Abstract
Photothermal therapy is an alternative cancer therapy that uses a photothermal agent with light irradiation to induce fatal hyperthermia in cancer cells. In a previous study, we found that ex vivo photothermal (PT) treatment induced expression of heat shock proteins (HSPs), such as HSP70, HSP27, and HSP90, in cancer cells; moreover, immunization with lysates from PT-treated tumor cells resulted in significant tumor growth inhibition in tumor-bearing mice. In this study, we hypothesized that sublethal PT treatment of antigen-presenting cells regulates their immunogenicity. We observed the upregulation of expression of intracellular HSP70 and surface activation markers, such as CD40, CD80, CD86, and MHC class II, in sublethal PT-treated cells. The protumoral activity of myeloid-derived suppressor cells (MDSCs) was reduced by sublethal hyperthermia. Furthermore, poorly immunogenic MDSCs were converted into immunogenic antigen-presenting cells by PT treatment. The differences in immunogenicity between MDSCs untreated or treated with the PT technique were evaluated using the Student’s t-test or Mann–Whitney rank sum test. Collectively, direct hyperthermic treatment resulted in phenotypic changes and the functional regulation of immune cells.
Similar content being viewed by others
Introduction
Photothermal therapy (PTT) is an alternative strategy that compensates for the limitations of conventional cancer therapies1. The advantages of PTT include greater selectivity of cancer cells, minimal invasiveness, and distinct mechanisms of action in comparison to conventional therapy2,3. Irradiation with an appropriate laser wavelength generates heat energy in photothermal molecules. Due to fatal heat stress, photothermal agents containing cancer cells are aborted4. Cellular hyperthermia induces endoplasmic reticulum stress and generation of reactive oxygen species (ROS), ATP release and damage-associated molecular patterns (DAMPs), protein denaturation, and cell death5,6,7,8. This phenomenon triggers immune responses by releasing immune stimulating agents such as DAMPs, and therefore, is regarded as immunogenic cell death (ICD)9. PTT has direct and indirect anticancer effects; the direct effect is based on the cytotoxicity of heat stress to cancer cells, and the indirect effect is based on the activation of immune responses via ICD.
In a previous study, we demonstrated the application of ex vivo photothermal (PT) treatment in cancer cells to improve immunogenicity10. Heat shock proteins (HSPs) were increased in PT-treated cells and contributed to the induction of antitumor immune responses. Based on these results, we developed a dendritic cell (DC) vaccine pulsed with the lysates of PT-treated cells and confirmed its efficacy to be higher than that of the conventional DC vaccine.
In this study, we hypothesized that mild PT treatment of immune cells results in immunogenic conversion through the induction of DAMPs, such as HSPs. HSPs have reportedly contributed to DC maturation11,12,13,14,15. Furthermore, HSP-peptide complexes efficiently prime cytotoxic T lymphocyte (CTL) responses through cross-presentation. HSPs also activate signal transduction pathways and myeloid differentiation primary response 88 (MyD88) and nuclear factor kappa B (NF-κB), as well as induce cytokine production16,17. We performed a mild PT treatment on DCs as professional antigen-presenting cells (APCs), and myeloid-derived suppressor cells (MDSCs) as representative immune-suppressor cells. MDSCs are key regulators in tumor microenvironments through various mechanisms, including the induction of regulatory T cells and suppression of T cell responses18,19. Therefore, various agents that target MDSCs to overcome immunosuppressive tumor microenvironments have been evaluated in preclinical and clinical trials20. These agents include cytotoxic anticancer agents, tyrosine-kinase inhibitors, vitamins, and antioxidants. Here, we found that physical cue, hyperthermia induced by PT treatment resulted in DC activation, and the conversion of MDSCs to immunogenic APCs. Collectively, mild hyperthermia at a sublethal grade renders APCs more immunogenic.
Methods
Mice and cell lines
Six-week-old female BALB/c mice of a specific pathogen-free grade (Orient Bio and Hana Bio, Korea) were purchased and kept at the Inje University, Animal Resource Center. The Inje University, Institutional Animal Care and Use Committee (IACUC) approved all experiments (approval number: 2018-006 and 2020-013). All methods were performed according to IACUC guidelines and regulations. Mice had ad libitum access to water and chow. At the end of the experiments, mice were euthanized by gradually filled with CO2 at a rate of 30–70% of chamber volume per minute. We performed all experiments in accordance with Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines.
Her-2/neu-expressing Her-2/CT26 cells (provided by Dr. Chang-Yuil Kang) were previously developed by transduction of CT26 cells with retroviral vector including the cDNA for Her-2/neu21. Her-2/CT26 were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 500 µg/mL G-418 solution (Roche, USA), 10% fetal bovine serum (FBS), and 1% penicillin–streptomycin solution (all from Gibco, USA).
DC preparation
DCs were differentiated from the bone marrow cells of naïve BALB/c or C57BL/6 mice as previously described22. The bone marrow cells were incubated in 20 ng/mL mouse granulocyte–macrophage colony-stimulating factor (GM-CSF) and 20 ng/mL interleukin-4 (both from BioLegend, USA) in Roswell Park Memorial Institute 1640 (RPMI1640) medium supplemented with 10% FBS and 1% penicillin–streptomycin solution (all from Gibco, USA) for 6 days. DC differentiation media were replaced with fresh media every 2–3 days.
MDSC isolation
For MDSC isolation, BALB/c mice were subcutaneously inoculated with Her-2/CT26 syngeneic tumor cells (3 × 105 cells/mouse). After approximately 5–6 weeks, Her-2/CT26-bearing mice were sacrificed. Splenic CD11b+ MDSCs were then separated using anti-CD11b microbeads and a MACS LS column (both from Miltenyi Biotec, Germany), according to the manufacturer’s recommendations.
