly higher heritability than women for multiple cardiac traits, although these sex differences were not statistically significant after accounting for multiple testing. Sex differences in the genetic contribution to cardiac trait variation were more evident in inter-trait analyses; for genetic correlations, significant MAPSE-log(LVMI) and log(LSS)-LAD associations were seen in men, but not women. Second, all sex differences were observed for cardiac structure–function relations, not for cardiac structure–structure or function–function relations. Most associations involved specific measures of cardiac myocardial mechanical motion such as LV longitudinal shortening (LSS) and with four of the six differences involving MAPSE. Finally, and notably, there was one association that demonstrated a particularly significant and opposite sex difference, wherein the environmental correlation of LVMi and MAPSE was significantly positive for women and significantly negative for men.
Recognizing that we are unable to infer the directionality of the observed cardiac structure–function associations, our results are both illuminating and hypothesis generating regarding the potential timing and pathways by which sex-specific HF phenotypes arise. The findings that inter-related structure–function measures could be more genetically influenced in men than women suggests that if men develop relations between structure and function resulting in subsequent HF, they may do so at a younger age (i.e., less influenced by cumulative risk exposures including environmental factors over the life course). In turn, the inter-relations of LV structural and functional traits appearing more environmentally influenced in women suggests that if women are likely to develop integrated LV structural and functional abnormalities and subsequent HF, these may occur after longer duration of cumulative risk exposures including environmental factors over the life course. Interestingly, there is concordant evidence for female compared to male vascular physiology being potentially more susceptible to cardiometabolic risk burden over the adult life course11. In the current analysis, the finding that women and men had actually directionally opposite as well as significant findings regarding the environmentally mediated correlations between LVMi and MAPSE underscores how environment influences on both sexes likely drive the classic pathological remodeling patterns seen in men versus women—with women showing more concentric remodeling and men with eccentric remodeling. Increases in LVMi associated with increased longitudinal movement are more consistent with a concentric pathway, as commonly seen in aging women, whereas increases in LVMi associated with decreased longitudinal movement are more consistent with an eccentric pathway, as more often seen in men. Although apparently paradoxical, the inverse LVMi and MAPSE association in women is concordant with the LVDD and MAPSE association in women—given that the most commonly seen pattern of age-related concentric remodeling in women typically involves decreasing LVDD (and, in turn, LVMi) in association with decreasing MAPSE. Notwithstanding the subtleties required to interpret these results, it is notable that each of the sex-divergent patterns appears responsive to environmental effects, particularly the more clinically adverse form of eccentric remodeling in men. Overall, these findings highlight the continued importance of assessing measures of longitudinal ventricular motion in the context of all aspects that govern longitudinal motion (e.g. relative fiber orientation and synchrony) when investigating sex differences in HF phenotypes. These findings also underscore the potential for further discovery of the mediators and pathways by which observed sex differences develop over time into divergent subclinical HF phenotypes as well as divergent clinical HF outcomes.
Our study expands from the previously established body of evidence demonstrating heritability of echocardiographic traits. In particular, our results underscore the need for sex-specific analyses in future genome-wide association studies to further interrogate sex differences in cardiac structure and function that are consistently observed in clinical practice. Intriguingly, our finding of sex-specific heritability of aortic root size coincides with recently reported sex-specific polygenic risks score associations with hypertension traits in a separate cohort12. Taken together, these emerging data suggest that sex differences in vascular as well as associated cardiac traits and outcomes may have discernible sex-specific genetic underpinnings that could eventually, pending future follow-up work, serve as a premise for considering sex-specific approaches to cardiovascular risk assessment and management.
There are limitations of our study that are worth noting. Our analyses focused on sex differences in overall genetic contribution and in the overall environmental contribution to cardiac traits; thus, future follow-up work is also needed to examine the contributions of specific individual genetic variants and individual environmental factors underlying our findings. While our analyses focused on established left heart measures, it is now recognized that left- and right-heart cardiac alterations tend to be inter-related given their physiologic as well as anatomic inter-connectedness within the closed pericardial space. Historically fewer echocardiography studies have focused on right heart measures, and so additional investigations are needed to determine whether similar or different sex-specific genetic or environmental effects may be seen in relation to right heart traits. Importantly, given that the participants of our study are predominantly white individuals of European ancestry, replication work in more diverse study samples are needed to validate our findings. We also recognize that the sensitivity of identifying additional sex-specific associations may have been limited by our study sample size. Therefore, additional follow-up studies in large-sized samples are needed to not only validate but also potentially expand our findings. Although heritability estimates are challenging to validate outside of studies that have multi-generational family structure similar to that of the Framingham Heart Study, our results suggest that follow-up genome wide association studies in separate community-based cohorts would be useful for confirming that sex-specific associations with cardiac traits exist for individual or groups of genetic variations.
