Abstract
Low body mass index is closely related to a high risk of Alzheimer’s disease (AD) and related biomarkers including amyloid-β (Aβ) deposition. However, the association between sarcopenia and Aβ-confirmed AD remains controversial. Therefore, we investigated the relationship between sarcopenia and the AD continuum. We explored sarcopenia’s association with clinical implications of participants on the AD continuum. We prospectively enrolled 142 participants on the AD continuum (19 with preclinical AD, 96 with mild cognitive impairment due to AD, and 28 with AD dementia) and 58 Aβ-negative cognitively unimpaired participants. Sarcopenia, assessed using dual-energy X-ray absorptiometry and hand grip measurements, was considered a predictor. AD continuum, defined by Aβ deposition on positron emission tomography served as an outcome. Clinical severity in participants on the AD continuum assessed using hippocampal volume, Mini-Mental State Examination (MMSE), Seoul Verbal Learning Test (SVLT), and Clinical Dementia Rating Scale Sum of Boxes Scores (CDR-SOB) were also considered an outcome. Sarcopenia (odds ratio = 4.99, p = 0.004) was associated independently with the AD continuum after controlling for potential confounders. Moreover, sarcopenia was associated with poor downstream imaging markers (decreased hippocampal volume, β = − 0.206, p = 0.020) and clinical outcomes (low MMSE, β = − 1.364, p = 0.025; low SVLT, β = − 1.077, p = 0.025; and high CDR-SOB scores, β = 0.783, p = 0.022) in participants on the AD continuum. Sarcopenia was associated with the AD continuum and poor clinical outcome in individuals with AD continuum. Therefore, our results provide evidence for future studies to confirm whether proper management of sarcopenia can effective strategies are required for sarcopenia management to prevent the AD continuum and its clinical implications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
A growing body of evidence displays that low body mass index (BMI) is associated with the deposition of amyloid-β (Aβ)1,2, acceleration of neurodegeneration3,4, and development of Alzheimer’s disease (AD)5,6. Additionally, low BMI in patients with AD is associated with disease severity and poor prognosis7. However, previous studies were conducted using BMI measures, and, therefore, failed to cover the specific body composition including muscle mass and fat mass.
Sarcopenia is characterized by the loss of skeletal muscle mass and strength8. The disease is closely related to physical disability, cardiovascular diseases, and mortality9,10. Recently, several studies have identified that sarcopenia is predictive of incident mild cognitive impairment (MCI) and/or dementia of the Alzheimer type (DAT)11,12. The diagnosis of MCI and AD in previous studies mostly relied on clinical presentation rather than biomarkers. Therefore, the relationship between sarcopenia and biomarker-based diagnosis of AD remains controversial. As the importance of biomarkers for AD diagnosis was emphasized, the National Institute on Aging and Alzheimer’s Association proposed the term “AD continuum” that encompasses preclinical AD, MCI due to AD, and AD dementia13,14,15.
Therefore, this study aimed to investigate the relationship between sarcopenia and the AD continuum (preclinical AD, MCI due to AD, and AD dementia). Moreover, we explored whether sarcopenia was associated with clinical implications (hippocampal volume and cognitive function) in participants on the AD continuum. Considering that normal aging involves decreased muscle mass and increased fat mass, sarcopenia may contribute to the association between low BMI, AD, and related biomarkers. Thus, we hypothesized that sarcopenia is associated with the AD continuum and can predict poor clinical outcomes in patients with the AD continuum.
Methods
Study participants
We prospectively recruited 142 participants on the AD continuum aged 50–90 years (19 with preclinical AD, 96 with MCI due to AD, and 28 with AD dementia) at the memory clinic in the Department of Neurology at Korea University Guro Hospital in Seoul, Korea, between July 2021 and February 2023. All participants underwent comprehensive neuropsychological battery, high-resolution T1-weighted magnetic resonance imaging (MRI) scan, Aβ positron emission tomography (PET), and sarcopenia evaluation including dual-energy x-ray absorptiometry (DXA) and measurement of hand grip. According to the National Institute on Aging-Alzheimer’s Association criteria13,14,15, Aβ-positive (Aβ+) subjective cognitive decline (SCD), MCI, and dementia were defined as preclinical AD, MCI due to AD, and AD dementia, respectively. We excluded participants with the following conditions: (1) severe white matter hyperintensities (WMH) burden on MRI (cap or band > 10 mm and longest diameter of deep white matter lesion > 25 mm)16; (2) structural lesions on MRI including territorial infarction, lobar hemorrhage, brain tumor, and hydrocephalus; (3) abnormal laboratory findings on complete blood count, electrolyte levels, thyroid/liver/kidney/function tests, vitamin B12 levels, and syphilis serology; and (4) a recent history of cancer.
