Abstract
This study aimed to estimate the prevalence of asymptomatic and subpatent P. falciparum infections in the city of Bouaké, Central Côte d’Ivoire, to compare the performance of three tests, and to investigate potential P. falciparum histidine-rich protein 2 (pfhrp2) gene deletions. A cross-sectional survey was conducted in nine neighborhoods in Bouaké in 2016. Matched light microscopy (LM), rapid diagnostic test (RDT), and quantitative PCR (qPCR) data were used to determine the prevalence of P. falciparum infection and compare the performance of the three diagnostic tests. Pfhrp2/3 deletions were genotyped by digital PCR. Among 2313 individuals, 97.2% were asymptomatic and 2.8% were symptomatic. P. falciparum prevalence among symptomatic individuals was 25.8%, 30.3%, and 40.9% by LM, RDT, and varATS qPCR, respectively, and among asymptomatic individuals, it was 10.3%, 12.5%, and 34.9%. Asymptomatic infections comprised 96.4% of all malaria infections, with 58.2% detectable only by varATS qPCR. Although the prevalence of asymptomatic P. falciparum infections was higher in school-age children (5–14 years: 42.0%) compared to < 5 years (17.3%) and ≥ 15 years (35.9%), subpatent infections were more likely in ≥ 15 years (70.4%) than in < 5 years (39.7%) and school-age children (41.2%). LM and RDTs were reliable only at parasite densities > 10,000 parasites/µL. Individuals who were positive according to all three tests had significantly greater parasite density (856.8 parasites/µL; 95% CI 707.3–1,038) than did those who were positive by varATS qPCR only (13.7 parasites/µL; 95% CI 11.4–16.3) (p < 0.0001). No pfhrp2 deletions were observed. The high prevalence of asymptomatic and subpatent infections highlights the need for targeted strategies to reduce malaria in urban Côte d’Ivoire.
Similar content being viewed by others
Introduction
The overall number of malaria infections is undeniably much greater than the estimated 249 million clinical cases in 20221 owing to the large proportion of asymptomatic infections among people living in endemic areas2. Asymptomatic (or subclinical) malaria infection refers to malaria infection of any parasite density in the absence of fever or other acute symptoms in individuals who have not received recent antimalarial treatment3. While some asymptomatic infections may have parasite density levels that are detectable by light microscopy (LM) or rapid diagnostic tests (RDTs), others can be detected only by molecular methods and are termed subpatent infections4. Asymptomatic individuals remain untreated in countries where control focuses on clinical malaria cases and serve as reservoirs for onward transmission5. Quantifying the extent of subpatent infections and the underlying true prevalence of parasites in different settings is central to understanding the dynamics of malaria transmission and adapting control measures accordingly.
Côte d'Ivoire is one of the five countries accounting for more than 80% of estimated cases of malaria in West Africa, with Nigeria, Burkina Faso, Niger, and Mali1. The incidence of the disease has been constantly increasing, rising from 155 in 2016 to 230‰ in 2021 in the general population and from 287 to more than 580‰ among children under 5 years old during the same period6. Plasmodium (P.) falciparum is the predominant Plasmodium species, accounting for more than 95% of all malaria cases. P. malariae, P. ovale, and more rarely, P. vivax, account for less than 5% of all confirmed malaria cases. The main malaria vector incriminated in Cote d’Ivoire is Anopheles (An.) s.l. followed by An. funestus s.l. and An. nili s.l.7. Malaria transmission occurs throughout the year, with a peak during the rainy season. The social and economic burden of malaria is considerable for households; the direct costs associated with malaria represent, on average, 12 to 14% of the income of households8. A total of 4.9 million cases and 1400 deaths were reported due to malaria by the National Malaria Control Program (NMCP) in 2020.
Although malaria is traditionally more prevalent in rural compared to urban areas, urban malaria represents a serious threat owing to the growing number of people living in urban areas9. In Côte d’Ivoire, 51.2% of the population currently lives in urban areas10. Like in many cities in sub-Saharan Africa, the rapid and unplanned urbanization observed in Côte d'Ivoire, the colonization of lowlands for housing and agriculture11 contribute to creating environments conducive to vector breeding and sustained malaria transmission11. Entomological inoculation rates (EIR) ranging from 0 to more than 400 infectious bites per human per year have been reported in several cities in Côte d’Ivoire7,12. This suggests the existence of reservoirs of the malaria parasite which maintain parasite transmission13.
