Abstract
To identify metal adapted bacteria equipped with traits positively influencing the growth of two hyperaccumulator plant species Arabidopsis arenosa and Arabidopsis halleri, we isolated bacteria inhabiting rhizosphere and vegetative tissues (roots, basal and stem leaves) of plants growing on two old Zn–Pb–Cd waste heaps in Bolesław and Bukowno (S. Poland), and characterized their potential plant growth promoting (PGP) traits as well as determined metal concentrations in rhizosphere and plant tissues. To determine taxonomic position of 144 bacterial isolates, 16S rDNA Sanger sequencing was used. A metabolic characterization of isolated strains was performed in vitro using PGP tests. A. arenosa and A. halleri accumulate high amounts of Zn in their tissues, especially in stem leaves. Among in total 22 identified bacterial taxa, the highest level of the taxonomical diversity (H’ = 2.01) was revealed in A. halleri basal leaf endophytes originating from Bukowno waste heap area. The 96, 98, 99, and 98% of investigated strains showed tolerant to Cd, Zn, Pb and Cu, respectively. Generally, higher percentages of bacteria could synthesize auxins, siderophores, and acetoin as well as could solubilize phosphate. Nine of waste heap origin bacterial strains were tolerant to toxic metals, showed in vitro PGP traits and are potential candidates for bioremediation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Anthropogenic activities related release of metals has strongly contributed to environmental pollution on a wide world scale1. Metals as non-degradable elements can only be transformed into chemical forms of altered toxicity and/or mobility. They can enter into food chains and cause toxicity to organisms2. Implementation of diverse physical methods of remediation such as soil excavation and land filling, soil washing, electroremediation, vitrification, and chemical treatments such as precipitation, leaching, extraction, ion exchange, encapsulation or immobilization usually result in a reduction of metal reactivity but often produce byproducts3,4. Physicochemical remediation methods have negative effects on microbial life as well as on several soil parameters, like pH, clay and organic matter5,6. They also cannot be applied at large scale since they are generally too expensive, and their public acceptace is low3. As an alternative, plant-based strategies (often termed ‘phytoremediation’) have been proposed as more eco-friendly methods for the restoration of degraded soils7,8,9. Plants that avoid metal toxicity by storing metal ions in their underground tissues are used as phytostabilizers, whereas plants that accumulate metals in aboveground tissues can be applied as phytoextractors10. When a phytoextractor plant is taking up metals from soils and reaches certain threshold leaf metal concentrations it is classified as hyperaccumulator11. Actually, more than 500 metal hyperaccumulators have been described; a majority of them are obligate metallophytes restricted to metalliferous soils, whereas a smaller group involves facultative hyperaccumulators inhabiting both non-metalliferous and metalliferous soils12,13. In spite of an extensive understanding about the mechanisms of adaptation of metallophytes, it is clear that using such plants only for phytoextraction is not economically feasible because of their generally low growth rate and limited biomass production14,15. It has been recently shown that a combined use of plants with specific microorganisms may substantially increase the efficiency of remediation, for both organic pollutants and metals16,17,18,19. Plant endophytes can alleviate various types of stresses, e.g. salinity20, drought21, osmotic stress22, temperature23, and metal toxicity24,25,26,27. Sánchez-López et al.28, for example, demonstrated that the metal tolerant Methylobacterium sp. strain Cp3 isolated from seeds of Crotolaria pumila growing on Zn-polluted soil showed in in vitro tests multiple traits that can have beneficial effect on plant growth and thus can be potentially useful in phytoremediation. Microbes under metal stress conditions can interact with ions directly29,30,31 or can beneficially influence the fitness of their host plants including those that accumulate toxic metals in their tissues, reducing metal toxicity32,33,34,35. The knowledge about bacterial strains potentially useful in remediation of metal polluted soils is of significant importance.
The about 100-yrs old waste heaps in Bolesław and Bukowno in Southern Poland are post-mining deposits containing high metal concentrations (50,000 and 20,159 mg Zn kg−1; 5000 and 35 mg Pb kg−1; 500 and 18 mg Cd kg−1 respectively)36, that exceed to a significant extent the permissible metal concentrations proposed by WHO: 50 mg Zn kg−1, 85 mg Pb kg−1, 0.8 mg Cd kg−137,38,39. They harbor quite a number of plants and bacteria that adapted to metal toxicity40,41,42,43. For example, Arabidopsis halleri (L.) O’Kane and Al-Shehbaz (formerly Cardaminopsis halleri Hayek s. l.) and closely related Arabidopsis arenosa (L.) Lawalrée (formerly Cardaminopsis arenosa (L.) Hayek s. l.) along with associated microorganisms are natural inhabitants of these waste heaps. A halleri is an thoroughly investigated hyperaccumulator of Zn44,45,46,47,48,49, whereas A. arenosa (sand rock-cress) is recently recognized as hyperaccumulator of Zn and Cd50. It was shown that endophytes of Trifolium repens L., inhabiting calamine waste heaps in Southern Poland, contribute significantly to the phytostabilization potential of this plant species36,51,52. Significantly more white clover endophytes of the Zn–Pb–Cd waste heap origin than of the reference one revealed as metal tolerant an able to synthesize ACC-deaminase, acetoin, and siderophores. Moreover, two strains, Bacillus megaterium BolR EW3_A03 and Stenotrophomonas maltophilia BolN EW3_B03 were proposed as promising for application in phytostabilization of Zn, Pb or Cd polluted areas36. Therefore, it sounds likely that the hyperaccumulators A. halleri and A. arenosa, growing on about 100-yrs old waste heaps in Southern Poland, are natural reservoirs of adapted bacterial strains equipped with traits that can be beneficial to plants that might have potential for application as a tool in remediation. In order to identify metal adapted bacterial strains equipped with potential plant growth promoting traits, we studied the metal concentrations in soils and plants and the bacteria isolated from the rhizosphere and different parts (roots, rosette/basal leaves, and stem leaves) of A. halleri and A. arenosa growing on the above-mentioned Zn–Pb–Cd polluted waste heaps in Bolesław and Bukowno. The taxonomic position of bacterial isolates was determined using 16S rRNA gene Sanger sequencing. Also their in vitro potential for synthesizing siderophores, organic acids, acetoin, and indole-3-acetic acid (IAA, auxin), the activity of 1-aminocyclopropane-1-carboxylate (ACC)-deaminase (ACCD), and their ability for phosphate solubilization and fixation of atmospheric nitrogen were examined.
Results
The examination of 144 cultivable bacterial strains isolated from vegetative tissues and rhizosphere of A. arenosa and A. halleri revealed significant differences in bacterial species diversity depending on host plant species, plant habitat and type of the tissue. For A. arenosa, the highest value of strain diversity index (ISD = 100%) was found for the endophytes of stem leaves of plants growing on the Bolestraszyce reference area, while the lowest one (23%) was found for A. arenosa root endophytes from the Bolesław area (Table 1). For A. halleri endophytes, the highest value of strain diversity index (89%) was noticed in rosette leaves of plants growing on the Bukowno waste heap, the lowest one (50%) in rosette leaves of plants originating from the Bolesław waste heap (Table 1). The index of strain diversity of studied A. arenosa and A. halleri rhizosphere communities, except A. arenosa rhizosphere bacteria of Bukowno origin (80%), was 100%. The Shannon’s diversity index (H’) of the A. arenosa endophyte community was the highest in the rosette leaves from Bukowno (H’ = 1.67), while the lowest level (H’ = 0) was detected for the stem leaf endophytic community from the reference Bolestraszyce area (Table 1). Both A. halleri rhizosphere bacterial communities showed significantly lower levels of strain richness (H’ = 1.09) compared to the A. arenosa reference and waste heap communities (Table 1).
16S rRNA gene analysis revealed the diversity of the endophytic and rhizosphere microorganisms of A. arenosa and A. halleri. 62 and 51% of the endophytes of A. arenosa and A. halleri belonged to Firmicutes, 29 and 46.5% to Proteobacteria, and 9 and 2.5% to Actinobacteria, respectively (Fig. 1A). 62% of the endophytic strains of A. arenosa were Bacilli, the classes α- and β-Proteobacteria were represented by approximately 2.5% of the isolated strains, the γ-Proteobacteria by 24%, and the Actinomycetia by 9%. 51% of the isolated endophytes from A halleri were classified as Bacilli, 37% γ-Proteobacteria, 9.5% α-Proteobacteria, and 2.5% Actinomycetia. In both, A. arenosa and A. halleri, the Bacillales were found to be the most abundant order, the least abundant in A. arenosa were Burkholderiales (2.5%), Sphingomonadales (2.5%), and Corynebacteriales (2.5%), whereas in A. halleri the members of Caulobacterales (2.5%), Hyphomicrobiales (2.5%), and Micrococcales (2.5%) showed the lowest abundancies. Bacteria belonging to the genera Priestia sp. (32%), Bacillus sp. (24%), and Pseudomonas sp. (14%) were found as dominant endophytes of A. arenosa, while in A. halleri the prevailing bacterial endophytes were Bacillus sp. (37%), Pseudomonas sp. (16%), and Stenotrophomonas sp. (12%) (Fig. 1A).