In summary, we found similar overall heritability between men and women with respect to cardiac function and structural traits, with some notable sex differences in cardiac inter-trait correlations. In particular, men tended to demonstrate more genetically linked associations between cardiac structure and function, and women tended to demonstrate more environmentally influenced cardiac structural–functional associations. These findings from a single limited-sized cohort study need to be replicated, expanded, and further investigated in larger-sized and more diverse cohorts. Nonetheless, the results are intriguing and underscore the potential to identify potentially discordant gene-environment determinants that may be contributing to the sex differences in subclinical and clinical cardiac disease conditions and outcomes that are consistently observed in population studies and that are commonly encountered among individual patients in practice.
Methods
We studied the sex-specific relations of cardiac structure and function within the Framingham Heart Study (FHS) participants of Offspring Cohort examination 8 (2005–2008) and Third Generation Cohort examination 1 (2002–2005). We measured cardiac traits using standardized echocardiography with measurements performed in the FHS laboratory according to American Society of Echocardiography guidelines13. As in prior genetic analyses of echocardiographic traits14, we excluded individuals with prevalent HF (n = 71) or any missing echocardiographic measure or covariates (n = 1351). Given that individuals with prevalent HF can have extreme forms of cardiac remodeling and dysfunction related to dynamic disease processes or their treatments14, we excluded individuals with prevalent HF identified prior to or at the time of echocardiography. Prevalent HF was defined according to published criteria including signs and symptoms or a documented history of HF15. For our linkage analyses, we included five cardiac structural traits (left ventricular mass index [LVMi], LV diastolic dimension [LVDD], LV wall thickness [LVWT], left atrial [LA] systolic dimension, and aortic root diameter [AoD])16 and six functional traits (LV ejection fraction [LVEF], global circumferential and longitudinal strain [GCS, GLS], longitudinal synchrony [LSS], mitral annular plane systolic excursion [MAPSE], and mitral E/e’ ratio). LVWT was calculated as the sum of septal wall thickness and posterior wall thickness, consistent with prior similar genetic analyses of echocardiographic traits14”.
We used the Sequential Oligogenic Linkage Analysis Routines (SOLAR) program to perform the singular and bivariate quantitative trait linkage analysis. SOLAR is a flexible software which uses familial structures from pedigrees to perform linkage analysis between quantitative traits. It can account for multiple loci, dominance and household effects, and interactions8. For the current analyses, the input data into SOLAR required two files. The first file was the pedigree file, which listed the pedigree structure of study participants, which includes information on parents and family as detailed previously17. The second file was the phenotype file, which included information on the phenotype to be studied. The genetic heritability was estimated using the SOLAR software “polygen” function; the genetic and environmental correlation between any two traits was estimated using the “polygen–testrhoe–testrhog” function. For each echocardiographic trait, a linear regression model was used to adjust for the effect of age, sex and height. The residuals were then transformed by the rank-based inverse normal transformation. Due to the skewed distributions of LVMi, LSS and E/e′, these traits were natural log-transformed before the linear regression adjustment. We used the variance components method to estimate the genetic and environmental correlations between echocardiographic traits10. Sex-differences were assessed by t-test, and Bonferroni correction was used to adjust for multiple testing.
Data availability
The data used in the current study could be requested through the dbGaP database with Study Accession: phs000007.v32.p13.
Abbreviations
- AoD:
-
Aortic root diameter
- GCS:
-
Global circumferential strain
- GLS:
-
Global longitudinal strain
- LAD:
-
Left atrial systolic dimension
- LSS:
-
Longitudinal synchrony
- LV:
-
Left ventricle/left ventricular
- LVDD:
-
Left ventricular diastolic dimension
- LVEF:
-
Left ventricular ejection fraction
- LVM:
-
Left ventricular mass
- LVMi:
-
Left ventricular mass index
- LVWT:
-
Left ventricular wall thickness
- MAPSE:
-
Mitral annular plane systolic excursion
- SOLAR:
-
Sequential Oligogenic Linkage Analysis Routines
References
Lam, C. S. P. et al. Sex differences in heart failure. Eur. Heart J. 40, 3859–3868c (2019).