The NMCP is committed to reducing malaria incidence and mortality rates by at least 75% by 203014. To achieve this goal, detection of symptomatic infections and promptly treating them with Artemisinin-based Combination Therapies (ACTs) is very important for effective malaria control and prevention programs15. This strategy typically uses rapid diagnostic tests (RDTs), the only diagnostic method that can be easily deployed in field settings as they do not require expertise or specialized instrumentation. However, the sensitivity of RDTs is limited. Compared with highly sensitive PCR, RDT often misses more than half of all infections16. The most sensitive RDTs for P. falciparum detect the histidine-rich proteins 2 and 3 (pfhrp2/3). Deletions of the pfhrp2/3 genes result in false-negative RDT results, even if the parasite density is high. Surveillance of pfhrp2/3 deletions in Côte d’Ivoire is then required, as the neighboring Ghana has reported parasites carrying pfhrp2/3 deletions17. In addition to reducing symptomatic infections, tackling the transmission of malaria from asymptomatic infections will be critical for a longer-lasting and significant impact. Asymptomatic malaria is recognized as an obstacle to malaria control and elimination. Despite some studies on asymptomatic infections in the Northern18 and Southern19,20 cities of Côte d’Ivoire, there is no study in urban Bouaké evaluating the accuracy of malaria diagnostic methods and only one documented study assessing malaria infection in children < 15 years, using LM21. Therefore, it is crucial to understand the present prevalence of both asymptomatic and subpatent malaria infection in the general population as well as the risk factors in the communities in order to scale up targeted intervention programs. The objectives of this study were to determine the prevalence of asymptomatic and subpatent P. falciparum infections by LM, RDT, and qPCR in the highly endemic city of Bouaké, to compare the performance of the three tests, and to investigate potential pfhrp2/3 gene deletions.
Results
Study population demography
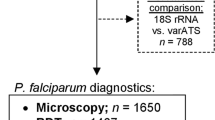
Blood samples were collected from 2725 participants during a cross-sectional field malaria survey performed in nine neighborhoods of the city of Bouaké. While LM data were available for all study participants, RDT data were not available for some participants (n = 278) either by errors in the electronic data entry method, or by failure to carry out the RDT assay, especially in younger children. Thirty (n = 30) blood samples were lost in transit to the United States for molecular analyses. Matched LM, RDT, and varATS qPCR data were available for 2313 individuals (Fig. 1) and used for the determination of the prevalence of P. falciparum infections and the comparison of diagnostic methods. The median age was 18 years (interquartile range: 8–33). Females accounted for 57.2% (1324/2313) of the study population. The majority of individuals (1263/2090, 60.4%) reported using an LLIN on a daily basis within the last 15 days, which varied between neighborhoods.
Symptomatic and asymptomatic P. falciparum infections
The mean body temperature was 37.1 (35.9–38.4) and fever is considered at body temperature > 37.5 °C. From the total 2313 study participants, 2247 (97.2%) individuals were asymptomatic, i.e. did not report any fever or history of fever within 48 h prior to blood sampling, and 66 (2.8%) individuals were symptomatic. The overall prevalence of P. falciparum infections diagnosed by LM, RDT, and varATS qPCR varied significantly and was 10.8% (249/2313), 13% (300/2313), and 35.1% (812/2313), respectively (Cochran’s Q = 823.9, DF = 2, p < 0.001). The prevalence of P. falciparum infections among symptomatic individuals was 25.8% (17/66), 30.3% (20/66), and 40.9% (27/66) by LM, RDT, and varATS qPCR, respectively. Symptomatic school-aged children (5–14 years) were more likely to present P. falciparum infections than younger (0–4 years) and older (≥ 15 years) individuals (all p < 0.05), irrespective of the diagnostic method (Table 1). The prevalence of P. falciparum infections seemed higher in symptomatic males than females for RDT and varATS qPCR (STAT) and individuals who reported occasionally or not using bed nets as compared to those using bed nets on a daily basis, but the differences were not significant (all p > 0.05).
The prevalence of asymptomatic P. falciparum infections was 10.3% (232/2247) by LM, 12.5% (280/2247) by RDT, and 34.9 (785/2247) by varATS qPCR. Asymptomatic P. falciparum infections represented the vast majority (96.4%; 832/863) of malaria infections in the study population. As seen for symptomatic, school-age children (5–14 years) were also more likely to present asymptomatic P. falciparum infections than < 5 and ≥ 15 year-old individuals (all p < 0.001), irrespective of the diagnostic method (Table 1). Although the prevalence of asymptomatic P. falciparum infections tended to be similar or lower in individuals aged ≥ 15 years than in those aged < 5 years by LM and RDT, it was twofold higher in individuals aged ≥ 15 years than in those aged < 5 years by qPCR. No difference in asymptomatic P. falciparum infections was observed by gender or bed net use.
Considering only the asymptomatic study population (n = 2247), the prevalence of varATS qPCR-detectable P. falciparum infections was highly heterogeneous across the city of Bouaké and within health districts (Fig. 2, Supplementary File Table S1). It varied from 15.1% in Sokoura to 55.2% in Attienkro within the health district of Northwestern Bouaké, and from 21.4% in Dar es Salam to 49.2% in Djézoukouamékro, within the health district of Northeastern-Bouaké. The prevalence in the health district of Southern-Bouaké was quite similar between the neighborhoods of Kennedy, Air France, and Ngouatanoukro (Fig. 2, Supplementary File Table S1).
Distribution of asymptomatic P. falciparum infections by neighborhood in the city of Bouaké, August 2016, as determined by varATS qPCR. Prevalence of asymptomatic P. falciparum infections was also reported by neighborhood using LM and RDT results. QGIS software (version 3.16) was used to carry out the mapping. Database was imported into this software to represent the data to be mapped in a Pie chart. Then, the map obtained was imported into the computer graphics software Adobe Illustrator CS6 to refine the cartographic representation.