Ex vivo PT treatment and heating in water bath
In our previous study, we established the PT treatment conditions applied to the cells and confirmed that 100 μg/ml of indocyanine green (ICG; MPbio, France) was required to induce hyperthermia in PT-treated cells10. DCs or MDSCs at a concentration of 1 ~ 2 × 106 per well were seeded in 96-well flat bottom plates (SPL, Korea). We centrifuged the culture plate for 5 min at 400 × g, and discarded the supernatant. Cells were incubated in the presence of 100 μg/mL of ICG in PBS for 90 min. Thereafter, the cells were washed twice with warm PBS (Gibco, USA) and resuspended in 50 μL/well of fresh RPMI1640. At room temperature, the cells were then irradiated with an 808 nm laser (CNI laser, China) at continuous wave intensities of 3 W/cm2, 6 W/cm2, and 12 W/cm2, respectively, for durations ranging from 100 to 300 s. All the samples were irradiated with a laser at a constant distance from the cells. Immediately after laser irradiation, 200 μL/well of warm RPMI1640 with 10% FBS were added to each well. PT-treated cells were allowed to recover for 1 h at 37 °C in a humidified CO2 incubator (Thermo Fisher Scientific Forma, USA).
We utilized a water-based heating (WH) system as a control. Several studies have examined the effects of hyperthermia using WH23,24,25. For WH treatment, 0.5 ml of cells at a density of 2 × 107 cells/ml were transferred into microcentrifuge tubes (SPL, Korea). Each tube was immersed in the water bath (DAIHAN Scientific, KOREA) for the indicated period.
Analysis of temperature and cell viability
The cell temperatures were measured using an infrared thermal imaging camera (FLIR E60, Wilsonville, USA) immediately after the PT treatment or WH.
To assess cell viability, cells exposed to hyperthermic conditions were transferred to 96-well plates, and a water-soluble tetrazolium (WST) solution (EZ-Cytox, Dogen) was added. Cell viability was determined by measuring optical density (OD) at 450 nm using an ELISA reader (Tecan, Switzerland).
Flow cytometry
To detect intracellular HSP70, DCs or MDSCs were fixed/permeabilized using the Fix/Perm kit (eBioscience, CA, USA) in accordance with the manufacturer’s protocol. Briefly, cells were resuspended with Fixation/Permeabilization working solution for 60 min and then washed with Permeabilization buffer. They were stained with phycoerythrin (PE)-labeled anti-HSP70 antibodies (Santa Cruz) in Permeabilization buffer for 30 min. After washing, cells were analyzed using FACSCalibur (BD Biosciences, CA, USA).
To analyze phenotypes, DCs or MDSCs were stained with allophycocyanin-labeled anti-CD40, anti-CD80, anti-CD86, or anti-IA/IE antibodies (all from BioLegend, CA, USA) in FACS buffer (PBS containing 1% FBS) for 60 min. After washing, stained cells were detected via flow cytometry and analyzed using CELLQuest Pro software (BD, CA, USA).
Reverse transcription quantitative real-time polymerase chain reaction (RT-qPCR)
PT-treated or WH-treated MDSCs were recovered for 24 h in culture media at 37 °C. The cells were then harvested, and RNA was purified using the RNeasy mini kit (Qiagen, Germany). We used the M-MLV cDNA Synthesis Kit for cDNA synthesis and the TopReal™ qPCR Kit (both from Enzynomics, Korea) for RT-qPCR. Primers for MDSC functional mediators (Cosmogenetech, Korea) were used, as previously reported26: Glyceraldehyde-3-phosphate dehydrogenase (GAPDH): Forward 5–CCT GGA GAA ACC TGC CAA GTA–3, Reverse 5–GGA AGA GTG GGA GTT GCT GTT G–3; Arginase 1 (ARG1): Forward 5–AAC ACG GCA GTG GCT TTA ACC T–3, Reverse –GTG ATG CCC CAG ATG GTT TTC–3; NADPH oxidase 2 (NOX2): Forward 5–GAC CCA GAT GCA GGA AAG GAA–3, Reverse 5–TCA TGG TGC ACA GCA AAG TGA T–3; Inducible Nitric Oxide Synthase (iNOS): Forward 5–AGG AAG TGG GCC GAA GGA T–3, Reverse 5–GAA ACT ATG GAG CAC AGC CAC AT–3.
Immunization with MDSC vaccine
Isolated MDSCs were untreated or treated with PT treatment under mild condition (6 W/cm2 of 808 nm laser irradiation for 100 s) at room temperature to ensure MDSC viability. Control MDSCs or PT-treated MDSCs were recovered for 24 h, including a final 2-h pulse with 2.5 μg/mL of Her-2/neu CTL epitope peptide, hP63 (AnyGen, Korea)27. Peptide-loaded MDSCs (2 × 106 cells/mouse) were injected into the mice intravenously.
In vivo CTL assay
As described previously, target cells were prepared from syngeneic naïve splenocytes28. Her-2/neu CTL epitope (hp63) pulsed and unpulsed cells were labeled with 20 and 2.5 μM carboxyfluorescein diacetate succinimidyl ester (CFSE) (Thermo Fisher Scientific, MA, USA), respectively. Seven days after immunization, the target cells (2 × 107 cells/mouse) were intravenously transferred to immunized mice. After 24 h, the mice were sacrificed, and each spleen was isolated. CFSE+ target cells in the splenocytes were detected via flow cytometry. The specific lysis (%) was obtained as follows:
Adoptive transfer of MDSCs
PT-treated MDSCs (1.5 × 107 cells/mouse) were intravenously transferred into Her-2/CT26 tumor-bearing mice at a tumor size of approximately 50 mm3. The tumor size was measured three times per week as follows: tumor size (mm3) = 1/6 × π × height × width × length.