Additionally, we recruited 58 Aβ-negative (Aβ−) cognitively unimpaired (CU) participants for the control group. These participants comprised participants with subjective cognitive decline; volunteers who applied for comprehensive dementia screening advertised in the local community, memory clinic, and dementia prevention center; and spouses of patients who visited the memory clinic. They visited the memory clinic in the Department of Neurology at Korea University Guro Hospital and underwent a comprehensive dementia screening. All Aβ-CU participants met the following criteria: (1) no medical history that has the potential to affect cognitive impairment based on Christensen's health screening criteria17; (2) no objective cognitive impairment in any cognitive domain on a comprehensive neuropsychological battery (above at least − 1.0 standard deviation of age-adjusted norms on any cognitive test); (3) independence in activities of daily living; (4) no structural lesions; (5) no severe WMH burden on MRI; and (6) no Aβ deposition on PET.
This study was approved by the Institutional Review Board of Korea University Guro Hospital. Written informed consent was obtained from all participants.
Sarcopenia evaluation
Sarcopenia is defined as having both decreased muscle mass and reduced handgrip strength. Given that Asian have lower muscle mass and weaker grip strength than Non-Hispanic White18,19,20,21, a guideline in the Asian Working Group for Sarcopenia was used to define the decreased muscle mass and reduced handgrip strength.
Skeletal muscle mass was evaluated using whole-body DXA (Discovery A; Hologic, Bedford, MA, USA). The appendicular skeletal mass index (ASMI) was calculated by dividing the sum of the lean masses in the bilateral upper and lower limbs by the square of height (lean mass in the bilateral upper and lower extremities/[height]2). According to the guideline in the Asian Working Group for Sarcopenia, decreased muscle mass was defined as ASMI < 7.0 kg/m2 in males and < 5.4 kg/m2 in females22.
Grip strength was measured using a digital grip dynamometer (TKK-540 Grip-D; Takei, Niigata, Japan). Low handgrip strength was defined as grip strength < 26 kg in males and < 18 kg in females22,23.
MRI acquisition and hippocampal volume
We acquired standardized three-dimensional T1 Turbo Field Echo images using a 3.0 T MRI scanner (Philips 3.0 T Ingenia Elition X; Philips Healthcare, Andover, MA, USA) with the following imaging parameters: sagittal slice thickness, 1.0 mm; no gap; TR, 8.2 ms; TE, 4.0 ms; flip angle, 8°; and matrix size, 256 × 256 pixels.
We used hippocampal volume as a downstream imaging marker. For the acquisition of the hippocampal volume, we used freesurfer (version 7.3.2), which can automatically segment T1 MRI volumes and generate 3D surfaces based on them. The freesurfer pipeline transformed the T1 MRI of individual spaces into an MNI-305 template, resampled it into a 256 × 256 × 256 volume image, and completed the tissue segmentation of white matter through registration and segmentation step by step. The completed segmentation image could be visualized and validated through 'aparc + aseg.mgz', and each hippocampal volume (mm3) was obtained through the 'aseg.stats'. The ICV value for statistical analysis could be also obtained in the same process.
Aβ PET acquisition and visual reading
All participants underwent 18F-florbetaben PET using a Discovery MI PET/computed tomography (CT) scanner (GE Medical Systems, Milwaukee, WI, USA). A 20-min emission PET scan in the dynamic mode (comprising 4 × 5 min frames) was performed 90 min after injecting a mean dose of 296 MBq 18F-florbetaben16. Three-dimensional PET images were reconstructed in a 384 × 384 matrix with 0.65 × 0.65 × 2·79 mm voxel size using the ordered-subsets expectation maximization algorithm (iteration = 8 and subset = 34)16.