Specificity, sensitivity and performance of LM and RDT
Compared to the highly sensitive qPCR, the sensitivity of RDT and LM in detecting asymptomatic P. falciparum infections was low at 33.25% (95% CI 29.96–36.67) and 23.57% (95% CI 20.64–26.70), respectively (Table 2). However, both methods were highly specific: 98.70% (95% CI 97.98–99.22) for RDT and 96.79% (95% CI 95.75–97.63) for LM. The measurement of agreement (kappa) was high between RDT and microscopy (60.6%, p < 0.001).
Parasite density, as determined by varATS qPCR, was analyzed according to LM and RDT to see how it impacted diagnostic results. The overall parasite density, expressed as the geometric mean parasite density, was 40 parasites/µL (95% CI 33.7–47.5; Fig. 3A) and varied significantly by age group and was lower in ≥ 15-year-olds (27.2 parasites/µL; 95% CI 22.2–33.5) than in < 5-year-olds (73.5 parasites/µL; 95% CI 36.9–146.2; p = 0.004) and 5–14-year-olds (76.5 parasites/µL; 95% CI 55.9–104.7; p < 0.001; Fig. 3B). Parasite density strongly influenced the test performance of LM and RDT for diagnosing asymptomatic P. falciparum infections. Individuals who were positive according to all three tests had a significantly greater parasite density (856.8 parasites/µL; 95% CI 707.3–1,038) than did those who were positive by varATS qPCR only (13.7 parasites/µL; 95% CI 11.4–16.3) (p < 0.0001) (Fig. 3A). When the proportion of positive samples by varATS qPCR detected by LM and RDT was analyzed according to parasite density, the sensitivity of both methods increased with parasite density (Fig. 3C). LM and RDT did not reliably detect P. falciparum infections at a density below 100 parasites/µL (both methods had a sensitivity < 20%). However, RDT outperformed LM for densities ranging between 100 and 1000 parasites/µL (p < 0.0001, McNemar’s exact test), with 58.3% sensitivity compared to 39.6% for LM. Both methods achieved more than 80% sensitivity for densities between 1000 and 10,000 parasites/µL and reached 100% for densities above 10,000 parasites/µL (Fig. 3C). Precise sensitivity ratings for these methods should, however, be evaluated cautiously due to the small number of LM and RDT positive samples.
P. falciparum density (as determined by varATS qPCR) and diagnostic sensitivity of LM and RDT in asymptomatic individuals. (A) P. falciparum density in individuals tested positive (+) or negative (−) by microscopy and/or RDT. (B) P. falciparum density by age group (0–4, 5–14, and ≥ 15 years). Dots represent the parasite density of each individual. The red bars indicate geometric means with 95% confidence intervals. P values from Dunn’s test with Bonferroni adjustment for multiple pairwise comparisons are given. (C) Diagnostic performance of LM and RDT calculated in varATS qPCR positive individuals and divided by parasite density (0.1–1, 1–10, 10–100, 100–1000, 1000–10,000, and > 10,000 parasites/µL). The average parasite density found within each fold range is displayed on the graph.
Prevalence and risk factors for subpatent infections
Subpatent infections (P. falciparum infections only detectable by varATS qPCR) represented the majority of asymptomatic P. falciparum infections (484/832, 58.2%). Age group and neighborhood of residency were associated with an increased risk of subpatent malaria infections (Table 3). The proportions of subpatent infections were comparable between school-aged (5–14 years) and younger (0–4 years) children (41.2% vs. 39.7%, p > 0.05). In individuals aged ≥ 15 years, most P. falciparum infections were subpatent (70.4%). The proportions of subpatent infections also varied significantly according to the neighborhood of residence (specifically for Dar es Salam) (aOR: 3.97; 95% CI 1.91–8.27; p < 0.001). However, gender and bed net use did not correlate with the prevalence of subpatent P. falciparum infections (all p > 0.05) (Table 3).
Plasmodium falciparum hrp2/3 gene deletion genotyping
A total of 348 varATS qPCR positive blood samples with a density > 5 parasites/µL were randomly selected for pfhrp2 and pfhrp3 genotyping, of which 344 samples were successfully genotyped for pfhrp2 and pfhrp3 gene deletions. No pfhrp2 deletion was observed. However, two samples carried pfhrp3 deletion/wild-type mixed infections. The first was from an 8-year-old asymptomatic male living in the neighborhood of Air France (health district of Southern-Bouaké), who was negative by RDT but positive by LM and qPCR, with a parasite density of 149 parasites/µL. The second was from a 9-year-old asymptomatic female from the neighborhood of Ngattakro (health district of Northeastern Bouaké) and was also positive by LM and qPCR only, with a parasite density of 190 parasites/µL.