Statistical analysis
Student’s t-test was performed using SigmaPlot 14.5 (Systat Software, CA, USA) to compare the significant differences between the two groups. If the normality test (Shapiro–Wilk) failed, Mann–Whitney rank sum test was performed. Statistical significance was set at p < 0.05.
Results
Changes in temperatures and viabilities of DCs and MDSCs after ex vivo PT treatment
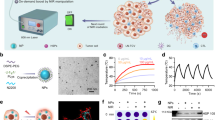
To analyze the effects of the ex vivo PT treatment in APCs, bone marrow-derived DCs were incubated in 100 μg/mL of ICG for 90 min, following which they were irradiated with an 808-nm laser at 3 W/cm2, 6 W/cm2, or 12 W/cm2 for 100–300 s. After irradiation with a 12 W/cm2 laser for 300 s, the temperature of the DCs increased to 62 °C (Fig. 1A). In the mild PT treatment condition, the temperatures of the DCs reached 31 °C and then reached 40 °C. Next, we performed ex vivo PT treatment of the MDSCs isolated from the splenocytes of tumor-bearing mice. Similar temperature increases were observed in MDSCs after ICG treatment and a laser irradiation (Fig. 1B). As the conventional method to induce hyperthermia, cells were treated with WH. It was reported that the patterns of cell death were temperature-dependent29, and cells underwent necrotic death predominantly at 49 °C. HSPs may be maximally induced in cells incubated at 41°C24,30,31,32. Therefore, we set the water temperatures at 41 °C, 49 °C, and the median, 45 °C. The cells were soaked for 60 min, and the cell temperature was increased to a level comparable to that of the water (Fig. 1C,D).
Ex vivo photothermal treatment of DCs and MDSCs resulted in an increase in cell temperature and decrease in cell viability. DCs were differentiated from the bone marrow cells of naïve BALB/c mice. Splenic MDSCs were isolated from Her-2/CT26 tumor-bearing mice. For PT treatment, cells were incubated in the presence of 100 µg/mL of ICG for 90 min and irradiated with an 808-nm laser. The control cells were heated in water at the indicated temperature of water for 60 min. (A-D) Cell temperatures were analyzed immediately after ex vivo PT treatment or heating in a water bath. (E–H) The cell viability test was performed after 1 h of recovery in a 37 °C CO2 incubator. (A and E) PT-treated DCs under the indicated conditions. (B and F) PT-treated MDSCs under the indicated conditions. (C and G) Heated DCs under the indicated conditions. (D and H) Heated MDSCs under the indicated conditions. The representative data are from three independent experiments.
Next, we analyzed the effects of the PT and WH treatments on cell viability. Irradiation with a laser at 12 W/cm2 was fatal to ICG-containing cells, while irradiation with a laser at 6 W/cm2 or 3 W/cm2 was sublethal; approximately 80% of the cells were still alive after the ex vivo PT treatment (Fig. 1E,F). In WH-treated cells, 53% of the DCs and 70% of the MDSCs were viable after heating at 49 °C (Fig. 1G,H). When the DCs or MDSCs were heated in water at 45 °C or 41 °C, almost all cells were viable.
Hyperthermia induced an increase in activation marker levels on DCs
To determine the effects of hyperthermia on immune cells, we analyzed the levels of intracellular HSPs and surface activation markers. The strongest PT treatment (12 W/cm2 laser irradiation for 300 s) resulted in significant reduction of HSP70 induction and upregulation of CD40, CD80, CD86, and IA:IE expression, within 1 h of recovery (Fig. 2A and C). Irradiation with the 6 W/cm2 laser for 300 s and 12 W/cm2 laser for 100 s induced comparable levels of the phenotypic changes in PT-treated DCs. Mild PT treatment also induced a more significant upregulation of HSP70, CD40, and CD86 expression, than that in unirradiated DCs. A similar phenomenon was observed in DCs obtained from C57BL/6 mice (Supplementary Fig. 1).
Phenotypic changes of DCs were induced by hyperthermia. (A–D) DCs were treated with 100 μg/mL of ICG for 90 min. For PT treatment, cells were irradiated with a laser for the indicated period. Intracellular HSP70 and surface molecules of DCs were analyzed after a 1-h (A and C) or 24-h (B and D) recovery in a 37 °C CO2 incubator. Gray fill, unirradiated cells; bold line, PT-treated cells. (C) Data are representative of three independent experiments, and relative mean fluorescence intensities (MFIs) are shown in mean ± standard error (S.E). The relative MFI was calculated from the MFI of the PT-treated cells divided by that of the unirradiated cells. Student’s t-test was performed to assess significant differences between the unirradiated group and PT-treated group. *p < 0.05, **p < 0.01. (D) Data are representative of two independent experiments, and relative MFIs are shown in mean ± standard error (S.E). E–H: DCs were soaked in the indicated water temperature for 1 h. DCs after 1-h recovery (E and G); DCs after 24-h recovery (F and H). Gray fill, incubated cells at 37 °C; bold line, heated cells in water bath. Representative data from two independent experiments are shown. (G) Data are representative of two independent experiments and relative MFIs are shown in mean ± standard error (S.E). (H) Relative MFIs are depicted in a bar graph.
To analyze the effect of hyperthermia on DC maturation, we incubated the PT-treated cells for 24 h (Fig. 2B and D). HSP levels increased after further incubation in the strongest PT-treated cells (a 12 W/cm2 laser irradiation for 300 s). PT treatment using a 6 W/cm2 laser for 300 s or a 12 W/cm2 laser for 100 s resulted in minimal changes in the levels of the DC activation markers CD40, CD80, and CD86. We found that the levels of MHC class II molecules decreased significantly in PT-treated DCs.