Amyloid PET images were reviewed by three experienced physicians (one neurologist and two nuclear medicine doctors) blinded to clinical information and dichotomized as either Aβ positive or negative using visual reads24. 18F-florbetaben PET was classified as positive when interpreters scored the visual assessment as 2 or 3 based on the brain amyloid plaque load (BAPL) score24,25. After the physicians independently rated, we determined the final Aβ positivity based on the majority of visual reading results.
Assessment of cognitive function
All participants underwent a comprehensive neuropsychological test battery, including the Mini-Mental State Examination (MMSE), delayed recall task of the Seoul Verbal Learning Test (SVLT), and the Clinical Dementia Rating Scale Sum of Boxes Scores (CDR-SOB)26.
Statistical analyses
Independent t-tests and chi-square tests were used to compare the demographic and clinical characteristics of participants. The normality of the continuous variables was checked based on skewness and kurtosis.
To investigate the association between sarcopenia and AD continuum (preclinical AD, MCI due to AD, and AD dementia), we performed logistic regression analysis with sarcopenia as a predictor and AD continuum as an outcome, after controlling for age, sex, education years, hypertension, and diabetes in total participants (AD continuum and Aβ-CU groups). Furthermore, to identify the association between sarcopenia and Aβ deposition before cognitive decline occurs, we performed logistic regression analysis with sarcopenia as a predictor and AD continuum as an outcome, after controlling for age, sex, education years, hypertension, and diabetes in CU participants (Aβ-CU and preclinical AD groups).
To explore the association between sarcopenia and neurodegeneration in the AD continuum, we performed a linear regression analysis with sarcopenia as a predictor and hippocampal volume as an outcome after controlling for age, sex, years of education, hypertension, diabetes, and intracranial volume (ICV) in participants on the AD continuum.
To explore the association between sarcopenia and cognitive function in the AD continuum, we performed linear regression analyses with sarcopenia as a predictor and MMSE, SVLT, and CDR-SOB scores as outcomes after controlling for age, sex, years of education, hypertension, and diabetes in participants on the AD continuum.
To determine whether neurodegeneration mediated the effects of sarcopenia on cognitive function, we used mediation analyses with sarcopenia as a predictor, hippocampal volume as a mediator, and MMSE, SVLT, and CDR-SOB scores as outcomes controlling for age, sex, years of education, hypertension, diabetes, and ICV in participants on the AD continuum.
All reported p values were two-sided and the significance level was set at 0.05. All analyses were performed using the R software (version 3.6.1; Institute for Statistics and Mathematics, Vienna, Austria; www.R-project.org).
Ethics approval and consent to participate
Approval was obtained from the Ethics Committee of Korea University Guro Hospital. The study procedures were conducted in accordance with the tenets of the Declaration of Helsinki. Written informed consent was obtained from all the patients and caregivers.
Results
Clinical characteristics of participants
Among the 200 participants, 44 (22%) were sarcopenic (Table 1). There were no differences between participants with sarcopenia and without sarcopenia in female ratio (75.0% and 72.4%, p = 0.884) and Apolipoprotein E4 carrier (56.8% and 41.7%, p = 0.107). Participants with sarcopenia are more likely to older (73.8 ± 5.7 and 71.8 ± 6.8, p = 0.078), lower education years (8.2 ± 3.1 and 9.4 ± 4.7, p = 0.052), and higher frequency of hypertension (61.4% and 43.6%, p = 0.056) and diabetes (34.1% and 19.2%, p = 0.060) than those without sarcopenia, while the differences were not statistically significant.
Sarcopenia and AD continuum
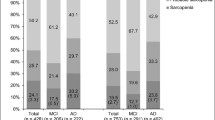
Participants with AD continuum had higher proportion of sarcopenia than those with Aβ-CU (28.2% and 6.9%, p < 0.001, Fig. 1). Specifically, the proportions of sarcopenia were 26.3%, 27.1%, and 33.3% in participants with preclinical AD, MCI due to AD, and AD dementia, respectively.
Sarcopenia (odds ratio [OR] 4.99, 95% confidence interval [CI] 1.84–17.53) was independently associated with the AD continuum (Table 2). Among CU participants, sarcopenia (odds ratio [OR] 5.07, 95% confidence interval [CI] 0.95–28.81) was marginally associated with Aβ deposition, while the association was not statistically significant.