Discussion
This study provides a comprehensive assessment of the prevalence of asymptomatic and subpatent P. falciparum infections in Bouaké, Central Côte d’Ivoire. Asymptomatic P. falciparum infections represented the vast majority of infections, and school-age children had the highest prevalence of malaria infections. A substantial portion of these asymptomatic P. falciparum infections were subpatent (only detected by varATS qPCR) and were more frequent in individuals ≥ 15 years old. Asymptomatic and subpatent infections also varied significantly according to neighborhoods. Light microscopy (LM) and malaria rapid diagnostic tests (RDTs) were reliable at diagnosing asymptomatic infection only at high parasite densities. These results underscore the significant burden of these hidden infections and highlight the need for improved diagnostic strategies and targeted interventions in urban settings.
Our findings revealed that the prevalence of symptomatic P. falciparum infections was 25.8%, 30.3%, and 40.9% by LM, RDT, and varATS qPCR, respectively. Different symptomatic malaria prevalences have been reported in Côte d’Ivoire. In Bouaké, a previous study reported a high prevalence of symptomatic malaria infections (70%) by LM21. Another study in the urban areas of Adzopé (southern Côte d’Ivoire) has reported a lower prevalence of symptomatic malaria (16%) during the rainy season19. This difference might be due to variations in study design, setting, or vector control use. For example, the previous studies conducted in Bouaké and Adzopé only involved children under 14 years old. In addition, LLIN use in Bouaké was lower (54%) compared to our study, where at least 60% used their LLINs on a daily basis. Other factors, such as travel to rural areas, and agricultural practices in the neighborhood of residence, may explain the difference in symptomatic malaria prevalence between urban areas. Moreover, outside Côte d’Ivoire, much higher prevalence rates have been reported by LM, RDTs, or qPCR in Tanzania (64.5–89%)22 and Ethiopia (44.5–48.4%)23 The characteristics of the research population may have differed in Tanzania, where children aged 5 to 14 years old are at a higher risk of developing symptoms of malaria. Other factors that may have contributed to this variance include the epidemiology of malaria in the two countries.
The present study showed that the prevalence of asymptomatic P. falciparum infections was 10.3% by LM, 12.5% by RDT, and 34.9 by varATS qPCR. The prevalence reported here is much lower than the approximately 80 and 60% microscopy-positive rates reported in the cities of Bouaké21 and Adzopé (southern Côte d’Ivoire)19, respectively. However, prevalence rates lower (4.0, 5.2, and 18.8% by RDT, LM, and loop‑mediated isothermal amplification, respectively)24 or similar (10.5% and 9.3% by LM and RDT, respectively)18 to the present study have been reported in Korhogo, Northern Côte d’Ivoire. There have also been reports of greater rates of asymptomatic malaria infections in other countries, such as Ethiopia (4.2%)25 and Ghana (27%)26. Given that the endemicity of malaria is high in the study area and its population is constantly exposed to the disease, this difference may be explained by geographic differences between study areas. The population may have developed immunity, resulting in asymptomatic carriage of the parasite3. The implementation of malaria control and preventive strategies in Bouaké could also explain the sharp decrease in malaria prevalence between 2014 and 2016. Indeed, intensive malaria control efforts have been undertaken by the NMCP since the end of the crisis in Bouaké that occurred from 2000 to 2011. Two rounds of large-scale distribution of LLINs were performed in 2011 and 2014, leading to coverage and usage rates of 95% and 68%, respectively27. In addition, the wide availability of RDTs and ACTs for testing and treating symptomatic individuals, respectively, may have helped decrease the burden of malaria in Bouaké. Additional potential causes might include variations in research design, study population, sample size, and study period28.
The prevalence of asymptomatic P. falciparum infections (17.6, 22.9, and 42% by LM, RDT, and qPCR, respectively) in school-aged children (5–14 years) reported in our study was comparable to the prevalence rate reported in Korhogo (16% by LM)18. However, the prevalence rates reported in our study were higher than those reported in Malawi (31%)29 and Kenya (34%)30. The high number of malaria infections detected in school-aged children raises concern because they serve as a source of onward parasite transmission31, as this age group has been shown to be the least likely to benefit from universal malaria interventions such as bed nets and access to prompt diagnosis and treatment32,33. The implementation of strategies that specifically target this age group could help further decrease malaria transmission in this highly endemic area and improve the health of schoolchildren34,35. The proportion of subpatent infections was higher in individuals older than 15 years of age (42%). This is in line with a previous study in Kenya, where more than 70% of subpatent infections occurred in adults30. These findings align with the current theory that, in areas with stable transmission, older individuals will have sufficient immunity to tolerate infections and maintain parasite densities below the limit of detection of RDTs28.
The study also highlights the limitations of LM and RDTs in detecting low-density malaria infections. Both methods were comparable to qPCR only when parasite density exceeded 10,000 parasites/µL, but their accuracy decreased at lower parasite densities. Similar findings have been reported in The Gambia36 and Ghana37 where the sensitivity of RDTs and LM increased with increased parasite density. Although RDT outperformed LM for detecting P. falciparum infections with parasitemia < 1000 parasites/µL, it missed substantial low-density infections. Highly sensitive RDTs38 or loop-mediated isothermal amplification (LAMP)24 could be promising options for improving the detection of asymptomatic and subpatent infections in areas where they are common. In Northern Côte d’Ivoire, for example, LAMP has been shown to be a relevant field diagnostic alternative to standard RDTs for identifying asymptomatic infections, with a sensitivity and specificity of 93.3% (95% CI 85.7–100) and 95.4% (95% CI 92.2–100), respectively24.