Next, we analyzed the effect of hyperthermia originating from an external source, namely, warmed water. DCs were heated under the indicated conditions for 1 h and recovered for 1 h (Fig. 2E and G) or 24 h (Fig. 2F and H). HSP70 and CD40 expression was induced by heating in a temperature-dependent manner within 24 h. After incubating for 24 h, the CD86 and IA:IE levels of heated DCs increased similarly among groups, unlike in untreated DCs. However, the levels of CD40, CD80, CD86, and MHC class II molecules decreased in WH-treated DCs with a 1-h recovery time. Collectively, hyperthermia induced by either the PT or WH treatment affected the activation status of DCs.
Poorly immunogenic phenotype of MDSCs were converted into immunogenic phenotype by PT treatment
MDSCs have a poor immunogenic phenotype, especially compared to DCs. When we performed PT treatment on MDSCs, significant levels of HSP70, CD40, CD80, CD86 and MHC class II molecules were induced within 1 h under the strongest PT-treated cells (a 12 W/cm2 laser irradiation for 300 s). Even under mild PT treatment conditions (6 W/cm2 laser for 300 s), their levels were increased, compared to those of the unirradiated MDSCs. (Fig. 3A and C). In the WH-treated MDSCs, a slight HSP70 induction was detected, and the levels of other activation markers decreased in comparison to those of the untreated cells (Fig. 3B and D). In the MDSCs, PT treatment was an efficient method for immunogenic phenotype conversion, but WH treatment was not.
The poorly immunogenic phenotype of MDSCs was converted into the immunogenic phenotype by PT treatment. CD11b+ MDSCs were isolated from Her-2/CT26 tumor-bearing mice using the MACS system. (A and C) For PT treatment, cells were treated with 100 μg/mL of ICG for 90 min and irradiated with a laser. (B and D) MDSCs were heated in the indicated water temperature. After 1 h of recovery, they were analyzed using flow cytometry. Gray fill, no treated cells; bold line, PT-treated or heated cells. (C) Data are representative of two or three independent experiments and relative MFIs are presented in mean ± standard error (S.E). Differences between the unirradiated MDSCs and PT-treated MDSCs were evaluated using Student’s t-test or Mann–Whitney rank sum test. *p < 0.05, **p < 0.01, ***p < 0.001. (D) Relative MFIs are depicted in a bar graph.
Changes of functional mediators in PT-treated MDSCs
To analyze the immunomodulatory effect of hyperthermia on MDSCs, we detected the expression of the functional mediators, ARG1, iNOS, and NOX2 in hyperthermia-treated MDSCs. The NOX2 gene expression levels were reduced by 30.5-fold through PT treatment, whereas the levels of the other target genes did not decrease (Fig. 4A). The NOX2 gene expression levels were also reduced by 30.1-fold through WH treatment, whereas ARG1 expression was increased by 36% with WH treatment (Fig. 4B). These data suggest that NOX2 gene expression may be critically regulated by heat stress.
PT treatment regulates the expression of the functional mediator of MDSCs. MDSCs were isolated from the spleens of tumor-bearing mice and treated with PT (A) and WH (B). After 24 h of incubation, RNA was extracted from the MDSCs, and the expression of the indicated genes was evaluated by quantitative real-time PCR. The data are representative of three experiments.
Contribution of PT-treated MDSCs in tumor growth
To analyze the in vivo function of PT-treated MDSCs in the tumor environment, we monitored tumor growth in MDSC-transferred tumor-bearing mice (Fig. 5). The strongest PT treatment (a laser irradiation at 12 W/cm2) led to significant cell death; therefore, we applied the mild PT treatment (a laser irradiation at 6 W/cm2) to induce sub-lethal hyperthermic conditions in the MDSCs. Tumor growth was not significantly affected by the PT-treated MDSCs. PT treatment of the MDSCs reduced their protumoral activity in tumor-bearing mice.
The contribution of PT-treated MDSC to tumor growth. MDSCs were isolated from the spleens of tumor-bearing mice and treated with PT. After 1 h of recovery, MDSCs were adoptively transferred via intravenous injection to tumor-bearing mice (n = 3/group) at 14 days after the tumor challenge. Tumor size was monitored every 2 to 3 days. The data are representative of three experiments.
Immunogenicity of heat-stressed MDSCs in inducing CTL responses
Phenotypic changes and downregulation of the suppressive functional mediator, NOX2 may be followed by functional changes in MDSCs. We analyzed the immunogenicity of heat-stressed MDSCs to induce CTL activity in vivo. Administration of PT-treated MDSCs pulsed with Her-2/neu CTL peptide, hP63, efficiently induced CTL response, with 65.7% of hP63-loaded target cells significantly removed in MDSC-transferred mice (Fig. 6A,B). In contrast, poorly immunogenic MDSCs loaded with hP63 induced only 30.7% peptide-specific target lysis. PT-treated MDSC with hP63 activated an antigen(Ag)-specific CTL response significantly. Collectively, hyperthermia-exposed MDSCs were converted into immunogenic APCs, which was sufficient to generate CTL response without additional immunostimulatory agents.
PT-treated MDSCs can function as immunogenic APCs to induce CTL responses. PT-treated MDSCs were pulsed with the CTL epitope peptides. They were then injected into naïve mice. After seven days, CTL epitope peptide-pulsed (CFSEhigh) syngeneic splenocytes and unpulsed (CFSElow) syngeneic splenocytes were intravenously injected into recipient mice. (A) After 24 h, CFSE positive target cells in the spleens of recipient mice were analyzed via flow cytometry. Upper panels: untreated mice; Middle panels: MDSC/hP63 injected mice; Lower panels: PT-MDSC/hP63 injected mice (B) Percentages of antigen specific lysis were shown in bar graph (mean ± standard error). The data are representative of two experiments. ***p < 0.001.