Sarcopenia, downstream imaging marker, and clinical implications in the AD continuum
Sarcopenia (β = − 0.206, p = 0.020) was negatively associated with hippocampal volume. Specifically, muscle mass (β = − 0.274, p = 0.001) and hand grip (β = − 0.202, p = 0.015) were negatively associated with hippocampal volume (Table 3).
Regarding cognitive function, sarcopenia was associated with MMSE (β = − 1.364, p = 0.025), SVLT (β = − 1.077, p = 0.025), and CDR-SOB (β = 0.783, p = 0.022) scores (Table 4).
Specifically, low muscle mass was associated with MMSE (β = − 1.255, p = 0.025), SVLT (β = − 1.286, p = 0.003), and CDR-SOB (β = 0.667, p = 0.035) scores (Table 4). However, low hand grip was not associated with MMSE (β = − 0.942, p = 0.098), SVLT (β = − 0.594, p = 0.186), and CDR-SOB (β = 0.617, p = 0.053) scores (Table 4).
The results of the mediation analyses are illustrated in Fig. 2. Decreased hippocampal volume fully mediated the relationship between sarcopenia and poor cognitive function. Specifically, sarcopenia was associated with decreased hippocampal volume, which was further associated with low MMSE (indirect pathway β = − 0.438, p = 0.046), low SVLT (indirect pathway β = − 0.519, p = 0.030), and high CDR-SOB scores (indirect pathway β = 0.277, p = 0.037). However, sarcopenia was not associated with MMSE (direct pathway β = − 0.892, p = 0.115), SVLT scores (direct pathway β = − 0.527, p = 0.199), and CDR-SOB (direct pathway β = 0.510, p = 0.106) without mediation of hippocampal volume.
Schematic diagram of the mediation analyses among sarcopenia, hippocampal volume, and cognitive function. Mediation analyses were performed using sarcopenia as a predictor, hippocampal as a mediator, and each cognitive function (MMSE, SVLT, or CDR-SOB) as an outcome after controlling for age, sex, years of education, hypertension, diabetes, and intracranial volume in participants on the AD continuum. AD Alzheimer’s disease; CDR-SOB clinical dementia rating scale sum of boxes scores; MMSE mini-mental state examination; SVLT delayed recall task of the Seoul verbal learning test.
Discussion
In this study, we investigated the relationships of sarcopenia with the presence of the AD continuum and clinical outcomes in participants on the AD continuum. Our major findings were as follows: First, sarcopenia was associated with the presence of the AD continuum. Second, sarcopenia was associated with poor downstream image marker and clinical outcomes in participants on the AD continuum. Specifically, sarcopenia was associated with decreased hippocampal volume and severe cognitive impairment (low MMSE and SVLT scores, and high CDR-SOB scores), which were fully mediated by hippocampal atrophy in participants on the AD continuum. Taken together, our findings suggest that sarcopenia may be an important predictor of the AD continuum and poor clinical outcomes.
Our first major finding was the association of sarcopenia with the presence of the AD continuum. Our findings are consistent with previous studies demonstrating that being underweight is a risk factor or an early symptom of brain Aβ deposition and extends the findings by suggesting that Aβ-related underweight may be predominantly related to loss of muscle mass1,27,28,29. Additionally, considering muscle function and physical performance as major components of sarcopenia, our findings align with those of prior studies conducted in the Non-Hispanic White population. Previous studies have demonstrated that low grip strength and slow gait speed were associated with Aβ deposition30,31,32,33. Due to the differences in body composition and prevalence of AD across ethnicities18,19,20,21, the association between sarcopenia and Aβ deposition may be different across ethnicities. However, to the best of our knowledge, the association between sarcopenia and Aβ-confirmed AD has not been identified in the Asian population. Although further studies are required to elucidate the mechanisms underlying the relationship between sarcopenia and the AD continuum, neuroinflammation may mediate this association. Muscles are known to act like endocrine organs, secreting various myokines, peptides, and growth factors34,35. Thus, sarcopenic condition results in decreased insulin-like growth factor I, which plays a key role in anti-inflammatory responses36. Moreover, sarcopenia is closely related to chronic inflammation and increased oxidative stress37, which in turn aggravates the Aβ-related neuroinflammatory process38,39. Furthermore, sarcopenia and AD share several genetic risk factors40,41. Additionally, a mutation in the amyloid precursor protein gene that causes familial AD is also associated with a decrease in muscle mass and grip strength in animal studies42,43. Alternatively, it is possible that sarcopenia and AD continuum co-occur with aging, underlying medical conditions such as cardiometabolic syndrome, or poor lifestyle factors including malnutrition, physical inactivity, and smoking.