The prevalence of asymptomatic malaria infection was highly heterogeneous across neighborhoods, ranging from 15% to more than 55%. Similarly, the prevalence of subpatent infections also varied across neighborhoods. These findings are supported by studies in Yemen39 and Kenya40. The ecological environment and Anopheles density variability between neighborhoods could be considerable factors that explain this variation. Bouaké is characterized by the presence of lowlands used for agricultural activities, depending on the neighborhood41. This agricultural activity has been shown to have a significant impact on vector diversity and density12 and consequently on malaria transmission11, which is highly heterogeneous across neighborhoods. However, there are still unanswered questions regarding the other factors that may contribute to the variation in P. falciparum asymptomatic carriage in our study area. These factors could include variations in the distance to the closest mosquito breeding site42, features of household structure, usage of protective measures, and human behavior that could also lead to differential attractiveness to mosquitoes.
For the first time, in Côte d’Ivoire, parasites were typed for pfhrp2/3 gene deletions. While no pfhrp2 deletions were detected in the study parasite population, two samples carried pfhrp3/wild-type mixed infection. Pfhrp3 deletion is not a threat for HRP2-based RDTs as long as pfhrp2 is present43. Thus, the use of HRP2-based RDTs for the diagnosis of P. falciparum is still recommended. However, future monitoring of pfhrp2 deletion is warranted owing to the circulation of parasites carrying pfhrp2 in the neighboring country, Ghana17.
The high prevalence of asymptomatic and subpatent infections underscores the critical need for strategies targeting these silent reservoirs of infection. Mass screening and treatment, improved diagnostic tools, and community-based interventions could significantly reduce malaria transmission. Given the heterogeneous distribution of infections within the city, localized interventions tailored to specific high-risk areas and populations are recommended.
Conclusions
The prevalence of P. falciparum infection was underestimated when microscopy and RDT were used for diagnosis because a substantial proportion of asymptomatic and subpatent malaria carriers were present in the population living in the city of Bouaké and could be diagnosed using only the ultrasensitive PCR diagnostic method (varATS qPCR). The prevalence of malaria infection was highly heterogeneous across the city, and older children and adults constitute the reservoirs of the parasite. These hidden parasite reservoirs are a major challenge for malaria control programs in urban areas. Various strategies, such as intermittent preventive treatment, periodic mass parasite screening and treatment, or mass drug administration, could be suggested to further reduce the burden of malaria in this city. However, before that, additional follow-up cohort studies will be needed to identify the role of asymptomatic cases in the dynamics of malaria transmission, its seasonal variability, and its contribution to the incidence of malaria in the region and to provide support in planning an effective and appropriate malaria elimination strategy.
Materials and methods
Study area and demographic data collection
A cross-sectional study was performed in August 2016 in nine neighborhoods of the city (three neighborhoods per health district): Dar-es-Salam, Djezoukouamekro, and N’gattakro in Northwestern-Bouaké; Sokoura, Belle-ville, and Attienkro in Northeastern-Bouaké; and Kennedy, Air-France, and N’gouattanoukro in Southern-Bouaké. Located in central Côte d’Ivoire, Bouaké is the second most populous city in the country, with more than 750,000 inhabitants in 20156. Like in many cities in sub-Saharan Africa, urban agriculture is widespread within and around the city to increase food security. This agricultural activity has been shown to significantly affect malaria transmission through the creation of numerous larval habitats and vector proliferation. This area has year-round malaria transmission, which peaks during the wet season (April–October). P. falciparum is the dominant species identified in more than 95% of malaria cases21. Malaria is highly endemic in the city with > 160,000 malaria cases recorded each year (annual incidence > 200 per 1000 inhabitants) and more than 100 deaths 20. Bouaké suffered from an armed conflict and a sociopolitical crisis from 2000 to 2011, leading to large population moves, environmental modifications, and interruptions in the implementation and/or maintenance of malaria control strategies for the whole period 21. Following the crisis, the population reinvested in the city and resettled in formerly abandoned neighborhoods.
This study was part of a large operational research project named PALEVALUT (http://www.mesamalaria.org/mesa-track/palevalut), which aimed to evaluate the effectiveness of malaria control resources currently deployed and identify the barriers to their effectiveness in five different sub-Saharan countries (Benin, Cameroon, Côte d’Ivoire, Niger, and Madagascar). The study design has already been published44. Briefly, 50 households were randomly selected from each neighborhood, and all family members were invited to participate in the study. After enrollment, all participating households were geo-located using GPS instruments (Garmin, Switzerland) with an accuracy of up to 5 m. A sociodemographic questionnaire was completed by the head of the family to collect information about the sanitary conditions of the household and household characteristics (house type and construction materials, bathroom type, access to public services, etc.). Information on age, sex, history of fever, and LLIN use was recorded for each participant.
