Abstract
Mitochondrial dysfunction, characterized by elevated oxidative stress, impaired energy balance, and dysregulated mitochondrial dynamics, is a hallmark of metabolic syndrome (MetS) and its comorbidities. Ferulic acid (FA), a principal phenolic compound found in whole grains, has demonstrated potential in ameliorating oxidative stress and preserving energy homeostasis. However, the influence of FA on mitochondrial health within the context of MetS remains unexplored. Moreover, the impact of FA on autophagy, which is essential for maintaining energy homeostasis and mitochondrial integrity, is not fully understood. Here, we aimed to study the mechanisms of action of FA in regulating mitochondrial health and autophagy using palmitate-treated HepG2 hepatocytes as a MetS cell model. We found that FA improved mitochondrial health by restoring redox balance and optimizing mitochondrial dynamics, including biogenesis and the fusion/fission ratio. Additionally, FA was shown to recover autophagy and activate AMPK-related cell signaling. Our results provide new insights into the therapeutic potential of FA as a mitochondria-targeting agent for the prevention and treatment of MetS.
Similar content being viewed by others
Introduction
Metabolic syndrome (MetS) is characterized by a cluster of metabolic conditions including hypertension, hyperglycemia, central obesity, dyslipidemia, and insulin resistance1. As one of the most common non-communicable diseases, it poses health threats to around 1/3 of the world population2. According to the most recent epidemiological studies, MetS raised the risk of stroke by 2–4 times3, increased the risk of type 2 diabetes mellitus (T2DM) by five times 4, doubled the risk of cardiovascular disease (CVD) in the next 5–10 years3, and also elevated the risks of many other chronic diseases including non-alcholic fatty liver disease (NAFLD)5,6, which is now replaced by the terminology metabolic dysfunction-associated fatty liver disease (MAFLD), emphasizing metabolic dysfunction over the absence of alcohol consumption as the primary factor7,8, and many cancers such as liver, bladder and colon cancer2,9. Due to the complicated pathogenesis and mechanisms, there are ongoing debates about the diagnostic criteria of MetS and its interconnected risk factors. However, one theory widely accepted is that the lifestyle changes in the past decades especially the elevated high-fat diet (HFD) are the major contributing factors of the emerging epidemic10. Diets rich in unhealthy saturated fats lead to energy imbalance and oxidative stress which play important roles in developing metabolic disorders. Excessive free fatty acids (FFAs) produced from HFD are taken by the liver, progressively resulting in the physio-pathological features commonly seen in metabolic syndrome patients such as elevated waist circumference, increased low density lipoprotein (LDL) cholesterol level, high blood pressure level and insulin resistance.
Recently, mitochondria-targeting therapeutics with the use of natural plant-derived ingredients have gained attention to treat chronic diseases like type 2 diabetes, cancers, and neurodegenerative diseases such as Alzheimer’s disease11,12,13. Mitochondria, as the powerhouse of the cells where most of the ATP is produced, play an important role in energy homeostasis14. HFD produces excessive FFAs which cause an extra burden for the mitochondria to process via fatty acid oxidation, leading to an overexpression of acetyl coenzyme A that drives the generation of NADH and FADH2 via the tricarboxylic acid (TCA) cycle15. This surge in reducing equivalents enhances electron flux to the electron transport chain (ETC) within mitochondria. Consequently, a fraction of these high-energy electrons deviates from the intended redox reactions, resulting in the formation of reactive oxygen species (ROS). HFD leads to nutrient overload and insulin resistance which have been shown to trigger mitochondrial dysfunction involving reduced mitochondrial biogenesis and impaired mitophagy- the two essential processes in maintaining mitochondria homeostasis14. Mitochondrial biogenesis, as the process by which cells increase mitochondria mass, is regulated by the peroxisome proliferator-activated receptor-gamma coactivator-1alpha (PGC-1α), which coordinates with transcriptional factors such as nuclear respiratory factors (NRFs) and mitochondrial transcriptional factor A (TFAM). Mitophagy is a specific process of autophagy, which involves the turnover of damaged mitochondria16. In the HFD-fed mice and db/db mice with MAFLD phenotype, both dysregulated mitochondrial biogenesis and mitophagy were observed17,18. Additionally, mitochondrial dynamics, which encompass the processes of fission and fusion that govern the separation and merging of mitochondria, are important indicators of mitochondrial health19,20. A shift towards fission over fusion was consistently found in the adipose and skeletal muscle tissues of HFD-fed mice by favoring the expression or activity of dynamin-related protein-1 (DRP1), resulting in imbalanced mitochondrial dynamics and elevated mitochondrial fragmentation21,22. Mitochondria and biomarkers related to mitochondrial function have emerged as important therapeutic targets in regulating cell metabolisms in treating metabolic diseases23,24.
AMP-activated protein kinase (AMPK) has been widely studied as a key regulator sensing metabolic stress and maintaining energy homeostasis25. In diabetic or MAFLD models and patients, AMPK activity was always decreased in adipose tissues, liver, and heart, leading to increased fatty acid and cholesterol generation, which require ATP consumption, insulin resistance, and systematic inflammation16,26,27,28,29,30,31. Improving the AMPK activity has been a core strategy in terms of cell signaling regulation for MetS prevention32. Progressive research found AMPK promoted autophagy via inhibiting mammalian target of rapamycin (mTOR) and upregulating unc-51 like autophagy activating kinase 1 (ULK1)33,34. It works as a cellular defensive mechanism in response to metabolic stress by degrading damaged organelles such as mitochondria and other cell debris. Besides, AMPK controls mitochondrial functions by regulating the downstream regulatory factors. It regulates mitochondrial biogenesis by phosphorylating and activating PGC-1α, which in turn induces the expression of genes involved in mitochondrial biogenesis, such as NRF1 and TFAM35. Besides, AMPK directly phosphorylates mitochondrial fission factor (MFF) to regulate mitochondrial fission, recruits DRP1 to the outer mitochondrial membrane, and phosphorylates optic-atrophy-1 (OPA1) to enhance mitochondrial fusion36. These regulatory mechanisms are crucial for maintaining mitochondrial homeostasis and preventing the accumulation of damaged mitochondria.
Phenolics from fruits, vegetables, and whole grains are natural antioxidants and bioactive compounds that have shown protective effects in preventing and treating chronic diseases with metabolic disorders37,38,39. Ferulic acid (FA) is one of the most abundant phenolics in whole grains. In our previous study, we already showed that FA alleviated palmitate-induced lipid accumulation, enhanced glucose consumption, and regulated the hepatic glucose and lipid metabolism via the Insulin/IGF-1 receptor-PI3K-AKT pathway40. Considering the key roles that mitochondria play in the development and progression of MetS and the potential capability of FA in preventing metabolic disorders, we aimed to further study whether FA regulated the mitochondrial dynamics and autophagy via the intrinsic cell AMPK-regulated signaling pathway in the palmitic acid-induced MetS cell model. We discovered that FA not only rebalances redox states but also positively influences mitochondrial biogenesis, fusion, and fission, emphasizing its promise as a targeted mitochondrial therapeutic for MetS, acting through the enhancement of autophagy and activation of AMPK signaling.
