Abstract
Cervical cancer (CC) remains a major health concern globally, much of the brunt of which is experienced by the low- and middle-income countries where screening in terms of cytology and DNA genotyping for the high-risk oncogenic subtypes of the human papilloma virus (hr-HPV) is either inadequate or performed rather late. In this study, we aimed to determine biomarkers or panels of biomarkers that are capable of diagnosing the precancerous cervical intraepithelial neoplasia (CIN) stages from healthy and CC patients via untargeted gas chromatography–mass spectrometry-based metabolomics. Various cross-comparisons were conducted from which differential metabolites were identified. The underlying metabolic pathways based on the differential metabolites identified from the various cross-comparisons mainly related to amino acids biosynthesis and metabolism and steroid hormone biosynthesis. From all cross-comparisons, two common metabolites namely, 2-methyl-1-propylamine (also known as isobutylamine) and estrone were found to possess excellent to good diagnostic abilities, especially in distinguishing the early stages of CIN (CIN I, CIN II) from healthy women and CC patients. These findings have clinical significance in the sense that, once validated the 2-biomarker panel could be adopted in clinical practice for early diagnosis of CIN and invasive carcinoma. This would therefore inform the choice of treatment to be initiated by the clinician.
Similar content being viewed by others
Introduction
Cervical cancer (CC) is a major health concern globally, and much of the brunt of this disease is experienced by the low- and middle-income countries where the mortality rate could be as high as 18 times relative to the developed countries1,2,3. It is estimated that of the total deaths due to CC in 2015, about 90% the cases were traced to the low- and middle-income countries1,2,3,4. CC is the fourth most common cancer type in the world that plagues the female gender and almost all manifestations of the disease are associated with long-term infections by the high-risk oncogenic subtypes of the human papilloma virus (hr-HPV)4. Hence, the main risk factors of this disease are linked to HPV infection, impaired immune response to its infection or a combination of the two. Examples include, early age of first sexual experience, multiple sex partners, compromised immunity (as in the case of organ transplant individuals, and persons with HIV), etc1,4. The most common histological types of CC are squamous cell carcinoma and adenocarcinoma that respectively account for nearly 70% and 25% of all cases1,3,5. These are mainly the result of progressive pathological transformations of the squamocolumnar junction of the endocervical canal from low- and high-grade cervical intraepithelial neoplasia (CIN) to malignancy6.
CC as a disease however to a large extent can be prevented by virtue of early screening using the Papanicolaou test (popularly known as the Pap smear) or any other cytology testing plus HPV DNA genotyping and early intervention7,8. Available screening methods not only cause discomfort due to their invasiveness but suffer from generally low sensitivities and specificities7,8. The choice and success of the best treatment method largely depends on the extent of disease at the time of diagnosis3,9. In this regard, early and accurate diagnosis of the disease is very crucial since that could inform the choice of treatment method, thus, surgery, chemotherapy, radiotherapy or their combinations9.
The concept of metabolomics is gradually gaining preeminence and acceptance in the circles of clinical medicine as a prospective diagnostic and/or prognostic tool for diverse diseases, as well as to assess treatment successes or otherwise10,11. This can be achieved via the identification and use of biomarker(s). With respect to CC, the use of biomarkers could aid in the early diagnosis of the disease and better inform the clinician on the best treatment approach12,13. Since the precancerous stage of CC usually presents with asymptomatic conditions, its accurate diagnosis at the early stage is usually dependent on early screening of the individual on a voluntary basis. Hence, much of the diagnosis is usually done at the late stages of the disease in places where routine HPV screening is not instituted thereby narrowing the success of the best treatment method(s)9. Various researchers have to sought to utilize metabolomics to distinguish the various stages of precancer (i.e., prior to CC) from CC. For instance, by virtue of plasma metabolomics using the liquid chromatography quadrupole-time-of-flight mass spectrometry (LC-QTOF-MS) and multivariate analysis, Yang et al. found 5 metabolites to accurately differentiate CC patients from normal persons with good accuracy (AUC, 0.99)14. This high AUC value was due to the fact that the CC patients were actually confirmed as such and did not include persons with precancerous CIN. When samples of plasma were gotten from patients with CIN, CC and healthy individuals, Khan et al. reported a 7-biomarker panel that gave lower AUC values, 0.82 and 0.83 for the comparison of CIN versus healthy and CC versus healthy respectively12. A much lower AUC value of about 0.75 was found for a 5-biomarker panel for cervical squamous cell carcinoma by Zhou et al. using plasma-based LC–MS metabolomics13. Aside from plasma, others have sought to use tissue and urine samples to aid in the accurate diagnosis of CC15,16.