Among 21 bacterial strains isolated from the rhizosphere of A. arenosa (15 isolates) and A. halleri (6 isolates) 95% of them were Proteobacteria, while 5% were Firmicutes. The members of the class Bacilli, represented by 5% of the isolated rhizosphere bacteria were members of Bacillales, and Bacillaceae. Almost 95% of the γ-Proteobacteria belonged to the orders Pseudomonadales (29%), Enterobacterales (43%), and Xanthomonadales (23%), and were members of the families Pseudomonadaceae (29%), Enterobacteriaceae (33%), Xanthomonadaceae (23%), Erwiniaceae (5%), and Yersiniaceae (5%). A. arenosa endophytes consisted of γ-Proteobacteria (93%) and Firmicutes (7%) (Fig. 1B). The γ-Proteobacteria from the rhizosphere of A. arenosa belonged to the orders Enterobacterales (46%), Pseudomonadales (27%), and Xanthomonadales (20%), and families: Enterobacteriaceae (32%), Pseudomonadaceae (27%), Xanthomonadaceae (20%), Yersiniaceae (7%), and Erwiniaceae (7%), while the strains belonging to the Bacilli class (7% of the rhizosphere inhabitants) were members of Bacillales, and family of Bacillaceae. All cultivable bacterial inhabitants of the rhizosphere of A. halleri belonged to the class γ-Proteobacteria (Fig. 1B). 33% of them were members of the order Enterobacterales and family Enterobacteriaceae, 33% Pseudomonadales and Pseudomonadaceae, and 33% were members of order Xanthomonadales and family Xanthomonadaceae (Fig. 1B).
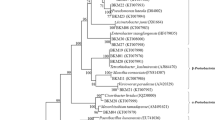
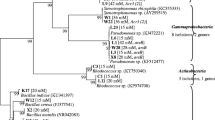
The Neighbor-Joining analysis of the 16S rDNA of the cultivable bacteria showed that their taxonomic position differs between the host-plant species, type of the tissue, and origin of the plant (Fig. 2). For instance, only in rosette leaves of A. arenosa Paenibacillus sp. and Micrococcus sp. were found, whereas in stem leaves Frigoribacterium sp. or Rhodococcus sp. were identified (Fig. 3). In roots of A. arenosa, Variovorax sp. was found, while in its rhizosphere Lelliottia sp. or Pantoea sp. were noticed. Xanthomonas sp. was found only in A. arenosa tissues from waste heap origin. In spite of these differences, also common taxa were identified in A. arenosa samples from the waste heap and the reference area, i.e., Priestia sp. in rosette leaves, stem leaves and roots or Stenotrophomonas sp. in rosette leaves and rhizosphere.
Phylogenetic Neighbor-Joining tree based on 16S rRNA gene sequences showing the relationship of studied endohytic and rhizosphere A. arenosa and A. halleri bacterial strains and reference bacteria (GenBank). Numbers at nods indicate levels of bootstap index based on analysis of 1000 resampled datasets. The scale bar indicates the number of substitutions per site. Accession numbers are shown in parentheses. Bacterial taxa proposed as potentially useful in bioremediation are marked in red rectangles.
In the rosette leaves of A. halleri Brevundimonas sp., Sphingomonas sp., Neobacillus sp. or Methylobacterium sp. were identified, whereas in its stem leaves Stenotrophomonas sp. or Pseudomonas sp. were found (Fig. 4). In the roots of A. halleri Arthrobacter sp. or Serratia sp. were detected, while in its rhizosphere Lelliottia sp., Pseudomonas sp. or Stenotrophomonas sp were identified. Only in A. halleri tissues Neobacillus sp., Methylobacterium sp., Arthrobacter sp. were found. Priestia sp. and Pseudomonas sp. were noted as common genera of rosette leaf endophytes of A. halleri from both waste heaps. Pseudomonas sp. was found in the stem leaves of A. halleri, Bacillus sp. and Serratia sp. in the roots, while Stenotrophomonas sp., Pseudomonas sp., and Lelliottia sp. in its rhizosphere (Fig. 4).
In vitro tests of potential plant growth promoting traits revealed differences between bacterial strains inhabiting tissues and rhizosphere of A arenosa (Fig. 5) and A. halleri (Fig. 6) depending on the host plant species, the type of the tissue as well as the origin of the plants (Table S1). Among A. arenosa endophytes of waste heap origin, auxins, siderophores, and acetoin could be produced by a significantly higher percentage of strains isolated from rosette and stem leaves than from roots; organic acids were produced by significantly more rosette leaf endophytes than stem leaf and root endophytes. Phosphate solubilization was performed by all isolated stem leaf endophytes while a significantly higher percentage of rosette leaf and root endophytes than stem leaf endophytes could perform atmospheric nitrogen fixation (Fig. 5A). 100% of the rhizosphere bacteria from A. arenosa of the waste heap origin showed able to synthesize auxins, siderophores, ACC, and to solubilize phosphates, 92% produced organic acids, 83% of the strains synthesized acetoin, and 64% could fix atmospheric nitrogen (Fig. 5A). In A. arenosa originating from the reference area, significantly more (100%) endophytic strains from rosette leaves and roots and also from the rhizosphere could synthesize auxins and siderophores in comparison with the strains from the stem leaves (60%) (Fig. 5B). About 60% of the endophytes from rosette and stem leaves as well as from the roots of A. arenosa from the Bolestraszyce reference area synthesized organic acids, but rhizosphere bacteria did not produce them. Bacteria associated with A. arenosa of reference area origin showed efficient in producing ACC deaminase, phosphate solubilization and atmospheric nitrogen fixation; all strains isolated from roots, rosette leaves and rhizosphere were effective in ACC deamination; 100% of the stem leaf endophytes and rhizosphere inhabitants could solubilize phosphate; 80% of the stem leaf endophytes could fix atmospheric nitrogen (Fig. 5B). No significant differences were observed in Cd, Zn, Cu, and Pb tolerance of bacteria associated with A. arenosa originating from the waste heap area, but significantly lower tolerances to Zn and Cd were found in rhizosphere strains isolated from A. arenosa growing on the reference area (Fig. 5, Table S1).
In vitro studies testing plant growth properties: indole-3-acetic acid (IAA), siderophores (Sid), organic acids (OA), and acetion (Acet) production, 1-aminocyclopropane-1-carboxylate (ACC)-deaminase activity, phosphate solubilization (P), atmospheric nitrogen fixation (N2) as well as cadmium (Cd), zinc (Zn), copper (Cu), and lead (Pb) tolerance of bacterial endophytes isolated from roots (R), rosette leaves (RL), stem leaves (SL), and rhizosphere (RS) of A. arenosa growing on Zn–Pb–Cd waste heaps (A) and the reference area (B).
In vitro studies testing plant growth properties: indole-3-acetic acid (IAA), siderophores (Sid), organic acids (OA), and acetion (Acet) production, 1-aminocyclopropane-1-carboxylate (ACC)-deaminase activity, phosphate solubilization (P), atmospheric nitrogen fixation (N2) as well as cadmium (Cd), zinc (Zn), copper (Cu), and lead (Pb) tolerance of bacterial endophytes isolated from roots (R), rosette leaves (RL), stem leaves (SL), and rhizosphere (RS) of A. halleri growing on Zn–Pb–Cd waste heaps.
All endophytes isolated from the stem leaves and rhizosphere of A. halleri could produce indole-3-acetic acid; siderophores were produced by all studied root, rosette leaves, and rhizosphere bacteria; ACC deaminase was produced by all isolated root endophytes and rhizosphere bacteria; organic acids were synthesized by 46% of the stem leaf endophytes and were not produced by all rhizosphere bacteria; acetoin was produced by 90% of the root endophytes; the highest frequency of phosphorus solubilization was found for root and stem leaf endophytes and rhizosphere bacteria; the highest percentage of atmospheric nitrogen fixation (45%) was found for the root endophytes (Fig. 6).
A significantly higher percentage of A. arenosa rosette leaf endophytes synthesized IAA, organic acids, acetoin, and fixed atmospheric nitrogen in comparison with those of the A. halleri rosette. Significantly more A. halleri stem leaf endophytes produced auxins compared to A. arenosa (Figs. 5 and 6). All A. halleri endophytes and rhizosphere inhabitants were tolerant to Pb and Cu. A higher percentage of A. halleri endophytes were tolerant to Zn in comparison to the rhizosphere inhabitants; more rosette leaves endophytes were tolerant to Cd in comparison to strains isolated from other tissues (Fig. 6).
The results from in vitro studies revealed differences in plant growth promotion traits between the bacterial endophytes of the hyperaccumulators originating from the waste heap and the reference area. Significantly more endophytes of roots, stem and rosette leaves and rhizosphere from the reference area could fixate atmospheric nitrogen compared to the ones from waste heap origin. Significantly more stem leaf endophytes of the waste heap origin could produce auxins, siderophores, and acetoin than the stem leaf endophytes of plants from the reference grassland; more rosette leaf endophytes of waste heap origin could solubilize phosphate in comparison with those originating from the reference area (Fig. 7). Bacterial communities associated with A. arenosa and A. halleri growing on the Bolesław and Bukowno waste heaps and the reference area did not show significant differences concerning IAA production, ACCD activity and SI (Table S2).