Case definitions
An asymptomatic malaria infection was defined as a P. falciparum-positive test result in an afebrile individual (temperature ≤ 37.5 °C) at the time of sampling with no history of fever in the previous 48 h. A clinical malaria case was defined as a positive test result with a history of fever or temperature > 37.5 °C. Subpatent infections were defined as infections that were only positive by quantitative Polymerase Chain Reaction (qPCR).
Blood sample collection
During the house visit, blood samples were collected from each study participant using a 1 mL insulin syringe (Luer-Lok™, BD, Franklin Lakes, NJ, USA) to prepare thick and thin blood smears (both thick and thin smears were collected on the same slide), one RDT, and two spots on Whatman qualitative filter paper N°3. The remaining blood was poured into a 1.5-mL microcentrifuge tube. In the laboratory, the microcentrifuge tubes were centrifuged, and the plasma was removed and stored at − 20 °C. The red cell pellets were stored at − 20 °C until DNA extraction. All samples from each study participant were labeled with a unique code and stored until use.
RDT-based diagnosis and treatment
HRP2-based RDTs (Standard Diagnostics, Inc.; Yongin, Republic of Korea) recommended by the NMCP of Côte d’Ivoire were used in this study. These RDTs are based on HRP2 detection and are specific to P. falciparum. All febrile individuals who tested positive for RDT were treated with antimalarial drugs according to the national drug policy. Those individuals found to be positive by microscopy or qPCR were not treated because microscopic examination and qPCR assays were not performed at the time of collection.
Microscopic examination
Giemsa-stained peripheral blood smears were examined by trained technicians under a compound microscope (Olympus CX21 LED) at 1000× magnification to detect malaria parasites. The ring, trophozoite, and gametocyte stages were identified and assessed for parasite density (parasites/µL blood) against 200 white blood cells (WBCs), and densities were calculated considering an average of 8000 WBCs/µL.
All positive slides and 10% of the randomly chosen negative blood slides were cross-examined by independent laboratory personnel as part of a standard protocol to monitor the examination quality. The microscopists who examined the participants' blood slides were unaware of the RDT results.
DNA extraction and qPCR detection of P. falciparum infections
DNA was extracted from 100 μl of blood using a Genomic DNA Extraction Kit (Macherey–Nagel, Düren, Germany) and eluted in 100 µl of elution buffer. Four (4) microliters of extracted DNA was screened for P. falciparum using ultrasensitive qPCR, which amplifies a conserved region of the var gene Acidic Terminal Sequence (varATS) according to a previously published protocol45. The qPCR results were converted to parasites/μL using an external standard curve of tenfold serial dilutions (5 steps) of 3D7 P. falciparum parasites quantified by digital Polymerase Chain Reaction (dPCR).
Pfhrp2/3 genotyping by digital PCR
Plasmodium falciparum-positive samples at a density of approximately > 5 parasites/μL according to varATS qPCR were typed for pfhrp2/3 deletions by dPCR. To this end, the assay was successfully transferred from the Bio-Rad QX 200 dPCR system to the Qiagen QIAcuity dPCR system. The assay conditions were maintained as previously published46. In this assay, pfhrp2/3 and a control gene (serine-tRNA ligase) were quantified in a single tube with very high specificity, thus providing highly accurate data on the deletion status. The assay amplified parts of pfhrp2 exon 2, which encodes the antigen detected by the RDT.
Statistical analysis
Field data were collected and stored using the Open Data Kit toolkit. The electronic data were exported to MS Excel for analysis. The data were summarized using descriptive statistics.
The prevalence of malaria was calculated for each diagnostic test (light microscopy and RDT) and compared to that of the gold standard, varATS qPCR, using McNemar’s or Cochran’s test. The comparison of malaria prevalence by age group, sex, neighborhood, symptoms, and LLIN use was performed using the Pearson χ2 test or Fisher’s exact test, as appropriate. Differences in parasite densities between age groups were determined by ANOVA with Dunn’s multiple comparison test. Univariate and multivariate logistic regression models were used to investigate the associations between subpatent malarial infections and risk factors. Variables significant at a p value of 0.30 in the univariate logistic regression were selected for the multivariate logistic regression analysis model. A significance level of 5% was used for all the statistical tests. Odds ratios (ORs) with 95% confidence intervals (CIs) are reported. All the statistical analyses were performed using GraphPad Prism 9.5 (Graph Pad Software, San Diego, CA, USA) and MedCalc 20.218 (MedCalc Software Ltd., Ostend, Belgium).
Ethics approval and consent to participate
This study followed the ethical principles recommended by the Edinburgh revision of the Declaration of Helsinki and was approved by the Ethics Committee of the Ministry of Health of Côte d’Ivoire (submission numbers 029/MSLS/CNER-dkn, 110/MSHP/CNER-kp) and the University of Notre Dame Institutional Review Board (18-08-4803). Written informed consent was obtained from all participants and/or guardians of juveniles < 18 years.