Of all the plasma-based metabolomics studies reported so far, to our best knowledge, none used the gas chromatography–mass spectrometry (GC–MS) analytical platform in a bid to capture as much as possible the most appropriate biomarkers that could enhance the diagnosis and prognosis of CC. GC–MS is a standardized high-throughput analytical method that is ideal for the identification and quantification of small molecular weight metabolites such as amino acids, alcohols, fatty acids, small acids, hydroxyl acids, and other metabolic intermediates17. The availability of public libraries such as the Human Metabolome Database (HMDB) makes GC–MS a very important analytical platform in metabolomics, where the concept is to as much as practicable identify all metabolites within a given biological system17,18.
In this study, we sought to find alternative biomarkers (or a panel of biomarkers) that could distinguish the various precancerous stages (CIN I, II, III) from CC and CIN I, II, III and CC from healthy individuals with a good level of accuracy, selectivity and specificity using GC–MS-based metabolomics. A comparison of the metabolomes of CIN and CC vs healthy persons could aid in the early diagnosis of the various stages of CIN that could eventually lead to the development of CC.
Materials and methods
Patients
The study involved women diagnosed with the various stages of CIN (i.e., CIN I, II, III) and healthy volunteers after routine thin-prep cytology test (TCT) and HPV genotyping. Positive cases were then subjected to colposcopy and biopsies taken for determinations of the CIN-specific cases. Patients with cervical lesions were biopsied and cancer confirmed thereafter. In all, 45 CC, 18 CIN I, 12 CIN II, 84 CIN III patients, 39 healthy volunteers without HPV infection and 30 individuals who were hr-HPV+ with negative cytology (HHPV+) were recruited at the Gynecology Department of Nanjing Medical University (Nanjing, China). Persons with co-morbid conditions such as kidney, liver or other metabolic diseases including other types of cancer were excluded from the study. Also, any participant that received any form of treatment, chemotherapy, radiotherapy or surgical intervention was excluded from the study. All participants signed the informed consent form and the study design and execution followed the Helsinki Declaration with the approval of the Ethics Committee of Nanjing Medical University (Nanjing, China).
Sample collection and preparation
About 5 mL of blood was collected from each participant into plasma vials, centrifuged and the plasma stored at − 80 °C till further analysis. To a 200 µL aliquot of each sample was added 10 µL of myristic acid in methanol (1 mg/mL) as internal standard and 500 µL of a 3:1 mixture (v/v) of methanol and chloroform, vortexed for 2 min and centrifuged at 13,000 rpm for 10 min at 4 °C. 300 μL aliquots of the supernatants were then transferred to Eppendorf tubes for drying under nitrogen gas at room temperature. The dried residue was then redissolved in 40 μL of methoxyamine solution (15 mg/mL in pyridine), vortexed for 30 s and kept in an oven at 37 °C for 1 h 30 min for oximation to occur. An 80 μL aliquot of BSTFA (containing 1% TMCS) was then added to the solution and vortex for 30 s and the samples again kept in an oven preheated to 70 °C for 1 h. 40 µL aliquots of acetonitrile were then added to the resultant solutions, vortexed for 10 s and finally centrifuged at 13,000 rpm for 10 min at 4 °C. The supernatants of each sample were then transferred to sample vials for GC–MS analysis.