Methods
Cell culture and treatment
Human hepatic cancer HepG2 cells were obtained from the American Type Culture Collection (ATCC) (Manassas, VA). The cells were cultured in William’s Medium E supplemented with 5% FBS, 2 mM l-glutamine, 0.05 μg/mL hydrocortisone, 5 μg/mL insulin, and 10 mM Hepes as described previously40. They were maintained at 37 °C in an atmosphere containing 5% CO2, following previously established protocols41. For the negative controls, cells received 0.3 mM BSA and 0.1% DMSO as vehicles. For the MetS model (positive control), cells were treated with 0.1% DMSO and 0.3 mM PA-BSA, both pre-heated to 37 °C, to induce metabolic disorders. In the treatment groups, concentrations of 50, 100, and 200 μM FA (with 0.1% DMSO, designated as LFA, MFA, and HFA) were introduced to the cells in conjunction with 0.3 mM PA-BSA.
Detection of total cellular and mitochondrial ROS
Total cellular ROS levels were detected using the cell-permeant reagent 2′,7′-dichlorofluorescin diacetate (DCFH-DA). HepG2 cells were seeded at a density of 5 × 104 cells/well in a 96-well plate and allowed to attach overnight. After seeding, cells were treated with 0.3 mM of PA-BSA or different doses of FA as described for 24 h. The cells were then washed with PBS and incubated with 25 µM DCFH-DA in a serum-free medium for 30 min at 37 °C in the dark. Following incubation, ROS production was determined immediately by measuring the formation of fluorescent dichlorofluorescein (DCF) using BioTek Cytation 5 plate reader (Agilent Technologies, Santa Clara, CA) with excitation at 485 nm and emission at 535 nm. The total ROS level is proportional to the fluorescence and expressed as the percentage relative to the control.
Mitochondrial ROS level was detected using Mitochondrial ROS detection kit (701,600) purchased from Cayman Chemical (Ann Arbor, MI), following the manufacture’s instruction. Briefly, HepG2 cells were seeded in a dark 96 well plate at a density of 2 × 104 cells/well. After seeding, cells were treated with 0.3 mM of PA-BSA or different doses of FA as described for 24 h. Antimycin A (1 μM) was included in the kit and used to treat the cells for 1 h as a positive control that caused superoxide production from complex III of the mitochondrial electron transport chain. The mitochondrial ROS detection reagent was used to primarily target the mitochondrion and the fluorescence was visualized and quantified by a BioTek Cytation 5 plate reader.
ATP measurement
Cellular ATP levels were measured using the ATP Determination Kit purchased from Thermo Fisher Scientific (Waltham, MA). Briefly, HepG2cells were plated in a dark 96 well plate at a density of 2 × 104 cells/well. After seeding, cells were treated with PA-BSA or different doses of FA as described for 24 h. Since ATP is necessary for D-luciferin to produce light, the luminescence is proportional to the ATP concentration presented. The reaction was carried out and allowed for 15 min before measuring the luminescence. Luminescence was measured using a BioTek Cytation 5 plate reader (Agilent Technologies, Santa Clara, CA) at an emission wavelength of 560 nm. The ATP concentration is calculated by subtracting the background luminescence from the ATP content.
Autophagy level detection
Autophagy levels of the cells were determined using the Autophagy Assay Kit ab139484 (Abcam, Cambridge, UK). HepG2 cells were plated in a dark 96 well plate at a density of 2 × 104 cells/well. After seeding, cells were treated with PA-BSA or different doses of FA as described for 24 h. Rapamycin (500 nM) as an autophagy inducer was included in the kit and was used to treat the cells for 18 h (as a positive control). After removing the cell medium, the cells were stained with Green Detection Reagent and Nuclear Stain. The fluorescence of the stained cells was detected using a spinning disk confocal microscope using green (excitation/emission spectra 463/534 nm) and blue (excitation/emission spectra 350/461 nm for nuclear stain) channels (SDCM; Dragonfly, Andor) and the intensity was quantified by a BioTek Cytation 5 plate reader (Agilent Technologies, Santa Clara, CA). The autophagy level was calculated as the signal reading of green channel/ blue channel and expressed as a percentage over the control.
Mitochondrial membrane potential determination
Mitochondrial membrane potential (MMP) was determined using a JC-1 Mitochondrial Membrane Potential Assay Kit ab 113,850 (Abcam, Cambridge, UK). HepG2 cells were plated in a dark 96 well plate at a density of 1.5 × 104 cells/well. After seeding, cells were treated with PA-BSA or different doses of FA as described above for 24 h. FCCP (Carbonyl cyanide 4- (trifluoromethoxy) phenylhydrazone) was included in the kit as a negative control, which was added 4 h at a final concentration of 100 μM prior to the end of the treatment. The cells were then treated with JC-1 solution for 20 min and the medium was taken. The cells were washed, and the fluorescence was measured in a BioTek Cytation 5 plate reader at excitation/emission spectra 535/590 nm (for aggregates) and 475/530 nm (for monomers). The fluorescence was detected and visualized using a spinning disk confocal microscope (SDCM; Dragonfly, Andor). MMP was calculated as the ratio of red fluorescence counts/ green fluorescence counts.
Western blot analysis
The protein levels of cell signaling regulators were measured using Western blot analysis as described previously42. After treatments, cells were harvested in PBS solution and lysed in RIPA buffer with Halt™ Protease and Phosphatase Inhibitor Cocktail. The proteins were extracted as supernatants after centrifugation at 10,000g for 15 min at 4 °C. The protein concentrations were determined using the Lowry assay and adjusted to the same level. After mixing the sample with sample buffer and boiling the samples for 5 min, 20 μL of samples were loaded onto the 4 − 20% Mini-PROTEANTGX stain-free precast gel (Bio-Rad Laboratories, Inc., Hercules, CA) to run electrophoresis and then transferred to 0.22 μM polyvinylidene fluoride (PVDF) membranes. The membranes were then blocked using the EveryBlot blocking buffer for 5 min and incubated with diluted primary antibodies on a rotator at 4 °C overnight. The membranes were cut prior to hybridization with antibodies during blotting. Next, the membranes were washed three times for 5 min each with TBST and then incubated in corresponding secondary antibodies for 2 h under room temperature. After washing using TBST, the membranes were incubated with Clarity Western ECL substrate for 5 min and the protein bands were visualized using a Bio-Rad ChemiDoc MP Imager (Bio-Rad Laboratories, Inc., Hercules, CA). The protein expression of β-Actin was used as an internal reference. The data were analyzed by the Image Lab software (Bio-Rad Laboratories, Inc., Hercules, CA). Antibodies were purchased from Cell Signaling Technology Inc. (Danvers, MA) with detailed information listed in the Supplementary Table 1.
Statistical analysis
GraphPad Prism 9 software (GraphPad Software Inc., San Diego, CA, USA) was used for data analysis and plotting. The measurements were conducted in triplicates and reported as mean ± standard deviation (SD) for at least three independent samples. Statistical analyses were performed using one-way ANOVA with Tukey’s multiple comparisons test. p < 0.05 indicates a significant difference.