Comparison of in vitro studied plant growth properties: indole-3-acetic acid (IAA), siderophores (Sid), organic acids (OA), and acetion (Acet) production, 1-aminocyclopropane-1-carboxylate (ACC)-deaminase activity, phosphate solubilization (P), atmospheric nitrogen fixation (N2) as well as cadmium (Cd), zinc (Zn), copper (Cu), and lead (Pb) tolerance of bacteria isolated from roots (R), rosette leaves (RL), stem leaves (SL), and rhizosphere (RS) of hyperaccumulator plants A. arenosa and A. halleri originating from Zn–Pb–Cd waste heaps (WH) and reference (R) area.
Among the bacterial strains from the waste heap, which were tolerant to Zn, Pb, and Cd and showed positive for all plant growth promotion traits that were tested in vitro, nine endophytic strains, six from A. arenosa origin as well three from A. halleri, might have significant potential for use in bioremediation (Table S1). The most promising endophytes of A. arenosa from the Bolesław waste heap area were: Priestia sp. strain EW1_D06 isolated from rosette leaves, Bacillus sp. strain EW1_B04, Pseudomonas sp. strain EW1_E01, and Stenotrophomonas sp. strain EW3_G06 that inhabited stem leaves. From the Bukowno waste heap it were: Priestia sp. strain EW1_H08 isolated from rosette leaves as well as Pseudomonas sp. strain 1.2 of rhizosphere origin. From the A. halleri endophytes the most promising were: Bacillus sp. strain EW2_B02 isolated from rosette leaves (Bolesław origin), Bacillus sp. strain EW2_H12 isolated from stem leaves (Bolesław origin), and Priestia sp. strain EW1_C08 isolated from roots (Bukowno origin).
Trace element analysis revealed significantly higher Zn, Pb, and Cd concentrations in rhizosphere soil as well as in rosette and stem leaves and roots of A. arenosa growing on the Bolesław and Bukowno waste heaps compared to those from the Bolestraszyce reference area (Table 2B). Zinc concentrations in leaves and roots of A. arenosa and A. halleri were significantly higher level than those of Pb and Cd. In leaves of plants of the Bolesław origin Zn concentrations were higher than in roots. The Pb concentrations in roots of A. arenosa and A. halleri were significantly higher than in the leaves (Table 2). The limits of metal detection (LOD) as well as limits of metal quantifications (LOQ) were detected as follows: LODZn = 0.040 and LOQZn = 0.13 μg × L−1, LODPb = 0.0031 and LOQPb = 0.0102 μg × L−1, LODCd = 0.0017 and LOQCd = 0.0056 μg × L−1.
Discussion
Our study of 144 cultivable bacterial endophytes isolated from roots, rosette and stem leaves as well as rhizosphere of the Zn-hyperaccumulators A. halleri and A. arenosa growing on calamine waste heaps in Bolesław and Bukowno and the unpolluted reference area in Bolestraszyce revealed different percentages of intra-community taxonomic diversity of bacteria, high tolerance to Zn, Pb, Cd, and Cu as well as a prominent potential to beneficially influence the fitness and growth of the host plants (Figs. 1, 2, 5, 6 and Table S1). Almost all A. arenosa and A. halleri rhizosphere bacterial communities, except A. arenosa rhizosphere bacteria of Bukowno waste heap origin, showed maximal 100% strain diversity. The highest level of the Shannon’s diversity index (H’ = 2.01) was found for the A. halleri rosette leaf endophyte community originating from the Bukowno waste heap while the lowest one (H’ = 0) was observed for the A. arenosa stem leaf endophytes from the reference area origin (Table 1).
Bacteria can develop different tolerance mechanisms to cope with metal toxicity, e.g. by modifications of cellular barriers, efflux of metals out of the cell, enzymatic conversion of metals, extracellular and intracellular sequestration29,31,32,53. Bacteria can positively stimulate plant growth via increasing the availability of essential nutrients, production and regulation of compounds regulating plant growth or protecting plants against diseases33,34,35. Among the bacteria found in the present study, several have been mentioned for possessing bioremediation potential, i.e., Arthrobacter sp., Bacillus sp., Brevundimonas sp., Enterobacter sp., Frigoribacterium sp., Lelliottia sp., Micrococcus sp., Methylobacterium sp., Neobacillus sp., Paenibacillus sp., Pantoea sp., Phytobacter sp., Plantibacter sp., Pseudomonas sp., Rhodococcus sp., Serratia sp., Sphingomonas sp., Stenotrophomonas sp., Variovorax sp., Xanthomonas sp.54,55,56,57,58,59. The adaptations of bacteria to metal toxicity are determined genetically on chromosomal genes and/or genes located on plasmids60,61. In general, the genetics of metal tolerance mechanisms in bacteria are quite well-studied. For example, several species of genus Bacillus comprise strains that are efficient in removal of Zn (e.g., B. subtilis, B. licheniformis, B. cereus, B. jeotgali, B. firmus), Cd (e.g., B. subtils, B. licheniformis, B. safensis, B. megaterium, B. catenulatus) or Pb (e.g., B. cereus)54,62. Cadmium resistance of Bacillus sp. is determined by the cad operon (3.5 kbp) localized on plasmid pI258, composed of cadA and cadC genes. Gene cadA encodes a 727-aminoacid protein that functions as Cd efflux ATP-ase, while cadC encodes a 122-aminoacid protein, which acts as a regulator of the cad operon63. In contrast, Pb2+ ions in Bacillus spp. cells are immobilized by phosphate, carboxyl, carbonyl, sulfhydryl, and hydroxyl groups that give cell walls a negative charge as well as in polypeptides, polysaccharides, and proteins via van der Waal’s forces, covalent and ion bonds. The resistance to lead in Bacillus sp. is determined by a plasmid pbr operon54,61.
Metal tolerant bacteria that can immobilize metal ions can have indirect beneficial effects on the growth of their host plants33,64,65. In vitro studies on plant growth promotion traits of A. halleri and A. arenosa endophytes and rhizosphere inhabitants showed that bacterial traits differ between plant tissues, plant species and their origin (Figs. 5, 6, 7 and Table S1). A significantly higher percentage of A. arenosa root endophytes originating from the waste heap area could solubilize phosphate compared to those originating from the reference area. Similarly, higher percentages of endophytes of basal leaves of plants from the waste heaps were producing acetoin and solubilizing phosphate compared to the ones from the reference grassland. Also higher percentages of stem leaf endophytes from waste heap origin could synthesize of auxins, siderophores, and acetoin in comparison to endophytes of A. arenosa growing on the reference grassland (Fig. 5). Metabolic traits were also different between host plant species. Higher percentages of root endophytes of A. halleri could produce siderophores, ACC-deaminase, and acetoin than root endophytes of A. arenosa of waste heap origin. Similarly, a higher percentage of A. halleri basal leaf endophytes could solubilize phosphates, as well as more A. halleri stem leaf endophytes could produce auxins and ACC-deaminase in comparison to endophytes of A. arenosa from the waste heaps (Figs. 5 and 6). 1-aminocyclopropane-1-carboxylate (ACC) deaminase (ACCD) converts the immediate precursor of ethylene in plant cells (ACC) into α-ketobutyrate and ammonia33,66,67, resulting in lowered levels of ethylene production. High levels of ethylene can negatively affect plant growth e.g. by inhibiting root and stem elongation, by inducing hypertrophy and accelerating senescence and abscission68. It is also known that, in case of metal stress, phosphate solubilizing bacteria (PSB), e.g. Bacillus spp., Enterobacter spp., Micrococcus spp., Pseudomonas spp., Rhizobium spp.69, are of the great importance for plant growth by enhancing the availability of phosphorous, which is an essential building block of several macromolecules e.g., DNA, RNA, ATP and phospholipids70,71,72. Stenotrophomonas spp. strain RC5, Serratia spp. strain RCJ6 and Enterobacter spp. strain RJAL6 that could solubilize phosphate by producing organic acids and phosphatases were demonstrated to assist ryegrass to overcome aluminium toxicity73, while Ensifer adhaerens strain OS3 with high acid and alkaline phosphatase activity turned out to be a chromium reducer74. Under metal stress conditions also bacteria synthesizing siderophores, low molecular (400–1500 Da) chelators of iron (FeIII)75, have positive effects on plant growth and health. Iron is essential for maintaining the structure and function of chloroplasts, DNA synthesis, cellular respiration, and is involved in many redox reactions, among others as a constituent of many enzymes’ prosthetic groups76. Presence of metals induces the synthesis of siderophores in bacteria. For example, under Cd(II) and Zn(II) stress Pseudomonas aeruginosa strain ZGKD3 produced pyoverdine77, under Cd (II) stress Streptomyces spp. isolated from Betula pendula and Alnus glutinosa rhizosphere produced hydroxamate, catecholate and phenolate siderophores, particularly ferrioxamine B78, while under exposure to toxic Pb(II) concentrations Bacillus spp. strain PZ-1 isolated from Brassica juncea synthesized siderophores of hydroxamate structure79,80. Bacteria associated with plants can synthesize phytohormones, e.g. auxins, and/or influence the hormone balance of their host plants, e.g. by, like already mentioned above, lowering the plants’ ethylene production by ACCD activity81. Under conditions of metal stress, the IAA-synthesizing B. megaterium strain MCR-8 enhanced the biomass production of Vinca rosea as well as increased the levels of phenols, flavonoids, and antioxidative enzymes82, while IAA-producing Leifsonia spp. and Bacillus spp. significantly increased the growth of Zea mays in comparison to non-polluted soils83. Acetoin (3-hydroxy-2- butanone), one of the volatile organic compounds (VOCs), that are low molecular weight (< 300 Da) hydrocarbons emitted in a gaseous phase or secreted into liquids84,85, provokes ISR (induced systemic resistance) and contributes to an improvement of plant growth86,87. Bacterial born VOCs influence the bacterial motility, antibiotic resistance or biofilm formation, as well as influence the plants, e.g. increase biomass, fruit yield, seed production, lateral root and root hair formation, nutrient uptake, and photosynthetic activity87,88,89,90. It is clear that there are some strains of bacteria active in deaminase ACC, phosphate solubilization as well as in production of organic acids, siderophores, auxins, and VOCs under metal stress conditions, and are beneficial to plants factors of the potential usage in restoration of degraded soils.