GC–MS instrumental analysis
The prepared samples were analyzed using an Agilent 7890 chromatograph coupled with a 5977B MS system (Agilent Technologies, USA). Separations were achieved on a DB-5 ms capillary column coated with 95% dimethyl/5% diphenyl polysiloxane (30 m × 0.25 mm i.d., 0.25 µm film). Initial GC oven temperature was set at 60 °C for 1 min, followed by 10 °C/min oven temperature ramp to 325 °C and kept for 10 min. The temperature of inlet, transfer line, and ion source was set to 250, 290, and 250 °C, respectively. The injection volume was 1 μL. Helium was used as the carrier gas with a constant flow rate of 0.87 mL/min. Measurements were made with electron impact ionization (70 eV) in the full scan mode (m/z 50–650). The precision and repeatability were validated by the reduplicate analysis of six injections of the same QC sample and six parallel QC samples prepared using the same preparation and method, respectively. From this QC sample, extracted ion chromatographic peaks of five ions (7.48_147.1, 9.91_299.1, 16.72_285.0, 20.28_339.3, 27.43_329.3) were selected for method validation. The RSD of peak area and retention time were below 4.1% and 0.036% respectively and the reproducibility and precision were satisfactory for metabolomic analysis. The stability of samples was tested by analyzing two QC samples kept in autosampler at room temperature for 12, 24 and 72 h. The RSD of peak areas of the serum sample for metabolomic analyses were 2.9–6.4%.
Data processing
The raw unprocessed GC–MS data were converted to the mzdata format using the MassHunter Workstation Software (Version B.06.00, Agilent Technologies). Pretreatment of the resultant data was performed. This included nonlinear retention time alignment, peak discrimination, filtering, alignment, matching and identification, which were accomplished using the XCMS package (http://metlin.scripps.edu/download/) in R-3.3.3. The pretreated data were subjected to analysis by MetaboAnalyst 5.0. However, prior to the multivariate analysis using MetaboAnalyst 5.0, the pretreated data were normalized as follows: for sample normalization, the option of “normalization by median” was selected; for data transformation, “log transformation” was used and for data scaling, the “auto scaling” option was chosen.
Multivariate analysis
The unsupervised model (principal component analysis, PCA) was used to elucidate the intrinsic similarities and differences between the various samples. Using the supervised model of orthogonal partial least squares discriminant analysis (OPLS-DA), subtle and minor differences in the metabolic phenotypes of the various samples were better captured. The fitting ability (R2Y) and predictability (Q2) of the models provided evidence of the quality of the various OPLS-DA models obtained. The variable importance in projection (VIP) values from the OPLS-DA models helped to identify metabolites with significant contributions to the group classifications (i.e., differential metabolites). Together with other parameters such as fold change, and adjusted p value, the differential metabolites (and possible biomarkers) were determined.
Identification of biomarkers and receiver operating characteristic (ROC) analysis
Based on 11 cross-comparisons including CC versus H, CC versus CIN I II III, CC versus CIN I, CC versus CIN II, CC versus CIN III, CC versus HHPV+, CIN I II III versus HHPV+, H versus CIN I II III, H versus CIN I, H versus CIN II, and H versus CIN III a set of differential metabolites were obtained based on an inclusion criterion. Metabolites with false discovery adjusted rate (FDR)-adjusted p value < 0.05, fold change (FC) > 2 or log2 (FC) and variable importance in projection (VIP) > 1 were considered differential metabolites. Their identities were ascertained via library search (the Human Metabolome Database, HMDB and Fiehn database) and confirmed using reference compounds. The diagnostic potentials of possible biomarkers or combinations of same from the lists of differential metabolites were assessed using the ROC analysis. Any biomarker or combinations of same that gave an area under the ROC curve (AUC) > 0.8 was considered to possess a good diagnostic ability.
Pathway and enrichment analyses
To determine the pathways that the various differential metabolites underlie, their HMDB IDs were input in MetaboAnalyst 5.0 and the specific pathway parameters selected: visualization method, scatter plot; enrichment method, Fisher’s exact test; topology analysis, relative-betweenness centrality; and pathway library, Homo sapiens (SMPDB). Similarly, for the enrichment analysis, the HMDB IDs of the differential metabolites were subjected to analysis in MetaboAnalyst 5.0. The feature type selected was “metabolites”. For pathway-based analysis, the SMPDB which has a wider coverage of about 99 metabolite sets based on human metabolic pathways. For the enrichment analysis based on disease signatures, the choice of blood was made since that is the biofluid that was used in this study.
Institutional review board statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of Nanjing Medical University (protocol code: 2023-KY-138-02).
Informed consent statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Results
Characteristics of study participants
Details of the study participants are summarized in Table 1. On the whole, the ages of the participants range between 23 and 58 years. The average ages were: 39.05 years for healthy volunteers, 49.56 years for CC patients, 35.33 years for CIN I patients, 36 years for CIN II patients, 43.96 years for CIN III patients and 35.33 years for hr-HPV+ persons.