Discussion
The immunomodulatory activity of hyperthermia has been reported in various models. Mild hyperthermia-experienced T cells exhibit enhanced Ag-specific activities33. Mild heating of DCs at 41 °C leads to their maturation34, and the heating of human monocyte-derived DCs at 39 °C leads to distinct gene expression profiles35. In this study, we demonstrated the effect of PT treatment-induced hyperthermia on DCs and MDSCs. This approach could be applied to immune cell-based immunotherapies, such as MDSC vaccines36. We found that PT-treated MDSCs were transformed into immunogenic cells without protumoral activity.
Heat stress leads to the upregulation of various DAMPs, including HSPs, in hyperthermia-experienced cells. In this study, we focused on HSP70. HSP70 is not only a chaperone to protect cells from heat stress, but it is also an immune response enhancer37. Extracellular HSP70 exerts immunostimulatory activity via the Toll-like receptors TLR2 and TLR438. The HSP70 of tumor-derived HSP70-peptide complexes delivers antigens and maturation signals to DCs, and it contributes to Ag-specific T cell priming39,40. Other HSPs, such as HSP27, HSP60, and HSP90, also show various immunological functions in the innate and adaptive immune systems41. In addition to HSPs, there are other DAMPs with relevant immune functions. High mobility group box 1 (HMGB1), a representative DAMP, can be released from necrotic cells, bind to TLR2, and enhance immune responses during vaccination42,43,44,45. Therefore, we cannot exclude the possibility that non-HSP70 DAMPs are critically involved in the immunogenic changes of hyperthermia-experienced cells. As many previous studies demonstrated that the immunological roles of extracellular DAMPs, further studies are needed to identify the mechanism of intracellular/extracellular DAMPs in the functional regulation of immune cells.
When cells are exposed to external heat, such as in WH, they produce low levels of HSP and exhibit a limited immunogenicity, even under optimal heating conditions. In contrast, under the PT treatment, heat stress generated internally from photoabsorber, resulting in maximum HSP expression and the regulation of MDSC immunosuppressive functions.
In the previous study, we established an ICG-based ex vivo PT treatment system for inducing ICD in cancer cells10. ICG is a well-characterized photoabsorbing agent that possesses photothermal conversion abilities46,47 and has been clinically approved as an imaging agent for diagnosis48. It is also a photodynamic agent that generates ROS when irradiated with a laser, leading to cancer cell ablation49. ICG molecules have a strong absorption property of approximately 800–810 nm (near infrared) light, and the absorbed energy is efficiently converted to heat inside the molecule50. In this study, we used an 808-nm laser for the irradiation of ICG in the PT treatment.
Here, we compared the intracellular- and extracellular-originated hyperthermia. Mild PT and WH treatment led to similar increases in cell temperature and comparable cell viabilities after treatment (Fig. 1). However, the PT treatment induced a significant increase in HSP70 and immunogenic surface molecules on MDSCs (Fig. 3). These data suggest that each treatment may be related to a distinct cellular pathway and is not simply a quantitative difference.
We analyzed the effects of hyperthermia on DCs and MDSCs. Cell viability after PT treatment was similar in both cell types. However, the level of HSP70 was slightly increased by mild PT treatment in immunogenic DCs within 1 h, but it was not maintained until 24 h (Fig. 2). After incubating for 96 h, we detected distinct phenotypic changes only in DCs treated with strong PT, but not in DCs treated with mild PT condition (Supplementary Fig. 2). The strong PT treatment is too harsh to analyze cellular responses against hyperthermia; therefore, we did not confirm the functional changes induced by PT treatment in DCs. However, we observed distinct phenotypic changes in the MDSCs under all experimental PT treatment conditions (Fig. 3). Furthermore, immunosuppressive function was eliminated by mild PT treatment of MDSCs. These data suggest that responsiveness to PT treatment may differ depending on cell type. The downregulation patterns of activation marker expression were similar between the DCs and MDSCs after WH treatment.
For successful antitumor immunotherapy, it is important to modulate the immunosuppressive tumor microenvironment. We focused on MDSC as a key player in establishing suppressive environment via various mediators, including ARG1, NOX2, and iNOS51. L-arginine depletion by ARG1 inhibits T cell responses52. Nitric oxide and ROS produced by iNOS and NOX2 in MDSCs contribute to oxidative stress, and suppress T cell activity53. Many attempts have been made to target MDSCs in preclinical and clinical settings54,55,56. Chemotherapy with gemcitabine57 and 5-fluoroiracil58 selectively deplete MDSCs, resulting in augmented antitumor immunity. MDSCs treated with all-trans-retinoic acid differentiate into more mature phenotypes with a reduced immunosuppressive function in patients with cancer59. Furthermore, with the help of natural killer T cells, MDSCs treated with all-trans-retinoic acid become immunogenic cells that prime antitumor T cell responses60. MDSCs can be functionally regulated by several pharmacological drugs, such as the phosphodiesterase-5 inhibitor, tadalafil61, rapamycin62, and celecoxib63, and dietary supplements, such as antioxidants64. Here, we showed that PT treatment can regulate the immunosuppressive function of MDSCs; therefore, it may be applied in cancer immunotherapies to overcome MDSC-mediated immune suppression.