In the present research we identified strains with high tolerance to Zn, Pb, Cd that also tested positive for all in vitro potential plant growth promotion parameters. Therefore, we propose them as potential candidates for bioremediation purposes. Among these strains of the waste heap origin, six strains associated with A. arenosa and three A. halleri endophytes meet these criteria. From A. arenosa originating from the Bolesław waste heap it are Priestia sp. strain EW1_D06 (isolated from rosette leaves), Bacillus sp. strain EW1_B04, Pseudomonas sp. strain EW1_E01, and Stenotrophomonas sp. strain EW3_G06 (from the stem leaves), and from the Bukowno waste heap origin it are Priestia sp. strain EW1_H08 (from basal leaves) as well as Pseudomonas sp. strain 1.2 (from the rhizosphere). From A. halleri orginating from Bolesław it are Bacillus sp. strain EW2_B02 (isolated from rosette leaves) and Bacillus sp. strain EW2_H12 (from stem leaves) and from Bukowno Priestia sp. strain EW1_C08 (from roots). Priestia sp. (formerly Bacillus sp.) was shown to be efficient to assist their host plant to remove Pb and Cd from polluted soil58,91,92 and equipped with plant growth promoting traits93. For example, P. megaterium strain R181 was reported as an effective stimulator of corn and wheat growth94 and also as an inhibitor of plant diseases due to synthesis of antibiotic-type compounds, e.g. lipopeptides similar to surfactins, lichenysins, itrurinA, and fengycins95,96. Also, biosorptive microbes like Stenotrophomonas maltophilia as well as Pseudomonas viridiflava97 were suggested as useful in metal bioremediation98,99. It was also reported that S. maltophilia producing ACC deaminase, gibberellic acid, indole-3-acetic acid, and siderophores can promote host plant growth100.
In conclusion, our studies revealed that A. arenosa and A. halleri are Zn-hyperaccumulators, which store high metal concentrations in leaves and in roots. Endophytic and rhizospheric bacterial communities associated with these hyperaccumulators differ in taxonomic composition and also in metabolic traits depending on the plant species and origin of the host-plant and the plant compartment. Due to a high tolerance to Zn, Pb, and Cd and the fact that they tested positive for all in vitro assessed plant growth promotion traits, bacterial strains originating from the waste heaps, i.e., Priestia sp., Stenotrophomonas sp., Pseudomonas sp., and Bacillus sp. are proposed as potential candidates for bioremediation purposes.
Materials and methods
In total 144 bacterial strains were isolated from plant tissues (123 strains), i.e., roots, rosette (basal) leaves, and stem leaves as well as from the rhizosphere (21 strains) of A. halleri and A. arenosa from two, about 100-yrs old Zn–Pb–Cd polluted waste heaps in Bolesław (50° 17′ N 19° 29′ E) and Bukowno (50° 16′ N 19° 28′ E) situated in the Olkusz Ore Region (Silesia-Krakow Upland), and from a reference grassland in Bolestraszyce (49° 48′ N 22° 50′ E, Przemyskie Foothills), in Southern Poland. The plants were randomly selected and dug out with a sterilized shovel, stored in sterile plastic bags that were kept and transferred to the laboratory in a temperature controlled cool box (4–8 °C). Collected A. halleri and A. arenosa individuals were identified by Dr. Ewa Oleńska and the voucher specimens were deposited in Faculty of Biology University of Bialystok (Poland) plant collection.
Isolation of plant associated bacteria
Rhizosphere bacteria
Approximately 5 g of soil was suspended in 30 mL liquid R2A medium (in w/v%, casein acid hydrolysate 0.05, yeast extract 0.05, protease peptone 0.05, dextrose 0.05, soluble starch 0.05, K2HPO4 0.03, MgSO4 × 7H2O 0.003, sodium pyruvate 0.03, agar 1.5, pH 7.2)101 in sterile Falcon tubes and incubated on a rotary shaker (140 rpm) at 28 °C. After seven days tubes were centrifuged at 2000 rpm for 15 min and 100 µL of supernatant diluted into 10−8 was plated on Petri dishes with solid R2A medium. Single bacterial colonies of a different morphology were transferred to plates with solid R2A medium. After 7-days of incubation at 28 °C in a liquid R2A medium they were transferred into sterile deionized water containing glycerol (15% w/v) and 0.85% w/v NaCl, and stored in a freezer at − 45 °C.
Plant tissue endophytes
Basal and stem leaves as well as roots were carefully rinsed with water and then surface sterilized with 0.1% NaOCl (6–14% active chlorine, Emplura®) for 10 s. Subsequently, the tissues were transferred into 75% ethanol for 30 s, and rinsed three times in sterile MilliQ water for 30 s. The effectiveness of sterilization was verified by plating 100 µL of the last rinsing water on a solid 1/10 869 medium (in w/v%, CaCl2 × 2H2O 0.035, D-glucose 0.1, NaCl 0.5, tryptone 1.0, yeast extract 0.5, agar 1.4, pH 7.0), which is an appropriate medium for studying the diversity of plant endophytes102. Leaves and roots of plants were homogenized in sterile 10 mM MgSO4 buffer solution using TissueLyser LT (Qiagen); thereafter, 100 µL of plant extract diluted into 10−4 was plated on 1/10 869 solid medium. After 7 days of incubation at 30 °C, single colonies that showed different morphologies were plated on separate 1/10 869 solid media. Pure colonies were transferred to sterile liquid glycerol medium, and stored in a freezer at − 45 °C.
The level of the intra-population taxonomic diversity was estimated using the index of strain diversity (ISD) that expresses population richness in bacterial strains and the Shannon’s diversity index (H’), which indicates a population bacterial strain variability. Parameters ISD and H’ were calculated according to equations described by Oleńska and Małek103.
Identification of bacteria using 16S rRNA gene analysis
DNA was isolated from each of the 144 bacterial strains using the Applied Biosystems MagMAX™ Total DNA Multi-Sample Ultra Kit according the manufacturer’s instructions (ThermoFisher Scientific) after lysis of the bacterial cells as described by Oleńska et al.36. The amplification of the 16S rRNA gene was performed using FastStart™ HF (High Fidelity) PCR System (Sigma-Aldrich) in a total volume 25 μL containing: 2.55 µL 10 × concentrated with 18 mM MgCl2 FastStart HF Reactive Buffer, 0.5 µL 10 mM PCR grade dNTP (deoxynucleoside triphosphate) mix, 0.5 µL 0.2 µM of each primer, 0.2 µL 5 U/µL Fast Start High Fidelity Enzyme Blend, 19.75 µL nuclease free water, and 1 μL of DNA as a template. Amplification of 16S rDNA was performed with the primers 27F 5’-AGAGTTTGATCMTGGCTCAG-3’ and 1492R 5’-TACGGYTACCTTGTTACGACTT-3’ (Macrogen, Netherlands)104 using the following conditions: initial denaturation at 94 °C for 5 min, 35 cycles of denaturation at 94 °C for 1 min, annealing at 52 °C for 30 s, and extension at 72 °C for 3 min, and a final extension at 72 °C for 10 min. Amplicons were inspected in a 1% agarose gel electrophoresis performed in 1 × TBE buffer, and documented with the Gel Doc System (Invitrogen). The Sanger sequence PCR reaction and removal of fluorescent labelled ddNTPs unbound with DNA were performed by Macrogen (Netherlands). The 16S rRNA gene sequences obtained in the present study were inspected using the Chromas 2.5 and BioEdit105 programs. The high-quality sequences were searched for the closest relative in the NCBI (National Center for Biotechnology Information) database using BLAST (Basic Local Alignment Search Tool).