Cheng, S. et al. Correlates of echocardiographic indices of cardiac remodeling over the adult life course: Longitudinal observations from the Framingham Heart Study. Circulation 122, 570–578 (2010).
Gebhard, C. et al. Age-and gender-dependent left ventricular remodeling. Echocardiography 30, 1143–1150 (2013).
Yoneyama, K. et al. Age, sex, and hypertension-related remodeling influences left ventricular torsion assessed by tagged cardiac magnetic resonance in asymptomatic individuals: The multi-ethnic study of atherosclerosis. Circulation 126, 2481–2490 (2012).
Gori, M. et al. Sex-specific cardiovascular structure and function in heart failure with preserved ejection fraction. Eur. J. Heart Fail. 16, 535–542 (2014).
Heidecker, B. et al. The gene expression profile of patients with new-onset heart failure reveals important gender-specific differences. Eur. Heart J. 31, 1188–1196 (2010).
Naqvi, S., Godfrey, A. K., Hughes, J. F., Goodheart, M. L., Mitchell, R. N. & Page, D. C. Conservation, acquisition, and functional impact of sex-biased gene expression in mammals. Science. 365(6450), eaaw7317. https://doi.org/10.1126/science.aaw7317 (2019).
Almasy, L. & Blangero, J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am. J. Hum. Genet. 62, 1198–1211 (1998).
Kochunov, P. et al. Multi-site study of additive genetic effects on fractional anisotropy of cerebral white matter: comparing meta and megaanalytical approaches for data pooling. Neuroimage 95, 136–150 (2014).
Lin, H. et al. Shared genetic and environmental architecture of cardiac phenotypes assessed via echocardiography: The Framingham Heart Study. Circ. Genom. Precis. Medi. 14, e003244 (2021).
Ji, H. et al. Cardiometabolic risk-related blood pressure trajectories differ by sex. Hypertension 75, e6–e9 (2020).
Kauko, A. et al. Sex differences in genetic risk for hypertension. Hypertension 78, 1153–1155 (2021).
Lang, R. M. et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J. Am. Soc. Echocardiogr. 18, 1440–1463 (2005).
Vasan, R. S. et al. Genetic variants associated with cardiac structure and function: A meta-analysis and replication of genome-wide association data. JAMA 302, 168–178 (2009).
Lloyd-Jones, D. M. et al. Lifetime risk for developing congestive heart failure: The Framingham Heart Study. Circulation 106, 3068–3072 (2002).
Adedinsewo, D.A.et al. Cardiovascular disease screening in women: Leveraging artificial intelligence and digital tools. Circulat. Res. 130(4), 673–690 (2022).
Pedigree file format for use in SOLAR analyses. https://www.mv.helsinki.fi/home/tsjuntun/autogscan/pedigreefile.html. Accessed February 1, 2023.
Funding
This work was supported by the National Institutes of Health [contracts 75N92019D00031, HHSN268201500001I, and N01‐HC 25195 to the Framingham Heart Study and Grant numbers R01HL093328, R01HL107385, R01HL126136, R01HL143227, R01HL142983, and U54AG065141. HL is also supported in part by U01AG068221. RSV is also supported in part by the Evans Medical Foundation and the Jay and Louis Coffman Endowment from the Department of Medicine, Boston University School of Medicine. CCD was supported by the Multidisciplinary Training Program (T32) in Cardiovascular Epidemiology (5T32HL125232). ACK is supported by the Doris Duke Charitable Foundation Grant 2020059. We acknowledge the dedication of the Framingham Heart Study participants without whom this research would not be possible.
Author information
Authors and Affiliations
Contributions
H.L., R.S.V. and S.C. conceived the study. H.L. performed the statistical analyses. H.L. and A.C.K. drafted the manuscript. C.C.D., M.I.S., V.X., I.M.Y., G.S., G.F.M., M.G.L., R.S.V. and S.C. critically reviewed the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lin, H., Kwan, A.C., Castro-Diehl, C. et al. Sex-specific differences in the genetic and environmental effects on cardiac phenotypic variation assessed by echocardiography. Sci Rep 13, 5786 (2023). https://doi.org/10.1038/s41598-023-32577-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-32577-6
- Springer Nature Limited