Our second major finding was that sarcopenia was associated with poor downstream image marker and cognitive performances in participants on the AD continuum. Specifically, sarcopenia was associated with decreased hippocampal volume and poor cognitive function (low MMSE and SVLT scores, and high CDR-SOB scores), which were fully mediated by hippocampal atrophy in participants on the AD continuum. These findings are consistent with those of previous studies involving participants with DAT. Previous studies have discovered that sarcopenia is associated with neurodegeneration and poor cognitive function in patients with DAT44,45,46,47, and extends the findings by suggesting that sarcopenia is independently associated with poor prognosis or accompanying symptoms in Aβ-confirmed AD. Additionally, our findings are supported by community-based studies demonstrating that sarcopenia is closely related to poor cognitive trajectories12,48.
Limitations
The strength of the present study is that we systematically investigated the association between sarcopenia and biomarker-defined AD, and its clinical implications. However, this study has several limitations. First, because this study had a cross-sectional design, determining the causal relationship between sarcopenia and the AD continuum was impossible. Thus, future longitudinal studies are needed to identify the possible directions of causality between sarcopenia and Aβ deposition and related clinical outcomes. Second, the number of individuals with preclinical AD was relatively small. Further studies with a larger number of CU participants (Aβ-CU and preclinical AD) are required to investigate the association between sarcopenia and Aβ deposition before cognitive decline occurs. Third, we used Asia–Pacific criteria for sarcopenia; therefore, caution is required when generalizing our findings to other ethnicities. Fourth, we did not assess several components of physical frailty, including gait velocity and physical activity, which are closely associated with sarcopenia. Finally, because our study was conducted in a memory clinic setting, the generalizability of the present study to community-based populations is limited. Nevertheless, our study is noteworthy, suggesting that proper management of sarcopenia may be important for the prevention of the AD continuum and its clinical implications. Alternatively, sarcopenia is an early marker related to the AD continuum and a poor prognosis in patients on the AD continuum. Thus, screening for the early detection of sarcopenia may be useful for identifying individuals at a high risk of AD continuum.
Conclusions
In conclusion, we highlighted that the presence of sarcopenia was associated with the AD continuum, downstream imaging marker, and poor cognitive performance in individuals with Aβ-confirmed AD. Therefore, given that sarcopenia is improved by strength exercises and diet, our results provide evidence for future studies to confirm whether proper management of sarcopenia can prevent the AD continuum and its clinical progression in individuals with AD.
Data availability
The anonymized data used for our analyses presented in this study are available upon request from the corresponding authors.
References
Vidoni, E. D., Townley, R. A., Honea, R. A. & Burns, J. M. Alzheimer disease biomarkers are associated with body mass index. Neurology 77, 1913–1920 (2011).
Kang, S. H. et al. Independent effect of body mass index variation on amyloid-β positivity. Front. Aging Neurosci. 14, 924550 (2022).
Kim, H. et al. Association between body mass index and cortical thickness: among elderly cognitively normal men and women. Int. Psychogeriatr. 27, 121–130 (2015).
Kim, S. E. et al. Sex-specific relationship of cardiometabolic syndrome with lower cortical thickness. Neurology 93, e1045–e1057 (2019).
Tolppanen, A. M. et al. Midlife and late-life body mass index and late-life dementia: Results from a prospective population-based cohort. J. Alzheimers Dis. 38, 201–209 (2014).
Bell, S. P. et al. Late-life body mass index, rapid weight loss, apolipoprotein E ε4 and the risk of cognitive decline and incident dementia. J. Nutr. Health Aging 21, 1259–1267 (2017).
White, H., Pieper, C. & Schmader, K. The association of weight change in Alzheimer’s disease with severity of disease and mortality: A longitudinal analysis. J. Am. Geriatr. Soc. 46, 1223–1227 (1998).
Fielding, R. A. et al. Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: Prevalence, etiology, and consequences. International working group on sarcopenia. J. Am. Med. Dir. Assoc. 12, 249–256 (2011).