Results
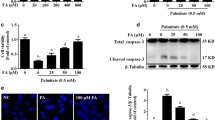
FA reduced total cellular and mitochondrial ROS overproduction and increased ATP level in the MetS hepatic cell model
Mitochondria are the main sites for the generation of ROS and ATP, which play pivotal roles in the development and progression of MetS. Here, we evaluated the effects of FA on total and mitochondrial ROS production, and cellular ATP levels (Fig. 1). The results indicated that mitochondrial ROS fluorescence intensity was significantly elevated in the MetS model and positive control model (Antimycin A) (Fig. 1A,B). Treatments of 50, 100, and 200 μM of FA all significantly reduced mitochondrial ROS levels in a dose-dependent manner. A similar trend was observed in the measurement of total cellular ROS where all doses of FA significantly alleviated the ROS levels evoked by palmitate (Fig. 1C). Lower ATP status is usually associated with a higher ROS level because electron leakage is more likely to happen via ETC, which results in elevated ROS production. Our results confirmed that the cellular ATP level was decreased in the MetS model and increased by FA treatments dose-dependently (Fig. 1D).
Ferulic acid (FA) reduced mitochondrial reactive oxygen species (ROS) production and increased ATP levels in the MetS human HepG2 hepatocyte model. (A) Representative fluorescent images of mitochondrial ROS accumulation in HepG2 cells treated with/without 0.3 mM PA-BSA, 1 μM antimycin (positive control, 1-h treatment), and 50, 100, and 200 μM of FA (24-h treatments); (B) Quantification of mitochondrial ROS levels; (C) Quantification of total cellular ROS (D) Quantification of cellular ATP levels. Data are expressed as mean ± standard deviation (SD) for three independent samples. Bars with no letters in common are significantly different (p < 0.05).
FA activated AMPK signaling pathway
AMPK is crucial for mitochondrial function and energy homeostasis. Upon energy deprivation, exercise, or antioxidant treatments, AMPK can be activated not only to increase ATP generation to maintain energy balance, but also to inhibit processes such as NADPH oxidase-derived ROS production to preserve redox balance33. As we observed the regulative effects of FA on mitochondrial ROS and ATP production, our next objective was to investigate if FA exerts functions via regulation of AMPK signaling. Our results indicated that FA efficiently increased the p-AMPKα /AMPKα ratio, indicating the activation of AMPK signaling (Fig. 2A,B). As the downstream protein that acts against AMPK, mTOR was highly phosphorylated at Ser 2448 in the MetS model and the p-mTOR/mTOR ratio was reduced by the FA treatments in a dose-dependent manner (Fig. 2A,C). ULK1 (UNC-51-like kinase 1) modulates the initiation of autophagy, a catabolic process that helps cell material recycling. ULK1 is activated by p-AMPK via phosphorylation at Ser 555. Our results showed the p-ULK1/ULK1 ratio was reduced in the MetS model when compared to the control, and upregulated by FA in a dose-dependent manner (Fig. 2A,D).
FA activated AMPK signaling in the MetS human HepG2 hepatocyte model. (A) Representative images of p-AMPKα, AMPKα, p-mTOR, mTOR, p-ULK, and ULK protein expressions; (B) Relative protein expression level of p-AMPKα /AMPKα ratio; (C) Relative protein expression level of p-mTOR /mTOR ratio; and (D) Relative protein expression level of p-ULK1 /ULK1 ratio. Data are expressed as mean ± standard deviation (SD) for three independent samples. Bars with no letters in common are significantly different (p < 0.05).
FA restored autophagy flux in the MetS model
Since the activation of p-ULK1 is an indicator of the initiation of autophagy, we further determined the autophagy levels of the HepG2 cells treated with/without PA-BSA and FA (Fig. 3). As shown in Fig. 3A,B, we found that autophagic vacuoles were decreased in the MetS model when compared to the control, meaning that autophagy was inhibited by PA-BSA administration. MFA and HFA significantly increased the autophagy level to a similar extent as the positive control rapamycin. Translocase of outer mitochondrial membrane 20 (Tom20) is responsible for the translocation of proteins into mitochondria. It plays an essential role in the early process of mitophagy, which is a part of autophagy involving the selective degradation of mitochondria. Our results showed that the protein expression of Tom20 was downregulated in the MetS model and elevated by FA treatments.
FA improved autophagy response in the MetS human HepG2 hepatocyte model. (A) Representative confocal fluorescent microscopy image showing autophagic vacuoles (green, FITC filter), nucleus (blue, DAPI filter), and the merge of the two channels in HepG2 cells treated with/without 0.3 mM PA-BSA, FA or rapamycin for 24 h; (B) Quantitative analysis of relative autophagy level calculated as a ratio between the fluorescence intensity of green and blue channels; (C) Representative image of Tom20 protein expression; (D) Relative protein expression level of Tom20. Data are expressed as mean ± standard deviation (SD) for three independent samples. Bars with no letters in common are significantly different (p < 0.05).
FA activated markers related to mitochondrial biogenesis and functions
In section "FA activated AMPK signaling pathway", we showed the activation of AMPK by FA treatments (Fig. 2). Next, we examined the protein expression regulators in the downstream of AMPK- SirT1, PGC-1α, NRF1 and TFAM. Sirtuin 1 (SirT1) is a NAD+-dependent deacetylase that can be mutually activated by AMPK43. It serves as a critical player in maintaining cellular energy homeostasis and aging related diseases. We found that SirT1 was upregulated significantly by medium and high doses of FA treatments compared to the model control (Fig. 4A,B). Both SirT1 and active AMPK are known to activate PGC-1α. Consistently, in Fig. 4A,C we showed that PGC-1α expression was dramatically decreased in MetS and increased by FA in a dose-dependent manner. As a transcription factor that helps the transcription of nuclear-encoded mitochondrial proteins, NRF1 was also found to be downregulated in the MetS model and upregulated by HFA treatment (Fig. 4A,D). TFAM is another transcription factor essential for the maintenance and biogenesis of mitochondrial DNA (mtDNA). FA was shown to elevate the protein expression of TFAM in a dose-dependent manner (Fig. 4A,E). Interestingly, we observed two bands of TFAM in the western blot results, which might indicate post-translational modifications or proteolytic cleavage happening to TFAM under metabolic stress44.
FA enhanced protein levels of mitochondrial biogenesis-related markers including SirT1 (A, B), PGC-1α (A, C), NRF1 (A, D), and TFAM (A, E) in MetS human HepG2 hepatocyte model. Data are expressed as mean ± standard deviation (SD) for three independent samples. Bars with no letters in common are significantly different (p < 0.05).
FA recovered mitochondrial membrane potential in the MetS model
Mitochondrial membrane potential (MMP) is an important indicator of mitochondrial health and was detected using the JC-1 dye (Fig. 5). At a high MMP, JC-1 presents as aggregates, which form green fluorescence. In contrast, at a low MMP, JC-1 dye presents as monomers which exhibit red fluorescence. In Fig. 5A, a decrease in the aggregate fluorescent intensity was detected in the MetS model, indicating the depolarization of the mitochondria. Whereas the increase of aggregates and decrease of monomers by FA is indicative of hyperpolarization of mitochondria. The ratio between the red and green fluorescence intensity was presented as the MMP. We reported that the MMP was significantly decreased by PA-BSA in the MetS model and the negative control FCCP, and was recovered by LFA, MFA, and HFA in a dose-dependent manner (Fig. 5B). This is direct evidence supporting the positive effects of FA on maintaining mitochondrial membrane potential and health.