Comparative global metabolome analysis and differential metabolites
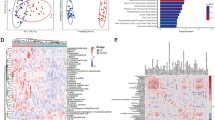
The global metabolomic phenotypes from the various cross-comparisons have been summarized in Figs. 1 and 2 (and Supplementary Figs. S1–S3). A visual analysis of the Figs. 1 and 2 exposes the weakness of the unsupervised PCA model in discriminating between the various metabolic phenotypes. There is an overlap in the metabolic features from the various comparisons using the PCA model. These apparent phenotypic similarities were however clearly distinguished using the supervised OPLS-DA model with good fitting (R2Y) and predictive abilities (Q2). From the 11 cross-comparisons and using the selection criterion earlier highlighted, various differential metabolites were identified. A total of 14 differential metabolites were identified from the comparison of CC versus H, while 6 differential metabolites were found from the comparison of CC versus CIN I II III. For CC versus CIN I; CC versus CIN II; CC versus CIN III; 9, 7, and 4 differential metabolites identified respectively. For the comparisons of H versus CIN I II III, H versus CIN I, H versus CIN II, H versus CIN III, 12, 10, 7, and 11 differential metabolites respectively were determined. All differential metabolites are summarized in Supplementary Tables S1–S10.
Global metabolome analysis from the comparison of cervical cancer (CC) patients versus hr-HPV+ normal cytology participants (HHPV+), hr-HPV+ (A,B) and hr-HPV+ normal cytology participants (HHPV+) versus CIN I II III subjects (C,D). Outcomes of multivariate analysis using unsupervised PCA (A,C) and supervised OPLS-DA (B,D) models.
Underlying perturbed metabolic pathways
On the basis of the differential metabolites identified from the comparison of CC versus H, the general idea of the perturbed metabolic pathways was obtained. As shown in Fig. 3 A, the disturbed metabolic pathways include: valine, leucine and isoleucine biosynthesis, histidine metabolism, purine metabolism, vitamin B6 metabolism, valine, leucine and isoleucine degradation, pyrimidine metabolism, beta-alanine metabolism, alanine, aspartate and glutamate metabolism, aminoacyl-tRNA biosynthesis, arginine biosynthesis, and steroid hormone biosynthesis. All these pathways can be generally summarized as perturbations in (1) amino acids biosynthesis and metabolism and (2) steroid hormone biosynthesis.
For the comparison of CC versus CIN I II III, three main perturbed metabolic pathways were found, hence, vitamin B6 metabolism, pyrimidine metabolism and amino sugar metabolism. This trend was observed for all other comparisons such as CC versus CIN I, CC versus CIN II and CC versus CIN III including steroid hormone biosynthesis for CC versus CIN I and CC versus CIN II. With regard to the metabolomic phenotypes of hr-HPV+ normal cytology persons in relation to CC patients, the main disturbed pathways were vitamin B6 metabolism, androstenedione metabolism and amino sugar metabolism. In the determination of the perturbed metabolic pathways of CIN I II III subjects relative to healthy volunteers, the following were found: steroid hormone metabolism, aminoacyl-tRNA biosynthesis, pyrimidine metabolism, arginine metabolism, arginine and proline metabolism, and tryptophan metabolism. These pathways mainly relate to amino acid biosynthesis and metabolism, and steroid hormone metabolism (Fig. 3B).
Enrichment analysis
Based on the set of differential metabolites identified from the CC versus H, the enrichment analysis by disease signatures and pathway-based analysis are summarized in Fig. 4. With respect to the enrichment analysis by disease signatures, the differential metabolites identified have been prior implicated in neurological, genetic, hormonal and metabolic disorders such as seizures, psychiatric disorders, Maple syrup urine disease, Sotos syndrome, hirsutism, pyruvate dehydrogenase deficiency, and metabolites affected by gender. The results of the pathway-based enrichment analysis support the fact that the main disturbed pathways are generally linked to amino acid biosynthesis and metabolism, and steroidal hormone metabolism. A similar trend was observed for the enrichment analyses based on the differential metabolites identified from the comparison of H versus CIN I II III (Fig. 4).