Conclusion
In the present study, we showed that sublethal PT treatment on antigen-presenting cells regulated the immunogenicity. In particular, poorly immunogenic MDSCs were converted into immunogenic APCs through ex vivo PT treatment. Immune modulation induced by physical methods, such as hyperthermia, might offer a novel approach for cancer immunotherapy. We hypothesized that induced DAMPs, such as HSP70, in stressed MDSCs play a role in the improved immunogenicity. Further studies may identify molecular targets involved in regulating the immunogenicity of PT-treated MDSCs. Additionally, agents that increase DAMP levels might have the capacity to modulate the function of MDSCs.
Data availability
All data generated or anlysed during this study are included in this published article (and its supplementary information files).
References
O’Neal, D. P., Hirsch, L. R., Halas, N. J., Payne, J. D. & West, J. L. Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer Lett. 209, 171–176. https://doi.org/10.1016/j.canlet.2004.02.004 (2004).
Jaque, D. et al. Nanoparticles for photothermal therapies. Nanoscale 6, 9494–9530. https://doi.org/10.1039/c4nr00708e (2014).
Xu, L., Cheng, L., Wang, C., Peng, R. & Liu, Z. Conjugated polymers for photothermal therapy of cancer. Polym. Chem. 5, 1573–1580. https://doi.org/10.1039/c3py01196h (2014).
Zou, L. et al. Current approaches of photothermal therapy in treating cancer metastasis with nanotherapeutics. Theranostics 6, 762–772. https://doi.org/10.7150/thno.14988 (2016).
Galluzzi, L. et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 25, 486–541. https://doi.org/10.1038/s41418-017-0012-4 (2018).
Zhou, F., Nordquist, R. E. & Chen, W. R. Photonics immunotherapy—a novel strategy for cancer treatment. J. Innov. Opt. Health Sci. 09, 1630001. https://doi.org/10.1142/s1793545816300019 (2016).
Gabor, A. et al. ITOC2–018. Hyperthermia induced immunogenic cell-death. Eur. J. Cancer https://doi.org/10.1016/j.ejca.2015.01.031 (2015).
Li, X. The inducers of immunogenic cell death for tumor immunotherapy. Tumori J. 104, 1–8. https://doi.org/10.5301/tj.5000675 (2018).
Zhou, J. et al. Immunogenic cell death in cancer therapy: Present and emerging inducers. J. Cell Mol. Med. 23, 4854–4865. https://doi.org/10.1111/jcmm.14356 (2019).
Yu, S. H., Yoon, I. & Kim, Y. J. Ex vivo photothermal treatment-induced immunogenic cell death for anticancer vaccine development. Int. Immunopharmacol. 127, 111450. https://doi.org/10.1016/j.intimp.2023.111450 (2024).
Milani, V. et al. Heat shock protein 70: Role in antigen presentation and immune stimulation. Int. J. Hyperth. 18, 563–575. https://doi.org/10.1080/02656730210166140 (2009).
Lianos, G. D. et al. The role of heat shock proteins in cancer. Cancer Lett. 360, 114–118. https://doi.org/10.1016/j.canlet.2015.02.026 (2015).
Welch, W. J. The role of heat-shock proteins as molecular chaperones. Curr. Opin. Cell Biol. 3, 1033–1038. https://doi.org/10.1016/0955-0674(91)90125-I (1991).
Kuppner, M. C. et al. The role of heat shock protein (hsp70) in dendritic cell maturation: Hsp70 induces the maturation of immature dendritic cells but reduces DC differentiation from monocyte precursors. Eur. J. Immunol. 31, 1602–1609. https://doi.org/10.1002/1521-4141(200105)31:5%3c1602::aid-immu1602%3e3.0.co;2-w (2001).
Somersan, S. et al. Primary Tumor tissue lysates are enriched in heat shock proteins and induce the maturation of human dendritic cells. J. Immunol. 167, 4844–4852. https://doi.org/10.4049/jimmunol.167.9.4844 (2001).
Beere, H. M. `The stress of dying’: the role of heat shock proteins in the regulation of apoptosis. J. Cell Sci. 117, 2641–2651. https://doi.org/10.1242/jcs.01284 (2004).
Schmitt, E., Gehrmann, M., Brunet, M., Multhoff, G. & Garrido, C. Intracellular and extracellular functions of heat shock proteins: Repercussions in cancer therapy. J. Leukocyte Biol. 81, 15–27. https://doi.org/10.1189/jlb.0306167 (2007).
Kusmartsev, S., Nefedova, Y., Yoder, D. & Gabrilovich, D. I. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J. Immunol. 172, 989–999. https://doi.org/10.4049/jimmunol.172.2.989 (2004).
Sinha, P., Clements, V. K., Bunt, S. K., Albelda, S. M. & Ostrand-Rosenberg, S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts Tumor immunity toward a type 2 response. J. Immunol. 179, 977. https://doi.org/10.4049/jimmunol.179.2.977 (2007).
Fleming, V. et al. Targeting myeloid-derived suppressor cells to bypass tumor-induced immunosuppression. Front. Immunol. https://doi.org/10.3389/fimmu.2018.00398 (2018).
Penichet, M. L. et al. In vivo properties of three human HER2/neu-expressing murine cell lines in immunocompetent mice. Lab. Anim. Sci. 49, 179–188 (1999).
Ko, H. J. et al. A combination of chemoimmunotherapies can efficiently break self-tolerance and induce antitumor immunity in a tolerogenic murine tumor model. Cancer Res. 67, 7477–7486. https://doi.org/10.1158/0008-5472.can-06-4639 (2007).
Gao, F., Ye, Y., Zhang, Y. & Yang, J. Water bath hyperthermia reduces stemness of colon cancer cells. Clin. Biochem. 46, 1747–1750. https://doi.org/10.1016/j.clinbiochem.2013.08.023 (2013).