FA treatments enhanced MMP in MetS human HepG2 hepatocyte model. (A) Representative confocal fluorescent microscopy images of HepG2 cells stained with JC-1 dye. JC-1 monomers yielded green fluorescence with emission of 530 nm, and JC-1 aggregates yielded red fluorescence with emission of 590 nm. The combination of the two displays was shown in the merge. HepG2 cells were treated with/without 0.3 mM PA-BSA, FA for 24 h. 100 μM of FCCP was administered to the cells for 4 h as the negative control. (B) Quantitative analysis of MMP calculated as the ratio of red fluorescence counts/ green fluorescence counts. Data are expressed as mean ± standard deviation (SD) for three independent samples. Bars with no letters in common are significantly different (p < 0.05).
FA regulated mitochondrial dynamics
Mitochondrial fission and fusion are two essential processes in maintaining mitochondrial integrity. In Fig. 6A,B, our results indicated that mitochondrial fission factor (MFF) was upregulated in the MetS model and downregulated by FA treatments in a dose-dependent manner. MFF serves as a mitochondrial receptor for DRP1. When phosphorylated at Ser 616, DRP1 is activated and facilitated mitochondrial fission. The p-DRP1/DRP1 ratio was increased in the MetS model, indicating its activation, and was deactivated in FA groups in a dose-dependent manner (Fig. 6A,C). OPA1 and mitofusins are located in the inner and outer membranes of mitochondria, respectively, and coordinate together to mediate mitochondrial tethering and fusion. In Fig. 7, we showed that the protein levels of the three key regulators related to mitochondrial fusion, including OPA1, Mitofusin-1, and Mitofusin-2, were all decreased in the MetS model and increased by FA treatments. Collectively, the evidence suggested FA could act as a mitochondria-targeted antioxidant that rescues metabolic disorders via restoring the mitochondrial dynamics.
FA suppressed mitochondrial fission through downregulating related protein markers in MetS human HepG2 hepatocyte model. (A) Representative images of MFF, p-DRP1(Ser616), DRP1; (B) Relative protein expression level of MFF; (C) Relative protein expression level of p-DRP1/DRP1. Data are expressed as mean ± standard deviation (SD) for three independent samples. Bars with no letters in common are significantly different (p < 0.05).
FA promoted mitochondrial fusion by activating its related regulators including OPA1 (A, B), Mitofusin-1 (A, C), and Mitofusin-2 (A, D) in MetS human HepG2 hepatocyte model. Data are expressed as mean ± standard deviation (SD) for three independent samples. Bars with no letters in common are significantly different (p < 0.05).
Discussion
Natural antioxidants and bioactive compounds such as dietary phenolics have been widely reported to restore metabolic functions45. Our study confronts the growing prevalence of MetS, exacerbated by high-fat diets and saturated fatty acids like palmitic acid (PA), via exploring the therapeutic potential of FA. Free fatty acid overload, particularly from saturated fatty acids like PA, is a key pathogenic trait shared by both MAFLD and metabolic syndrome46,47. Our previous research established that PA induced metabolic disorders in HepG2 hepatocytes as a simplified MetS cellular model with pathogenesis such as lipid accumulation, increased insulin resistance and disrupted energy metabolism29. In line with our results, other studies also demonstrated that PA led to fat accumulation, inflammation and insulin resistance in hepatocytes, allowing the exploration of molecular mechanisms that provide insights for MetS prevention48,49,50,51. FA, a prominent phenolic in whole grains, mitigates lipid accumulation and metabolic dysregulation via the insulin receptor-PI3K-AKT pathway, positioning it as a candidate for MetS intervention40,52,53. Previous studies suggested that FA was usually transported in its free form and taken by the cells through passive diffusion dependent on the environmental pH and monocarboxylate transporters in the cell membrane54.
The pivotal role of mitochondria in cellular metabolism has led to increased attention on how its dysfunctions may affect metabolic disorders like MetS55. In our current research, we further investigated the role of FA in regulating mitochondrial activities and dynamics. By elucidating the role of FA in regulating mitochondrial activities, dynamics, and reducing oxidative stress, the study not only provides novel insights into the cellular mechanisms of MetS intervention but also positions FA as a promising candidate for future therapeutic strategies against this increasingly prevalent condition.
A growing body of evidence now links ROS, commonly produced within mitochondria, to the onset and progression of MetS56. As a natural antioxidant and bioactive compound, FA has been reported to reduce oxidative stress in many cell lines and tissues such as H9c2 cells exposed to hypoxia, lipopolysaccharide (LPS)-treated bovine mammary epithelial cells, and injured rat heart tissues57,58,59.In vivo experiments also demonstrated that FA reversed ROS stress in the HFD-fed mice model and high fructose diet-induced rat model45. Our results indicated that both total cellular and mitochondrial ROS were increased in the MetS model and significantly reversed by the FA treatments in a dose-dependent manner. ROS overproduction leads to oxidative damage that adversely affects mitochondrial health, resulting in reduced ATP production, which was consistently observed in our disease model13. Previous studies have shown discrepancies in ATP levels stimulated by PA. It was indicated that 16-h and 24-h treatments of 200 μM of PA reduced cellular ATP levels in HepG2 cells60,61. However, another study by Eynaudi A et al. showed that 2–6 h treatment of PA of the same concentration increased the ATP level62. An explanation would be that the short-term treatment of PA provided more free fatty acid energy sources, while prolonged treatments led to mitochondrial dysfunction that further impaired ATP production. Consistently, we also found 24 h treatment of 0.3 mM of PA resulted in a significant decrease in cellular ATP which was reversed by 200 μM of FA treatments. The increase in ATP levels by FA can result from both elevated mitochondrial mass and function. On one hand, our results supported that FA stimulated mitochondrial biogenesis and fusion, which helped increase the number and size of mitochondria. On the other hand, by improving the MMP, reducing ROS level, and improving overall mitochondrial dynamics, FA may enhance the efficacy of mitochondria to produce energy. Further elucidation is needed to clarify the role of FA in regulating the mass and efficiency of mitochondria. Also, directly measuring the types of ROS and assessing mitochondrial transport chain activity may be needed to establish the specific relationship between ROS and ATP production.
AMPK is a key regulator in maintaining cellular homeostasis25. When activated, AMPK shifts energy metabolism from anabolism to catabolism, promoting ATP production over consumption64. Research using transgenic and high-fat diet HFD-induced MetS mouse models shows that AMPK inactivation correlates with disrupted energy metabolism65,66. Mice with prolonged inhibition of the AMPKα2 isoform struggle to maintain energy balance under stress, such as during muscle activity or heart ischemia65. Growing evidence suggests that natural bioactive compounds including phenolics such as resveratrol, quercetin, and curcumin are AMPK activators67,68,69. Recently, FA, as one of the most common phenolics, was also found to activate AMPK in rats with myocardial injury57. A similar trend was also observed in high-fat/cholesterol diet-induced vascular damage in mice and isolated primary human mononuclear cells63. It was shown that bioactive compounds from rice bran, especially FA, protected the vascular environment in ApoE − / − mice via regulating the interplay between mitochondrial biogenesis and dynamics and activation of AMPK signaling63. The activation of AMPK also enhances NAD + / NADH ratio, which in turn upregulates the expression of the NAD + -dependent deacetylase SIRT1. Both activated AMPK and SIRT1 activated or deacetylated the transcriptional factor PGC-1α, which further stimulated mitochondrial biogenesis70. Mitochondrial biogenesis is a fundamental process by which cells increase the number and mass of mitochondria. We found that FA successfully activated the AMPK and SIRT1 in MetS cell model, subsequently upregulating the PGC-1α-NRF1-TFAM pathway. TFAM entered the mitochondria, where it plays a pivotal role in mtDNA transcription, replication, and maintenance70. A recent study by Huang et al. also illustrated that 0.5% FA supplementation promoted mitochondrial health and reduced oxidative burden through activation of the AMPK/ PGC-1α/ NRF in the muscle of mice71. Similarly, enhancement of the AMPK/ SIRT1/ PGC-1α signaling was also observed in the muscle of weaned piglets fed with diets supplemented with FA72.