In vitro analysis of plant growth promotion traits
Each bacterial strain was in vitro tested for traits that can potentially improve plant growth by: (1) increasing the availability of nutrients, e.g. phosphate solubilization, synthesis of siderophores and organic acids, and atmospheric nitrogen fixation; (2) synthesis of potential plant growth regulators i.e., indole-3-acetic acid and 1-aminocyclopropane-1-carboxylate (ACC)-deaminase (ACCD) and acetoin production. Most in vitro phenotypic tests were qualitatively, colorimetrically assessed. A phosphate solubilization index (SI) was estimated by plating bacteria on selective NBRIP medium; IAA production as well as ACCD activity were quantitatively, spectrophotometrically assessed. The ability to synthesize organic acids was studied according to Cunnigham and Kuiack106, siderophore production was tested using chrome-azurol S according to Schwyn and Neilands107 using a 284 medium108. Bacterial acetoin production was examined according to Romick and Fleming109. The bacterial capacity to solubilize phosphate was tested according to Pikovskaya110 with the modifications of Nautiyal111, and the phosphate solubilization index (SI) was calculated using an equation described by Pande et al.112. The capability of bacteria to synthesize IAA was investigated according to the methods described by Gordon and Weber113 and Patten and Glick114. Quantification of the synthesized IAA by bacteria was performed according to Penrose and Glick115 and the bacterial ACCD activity was studied according to Belimov et al.116 and was described in detail in Oleńska et al.36.
Metal concentrations in plant tissues and soil
Zn, Pb, and Cd concentrations were examined in five samples of rhizosphere soil as well as roots, basal and stem leaves collected from A. arenosa and A. halleri growing on the Bolesław and Bukowno Zn–Pb–Cd waste heaps and on the reference Bolestraszyce area. The mineralization of plant tissues and soil samples was performed in a Mars 6 microwave oven (CEM Corporation, Matthews, NC, USA) according to the procedure described by Oleńska et al.36. The quality assurance procedures involving analysis of reagent blanks and reference materials (Montana II soil 2711a NIST® SRM® and Tomato leaves 1573a NIST® SRM®, Sigma-Aldrich) were performed in parallel. Metal concentrations were determined using ICP-MS 2030, Shimadzu, Japan equipped in mini-torch (quartz), 1.2 kW radio frequency power generator, under argon gas flow. Zn, Pb, and Cd concentrations were expressed as μg × L−1, and the quantified isotopes were used as follows Zn66, Pb208, and Cd111.
Statistical analysis
Metal as well as IAA concentrations, ACCD activity, and SI index results were presented as means ± SD. Values were analyzed with one-way analysis of variance (ANOVA), and significant differences between means were estimated with the multiple range Duncan’s test using Statistica version 13 (TIBCO). Differences in H’ and ISD values between populations were determined with the usage of non-parametric U Mann–Whitney statistical test at the significance level p < 0.05. The 16S rRNA gene based phylogenetic analysis of the bacterial taxa was performed using MEGA 7.0 software117. The phylogenetic Neighbor Joining tree construction was based on an analysis of 1000 resampled data sets according to the Maximum Composite Likelihood model.
Permissions or licences and legislation statement
The collection of, i.e., Arabidopsis halleri and Arabidopsis arenosa, being the source material of bacteria analysed in present study, does not need to obtain any permissions or licenses in Poland. Both species are not under species protection and were not sampled from protected areas.
I confirm that the experimental research and field studies presented in actual manuscript, including the collection of plant material and all used methods were carried out in accordance with institutional, national, and international guidelines and legislation.
Data availability
The datasets analysed during the current study are available in Supplementary Material of this manuscript. The 16S rRNA gene sequences were deposited in NCBI GenBank database under accession numbers OQ151829-OQ151972.
References
Briffa, J., Sinagra, E. & Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Helyon 6, e04691. https://doi.org/10.1016/j.heliyon.2020.e04691 (2020).
Tchounwou, P. B., Yedjou, C. G., Patlolla, A. K., Sutton, D. J. Heavy metal toxicity and the environment. In Molecular, Clinical and Environmental Toxicology (ed Luch, A.) (Experientia Suppl, 101, Springer, Basel, 2012).
Khalid, S. et al. A comparison of technologies for remediation of heavy metal contaminated soils. J. Geochem. Explor. 182, 247–268. https://doi.org/10.1016/j.gexplo.2016.11.021 (2017).
Li, Z. et al. Zeolite-supported nanoscale zero-valent iron: New findings on simultaneous adsorption of Cd(II), Pb(II), and As(III) in aqueous solution and soil. J. Hazard. Mater. 344, 1–11. https://doi.org/10.1016/j.jhazmat.2017.09.036 (2018).
Danh, L. T., Truong, P., Mammucari, R., Tran, T. & Foster, N. Vetiver grass, Vetiveria zizanioides: A choice plant for phytoremediation of heavy metals and organic wastes. Int. J. Phytoremediat. 11, 664–691. https://doi.org/10.1080/15226510902787302 (2009).
Shahid, M. et al. Long-term field metal extraction by Pelargonium: Phytoextraction efficiency in relation to plant maturity. Int. J. Phytoremediat. 14, 493–505. https://doi.org/10.1080/15226514.2011.604689 (2012).
Ani, E., Adekunle, A. A., Kadiri, A. B. & Njoku, K. L. Rhizoremediation of hydrocarbon contaminated soil using Luffa aegyptiaca (Mill) and associated fungi. Int. J. Phytoremediat. 23(14), 1444–1456. https://doi.org/10.1080/15226514.2021.1901852 (2021).
Mosa, K. A., Saadoun, I., Kumar, K., Helmy, M. & Dhankher, O. P. Potential biotechnological strategies for the cleanup of heavy metals and metalloids. Front Plant Sci. 7, 303. https://doi.org/10.3389/fpls.2016.00303 (2016).
Pilon-Smits, E. Phytoremediation. Annu. Rev. Plant Biol. 56, 15–39. https://doi.org/10.1146/annurev.arplant.56.032604.144214 (2005).
Suman, J., Uhlik, O., Viktorova, J. & Macek, T. Phytoextraction of heavy metals: A promising tool for clean-up of polluted environment?. Front. Plant Sci. 9, 1476. https://doi.org/10.3389/fpls.2018.01476 (2018).
Goolsby, E. W. & Mason, C. M. Toward a more physiologically and evolutionarily relevant definition of metal hyperaccumulation in plants. Front. Plant Sci. 6, 33. https://doi.org/10.3389/fpls.2015.00033 (2015).
Pollard, A. J., Reeves, R. D. & Baker, A. J. Facultative hyperaccumulation of heavy metals and metalloids. Plant Sci. 217–218, 8–17. https://doi.org/10.1016/j.plantsci.2013.11.011 (2014).
van der Ent, A., Baker, A. J. M., Reeves, R. D., Pollard, A. J. & Schat, H. Hyperaccumulators of metal and metalloid trace elements: Facts and fiction. Plant Soil 362, 319–334. https://doi.org/10.1007/s11104-012-1287-3 (2013).
McGrath, S. P. et al. Field evaluation of Cd and Zn phytoextraction potential by the hyperaccumulators Thlaspi caerulescens and Arabidopsis halleri. Environ. Pollut. 141, 115–125. https://doi.org/10.1016/j.envpol.2005.08.022 (2005).
Vangronsveld, J. et al. Phytoremediation of contaminated soils and groundwater: Lessons from the field. Environ. Sci. Pollut. Res. 16, 765–794. https://doi.org/10.1007/s11356-009-0213-6 (2009).
Gkorezis, P. et al. The interaction between plants and bacteria in the remediation of petroleum hydrocarbons: An environmental perspective. Front. Microbiol. 7, 1836. https://doi.org/10.3389/fmicb.2016.01836 (2016).
Singh, S. et al. Role of plant–microbe systems in remediation of petrochemical-contaminated water and soil environment. In Microbe Mediated Remediation of Environmental Contaminants (eds Kumar, A. et al.) 79–88 (Woodhead Publishing Series in Food Science, Technology and Nutrition, 2021). https://doi.org/10.1016/B978-0-12-821199-1.00008-0.
Thijs, S., Weyens, N., Gkorezis, P. & Vangronsveld, J. Plant-endophyte partnerships to assist petroleum hydrocarbon remediation. In Consequences of Microbial Interactions with Hydrocarbons, Oils, and Lipids: Biodegradation and Bioremediation. Handbook of Hydrocarbon and Lipid Microbiology (ed. Steffan, R.) (Springer, 2016). https://doi.org/10.1007/978-3-319-44535-9_9-1.
Thijs, S. et al. Tobacco, sunflower and high biomass SRC clones show potential for trace metal phytoextraction on a moderately contaminated field site in Belgium. Front. Plant Sci. 9, 1879. https://doi.org/10.3389/fpls.2018.01879 (2018).
Fan, D., Subramanian, S. & Smith, D. L. Plant endophytes promote growth and alleviate salt stress in Arabidopsis thaliana. Sci. Rep. 10, 12740. https://doi.org/10.1038/s41598-020-69713-5 (2020).
Pandey, V. et al. Dose-dependent response of Trichoderma harzianum in improving drought tolerance in rice genotypes. Planta 243, 1251–1264. https://doi.org/10.1007/s00425-016-2482-x (2016).
Sarma, M. V. R. K. Application of inorganic carrier-based formulations of fluorescent pseudomonads and Piriformospora indica on tomato plants and evaluation of their efficacy. J. Appl. Microbiol. 111, 456–466. https://doi.org/10.1111/j.1365-2672.2011.05062.x (2011).