Chen, H. et al. Localization and expression of heat shock protein 70 with rat myocardial cell damage induced by heat stress in vitro and in vivo. Mol. Med. Rep. 11, 2276–2284. https://doi.org/10.3892/mmr.2014.2986 (2015).
Zhao, Y. Y. et al. Microwave hyperthermia promotes caspase-3-dependent apoptosis and induces G2/M checkpoint arrest via the ATM pathway in non-small cell lung cancer cells. Int. J. Oncol. 53, 539–550. https://doi.org/10.3892/ijo.2018.4439 (2018).
Jeong, S. M. & Kim, Y. J. Astaxanthin treatment induces maturation and functional change of myeloid-derived suppressor cells in tumor-bearing mice. Antioxidants (Basel) https://doi.org/10.3390/antiox9040350 (2020).
Nagata, Y. et al. Peptides derived from a wild-type murine proto-oncogene c-erbB-2/HER2/neu can induce CTL and tumor suppression in syngeneic hosts. J. Immunol. 159, 1336–1343 (1997).
Kim, Y. J. et al. alpha-Galactosylceramide-loaded, antigen-expressing B cells prime a wide spectrum of antitumor immunity. Int. J. Cancer 122, 2774–2783. https://doi.org/10.1002/ijc.23444 (2008).
Zhang, Y. et al. Temperature-dependent cell death patterns induced by functionalized gold nanoparticle photothermal therapy in melanoma cells. Sci. Rep. 8, 8720. https://doi.org/10.1038/s41598-018-26978-1 (2018).
Hirayama, E., Atagi, H., Hiraki, A. & Kim, J. Heat shock protein 70 is related to thermal inhibition of nuclear export of the influenza virus ribonucleoprotein complex. J. Virol. 78, 1263–1270. https://doi.org/10.1128/jvi.78.3.1263-1270.2004 (2004).
Oehler, R. et al. Glutamine depletion impairs cellular stress response in human leucocytes. Br. J. Nutr. 87(Suppl 1), S17-21. https://doi.org/10.1079/bjn2001453 (2002).
Oehler, R. et al. Cell type-specific variations in the induction of hsp70 in human leukocytes by feverlike whole body hyperthermia. Cell Stress Chaperones 6, 306–315. https://doi.org/10.1379/1466-1268(2001)006%3c0306:ctsvit%3e2.0.co;2 (2001).
Mace, T. A., Zhong, L., Kokolus, K. M. & Repasky, E. A. Effector CD8+ T cell IFN-γ production and cytotoxicity are enhanced by mild hyperthermia. Int. J. Hyperth. 28, 9–18. https://doi.org/10.3109/02656736.2011.616182 (2012).
Matsumoto, K. et al. Optimization of hyperthermia and dendritic cell immunotherapy for squamous cell carcinoma. Oncol. Rep. 25, 1525–1532. https://doi.org/10.3892/or.2011.1232 (2011).
Liso, A. et al. Human monocyte-derived dendritic cells exposed to hyperthermia show a distinct gene expression profile and selective upregulation of IGFBP6. Oncotarget 8, 60826–60840 (2017).
Ko, H.-J. et al. Immunosuppressive myeloid-derived suppressor cells can be converted into immunogenic APCs with the help of activated NKT cells: An alternative cell-based antitumor vaccine1. J. Immunol. 182, 1818–1828. https://doi.org/10.4049/jimmunol.0802430 (2009).
Ito, A. et al. Heat shock protein 70 expression induces antitumor immunity during intracellular hyperthermia using magnetite nanoparticles. Cancer Immunol. Immunother. 52, 80–88. https://doi.org/10.1007/s00262-002-0335-x (2003).
Hulina, A. et al. Extracellular Hsp70 induces inflammation and modulates LPS/LTA-stimulated inflammatory response in THP-1 cells. Cell Stress Chaperones 23, 373–384. https://doi.org/10.1007/s12192-017-0847-0 (2018).
Binder, R. J., Blachere, N. E. & Srivastava, P. K. Heat shock protein-chaperoned peptides but not free peptides introduced into the cytosol are presented efficiently by major histocompatibility complex I molecules. J. Biol. Chem. 276, 17163–17171. https://doi.org/10.1074/jbc.M011547200 (2001).
Milani, V. et al. Heat shock protein 70: role in antigen presentation and immune stimulation. Int. J. Hyperth. 18, 563–575. https://doi.org/10.1080/02656730210166140 (2002).
Singh-Jasuja, H., Hilf, N., Arnold-Schild, D. & Schild, H. The role of heat shock proteins and their receptors in the activation of the immune system. Biol. Chem. 382, 629–636. https://doi.org/10.1515/bc.2001.074 (2001).
Kang, R., Zhang, Q., Zeh, H. J. 3rd., Lotze, M. T. & Tang, D. HMGB1 in cancer: good, bad, or both?. Clin. Cancer Res. 19, 4046–4057. https://doi.org/10.1158/1078-0432.ccr-13-0495 (2013).
Roh, J. S. & Sohn, D. H. Damage-associated molecular patterns in inflammatory diseases. Immune Netw. 18, e27–e27. https://doi.org/10.4110/in.2018.18.e27 (2018).
Sims, G. P., Rowe, D. C., Rietdijk, S. T., Herbst, R. & Coyle, A. J. HMGB1 and RAGE in inflammation and cancer. Annu. Rev. Immunol. 28, 367–388. https://doi.org/10.1146/annurev.immunol.021908.132603 (2010).
Fagone, P. et al. Molecular adjuvant HMGB1 enhances anti-influenza immunity during DNA vaccination. Gene Ther. 18, 1070–1077. https://doi.org/10.1038/gt.2011.59 (2011).