Autophagy is a cellular process that degrades and recycles damaged organelles and proteins, with autophagosomes playing a crucial role by enveloping and isolating these components for degradation, thereby maintaining mitochondrial function and metabolic balance73. Mitophagy is a specific type of autophagy where damaged mitochondria are enveloped by autophagosomes and delivered to lysosomes for degradation74. This process helps maintain mitochondrial health by removing damaged or superfluous mitochondria, thus being a trendy therapeutic target for MetS treatments75. AMPK and mTOR have an antagonistic relationship. Upon AMPK activation, mTOR was inhibited to ensure a shift from anabolic processes to catabolic processes such as autophagy76. Various roles of FA in regulating mitophagy/ autophagy have been found under different situations. For example, Luo et al. reported that FA suppressed mitophagy by downregulating the PINK1/Parkin pathway and reducing the autophagy marker- LC3-II/LC3-1 level, thus counteracting the hypoxia/ reoxygenation injury in the H9C2 cells59. However, other studies showed FA increased LC3-II levels, enhancing mitophagy or autophagy in both brain microvascular endothelial cells exposed to oxygen–glucose deprivation and PA-treated AML-12 mouse hepatocytes77,78. Our results were consistent with the results where increased autophagic vacuole levels were observed in FA-treated groups. Furthermore, we found the inhibition of mTOR by activating AMPK, indicating that AMPK was responsible for the phosphorylation of ULK1 at site Ser555, thus promoting autophagy initiation. The coordinated regulation of AMPK and mTOR to enhance autophagy was also found in the LPS-induced neuroinflammation in BV2 microglia79. Another indicator of mitophagy observed in our study was the elevated expression of TOM20. TOM20 helped with the recognition of damaged mitochondria in the early stages of mitophagy, and its ubiquitination and interactions with other proteins such as PINK1 and Parkin facilitated the engulfment of mitochondria by the autophagosomes80. The import of proteins into mitochondria via the TOM complex including TOM20 is an energy-dependent process that requires the driving force by MMP. The recovery of compromised MMP in the MetS model by FA was also a sign of improved mitochondrial health and a mutual trigger for AMPK activation32. Furthermore, ULK1 was reported to phosphorylate Parkin at Ser108 in its ACT domain, FUNDC1, and BNIP3 at Ser17, thus promoting mitophagy81,82. Our study recognized the elevated active ULK1 and TOM20 and the increased autophagosome level, indicating enhanced autophagy and possible mitophagy activation by FA. However, further research about the specific mitophagy pathways such as the PINK/Parkin cascade is desired for a validated mitophagy activation.
In models with metabolic disorders, mitochondrial dynamics were often disrupted70. Excessive nutrients and insulin resistance usually favor mitochondrial fission due to its function in segregating dysfunctional mitochondria22,83. A recent study by Xia et al. showed that the knockout of small GTPase RalA, an activator of fission protein DRP1, led to less mitochondrial fragmentation and resistance to weight gain induced by HFD, implying the potential of targeting DRP1 inactivation and preserving mitochondrial integrity in preventing metabolic diseases22. When faced with energy stress, AMPK targets the mitochondria and phosphorylates MFF, which attracts DRP1 to the mitochondrial outer membrane, subsequently triggering mitochondrial fission84. However, we found the fission markers MFF and DRP1 were both deactivated in FA groups despite AMPK activation. This could be explained by the alternative action of AMPK in upregulating mitochondrial fusion regulators including OPA1 and Mitofusins. OPA1, governs inner membrane fusion, meanwhile, outer membrane fusion is regulated by Mitofusin 1 and 285. Together, the three GTPases facilitated mitochondrial fusion that rescued mildly damaged mitochondria by merging their components. Consistently, the study by Anis et al. showed that FA enhanced mitochondrial fusion by upregulating the protein expressions of PGC-1α and Mitofusin-2, and inhibited fission by downregulating DRP1 in the 6-hydroxydopamine lesioned rat model86. The mRNA expressions of both PGC-1α and Mitofusin-1 were also observed to be elevated in the human mononuclear cells from the blood of healthy volunteers after consuming 500 mg FA for 15 days63.
Conclusion
In summary, our results demonstrated that the plant bioactive compound FA reduced ROS stress, recovered ATP production, increased MMP expression, and reinstated mitochondrial dynamics and autophagy in the PA-induced hepatocyte MetS model (Fig. 8). FA activated AMPK which regulated the following cell signaling pathways related to mitochondrial functions: (1) inhibited mTOR and activated ULK, thereby promoting autophagy and likely enhancing mitophagy (2) upregulated SIRT1/PGC-1α/NRF1/TFAM, which facilitated mitochondrial biogenesis; (3) deactivated MFF and DRP1 to suppress mitochondrial fission; and (4) stimulated Mitofusin-1,2 and OPA1 to enhance mitochondrial fusion. The elucidation of FA's role in modulating mitochondrial dynamics and autophagy underscores its potential as a significant therapeutic strategy for MetS, which may pave the way for innovative, mitochondria-focused treatments. Future investigations are warranted to further understand FA's therapeutic mechanisms in animal and clinical settings, and to develop protocols for its use that optimize the outcomes in MetS management.
Data availability
Data is provided within the manuscript or supplementary information files.
Abbreviations
- AMPK:
-
AMP-activated protein kinase
- BSA:
-
Bovine serum albumin
- CVD:
-
Cardiovascular disease
- FCCP:
-
Carbonyl cyanide 4- (trifluoromethoxy) phenylhydrazone
- Drp1:
-
Dynamin-related protein-1
- ETC:
-
Electron transport chain
- FA:
-
Ferulic acid
- FFAs:
-
Free fatty acids
- HFD:
-
High-fat diet
- LDL:
-
Low density lipoprotein
- LPS:
-
Lipopolysaccharide
- MetS:
-
Metabolic syndrome
- mTOR:
-
Mammalian target of rapamycin
- mtDNA:
-
Mitochondrial DNA
- MFF:
-
Mitochondrial fission factor
- TFAM:
-
Mitochondrial transcriptional factor A
- NAFLD:
-
Non-alcoholic fatty liver disease
- MAFLD:
-
Metabolic dysfunction-associated fatty liver disease
- NRFs:
-
Nuclear respiratory factors
- OPA1:
-
Optic-atrophy-1
- PA:
-
Palmitic acid
- PGC-1α:
-
Peroxisome proliferator-activated receptor-gamma coactivator-1alpha
- ROS:
-
Reactive oxygen species
- SirT1:
-
Sirtuin 1
- TCA:
-
Tricarboxylic acid
- T2DM:
-
Type 2 diabetes mellitus
- ULK1:
-
Unc-51 like autophagy activating kinase 1
References
Vaduganathan, M., Van Meijgaard, J., Mehra, M. R., Joseph, J., O’donnell, C. J., Warraich, H. J. Trends in the prevalence of metabolic syndrome in the United States, 2011–2016. https://doi.org/10.1001/jama.2020.9184.