Diagnostic biomarkers and biomarker panel
In a bid to find the best biomarkers or panel of biomarkers for the diagnosis of cervical cancer particularly ones with the ability to discriminate the precancerous CIN stages from CC, we subjected the individual differential metabolites or combinations of same to ROC analysis. A combination of 2-methyl-1-propylamine (isobutylamine) and estrone exhibited the best diagnostic abilities in terms of the AUC, sensitivity, and specificity values for all cross-comparisons. For instance, the AUC of the 2-biomarker panel in discriminating healthy women from precancerous CIN I II III patients was 0.908 with sensitivity and specificity values of 93.0% and 87.2% respectively. The diagnostic performance of this biomarker panel was higher for the early stages of CIN (i.e., CIN I, CIN II) relative to later stage (CIN III). The AUC for H versus CIN I was 0.973 at 94.4% sensitivity and 94.9% specificity. For H versus CIN II, an AUC of 0.927, sensitivity of 100% and specificity of 87.2% was obtained. However, for H versus CIN III, the AUC, sensitivity, and specificity values of 0.895, 88.1%, and 87.2% were obtained respectively. The diagnostic performance of the biomarker panel was also tested in the comparison of CC against CIN I II III cases. Also, the diagnostic strength of the 2-biomarker panel was found to be weaker with the progression of CIN. Holistically, the AUC, sensitivity and specificity of the biomarker panel in the comparison of CC versus CIN I II III were 0.624, 73.7% and 48.9% respectively. For CC vs CIN I, the AUC was 0.93 at 88.9% sensitivity and 95.6% specificity while the AUC, sensitivity and specificity values for CC vs CIN II were 0.885, 75.0% and 91.1% respectively. An AUC of 0.504, sensitivity of 42.9% and specificity of 48.9% were obtained for the biomarker panel in the comparison of CC vs CIN III (Fig. 5). Finally, the ability of the biomarker panel in distinguishing between healthy non-HPV+ women (H) from hr-HPV+ non-CIN, non-CC women (HHPV+) and CC patients from hr-HPV+ non-CIN, non-CC women. For the comparison of H versus HHPV+, an AUC of 0.834 was obtained at 94.9% sensitivity, and 76.7% specificity (Supplementary Fig. S4A). For CC vs HHPV+, an AUC of 0.967, sensitivity of 96.7% and specificity of 95.6% were obtained for the diagnostic biomarker panel (Supplementary Fig. S4B). The identities of the two diagnostic biomarkers were confirmed using reference compounds. As shown in Supplementary Fig. S5, the positions of the two diagnostic biomarkers in the various samples can be deduced from the retention times of the reference compounds. The retention times and detailed mass spectrometric data of the two biomarkers are however clearly shown in Supplementary Fig. S6. As evidenced from Fig. S6A, retention time of 2-methyl-1-propylamine (isobutylamine) is 6.928 min with a characteristic molecular ion of 73.10 while the retention time and molecular ion of derivatized estrone are 22.950 min and 371.30 respectively (Fig. S6B).
Receiver operating curves (ROC) showing the diagnostic potential of the 2-biomarker panel (2-Methyl-1-propylamine and estrone) in various comparisons: H versus CIN I II III (A), H versus CIN I (B), H versus CIN II (C), H versus CIN III (D), CC versus CIN I II III (E), CC versus CIN I (F), CC versus CIN II (G), CC versus CIN III (H). CC cervical cancer, CIN cervical intraepithelial neoplasia; H healthy.
Levels of diagnostic biomarkers in various pathological states
We sought to qualitatively assess the levels of the two diagnostic biomarkers in CC, and the three stages of CIN relative to the healthy individuals as shown in Fig. 6. On the whole, 2-methyl-1-propylamine was found to be upregulated in the various pathological states (CC, CIN I, CIN II, CIN III) relative to the healthy women. However, the level of estrone decreased in CC, CIN I, CIN II and CIN III compared to the healthy volunteers. It is worth noting that estrone is downregulated the most in CIN I and CIN II patients. The level of estrone in CC and CIN III patients was lower than that for the healthy women.
Discussion
With the general aim of finding a biomarker or panel of biomarkers capable of discriminating between the various stages of CIN from healthy women and CC patients, we sought in this work to uncover biomarkers that could aid in the accurate and early diagnosis of CC cases. Using the GC–MS analytical platform, we aimed to capture specifically low-molecular weight metabolites that could serve as diagnostic biomarkers for CC and CIN. Based on several cross-comparisons, various sets of differential metabolites were found from which two common metabolites (2-methyl-1-propylamine and estrone) exhibited good diagnostic abilities. The underlying metabolic pathways of the various differential metabolites from all cross-comparisons basically relate to amino acid biosynthesis and metabolism, and steroid hormone metabolism as reported in previous studies12,19.