Colombo, L. L., Vanzulli, S. I., Blázquez-Castro, A., Terrero, C. S. & Stockert, J. C. Photothermal effect by 808-nm laser irradiation of melanin: a proof-of-concept study of photothermal therapy using B16–F10 melanotic melanoma growing in BALB/c mice. Biomed. Opt. Express 10, 2932. https://doi.org/10.1364/boe.10.002932 (2019).
Chen, W. R. Photothermal effects on murine mammary Tumors using indocyanine green and an 808-nm diode laser an in vivo efficacy study. Cancer Lett. 98(2), 169–173 (1995).
Carr, J. A. et al. Shortwave infrared fluorescence imaging with the clinically approved near-infrared dye indocyanine green. Proc. Natl. Acad. Sci. USA 115, 4465–4470. https://doi.org/10.1073/pnas.1718917115 (2018).
Liu, B., Li, C., Xing, B., Yang, P. & Lin, J. Multifunctional UCNPs@PDA-ICG nanocomposites for upconversion imaging and combined photothermal/photodynamic therapy with enhanced antitumor efficacy. J. Mater. Chem. B 4, 4884–4894. https://doi.org/10.1039/c6tb00799f (2016).
Shafirstein, G. et al. Indocyanine green enhanced near-infrared laser treatment of murine mammary carcinoma. Int. J. Cancer 130, 1208–1215. https://doi.org/10.1002/ijc.26126 (2012).
Parker, K. H., Beury, D. W. & Ostrand-Rosenberg, S. Myeloid-derived suppressor cells: critical cells driving immune suppression in the Tumor microenvironment. Adv. Cancer Res. 128, 95–139. https://doi.org/10.1016/bs.acr.2015.04.002 (2015).
Gabrilovich, D. I., Ostrand-Rosenberg, S. & Bronte, V. Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 12, 253–268. https://doi.org/10.1038/nri3175nri3175[pii] (2012).
Corzo, C. A. et al. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J. Immunol. 182, 5693–5701. https://doi.org/10.4049/jimmunol.0900092182/9/5693[pii] (2009).
Gabrilovich, D. I. Myeloid-derived suppressor cells. Cancer Immunol. Res. 5, 3–8. https://doi.org/10.1158/2326-6066.cir-16-0297 (2017).
Farshidpour, M., Ahmed, M., Junna, S. & Merchant, J. L. Myeloid-derived suppressor cells in gastrointestinal cancers: A systemic review. World J. Gastrointest Oncol. 13, 1–11. https://doi.org/10.4251/wjgo.v13.i1.1 (2021).
Chaib, M., Chauhan, S. C. & Makowski, L. Friend or Foe? Recent strategies to target myeloid cells in cancer. Front. Cell Dev. Biol. 8, 351. https://doi.org/10.3389/fcell.2020.00351 (2020).
Suzuki, E., Kapoor, V., Jassar, A. S., Kaiser, L. R. & Albelda, S. M. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin. Cancer Res. 11, 6713–6721. https://doi.org/10.1158/1078-0432.ccr-05-0883 (2005).
Vincent, J. et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 70, 3052–3061. https://doi.org/10.1158/0008-5472.can-09-3690 (2010).
Mirza, N. et al. All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Res. 66, 9299–9307. https://doi.org/10.1158/0008-5472.can-06-1690 (2006).
Lee, J. M. et al. The restoration of myeloid-derived suppressor cells as functional antigen-presenting cells by NKT cell help and all-trans-retinoic acid treatment. Int. J. Cancer 131, 741–751. https://doi.org/10.1002/ijc.26411 (2012).
Weed, D. T. et al. Tadalafil reduces myeloid-derived suppressor cells and regulatory T cells and promotes tumor immunity in patients with head and neck squamous cell carcinoma. Clin. Cancer Res. 21, 39–48. https://doi.org/10.1158/1078-0432.ccr-14-1711 (2015).
Kim, Y. S. et al. Functional changes in myeloid-derived suppressor cells (MDSCs) during tumor growth: FKBP51 contributes to the regulation of the immunosuppressive function of MDSCs. J. Immunol. 188, 4226–4234. https://doi.org/10.4049/jimmunol.1103040 (2012).
Veltman, J. D. et al. COX-2 inhibition improves immunotherapy and is associated with decreased numbers of myeloid-derived suppressor cells in mesothelioma Celecoxib influences MDSC function. BMC Cancer 10, 464. https://doi.org/10.1186/1471-2407-10-464 (2010).
Jeong, S. M. & Kim, Y.-J. Astaxanthin treatment induces maturation and functional change of myeloid-derived suppressor cells in Tumor-bearing mice. Antioxidants 9(4), 350. https://doi.org/10.3390/antiox9040350 (2020).
Acknowledgements
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (Ministry of Science, ICT & Future Planning) (NRF2017R1D1A3B03029445 and NRF-2022R1I1A3053738).
Author information
Authors and Affiliations
Contributions
Conceptualization, Y-J. K. and M-S. L.; Investigation, M-S. L. and S M. P; Resources, Y-J. K; Writing—Original Draft Preparation, M-S. L.; Writing—Review & Editing, Y-J. K.; Project Administration, Y-J. K.; Funding Acquisition, Y-J. K.
Corresponding author
Ethics declarations
Competing interests
Yeon-Jeong Kim has a patent on ex vivo photothermal treated cells for immunotherapy. Min-Seob Lee and Seon Mi Park declare no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lee, MS., Park, S.M. & Kim, YJ. Photothermal treatment-based heat stress regulates function of myeloid-derived suppressor cells. Sci Rep 14, 18847 (2024). https://doi.org/10.1038/s41598-024-69074-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-69074-3
- Springer Nature Limited