Esposito, K., Chiodini, P., Colao, A., Lenzi, A. & Giugliano, D. Metabolic syndrome and risk of cancer: A systematic review and meta-analysis. Diabetes Care https://doi.org/10.2337/dc12-0336 (2012).
Lopez-Candales, A., Hernández Burgos, P. M., Hernandez-Suarez, D. F. & Harris, D. Linking chronic inflammation with cardiovascular disease: From normal aging to the metabolic syndrome HHS public access. J Nat Sci 3(4), e341 (2017).
Lee, M., Han, K., Kim, M., Koh, E. & Kim, E. changes in metabolic syndrome and its components and the risk of type 2 diabetes: A nationwide cohort study. Sci. Rep. 10, 2313 (2020).
Vanni, E.; Bugianesi, E.; Kotronen, A.; … S. D. M.-D. and liver; 2010, undefined. From the Metabolic Syndrome to NAFLD or Vice Versa? Elsevier.
Wainwright, P. & Byrne, C. D. Bidirectional relationships and disconnects between NAFLD and features of the metabolic syndrome. Int. J. Mol. Sci. 17(3), 367. https://doi.org/10.3390/ijms17030367 (2016).
Eslam, M. et al. MAFLD: A consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology https://doi.org/10.1053/j.gastro.2019.11.312 (2020).
Eslam, M. et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. https://doi.org/10.1016/j.jhep.2020.03.039 (2020).
Chen, H. et al. Metabolic syndrome, metabolic comorbid conditions and risk of early-onset colorectal cancer. Gut 70, 1147–1154. https://doi.org/10.1136/gutjnl-2020-321661 (2021).
Bovolini, A., Garcia, J., Andrade, M. A. & Duarte, J. A. Metabolic syndrome pathophysiology and predisposing factors. Int. J. Sports Med. 42(3), 199–214. https://doi.org/10.1055/A-1263-0898/ID/R8170-0020/BIB (2021).
Chodari, L. et al. Targeting mitochondrial biogenesis with polyphenol compounds. Oxid. Med. Cell Longev. https://doi.org/10.1155/2021/4946711 (2021).
Duchen, M. R. Mitochondria in health and disease: Perspectives on a new mitochondrial biology. Mol. Aspects Med. 25(4), 365–451. https://doi.org/10.1016/J.MAM.2004.03.001 (2004).
Singh Bhatti, J., Bhatti, G. K. & Hemachandra Reddy, P. Mitochondrial dysfunction and oxidative stress in metabolic disorders-a step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta Mol. Basis. Dis. https://doi.org/10.1016/j.bbadis.2016.11.010 (2016).
Prasun, P. Mitochondrial dysfunction in metabolic syndrome. Biochimica et Biophysica Acta (BBA) Mole. Basis Disease https://doi.org/10.1016/j.bbadis.2020.165838 (2020).
Kesh, S. B. High-fat diet-induced oxidative stress and its impact on metabolic syndrome: A review.
Zhong, G. et al. Arsenic causes mitochondrial biogenesis obstacles by inhibiting the AMPK/PGC-1α signaling pathway and also induces apoptosis and dysregulated mitophagy in the duck liver. Ecotoxicol. Environ. Saf. 230, 113117. https://doi.org/10.1016/J.ECOENV.2021.113117 (2022).
Liu, R. et al. High Ratio of ω-3/ω-6 polyunsaturated fatty acids targets MTORC1 to prevent high-fat diet-induced metabolic syndrome and mitochondrial dysfunction in mice. J. Nutr. Biochem. 79, 108330. https://doi.org/10.1016/J.JNUTBIO.2019.108330 (2020).
Wang, C. H. et al. Antioxidants rich herbal formula Ger-Gen-Chyn-Lian-Tang protects lipotoxicity and ameliorates inflammation signaling through regulation of mitochondrial biogenesis and mitophagy in nonalcoholic fatty liver disease mice. Front. Biosci. Landmark 27(8), 242. https://doi.org/10.31083/J.FBL2708242/2768-6698-27-8-242/FIG9.JPG (2022).
Chen, W., Zhao, H. & Li, Y. Mitochondrial dynamics in health and disease: Mechanisms and potential targets. Signal Trans. Target. Therapy. https://doi.org/10.1038/s41392-023-01547-9 (2023).
Liu, Y. J., McIntyre, R. L., Janssens, G. E. & Houtkooper, R. H. Mitochondrial fission and fusion: A dynamic role in aging and potential target for age-related disease. Mech. Ageing Dev. 186, 111212. https://doi.org/10.1016/J.MAD.2020.111212 (2020).
Heo, J. W. et al. Moderate aerobic exercise training ameliorates impairment of mitochondrial function and dynamics in skeletal muscle of high-fat diet-induced obese mice. FASEB J. https://doi.org/10.1096/fj.202002394R (2021).
Xia, W. et al. Obesity causes mitochondrial fragmentation and dysfunction in white adipocytes due to RalA activation. Nat. Metab. https://doi.org/10.1038/s42255-024-00978-0 (2024).
Yu, T., Wang, L., Zhang, L. & Deuster, P. A. Mitochondrial fission as a therapeutic target for metabolic diseases: Insights into antioxidant strategies. Antioxidants. https://doi.org/10.3390/antiox12061163 (2023).
Amorim, J. A. et al. Mitochondrial and metabolic dysfunction in ageing and age-related diseases. Nat. Rev. Endocrinol. https://doi.org/10.1038/s41574-021-00626-7 (2022).
Fang, C. et al. The AMPK pathway in fatty liver disease. Front. Physiol. 13, 970292. https://doi.org/10.3389/FPHYS.2022.970292/BIBTEX (2022).
Ren, G., Guo, J. H., Qian, Y. Z., Kong, W. J. & Jiang, J. D. Berberine improves glucose and lipid metabolism in HepG2 cells through AMPKα1 activation. Front. Pharmacol. 11, 647. https://doi.org/10.3389/fphar.2020.00647 (2020).
Zhou, Y. J. et al. Chrysin improves glucose and lipid metabolism disorders by regulating the AMPK/PI3K/AKT signaling pathway in insulin-resistant HepG2 Cells and HFD/STZ-induced C57BL/6J mice. J. Agric. Food Chem. https://doi.org/10.1021/acs.jafc.1c01109 (2021).
Sundarraj, K., Raghunath, A., Panneerselvam, L. & Perumal, E. Fisetin inhibits autophagy in HepG2 Cells via PI3K/Akt/MTOR and AMPK pathway. Nutr Cancer 73(11–12), 2502–2514. https://doi.org/10.1080/01635581.2020.1836241 (2021).
Forbes-Hernández, T. Y. et al. Lipid accumulation in HepG2 cells is attenuated by strawberry extract through AMPK activation. Nutrients 9(6), 621. https://doi.org/10.3390/NU9060621 (2017).
Fouqueray, P. et al. Pharmacodynamic effects of direct AMP kinase activation in humans with insulin resistance and non-alcoholic fatty liver disease: A phase 1b study. Cell Rep. Med. https://doi.org/10.1016/j.xcrm.2021.100474 (2021).