Arango-Lopera, V. E., Arroyo, P., Gutiérrez-Robledo, L. M., Pérez-Zepeda, M. U. & Cesari, M. Mortality as an adverse outcome of sarcopenia. J. Nutr. Health Aging 17, 259–262 (2013).
Chen, Y. Y. et al. Relationship between sarcopenia and cardiovascular disease risk among Taiwanese older adults. Public Health Nutr. 25, 1–21 (2022).
Beeri, M. S., Leugrans, S. E., Delbono, O., Bennett, D. A. & Buchman, A. S. Sarcopenia is associated with incident Alzheimer’s dementia, mild cognitive impairment, and cognitive decline. J. Am. Geriatr. Soc. 69, 1826–1835 (2021).
Salinas-Rodríguez, A., Palazuelos-González, R., Rivera-Almaraz, A. & Manrique-Espinoza, B. Longitudinal association of sarcopenia and mild cognitive impairment among older Mexican adults. J. Cachexia Sarcopenia Muscle 12, 1848–1859 (2021).
Sperling, R. A. et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 280–292 (2011).
Albert, M. S. et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 270–279 (2011).
McKhann, G. M. et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 263–269 (2011).
Kang, S. H. et al. Amyloid positivity in the Alzheimer/subcortical-vascular spectrum. Neurology 96, e2201–e2211 (2021).
Christensen, K. J., Multhaup, K. S., Nordstrom, S. & Voss, K. A cognitive battery for dementia: Development and measurement characteristics. Psychol. Assess. J. Consult. Clin. Psychol. 3, 168–174 (1991).
Bigman, G. & Ryan, A. S. Implications of race and ethnicity in sarcopenia US National prevalence of sarcopenia by muscle mass, strength, and function indices. Gerontol. Geriatr. Res. 4, 126 (2021).
Al Snih, S., Kaushik, V., Eschbach, K. & Markides, K. Ethnic differences in physical performance in older Americans: Data from the Third National Health and Nutrition Examination Survey (1988–1994). Aging Clin. Exp. Res. 20, 139–144 (2008).
Araujo, A. B. et al. Lean mass, muscle strength, and physical function in a diverse population of men: A population-based cross-sectional study. BMC Public Health. 10, 508 (2010).
Auyeung, T. W., Lee, S. W., Leung, J., Kwok, T. & Woo, J. Age-associated decline of muscle mass, grip strength and gait speed: A 4-year longitudinal study of 3018 community-dwelling older Chinese. Geriatr. Gerontol. Int. 14(Suppl 1), 76–84 (2014).
Chen, L. K. et al. Sarcopenia in Asia: Consensus report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 15, 95–101 (2014).
Lim, E. Y. et al. Clinical significance of physical frailty in subjects with subjective cognitive decline: A prospective study with amyloid PET data. J. Clin. Neurol. 19, 447–453 (2023).
Kim, S. E. et al. A Nomogram for predicting amyloid PET positivity in amnestic mild cognitive impairment. J Alzheimers Dis. 66, 681–691 (2018).
Barthel, H. et al. Cerebral amyloid-beta PET with florbetaben (18F) in patients with Alzheimer’s disease and healthy controls: a multicentre phase 2 diagnostic study. Lancet Neurol. 10, 424–435 (2011).
Kang, S. H. et al. Cortical neuroanatomical changes related to specific neuropsychological deficits in subcortical vascular cognitive impairment. Neuroimage Clin. 30, 102685 (2021).
Thirunavu, V. et al. Higher body mass index is associated with lower cortical amyloid-β burden in cognitively normal individuals in late-life. J. Alzheimers Dis. 69, 817–827 (2019).
Mathys, J., Gholamrezaee, M., Henry, H., von Gunten, A. & Popp, J. Decreasing body mass index is associated with cerebrospinal fluid markers of Alzheimer’s pathology in MCI and mild dementia. Exp. Gerontol. 100, 45–53 (2017).
Kang, S. H. et al. Gender-specific relationship between thigh muscle and fat mass and brain amyloid-β positivity. Alzheimers Res. Ther. 14, 145 (2022).