Our current understanding of the pathogenesis and etiology of CC suggests that it is the result of multiple pathophysiological processes that emanate from persistent high-risk HPV infections that eventually progress to mild dysplasia (CIN I) through moderate dysplasia (CIN II) and severe dysplasia (CIN III) to the invasive CC stage20. Even though these processes might exhibit unique and distinct metabolic phenotypes, they are usually asymptomatic to the patient. The focus of biomedical researchers now has been to diagnose the disease at its earliest precancerous stage so that early therapeutic intervention could be sought. In light of this, many have proposed several biomarkers or panels of biomarkers to aid in the accurate and early diagnosis of CC. These biomarkers with varied diagnostic performances have mainly been obtained from LC–MS-based plasma, serum or tissue derived metabolomic or lipidomic data16,20,21. In this study, we found that a combination of 2-methyl-1-propylamine and estrone exhibited good to excellent diagnostic abilities. This 2-biomarker panel could accurately diagnose CIN I II III from healthy non-HPV infected women with an AUC of 0.908 at 93.0% sensitivity and 87.2% specificity. For the diagnosis of the individual stages of CIN relative to the healthy women, the 2-biomarker panel exhibited excellent to good diagnostic power as earlier mentioned. With increase in the stage of CIN, the diagnostic ability of the biomarker panel decreased. This is clearly exemplified in the comparison of CC against CIN I, CIN II and CIN III where the lowest AUC of 0.504 at 42.9% sensitivity and 48.9% specificity was obtained for comparison of CC with CIN III. This could best be explained by the fact that, as the precancerous CIN progresses towards the invasive CC stage, the inherent metabolic phenotypic characteristics become more similar (rather than dissimilar for the early stages of CIN). The unique metabolic features in the early CIN (CIN I II) and late (CIN III) stages further lend credence to the need for early diagnosis of the disease so as to prevent eventual transition to CC.
Estrone is one of the three main mammalian estrogens (i.e., estrone, estradiol, and estriol) but with a relatively weaker activity. It is a steroid hormone that is produced in the placenta, ovaries and testis22. It is biosynthesized via conversion of natural C19 steroids, androstenedione and testosterone by cytochrome P450 aromatase or from estradiol-17β by 17β-hydroxysteroid dehydrogenase type 2. It is mainly synthesized in postmenopausal women from adrenal dehydroepiandrosterone. It is known to bind to ERα and ERβ, the two types of estrogen receptors22. These receptors have been implicated in different tumor cells including prostate, breast, gastric, ovarian, and cervical cancer cells23. In a recent study, Diaz-Ruano et al., who examined the effects of the estradiol and estrone in pre- and postmenopausal women on, epithelial-to-mesenchymal transition (EMT), inflammation and cancer stem cell enrichment in ER+ cervical cancer line HeLa, they found estrone to possess pro-inflammatory effects, increase the expression of embryonic stem transcription factors and induce EMT in ER+ HeLa cells24. 17β-estradiol has been implicated in CC pathogenesis in much the same way as 17β-hydroxysteroid dehydrogenase type 1, the enzyme that underlies the transformation of estrone to 17β-estradiol25,26, thereby establishing the indirect role of estrone in CC. Though preliminary studies have reported that sex hormone elevations do not predispose HPV+ premenopausal and postmenopausal women to CC, these findings require further investigations due to the weaknesses of the method used27. There is however evidence in support of the fact that some sex hormones (testosterone and estradiol) may be involved in the etiology of CC28. 2-methyl-1-propylamine (also known as isobutylamine) is a monoalkylamine with an almost ubiquitous distribution (i.e., exists in almost all living organisms). It has been reported as one of the intermediates in the production of the antibiotic, valanimycin from two amino acids, valine and serine29 and as a precursor for alkamide biosynthesis in the Echinacea genus through the biotransformation of valine and isoleucine30. It has also been reported in vaginal fluid, particularly vaginal fluids of women with nonspecific vaginitis31 and bacterial vaginosis32. We therefore report its direct link in CC for the first time in this study.