Subramanian, P., Mageswari, A., Kim, K., Lee, Y. & Sa, T. Psychrotolerant endophytic Pseudomonas sp. strains OB155 and OS261 induced chilling resistance in tomato plants (Solanum lycopersicum Mill.) by activation of their antioxidant capacity. Mol. Plant Microb. Int. 28, 1073–1081. https://doi.org/10.1094/MPMI-01-15-0021-R (2015).
Babu, A. G., Shea, P. J., Sudhakar, D., Jung, I. B. & Oh, B. T. Potential use of Pseudomonas koreensis AGB-1 in association with Miscanthus sinensis to remediate heavy metal (loid)-contaminated mining site soil. J. Environ. Manag. 151, 160–166. https://doi.org/10.1016/j.jenvman.2014.12.045 (2015).
Liu, S. et al. Role of two plant growth-promoting bacteria in remediating cadmium-contaminated soil combined with Miscanthus floridulus (Lab.). Plants 10, 912. https://doi.org/10.3390/plants10050912 (2021).
Luo, S. et al. Endophyte-assisted promotion of biomass production and metal-uptake of energy crop sweet sorghum by plant-growth-promoting endophyte Bacillus sp. SLS18. Appl. Microbiol. Biotechnol. 93, 1745–1753. https://doi.org/10.1007/s00253-011-3483-0 (2012).
Wang, J. L., Li, T., Liu, G. Y., Smith, J. M. & Zhao, Z. W. Unravelling the role of dark septate endophyte (DSE) colonizing maize (Zea mays) under cadmium stress: Physiological, cytological and genic aspects. Sci. Rep. 6, 22028. https://doi.org/10.1038/srep22028 (2016).
Sánchez-López, A. S. et al. Seed endophyte microbiome of Crotalaria pumila unpeeled: Identification of plant-beneficial Methylobacteria. Int. J. Mol. Sci. 19, 291. https://doi.org/10.3390/ijms19010291 (2018).
Ji, G. & Silver, S. Bacterial resistance mechanisms for heavy metals of environmental concern. J. Ind. Microbiol. 14(2), 61–75. https://doi.org/10.1007/BF01569887 (1995).
Oleńska, E. & Małek, W. Sequence analysis of hypothetical lysine exporter genes of Rhizobium leguminosarum bv. trifolii from calamine old waste heaps and their evolutionary history. Curr. Microbiol. 66, 493–498. https://doi.org/10.1007/s00284-013-0303-z (2013).
Silver, S. & Phung, L. T. Bacterial heavy metal resistance: New surprises. Annu. Rev. Microbiol. 50, 753–789. https://doi.org/10.1146/annurev.micro.50.1.753 (1996).
Glick, B. R. Using soil bacteria to facilitate phytoremediation. Biotechnol. Adv. 28, 367–374. https://doi.org/10.1016/j.biotechadv.2010.02.001 (2010).
Oleńska, E. et al. Beneficial features of plant growth-promoting rhizobacteria for improving plant growth and health in challenging conditions: A methodological review. Sci. Tot. Environ. 743, 140682. https://doi.org/10.1016/j.scitotenv.2020.140682 (2020).
Thijs, S., Sillen, W., Rineau, F., Weyens, N. & Vangronsveld, J. Towards an enhanced understanding of plant–microbiome interactions to improve phytoremediation: Engineering the metaorganism. Front. Microbiol. 7, 341. https://doi.org/10.3389/fmicb.2016.00341 (2016).
Thijs, S., Sillen, W., Weyens, N. & Vangronsveld, J. Phytoremediation: State-of-the-art and a key role for the plant microbiome in future trends and research prospects. Int. J. Phytoremediat. 19(1), 23–38. https://doi.org/10.1080/15226514.2016.1216076 (2016).
Oleńska, E. et al. Trifolium repens-associated bacteria as a potential tool to facilitate phytostabilization of zinc and lead polluted waste heaps. Plants 9, 1002. https://doi.org/10.3390/plants9081002 (2020).
World Health Organization (WHO). Permissible Limits of Heavy Metals in Soil and Plants. Geneva, Switzerland (1996).
Ogundele, D. T., Adio, A. A. & Oludele, O. E. Heavy metal congregations in plants and soil along heavy traffic roads in North Central Nigeria. J. Environ. Anal. Toxicol. 5(6), 1000334. https://doi.org/10.4172/2161-0525.1000334 (2015).
Raklami, A., Meddich, A., Oufdou, K. & Baslam, M. Plants—Microorganisms-based bioremediation for heavy metal cleanup: Recent developments, phytoremediation techniques, regulation mechanisms, and molecular responses. Int. J. Mol. Sci. 23, 5031. https://doi.org/10.3390/ijms23095031 (2022).
Babst-Kostecka, A. et al. Evolutionary dynamics of quantitative variation in an adaptive trait at the regional scale: The case of zinc hyperaccumulation in Arabidopsis halleri. Mol. Ecol. 27, 3257–3273. https://doi.org/10.1111/mec.14800 (2018).
Oleńska, E. & Małek, W. Genetic differentiation of Trifolium repens microsymbionts deriving from Zn–Pb waste-heap and control area in Poland. J. Basic Microbiol. 55, 462–470. https://doi.org/10.1002/jobm.201400604 (2015).
Słomka, A. et al. Increased genetic diversity of Viola tricolor L. (Violaceae) in metal-polluted environments. Chemosphere 83(4), 435–442. https://doi.org/10.1016/j.chemosphere.2010.12.081 (2011).
Wójcik, M., Dresler, S., Jawor, E., Kowalczyk, K. & Tukiendorf, A. Morphological, physiological, and genetic variation between metallicolous and nonmetallicolous populations of Dianthus cartusianorum. Chemosphere 90(3), 1249–1257. https://doi.org/10.1016/j.chemosphere.2012.09.068 (2013).
Honjo, M. N. & Kudoh, H. Arabidopsis halleri: A perennial model system for studying population differentiation and local adaptation. AoB Plants 11, 6. https://doi.org/10.1093/aobpla/plz076 (2019).
Meyer, C. L. et al. Variability of zinc tolerance among and within populations of the pseudometallophyte species Arabidopsis halleri and possible role of directional selection. New Phytol. 185, 130–142. https://doi.org/10.1111/j.1469-8137.2009.03062.x (2010).
Pauwels, M., Saumitou-Laprade, P., Holl, A. C., Petit, D. & Bonnin, I. Multiple origin of metallicolous populations of the pseudometallophyte Arabidopsis halleri (Brassicaceae) in central Europe: The cpDNA testimony. Mol. Ecol. 14, 4403–4414. https://doi.org/10.1111/j.1365-294X.2005.02739.x (2005).
Pauwels, M., Frérot, H., Bonnin, I. & Saumitou-Laprade, P. A broad-scale analysis of population differentiation for Zn tolerance in an emerging model species for tolerance study: Arabidopsis halleri (Brassicaceae). J. Evol. Biol. 19, 1838–1858. https://doi.org/10.1111/j.1420-9101.2006.01178.x (2006).
Pauwels, M., Willems, G., Roosens, N., Frérot, H. & Saumitou-Laprade, P. Merging methods in molecular and ecological genetics to study the adaptation of plants to anthropogenic metal-polluted sites: Implications forphytoremediation. Mol. Ecol. 17, 108–119. https://doi.org/10.1111/j.1365-294X.2007.03486.x (2008).
Pauwels, M. et al. Nuclear and chloroplast DNA phylogeography reveals vicariance among European populations of the model species for the study of metal tolerance, Arabidopsis halleri (Brassicaceae). New Phytol. 193, 916–928. https://doi.org/10.1111/j.1469-8137.2011.04003.x (2012).
Gieroń, Ż et al. Ecophysiology of Arabidopsis arenosa, a new hyperaccumulator of Cd and Zn. J. Hazard. Mater. 412, 125052. https://doi.org/10.1016/j.jhazmat.2021.125052 (2021).
Oleńska, E. et al. Exopolysaccharide carbohydrate structure and biofilm formation by Rhizobium leguminosarum bv. trifolii strains inhabiting nodules of Trifolium repens growing on an old Zn–Pb–Cd-polluted waste heap area. Int. J. Mol. Sci. 22, 2808. https://doi.org/10.3390/ijms22062808 (2021).
Oleńska, E. et al. An alliance of Trifolium repens-Rhizobium leguminosarum bv. trifolii-mycorrhizal fungi from an old Zn–Pb–Cd rich waste heap as a promising tripartite system for phytostabilization of metal polluted soils. Front. Microbiol. 13, 853407. https://doi.org/10.3389/fmicb.2022.853407 (2022).
Oleńska, E. & Małek, W. Mechanisms of heavy metal resistance in bacteria. Adv. Microbiol. 52, 363–371 (2013).
Alotaibi, B. S., Khan, M. & Shamim, S. Unraveling the underlying heavy metal detoxification mechanisms of Bacillus species. Microorganisms 9, 1628. https://doi.org/10.3390/microorganisms9081628 (2021).
Carlos, M. H. J., Stefani, P. V. Y., Janette, A. M., Melani, M. S. S. & Gabriela, P. O. Assessing the effects of heavy metals in ACC deaminase and IAA production on plant growth-promoting bacteria. Microbiol. Res. 188–189, 53–61. https://doi.org/10.1016/j.micres.2016.05.001 (2016).