Nadkarni, N. K. et al. Association of brain amyloid-β with slow gait in elderly individuals without dementia: Influence of cognition and apolipoprotein E ε4 genotype. JAMA Neurol. 74, 82–90 (2017).
Del Campo, N. et al. Relationship of regional brain β-amyloid to gait speed. Neurology 86, 36–43 (2016).
Kuo, K. et al. Associations of grip strength, walking pace, and the risk of incident dementia: A prospective cohort study of 340212 participants. Alzheimers Dement. 19, 1415–1427 (2023).
Jacob, M. E. et al. Grip strength and gait speed as early biomarkers of brain amyloid and tau deposition. Alzheimer's Dement. 16, e041178. https://doi.org/10.1002/alz.041178 (2020).
Trejo, J. L., Carro, E. & Torres-Aleman, I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J. Neurosci. 21, 1628–1634 (2001).
Lourenco, M. V. et al. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat. Med. 25, 165–175 (2019).
Morley, J. E., Baumgartner, R. N., Roubenoff, R., Mayer, J. & Nair, K. S. Sarcopenia. J. Lab. Clin. Med. 137, 231–243 (2001).
Migliavacca, E. et al. Mitochondrial oxidative capacity and NAD(+) biosynthesis are reduced in human sarcopenia across ethnicities. Nat. Commun. 10, 5808 (2019).
Maltais, M. et al. Association between brain β-amyloid and frailty in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 74, 1747–1752 (2019).
Yaffe, K. et al. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA 292, 2237–2242 (2004).
Pan, J. X. et al. Muscular Swedish mutant APP-to-Brain axis in the development of Alzheimer’s disease. Cell Death Dis. 13, 952 (2022).
Jones, G. et al. Genome-wide meta-analysis of muscle weakness identifies 15 susceptibility loci in older men and women. Nat. Commun. 12, 654 (2021).
Magara, F. et al. Genetic background changes the pattern of forebrain commissure defects in transgenic mice underexpressing the beta-amyloid-precursor protein. Proc. Natl. Acad. Sci. U.S.A. 96, 4656–4661 (1999).
Xu, H. et al. Age related changes in muscle mass and force generation in the triple transgenic (3xTgAD) mouse model of Alzheimer’s disease. Front. Aging Neurosci. 14, 876816 (2022).
Burns, J. M., Johnson, D. K., Watts, A., Swerdlow, R. H. & Brooks, W. M. Reduced lean mass in early Alzheimer disease and its association with brain atrophy. Arch Neurol. 67, 428–433 (2010).
Ogawa, Y. et al. Sarcopenia and muscle functions at various stages of Alzheimer disease. Front. Neurol. 9, 710 (2018).
Ohta, Y. et al. Female dominant association of sarcopenia and physical frailty in mild cognitive impairment and Alzheimer’s disease. J. Clin. Neurosci. 70, 96–101 (2019).
Güner Oytun, M. et al. Relationships of fall risk with frailty, sarcopenia, and balance disturbances in mild-to-moderate Alzheimer’s disease. J. Clin. Neurol. 19, 251–259 (2023).
Hu, Y., Peng, W., Ren, R., Wang, Y. & Wang, G. Sarcopenia and mild cognitive impairment among elderly adults: The first longitudinal evidence from CHARLS. J Cachexia Sarcopenia Muscle 13, 2944–2952 (2022).
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2022R1F1A1063966), and Korea University Guro Hospital (KOREA RESEARCH-DRIVEN HOSPITAL) Grant (No. O2400251), and a Korea University Grant (No. K2327171).
Author information
Authors and Affiliations
Contributions
S.H.K. conceived and designed the study. J.K., S-I.S., Y.J.P., M.K., S.J.C., E.S.L., H.N.J., J.S.E., S-B.K., K.O., and S.H.K. contributed to data collection. J.K., M.K., and S.H.K. performed the statistical analyses. J.K. and S.H.K. wrote the first draft of the manuscript. J.K., S-I.S., K.O., and S..H.K. contributed to data interpretation and manuscript revision. All the authors have read and approved the final version of this manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kim, J., Suh, SI., Park, Y.J. et al. Sarcopenia is a predictor for Alzheimer’s continuum and related clinical outcomes. Sci Rep 14, 21074 (2024). https://doi.org/10.1038/s41598-024-62918-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-62918-y
- Springer Nature Limited