Conclusions
We report in this work the probable applicability of 2-methyl-1-propylamine (isobutylamine) and estrone for the early diagnosis of CC. This 2-biomarker panel demonstrated good to excellent diagnostic abilities especially for the early stages of CIN (i.e., CIN I II) and CC. These findings have good clinical significance in the sense that, once this 2-biomarker panel is validated, they could be used as point of reference in laboratory tests for diagnosis of CIN stages and invasive CC. This would therefore inform the choice of treatment to be initiated by the clinician. Our study, however, has several limitations that need to be addressed in future studies in order to confirm or otherwise our findings. First, as a single center study, the sample size is small and could therefore have influenced the outcome of this study particularly the robustness of the statistical analysis. In future studies, a multicenter study could be conducted where more participants could be enrolled. Second, the levels of these biomarkers could be quantitatively determined in a multicenter study. Third, in addition to GC–MS analysis, other analytical platforms such as (LC–MS, LC-NMR) can be employed in order to validate the applicability of this 2-biomarker panel for diagnostic purposes. Finally, detailed demographic and personal characteristics of participants such as BMI, history of hormonal contraceptive use, diet, smoking status, etc., could be captured in future studies for risk-factor assessment.
Data availability
Experimental data is available and could be obtained from Raphael N. Alolga upon reasonable request.
References
Cohen, P. A. et al. Cervical cancer. Lancet 393(10167), 169–182 (2019).
Arbyn, M. et al. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health 8(2), e191–e203 (2020).
Hu, Z. & Ma, D. The precision prevention and therapy of HPV-related cervical cancer: New concepts and clinical implications. Cancer Med. 7(10), 5217–5236 (2018).
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68(6), 394–424 (2018).
Small, W. Jr. et al. Cervical cancer: A global health crisis. Cancer 123(13), 2404–2412 (2017).
Balasubramaniam, S. D. et al. Key molecular events in cervical cancer development. Medicina (Kaunas) 55(7), 384 (2019).
Peirson, L. et al. Screening for cervical cancer: A systematic review and meta-analysis. Syst. Rev. 2, 35 (2013).
Tsikouras, P. et al. Cervical cancer: Screening, diagnosis and staging. J. Buon 21(2), 320–325 (2016).
Arip, M. et al. Exploration of biomarkers for the diagnosis, treatment and prognosis of cervical cancer: A review. Discov. Oncol. 13(1), 91 (2022).
Pinu, F. R., Goldansaz, S. A. & Jaine, J. Translational metabolomics: Current challenges and future opportunities. Metabolites 9(6), 108 (2019).
Pinu, F. R. et al. Systems biology and multi-omics integration: Viewpoints from the metabolomics research community. Metabolites 9(4), 76 (2019).
Khan, I. et al. LC/MS-based polar metabolite profiling identified unique biomarker signatures for cervical cancer and cervical intraepithelial neoplasia using global and targeted metabolomics. Cancers (Basel) 11(4), 511 (2019).
Zhou, H. et al. Prognostic biomarkers of cervical squamous cell carcinoma identified via plasma metabolomics. Medicine (Baltimore) 98(26), e16192 (2019).
Yang, K. et al. A comprehensive analysis of metabolomics and transcriptomics in cervical cancer. Sci. Rep. 7, 43353 (2017).
Godoy-Vitorino, F. et al. Discriminating high-risk cervical Human Papilloma Virus infections with urinary biomarkers via non-targeted GC-MS-based metabolomics. PLoS ONE 13(12), e0209936 (2018).
Abudula, A. et al. Tissue-based metabolomics reveals potential biomarkers for cervical carcinoma and HPV infection. Bosn. J. Basic Med. Sci. 20(1), 78–87 (2020).
Fiehn, O. Metabolomics by gas chromatography–mass spectrometry: Combined targeted and untargeted profiling. Curr. Protoc. Mol. Biol. 114, 30.4.1-30.4.32 (2016).
Papadimitropoulos, M. P. et al. Untargeted GC–MS metabolomics. Methods Mol. Biol. 1738, 133–147 (2018).
Yin, Z., Hua, X. & Lu, M. Integrated network pharmacology and metabolomics to dissect the mechanisms of naringin for treating cervical cancer. Comb. Chem. High Throughput Screen 27, 754–764 (2023).
Nam, M. et al. Comparable plasma lipid changes in patients with high-grade cervical intraepithelial neoplasia and patients with cervical cancer. J. Proteome Res. 20(1), 740–750 (2021).