Data availability
The datasets supporting the conclusions of this article are included within the article. The raw data used for the analysis of the study are available from the corresponding author upon reasonable request.
Abbreviations
- aOR:
-
Adjusted odds ratio
- CI:
-
Confidence interval
- cOR:
-
Crude odds ratio
- dPCR:
-
Digital polymerase chain reaction
- LLIN:
-
Long-lasting insecticidal net
- LM:
-
Light microscopy
- NMCP:
-
National malaria control program
- PfHRP2/3:
-
Plasmodium falciparum Histidine-rich proteins 2 and 3
- qPCR:
-
Quantitative polymerase chain reaction
- RDT:
-
Rapid diagnostic test
- varATS:
-
Var gene acidic terminal sequence
References
World Health Organization. World Malaria Report 2023. https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2023 (2023).
Bousema, T., Okell, L., Felger, I. & Drakeley, C. Asymptomatic malaria infections: Detectability, transmissibility and public health relevance. Nat. Rev. Microbiol. 12, 833–840 (2014).
Lindblade, K. A., Steinhardt, L., Samuels, A., Kachur, S. P. & Slutsker, L. The silent threat: Asymptomatic parasitemia and malaria transmission. Expert Rev. Anti-infect. Ther. 11, 623–639 (2013).
Slater, H. C. et al. The temporal dynamics and infectiousness of subpatent Plasmodium falciparum infections in relation to parasite density. Nat. Commun. 10, 1433 (2019).
Coleman, R. E. et al. Infectivity of asymptomatic Plasmodium-infected human populations to Anopheles dirus mosquitoes in western Thailand. J. Med. Entomol. 41, 201–208 (2004).
Ministère de la Santé et de l’Hygiène Publique. Plan national de développement sanitaire 2016–2020. Abidjan, Côte d’Ivoire 88 (2016).
Assouho, K. F. et al. Vectorial transmission of malaria in major districts of Côte d’Ivoire. J. Med. Entomol. 57, 908–914 (2020).
Kouadio, A. S., Cissé, G., Obrist, B., Wyss, K. & Zingsstag, J. Fardeau économique du paludisme sur les ménages démunis des quartiers défavorisés d’Abidjan, Côte d’Ivoire. VertigO - la revue électronique en sciences de l’environnement https://doi.org/10.4000/vertigo.1776 (2006).
United Nations. World Urbanization Prospects 2018: Highlights. (2019).
World Bank. Urban Population - Cote d’Ivoire. https://data.worldbank.org/indicator/SP.URB.TOTL?locations=CI (2023).
Dossou-Yovo, J., Doannio, J. M., Diarrassouba, S. & Chauvancy, G. The impact of rice fields on malaria transmission in the city of Bouaké, Côte d’Ivoire. Bull. Soc. Pathol. Exot. 91, 327–333 (1998).
Adja, A. M. et al. Diversity of Anopheles gambiae s.l., Giles (Diptera: Culicidae) larval habitats in urban areas and malaria transmission in Bouaké, Côte d’Ivoire. Vector Borne Zoonotic Dis. 21, 593–601 (2021).
Alves, F. P. et al. Asymptomatic carriers of Plasmodium spp. as infection source for malaria vector mosquitoes in the Brazilian Amazon. J. Med. Entomol. 42, 777–779 (2005).
Ministère de la Santé et de l’Hygiène Publique. Plan National de Developpement Sanitaire 2021–2025. Abidjan, Côte d’Ivoire 593 (2021).
Landier, J. et al. The role of early detection and treatment in malaria elimination. Malar. J. 15, 363 (2016).
Yimam, Y., Mohebali, M. & Afshar, M. J. A. Comparison of diagnostic performance between conventional and ultrasensitive rapid diagnostic tests for diagnosis of malaria: A systematic review and meta-analysis. PLoS ONE 17, e0263770 (2022).
Amoah, L. E. et al. Contribution of P. falciparum parasites with Pfhrp 2 gene deletions to false negative PfHRP 2 based malaria RDT results in Ghana: A nationwide study of symptomatic malaria patients. PLoS ONE 15, e0238749 (2020).
Gbalégba, C. G. N. et al. Distribution of Plasmodium spp. infection in asymptomatic carriers in perennial and low seasonal malaria transmission settings in West Africa. Infect. Dis. Poverty 7, 39 (2018).
Assi, S. B. et al. Epidemiology of malaria in urban area (Adzopé city, Côte d’Ivoire). Am-Euras. J. Sci. Res. 5, 94–100 (2010).
Bassa, F. K. et al. Epidemiology of malaria in the Taabo health and demographic surveillance system, south-central Côte d’Ivoire. Malar. J. 15, 9 (2016).
Assi, S.-B. et al. Cross sectional study on prevalence of malaria case and malaria infection in children under 15 years after the military crisis in Bouake, Cte dIvoire. JPVB 14, 8–17 (2022).
Sumari, D. et al. Malaria prevalence in asymptomatic and symptomatic children in Kiwangwa, Bagamoyo district, Tanzania. Malar. J. 16, 222 (2017).