Fierros-Romero, G., Gómez-Ramírez, M., Arenas-Isaac, G. E., Pless, R. C. & Rojas-Avelizapa, N. G. Identification of Bacillus megaterium and Microbacterium liquefaciens genes involved in metal resistance and metal removal. Can. J. Microbiol. 62(6), 505–513. https://doi.org/10.1139/cjm-2015-0507 (2016).
Funes Pinter, I., Salomon, M. V., Berli, F., Bottini, R. & Piccoli, P. Characterization of the As(III) tolerance conferred by plant growth promoting rhizobacteria to in vitro-grown grapevine. Appl. Soil Ecol. 109, 60–68. https://doi.org/10.1016/j.apsoil.2016.10.003 (2017).
Pan, F. et al. Enhanced Cd extraction of oilseed rape (Brassica napus) by plant growth-promoting bacteria isolated from Cd hyperaccumulator Sedum alfredii Hance. Int. J. Phytoremediat. 19, 281–289. https://doi.org/10.1080/15226514.2016.1225280 (2017).
Paredes-Páliz, K. et al. Investigating the mechanisms underlying phytoprotection by plant growth-promoting rhizobacteria in Spartina densiflora under metal stress. Plant Biol 20, 497–506. https://doi.org/10.1111/plb.12693 (2018).
Rensing, C. & Grass, G. Escherichia coli mechanisms of copper homeostasis in a changing environment. FEMS Microbiol. Rev. 27, 197–213. https://doi.org/10.1016/S0168-6445(03)00049-4 (2003).
Silver, S. & Phung, L. T. A bacterial view of the periodic table: Genes and proteins for toxic inorganic ions. J. Ind. Microbiol. Biotechnol. 32, 587–605. https://doi.org/10.1007/s10295-005-0019-6 (2005).
Fakhar, A. et al. Heavy metal remediation and resistance mechanism of Aeromonas, Bacillus, and Pseudomonas: A review. Crit. Rev. Environ. Sci. Technol. 52(11), 1868–1914. https://doi.org/10.1080/10643389.2020.1863112 (2022).
Endo, G. & Silver, S. CadC, the transcriptional regulatory protein of the cadmium resistance system of Staphylococcus aureus plasmid pI258. J. Bacteriol. 177, 4437–4441. https://doi.org/10.1128/jb.177.15.4437-4441.1995 (1995).
Khanna, K., Jamwal, V. L., Gandhi, S. G., Ohri, P. & Bhardwaj, R. Metal resistant PGPR lowered Cd uptake and expression of metal transporter genes with improved growth and photosynthetic pigments in Lycopersicon esculentum under metal toxicity. Sci. Rep. 9, 5855. https://doi.org/10.1038/s41598-019-41899-3 (2019).
Oubohssaine, M., Sbabou, L. & Aurag, J. Native heavy metal-tolerant plant growth promoting rhizobacteria improves Sulla spinosissima (L.) growth in post-mining contaminated soils. Microorganisms 10, 838. https://doi.org/10.3390/microorganisms10050838 (2022).
Ong, H. B., Lee, W. S., Patterson, S., Wyllie, S. & Fairlamb, A. H. Homoserine and quorum-sensing acyl homoserine lactones as alternative sources of threonine: A potential role for homoserine kinase in insect-stage Trypanosoma brucei. Mol. Microbiol. 95, 143–156. https://doi.org/10.1111/mmi.12853 (2015).
Sugawara, M. et al. Rhizobitoxine modulates plant-microbe interactions by ethylene inhibition. Biotechnol. Adv. 24, 382–388. https://doi.org/10.1016/j.biotechadv.2006.01.004 (2006).
Singh, S., Parihar, P., Singh, R., Singh, V. P. & Prasad, S. M. Heavy metal tolerance in plants: Role of transcriptomics, proteomics, metabolomics and ionomics. Front. Plant Sci. 6, 1143. https://doi.org/10.3389/fpls.2015.01143 (2015).
De Boer, M. A., Wolzak, L. & Slootweg, J. C. Phosphorus: Reserves, production, and applications. In Phosphorus Recovery and Recycling (eds Ohtake, H. & Tsuneda, S.) (Springer, 2019). https://doi.org/10.1007/978-981-10-8031-9_5.
Emami, S. et al. Consortium of endophyte and rhizosphere phosphate solubilizing bacteria improves phosphorous use efficiency in wheat cultivars in phosphorus deficient soils. Rhizosphere 14, 100196. https://doi.org/10.1016/j.rhisph.2020.100196 (2020).
Razaq, M., Zhang, P., Shen, H.-L. & Salahuddin, S. Influence of nitrogen and phosphorous on the growth and root morphology of Acer mono. PLoS ONE 12(2), e0171321. https://doi.org/10.1371/journal.pone.0171321 (2017).
Suleman, M. et al. Phosphate solubilizing bacteria with glucose dehydrogenase gene for phosphorus uptake and beneficial effects on wheat. PLoS ONE 13(9), e0204408. https://doi.org/10.1371/journal.pone.0204408 (2018).
Barra, P. J. et al. Understanding the strategies to overcome phosphorus–deficiency and aluminum–toxicity by ryegrass endophytic and rhizosphere phosphobacteria. Front. Microbiol. 9, 1155. https://doi.org/10.3389/fmicb.2018.01155 (2018).
Oves, M., Khan, M. S. & Qari, H. A. Ensifer adhaerens for heavy metal bioaccumulation, biosorption, and phosphate solubilization under metal stress condition. J. Taiwan Inst. Chem. Eng. 80, 540–552. https://doi.org/10.1016/j.jtice.2017.08.026 (2017).
Kramer, J., Özkaya, Ö. & Kümmerli, R. Bacterial siderophores in community and host interactions. Nat. Rev. Microbiol. 18, 152–163. https://doi.org/10.1038/s41579-019-0284-4 (2020).
Rout, G. R. & Sahoo, S. Role of iron in plant growth and metabolism. Rev. Agric. Sci. 3, 1–24. https://doi.org/10.7831/ras.3.1 (2015).
Shi, P., Zing, Z., Zhang, U. & Chai, T. Effect of heavy-metal on synthesis of siderophores by Pseudomonas aeruginosa ZGKD3. Earth Environ. Sci. 52, 012103. https://doi.org/10.1088/1755-1315/52/1/012103 (2017).
Złoch, M., Thiem, D., Gadzała-Kopciuch, R. & Hrynkiewicz, K. Synthesis of siderophores by plant-associated metallotolerant bacteria under exposure to Cd2+. Chemosphere 156, 312e325. https://doi.org/10.1016/j.chemosphere.2016.04.130 (2016).
Yu, S. et al. Optimization of siderophore production by Bacillus sp. PZ-1 and its potential enhancement of phytoextration of Pb from soil. J. Microbiol. Biotechnol. 27, 1500–1512. https://doi.org/10.4014/jmb.1705.05021 (2017).
Jinal, H. N., Gopi, K., Prittesh, P., Kartik, V. P. & Amaresan, N. Phytoextraction of iron from contaminated soils by inoculation of iron-tolerant plant growth-promoting bacteria in Brassica juncea L. Czern. Environ. Sci. Pollut. Res. 26, 32815–32823. https://doi.org/10.1007/s11356-019-06394-2 (2019).
Shah, V. & Daverey, A. Phytoremediation: A multidisciplinary approach to clean up heavy metal contaminated soil. Environ. Technol. Innov. 18, 100774. https://doi.org/10.1016/j.eti.2020.100774 (2020).
Khan, W. U. et al. Application of Bacillus megaterium MCR-8 improved phytoextraction and stress alleviation of nickel in Vinca rosea. Int. J. Phytoremediat. 19, 813–824. https://doi.org/10.1080/15226514.2017.1290580 (2017).
Ahmad, I., Akhtar, M. J., Asghar, H. N., Ghafoor, U. & Shahid, M. Differential effects of plant growth-promoting rhizobacteria on maize growth and cadmium uptake. J. Plant Growth Regul. 35, 303–315. https://doi.org/10.1007/s00344015-9534-5 (2016).
Ali, G. S., Norman, D. & El-Sayed, A. S. Soluble and volatile metabolites of plant growthpromoting rhizobacteria (PGPRs): Role and practical applications in inhibiting pathogens and activating induced systemic resistance (ISR). Adv. Bot. Res. 75, 241–284. https://doi.org/10.1016/bs.abr.2015.07.004 (2015).
Audrain, B., Farag, M. A., Ryu, C. M. & Ghigo, J. M. Role of bacterial volatile compounds in bacterial biology. FEMS Microbiol. Rev. 39, 222–233. https://doi.org/10.1093/femsre/fuu013 (2015).
Ji, X.-J., Huang, H. & Ouyang, P.-K. Microbial 2,3-butanediol production: A stateof-the-art review. Biotechnol. Adv. 29, 351–354. https://doi.org/10.1016/j.biotechadv.2011.01.007 (2011).
Sharifi, R. & Ryu, C. M. Revisiting bacterial volatile-mediated plant growth promotion: Lessons from the past and objectives for the future. Ann. Bot. 122, 349–358. https://doi.org/10.1093/aob/mcy108 (2018).
Morcillo, R. J. L. et al. Rhizobacterium-derived diacetyl modulates plant immunity in a phosphate-dependent manner. EMBO J. 39(2), e102602. https://doi.org/10.15252/embj.2019102602 (2020).