Porcari, A. M. et al. Molecular signatures of high-grade cervical lesions. Front. Oncol. 8, 99 (2018).
Sato, T., Miyagawa, S. & Iguchi, T. Subchapter 94H—Estrone. In Handbook of Hormones (eds Takei, Y. et al.) 523–524 (Academic Press, 2016).
Li, Q. et al. Estrone-targeted PEGylated liposomal nanoparticles for cisplatin (DDP) delivery in cervical cancer. Eur. J. Pharm. Sci. 174, 106187 (2022).
Diaz-Ruano, A. B. et al. Estradiol and estrone have different biological functions to induce NF-κB-driven inflammation, EMT and stemness in ER+ cancer cells. Int. J. Mol. Sci. 24(2), 1221 (2023).
Tomaszewska, A. et al. Increased 17ß-hydroxysteroid dehydrogenase type 1 levels in primary cervical cancer. Biomed. Pharmacother. 72, 179–183 (2015).
Lutkowska, A., Roszak, A. & Jagodziński, P. P. 17β-hydroxysteroid dehydrogenase type Gene 1937 A > G polymorphism as a risk factor for cervical cancer progression in the Polish population. Pathol. Oncol. Res. 23(2), 317–322 (2017).
Shields, T. S. et al. A case-control study of endogenous hormones and cervical cancer. Br. J. Cancer 90(1), 146–152 (2004).
Rinaldi, S. et al. Endogenous sex steroids and risk of cervical carcinoma: Results from the EPIC study. Cancer Epidemiol. Biomark. Prev. 20(12), 2532–2540 (2011).
Garg, R. P. et al. Molecular characterization and analysis of the biosynthetic gene cluster for the azoxy antibiotic valanimycin. Mol. Microbiol. 46(2), 505–517 (2002).
Rizhsky, L. et al. Integrating metabolomics and transcriptomics data to discover a biocatalyst that can generate the amine precursors for alkamide biosynthesis. Plant J. 88(5), 775–793 (2016).
Chen, K. C. et al. Amine content of vaginal fluid from untreated and treated patients with nonspecific vaginitis. J. Clin. Investig. 63(5), 828–835 (1979).
Wolrath, H. et al. Analysis of bacterial vaginosis-related amines in vaginal fluid by gas chromatography and mass spectrometry. J. Clin. Microbiol. 39(11), 4026–4031 (2001).
Acknowledgements
Drs. Dou Xiaowei, Chen Xiaoxiao and Li Xiaozhao of the Prenatal Diagnostic Center of The Second Affiliated Hospital of Nanjing Medical University, who made available materials and basic equipment to undertake the initial separation and storage of blood samples. We also wish to sincerely thank Hui-Ying Wang (Public platform of the State Key Laboratory of Natural medicines, CPU) for the technical assistance.
Funding
This research was funded by the National Natural Science Foundation of China (Research Fund for Young International Scientist, Grant Number, 82150410450 and Key Project of Medical Science and Technology Development in Nanjing, Grant Number ZKX11178.
Author information
Authors and Affiliations
Contributions
G.F.N.A.., R.N.A., X.Y. and Z.Z. did the conceptualization, G.F.N.A., R.N.A., V.K.E., S.L., Z.L. and W.J. recruited patients and initial preparation of samples. R.N.A. and G.F.N.A. worked on the software for the experiments. G.F.N.A., X.Y. and Z.Z. validated the software. G.F.N.A., R.N.A., V.K.E., S.L, Z.L. and W.J. carried out the main experiment. G.F.N.A., B.X., Z.L. and W.J. did the raw data curation. G.F.N.A., R.N.A. and Z.J. did the formal analysis. G.F.N.A.., X.Y., B.X., Z.J. and S.L. provided resources. R.N.A. and G.F.N.A. wrote the manuscript. X.Y. and Z.Z. supervised the research. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Nuer-Allornuvor, G.F., Alolga, R.N., Liang, S. et al. GC–MS-based untargeted plasma metabolomics identifies a 2-biomarker panel for possible diagnosis of precancerous cervical intraepithelial neoplasia stages from cervical cancer. Sci Rep 14, 17649 (2024). https://doi.org/10.1038/s41598-024-64574-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-64574-8
- Springer Nature Limited