Entezari, M. et al. AMPK signaling in diabetes mellitus, insulin resistance and diabetic complications: A pre-clinical and clinical investigation. Biomed. Pharmacother. https://doi.org/10.1016/j.biopha.2021.112563 (2022).
Herzig, S. & Shaw, R. J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mole. Cell Biol. 19(2), 121–135. https://doi.org/10.1038/nrm.2017.95 (2017).
Ke, R., Xu, Q., Li, C., Luo, L. & Huang, D. Mechanisms of AMPK in the maintenance of ATP balance during energy metabolism. Cell. Biol. Int. 42(4), 384–392. https://doi.org/10.1002/CBIN.10915 (2018).
Li, Z., Miao, Z., Ding, L., Teng, X. & Bao, J. Energy metabolism disorder mediated ammonia gas-induced autophagy via AMPK/MTOR/ULK1-Beclin1 pathway in chicken livers. Ecotoxicol. Environ. Saf. 217, 112219. https://doi.org/10.1016/J.ECOENV.2021.112219 (2021).
Cantó, C. & Auwerx, J. PGC-1α, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr. Opin. Lipidol. https://doi.org/10.1097/MOL.0b013e328328d0a4 (2009).
Mikhail, A. I., Ng, S. Y., Mattina, S. R. & Ljubicic, V. AMPK is mitochondrial medicine for neuromuscular disorders. Trends Mole. Med. https://doi.org/10.1016/j.molmed.2023.03.008 (2023).
Liu, R. H. Dietary bioactive compounds and their health implications. J. Food Sci. 78(s1), A18–A25. https://doi.org/10.1111/1750-3841.12101 (2013).
Li, Y., Li, T. & Liu, R. H. Bioactive compounds of highland barley and their health benefits. J. Cereal Sci. https://doi.org/10.1016/j.jcs.2021.103366 (2022).
Li, Y., Chen, H. & Zhang, X. cultivation, nutritional value, bioactive compounds of morels, and their health benefits: A systematic review. Front. Nutr. https://doi.org/10.3389/FNUT.2023.1159029/FULL (2023).
Li, Y., Sair, A. T., Zhao, W., Li, T. & Liu, R. H. Ferulic acid mediates metabolic syndrome via the regulation of hepatic glucose and lipid metabolisms and the insulin/IGF-1 Receptor/PI3K/AKT pathway in palmitate-treated HepG2 Cells. Cite This J. Agric. Food Chem 2022, 14706–14717. https://doi.org/10.1021/acs.jafc.2c05676 (2022).
Jacob, J. R. & Tennant, B. C. Nitrite and nitrosamine synthesis by hepatocytes isolated from normal woodchucks (Marmota monax) and woodchucks chronically infected with woodchuck hepatitis virus. Cancer Res 52(15), 4139–4143 (1992).
Sair, A. T., Li, Y., Zhao, W., Li, T. & Liu, R. H. anticancer activity of apple peel extracts against human breast cancer cells through insulin-like growth factor-1 signal transduction pathway. J. Agric. Food Res. 11, 100507. https://doi.org/10.1016/j.jafr.2023.100507 (2023).
Haigis, M. C. & Sinclair, D. A. Mammalian sirtuins: biological insights and disease relevance. Ann. Rev. Pathol. Mech. Disease. https://doi.org/10.1146/annurev.pathol.4.110807.092250 (2010).
Antelman, J. et al. Expression of mitochondrial transcription factor a (TFAM) during porcine gametogenesis and preimplantation embryo development. J. Cell Physiol. https://doi.org/10.1002/jcp.21528 (2008).
Ibitoye, O. B. & Ajiboye, T. O. Dietary phenolic acids reverse insulin resistance, hyperglycaemia, dyslipidaemia, inflammation and oxidative stress in high-fructose diet-induced metabolic syndrome rats. Arch Physiol Biochem 124(5), 410–417. https://doi.org/10.1080/13813455.2017.1415938 (2018).
Radu, F. et al. The link between NAFLD and metabolic syndrome. Diagnostics. https://doi.org/10.3390/diagnostics13040614 (2023).
Lottenberg, A. M., Afonso, M. S., Lavrador, M. S. F., Machado, R. M. & Nakandakare, E. R. The role of dietary fatty acids in the pathology of metabolic syndrome. J. Nutr. Biochem. https://doi.org/10.1016/j.jnutbio.2012.03.004 (2012).
Malik, S. A., Acharya, J. D., Mehendale, N. K., Kamat, S. S. & Ghaskadbi, S. S. Pterostilbene reverses palmitic acid mediated insulin resistance in HepG2 cells by reducing oxidative stress and triglyceride accumulation. Free Radic Res https://doi.org/10.1080/10715762.2019.1635252 (2019).
Tong, T. et al. Theaflavins improve insulin sensitivity through regulating mitochondrial biosynthesis in palmitic acid-induced HepG2 cells. Molecules https://doi.org/10.3390/molecules23123382 (2018).
Zang, Y., Fan, L., Chen, J., Huang, R. & Qin, H. Improvement of lipid and glucose metabolism by capsiate in palmitic acid-treated HepG2 cells via activation of the AMPK/SIRT1 signaling pathway. J. Agric. Food Chem. https://doi.org/10.1021/acs.jafc.8b01831 (2018).
Joshi-Barve, S. et al. Palmitic acid induces production of proinflammatory cytokine interleukin-8 from hepatocytes. Hepatology https://doi.org/10.1002/hep.21752 (2007).
Gao, J. et al. Ferulic acid targets ACSL1 to ameliorate lipid metabolic disorders in Db/Db Mice. J. Funct. Foods 91, 105009. https://doi.org/10.1016/J.JFF.2022.105009 (2022).
Naowaboot, J., Piyabhan, P., Munkong, N., Parklak, W. & Pannangpetch, P. Ferulic acid improves lipid and glucose homeostasis in high-fat diet-induced obese mice. Clin. Exp. Pharmacol. Physiol. 43(2), 242–250. https://doi.org/10.1111/1440-1681.12514 (2016).
Antonopoulou, I., Sapountzaki, E., Rova, U. & Christakopoulos, P. Ferulic acid from plant biomass: A phytochemical with promising antiviral properties. Front. Nutrition. https://doi.org/10.3389/fnut.2021.777576 (2022).
Prasun, P. Mitochondrial dysfunction in metabolic syndrome. Biochimica et Biophysica Acta Mole. Basis Disease 1866(10), 165838. https://doi.org/10.1016/J.BBADIS.2020.165838 (2020).
Ando, K. & Fujita, T. Metabolic syndrome and oxidative stress. Free Radic. Biol. Med. 47(3), 213–218. https://doi.org/10.1016/J.FREERADBIOMED.2009.04.030 (2009).
Liu, X. et al. Ferulic acid alleviates myocardial ischemia reperfusion injury via upregulating AMPKa2 expression-mediated ferroptosis depression. J. Cardiovasc. Pharmacol. 79(4), 489–500 (2022).
Liu, M. et al. Ferulic acid inhibits LPS-induced apoptosis in bovine mammary epithelial cells by regulating the NF-ΚB and Nrf2 signalling pathways to restore mitochondrial dynamics and ROS generation. Vet. Res. 52(1), 104. https://doi.org/10.1186/S13567-021-00973-3 (2021).
Luo, C. et al. Ferulic acid attenuates hypoxia/reoxygenation injury by suppressing mitophagy through the PINK1/parkin signaling pathway in H9c2 cells. Front. Pharmacol. https://doi.org/10.3389/FPHAR.2020.00103/FULL (2020).