Morcillo, R. J. L. et al. Bacteria-derived diacetyl enhances Arabidopsis phosphate starvation responses partially through the DELLA-dependent gibberellin signaling pathway. Plant Signal Behav. 15, 1740872. https://doi.org/10.1080/15592324.2020.1740872 (2020).
Sharifi, R. & Ryu, C. M. Sniffing bacterial volatile compounds for healthier plants. Curr. Opin. Plant Biol. 44, 88–97. https://doi.org/10.1016/j.pbi.2018.03.004 (2018).
Esringü, A., Turan, M., Güneş, A. & Rüştü Karaman, M. Roles of Bacillus megaterium in remediation of boron, lead, and cadmium from contaminated soil. Commun. Soil Sci. Plant Anal. 45, 1741–1759. https://doi.org/10.1080/00103624.2013.875194 (2014).
Wang, Q., Zhang, W. J., He, L. Y. & Sheng, X. F. Increased biomass and quality and reduced heavy metal accumulation of edible tissues of vegetables in the presence of Cd-tolerant and immobilizing Bacillus megaterium H3. Ecotoxicol. Environ. Saf 148, 269–274. https://doi.org/10.1016/j.ecoenv.2017.10.036 (2018).
Ortíz-Castro, R., Valencia-Cantero, E. & López-Bucio, J. Plant growth promotion by Bacillus megaterium involves cytokinin signaling. Plant Signal. Behav. 3, 263–265. https://doi.org/10.4161/psb.3.4.5204 (2008).
Akinrinlola, R. J., Yuen, G. Y., Drijber, R. A. & Adesemoye, A. O. Evaluation of Bacillus strains for plant growth promotion and predictability of efficacy by in vitro physiological traits. Int. J. Microbiol. 2018, 5686874. https://doi.org/10.1155/2018/5686874 (2018).
Cochrane, S. A. & Vederas, J. C. Lipopeptides from Bacillus and Paenibacillus spp.: A gold mine of antibiotic candidates. Med. Res. Rev. 36, 4–31. https://doi.org/10.1002/med.21321 (2016).
Govindasamy, V. et al. Bacillus and Paenibacillus spp.: Potential PGPR for sustainable agriculture. In Plant Growth and Health Promoting Bacteria. Microbiology Monographs 18 (ed. Maheshwari, D. K.) 333–364 (Springer, 2010). https://doi.org/10.1007/978-3-642-13612-2_15.
Samad, A., Antonielli, L., Sessitsch, A., Compant, S. & Trognitz, F. Comparative genome analysis of the vineyard weed endophyte Pseudomonas viridiflava CDRTc14 showing selective herbicidal activity. Sci. Rep. 7, 17336. https://doi.org/10.1038/s41598-017-16495-y (2017).
Agarwal, M., Rathore, R. S., Jagoe, Ch. & Chauhan, A. Multiple lines of evidences reveal mechanisms underpinning mercury resistance and volatilization by Stenotrophomonas sp. MA5 isolated from the Savannah River Site (SRS), USA. Cells 8, 309. https://doi.org/10.3390/cells8040309 (2019).
Guzik, U., Hupert-Kocurek, K., Sałek, K. & Wojcieszyńska, D. Influence of metal ions on bioremediation activity of protocatechuate 3,4-dioxygenase from Stenotrophomonas maltophilia KB2. World J. Microbiol. Biotechnol. 29, 267–273. https://doi.org/10.1007/s11274-012-1178-z (2013).
Singh, R. P. & Jha, P. N. The PGPR Stenotrophomonas maltophilia SBP-9 augments resistance against biotic and abiotic stress in wheat plants. Front. Microbiol. 8, 1945. https://doi.org/10.3389/fmicb.2017.01945 (2017).
Reasoner, D. J. & Geldreich, E. E. A new medium for the enumeration and subculture of bacteria from potable water. Appl. Environ. Microbiol. 49, 1–7. https://doi.org/10.1128/aem.49.1.1-7.1985 (1985).
Eevers, N. et al. Optimization of isolation and cultivation of bacterial endophytes through addition of plant extract to nutrient media. Microbial Biotechnol. 8, 707–715. https://doi.org/10.1111/1751-7287915.12291 (2015).
Oleńska, E. & Małek, W. Genomic polymorphism of Trifolium repens root nodule symbionts from heavy metal-abundant 100-year-old waste heap in southern Poland. Arch. Microbiol. 201, 1405–1414. https://doi.org/10.1007/s00203-019-01708-x (2019).
Weisburg, W., Barns, S. M., Pelletier, D. & Lane, D. J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173, 697–703. https://doi.org/10.1128/jb.173.2.697-703.1991 (1991).
Hall, T. A. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acid Symp. Ser. 41, 95–98 (1999).
Cunningham, J. E. & Kuiack, C. Production of citric and oxalic acids and solubilization of calcium phosphate by Penicillium bilaii. Appl. Environ. Microbiol. 58, 1451–1458. https://doi.org/10.1128/aem.58.5.1451-1458.1992 (1992).
Schwyn, B. & Neilands, J. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 160, 47–56. https://doi.org/10.1016/0003-2697(87)90612-9 (1987).
Schlegel, H., Gottschalk, G. & Von Bartha, R. Formation and utilization of poly-β-hydroxybutyric acid by knallgas bacteria (Hydrogenomonas). Nature 191, 463–465. https://doi.org/10.1038/191463a0 (1961).
Romick, T. L. & Fleming, H. P. Acetoin production as an indicator of growth and metabolic inhibition of Listeria monocytogenes. J. Appl. Microbiol. 84, 18–24. https://doi.org/10.1046/j.1365-2672.1997.00302.x (1998).
Pikovskaya, R. I. Mobilization of phosphorus in soil connection with the vital activity of some microbial species. Microbiology 17, 362–370 (1948).
Nautiyal, C. S. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol. Lett. 170, 265–270. https://doi.org/10.1111/j.1574-6968.1999.tb13383.x (1999).
Pande, A., Pandey, P., Mehra, S., Singh, M. & Kaushik, S. Phenotypic and genotypic characterization of phosphate solubilizing bacteria and their efficiency on the growth of maize. J. Genet. Eng. Biotechnol. 15, 379–391. https://doi.org/10.1016/j.jgeb.2017.06.005 (2017).
Gordon, S. A. & Weber, R. P. Colorimetric estimation of indole-acetic acid. Plant Physiol. 26, 192–195. https://doi.org/10.1104/pp.26.1.192 (1951).
Patten, C. L. & Glick, B. R. Role of Pseudomonas putida indoleacetic acid in development of host plant root system. Appl. Environ. Microbiol. 68, 3795–3801. https://doi.org/10.1128/AEM.68.8.3795-3801.2002 (2002).
Penrose, D. M. & Glick, B. R. Quantifying the impact of ACC deaminase-containing bacteria on plants. In Plant Surface Microbiology (eds Varma, A. et al.) (Springer-Verlag, 2004). https://doi.org/10.1007/978-3-540-74051-3_26.
Belimov, A. A. et al. Cadmium-tolerant plant growth-promoting bacteria associated with the roots of Indian mustard (Brassica juncea L. Czern.). Soil Biol. Biochem. 37, 241–250. https://doi.org/10.1016/j.soilbio.2004.07.033 (2005).
Kumar, S., Stecher, G. & Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874. https://doi.org/10.1093/molbev/msw054 (2016).
Acknowledgements
Authors would like to express the gratitude to Carine Put, Ann Wijgaerts, Edyta Żuk-Kempa for laboratory assistance, and to Krzysztof Oleński for support in collecting the material used in present study. The research was funded by a BOF Special Research Fund grant from Hasselt University to E.O. and the UHasselt Methusalem project 08M03VGRJ to J.V. Support was also provided by the Ministry of Education and Science Republic of Poland bailout for University of Bialystok (E.O.). The Article Processing Charge was funded by Hasselt University.
Author information
Authors and Affiliations
Contributions
Conceptualization: E.O., J.V., S.T.; Methodology: S.T., S.Sz., T.W.; Formal analysis and investigation: E.O., S.T., S.Sz., O.A., W.S., W.Z.; Writing—original draft preparation: E.O.; Writing—review and editing: W.M., M.W., I.S., T.L., I.Ch., J.V.; Funding acquisition: E.O., J.V.; Resources: E.O.; Supervision: J.V., W.M.; Software: S.T.; Validation: W.M., J.V.; Data Curation: S.T., E.O.; Visualization E.O., S.T., W.S., M.W.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Oleńska, E., Małek, W., Wójcik, M. et al. Bacteria associated with Zn-hyperaccumulators Arabidopsis halleri and Arabidopsis arenosa from Zn–Pb–Cd waste heaps in Poland as promising tools for bioremediation. Sci Rep 13, 12606 (2023). https://doi.org/10.1038/s41598-023-39852-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-39852-6
- Springer Nature Limited
This article is cited by
-
Screening of heavy metal-resistant rhizobial and non-rhizobial microflora isolated from Trifolium sp. growing in mining areas
Environmental Monitoring and Assessment (2024)
-
Study on the physical and chemical properties of lead passivating agent in soil
Scientific Reports (2023)