Chou, I. P., Lin, Y. Y., Ding, S. T. & Chen, C. Y. Adiponectin receptor 1 enhances fatty acid metabolism and cell survival in palmitate-treated HepG2 cells through the PI3 K/AKT pathway. Eur. J. Nutr. https://doi.org/10.1007/s00394-013-0594-7 (2014).
García-Ruiz, I., Solís-Muñoz, P., Fernández-Moreira, D., Muñoz-Yagüe, T. & Solís-Herruzo, J. A. In vitro treatment of HepG2 cells with saturated fatty acids reproduces mitochondrial dysfunction found in nonalcoholic steatohepatitis. DMM Disease Model Mech. https://doi.org/10.1242/dmm.018234 (2015).
Eynaudi, A. et al. Differential effects of oleic and palmitic acids on lipid droplet-mitochondria interaction in the hepatic cell line HepG2. Front. Nutr https://doi.org/10.3389/fnut.2021.775382 (2021).
Perez-Ternero, C. et al. Ferulic acid, a bioactive component of rice bran, improves oxidative stress and mitochondrial biogenesis and dynamics in mice and in human mononuclear cells. J. Nutr. Biochem. 48, 51–61. https://doi.org/10.1016/j.jnutbio.2017.06.011 (2017).
Steinberg, G. R. & Hardie, D. G. New insights into activation and function of the AMPK. Nat. Rev. Mole. Cell Biol. https://doi.org/10.1038/s41580-022-00547-x (2023).
Russell, R. R. et al. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J. Clin. Investig. https://doi.org/10.1172/JCI19297 (2004).
Viollet, B. et al. AMPK: Lessons from transgenic and knockout animals. Front. Biosci. https://doi.org/10.2741/3229 (2009).
Jin, Z. et al. Curcumin exerts chondroprotective effects against osteoarthritis by promoting AMPK/PINK1/Parkin-mediated mitophagy. Biomed. Pharmacotherapy https://doi.org/10.1016/j.biopha.2022.113092 (2022).
Zhang, Q. et al. Quercetin attenuates diabetic peripheral neuropathy by correcting mitochondrial abnormality via activation of AMPK/PGC-1α pathway in vivo and in vitro. Front. Neurosci. https://doi.org/10.3389/fnins.2021.636172 (2021).
Park, S. J. et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting CAMP phosphodiesterases. Cell https://doi.org/10.1016/j.cell.2012.01.017 (2012).
Bhatti, J. S., Bhatti, G. K. & Reddy, P. H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochimica et Biophysica Acta (BBA) Mole Basis Disease 1863(5), 1066–1077. https://doi.org/10.1016/J.BBADIS.2016.11.010 (2017).
Huang, T. et al. Dietary Ferulic acid increases endurance capacity by promoting skeletal muscle oxidative phenotype, mitochondrial function, and antioxidant capacity. Mol. Nutr. Food Res. https://doi.org/10.1002/mnfr.202200719 (2024).
Wang, Y. et al. Effects of dietary ferulic acid supplementation on growth performance and skeletal muscle fiber type conversion in weaned piglets. J. Sci. Food Agric. https://doi.org/10.1002/jsfa.11157 (2021).
Hamacher-Brady, A. Autophagy regulation and integration with cell signaling. Antioxidants Redox Signal. https://doi.org/10.1089/ars.2011.4410 (2012).
Davinelli, S., De Stefani, D., De Vivo, I. & Scapagnini, G. Polyphenols as caloric restriction mimetics regulating mitochondrial biogenesis and mitophagy. Trends. Endocrinol. Metabol. 31(7), 536–550. https://doi.org/10.1016/J.TEM.2020.02.011 (2020).
Su, Z. et al. Phytochemicals: Targeting mitophagy to treat metabolic disorders. Front. Cell Dev. Biol. 2021, 9. https://doi.org/10.3389/FCELL.2021.686820/BIBTEX (2005).
Pezze, P. D. et al. A systems study reveals concurrent activation of AMPK and MTOR by amino acids. Nat. Commun. https://doi.org/10.1038/ncomms13254 (2016).
Chen, J. L. et al. Ferulic acid attenuates brain microvascular endothelial cells damage caused by oxygen-glucose deprivation via punctate-mitochondria-dependent mitophagy. Brain Res. 1666, 17–26. https://doi.org/10.1016/J.BRAINRES.2017.04.006 (2017).
Xu, T. et al. Ferulic acid alleviates lipotoxicity-induced hepatocellular death through the SIRT1-regulated autophagy pathway and independently of AMPK and Akt in AML-12 hepatocytes. Nutr. Metab. Lond. https://doi.org/10.1186/s12986-021-00540-9 (2021).
Chen, X. et al. Protective effect of Ferulic acid on lipopolysaccharide-induced BV2 microglia inflammation via AMPK/MTOR signaling pathway. Molecules https://doi.org/10.3390/molecules28083482 (2023).
Sulkshane, P. et al. Ubiquitination and receptor-mediated mitophagy converge to eliminate oxidation-damaged mitochondria during hypoxia. Redox Biol. https://doi.org/10.1016/j.redox.2021.102047 (2021).
Wang, S. et al. The mitophagy pathway and its implications in human diseases. Signal Trans. Target. Therapy. https://doi.org/10.1038/s41392-023-01503-7 (2023).
Hung, C. M. et al. AMPK/ULK1-mediated phosphorylation of Parkin ACT domain mediates an early step in mitophagy. Sci. Adv. https://doi.org/10.1126/SCIADV.ABG4544 (2021).
Natarajan, V. et al. Mitochondrial dysfunction in age-related metabolic disorders. Proteomics. https://doi.org/10.1002/pmic.201800404 (2020).
Toyama, E. Q. et al. Metabolism: AMP-activated protein kinase mediates mitochondrial fission in response to energy stress. Science https://doi.org/10.1126/science.aab4138 (2016).
Cipolat, S., De Brito, O. M., Dal Zilio, B. & Scorrano, L. OPA1 Requires Mitofusin 1 to promote mitochondrial fusion. Proc. Natl. Acad. Sci. US A https://doi.org/10.1073/pnas.0407043101 (2004).
Anis, E. et al. Ferulic acid reinstates mitochondrial dynamics through PGC1α expression modulation in 6-hydroxydopamine lesioned rats. Phytother. Res. 34(1), 214–226. https://doi.org/10.1002/PTR.6523 (2020).
Acknowledgements
The authors thank the Clover Center and Viviana Gradinaru's lab at California Institute of Technology for technical support in the imaging part. This work was partially supported by Cornell China Center, Dasong Agricultural Technology and YanGuFang Research Laboratory (2022-8950).
Author information
Authors and Affiliations
Contributions
Y.L.: conceptualization, methodology, investigation, data curation, writing original draft, writing-review and editing. R.H.L.: conceptualization, supervision, writing-review and editing. W.Z., A.S., T.L.: resources and review.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, Y., Zhao, W., Sair, A.T. et al. Ferulic acid restores mitochondrial dynamics and autophagy via AMPK signaling pathway in a palmitate-induced hepatocyte model of metabolic syndrome. Sci Rep 14, 18970 (2024). https://doi.org/10.1038/s41598-024-66362-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-66362-w
- Springer Nature Limited