Debash, H. et al. Burden and seasonal distribution of malaria in Ziquala district, Northeast Ethiopia: A 5-year multi-centre retrospective study. BMJ Open 13, e067103 (2023).
Benié, E. M. A. et al. Accuracy of a rapid diagnosis test, microscopy and loop-mediated isothermal amplification in the detection of asymptomatic Plasmodium infections in Korhogo, Northern Côte d’Ivoire. Malar. J. 21, 111 (2022).
Abebaw, A., Aschale, Y., Kebede, T. & Hailu, A. The prevalence of symptomatic and asymptomatic malaria and its associated factors in Debre Elias district communities, Northwest Ethiopia. Malar. J. 21, 167 (2022).
Mensah, B. A. et al. Prevalence and risk factors associated with asymptomatic malaria among school children: Repeated cross-sectional surveys of school children in two ecological zones in Ghana. BMC Public Health 21, 1697 (2021).
Ministère de la Santé et de l’Hygiène Publique. Rapport Annuel sur la situation Sanitaire (RASS) 2016. Abidjan, Côte d’Ivoire 354 (2017).
Carneiro, I. et al. Age-patterns of malaria vary with severity, transmission intensity and seasonality in sub-Saharan Africa: A systematic review and pooled analysis. PLoS ONE 5, e8988 (2010).
Walldorf, J. A. et al. School-age children are a reservoir of malaria infection in Malawi. PLoS ONE 10, e0134061 (2015).
Stresman, G. H. et al. High levels of asymptomatic and subpatent plasmodium falciparum parasite carriage at health facilities in an area of heterogeneous malaria transmission intensity in the Kenyan highlands. Am. J. Trop. Med. Hyg. 91, 1101–1108 (2014).
Coalson, J. E. et al. Simulation models predict that school-age children are responsible for most human-to-mosquito Plasmodium falciparum transmission in southern Malawi. Malar. J. 17, 147 (2018).
Olapeju, B. et al. Age and gender trends in insecticide-treated net use in sub-Saharan Africa: A multi-country analysis. Malar. J. 17, 423 (2018).
Coalson, J. E. et al. Challenges in treatment for fever among school-age children and adults in Malawi. Am. J. Trop. Med. Hyg. 100, 287–295 (2019).
Cohee, L. M. et al. School-based screening and treatment may reduce P. falciparum transmission. Sci. Rep. 11, 6905 (2021).
Staedke, S. G. et al. Assessment of community-level effects of intermittent preventive treatment for malaria in schoolchildren in Jinja, Uganda (START-IPT trial): A cluster-randomised trial. Lancet Glob. Health 6, e668–e679 (2018).
Mooney, J. P. et al. ‘Bouncing Back’ from subclinical malaria: Inflammation and erythrocytosis after resolution of P. falciparum infection in Gambian children. Front. Immunol. https://doi.org/10.3389/fimmu.2022.780525 (2022).
Opoku Afriyie, S. et al. Accuracy of diagnosis among clinical malaria patients: Comparing microscopy, RDT and a highly sensitive quantitative PCR looking at the implications for submicroscopic infections. Malar. J. 22, 76 (2023).
Niyukuri, D. et al. Performance of highly sensitive and conventional rapid diagnostic tests for clinical and subclinical Plasmodium falciparum infections, and hrp2/3 deletion status in Burundi. PLoS Glob. Public Health 2, e0000828 (2022).
Cook, J. et al. High heterogeneity of malaria transmission and a large sub-patent and diverse reservoir of infection in Wusab As Safil district, Republic of Yemen. Malar. J. 15, 193 (2016).
Idris, Z. M. et al. High and heterogeneous prevalence of asymptomatic and sub-microscopic malaria infections on islands in Lake Victoria, Kenya. Sci. Rep. 6, 36958 (2016).
Dahoui, M. M. C. et al. Entomological drivers of uneven malaria transmission in urban lowland areas in Bouaké, Côte d’Ivoire. Malar. J. 22, 34 (2023).
Zhou, G., Minakawa, N., Githeko, A. & Yan, G. Spatial distribution patterns of malaria vectors and sample size determination in spatially heterogeneous environments: A case study in the West Kenyan Highland. J. Med. Entomol. 41, 1001–1009 (2004).
Kong, A. et al. HRP2 and HRP3 cross-reactivity and implications for HRP2-based RDT use in regions with Plasmodium falciparum hrp2 gene deletions. Malar. J. 20, 207 (2021).
Traoré, D. F. et al. Operational evaluation of the effectiveness of long-lasting insecticidal nets on human-vector contact in an African urban malaria context. Open Forum Infect. Dis. 8, ofaa635 (2020).
Hofmann, N. et al. Ultra-sensitive detection of Plasmodium falciparum by amplification of multi-copy subtelomeric targets. PLoS Med. 12, e1001788 (2015).
Vera-Arias, C. A. et al. High-throughput Plasmodium falciparum hrp2 and hrp3 gene deletion typing by digital PCR to monitor malaria rapid diagnostic test efficacy. Elife 11, e72083 (2022).
Acknowledgements
We gratefully acknowledge the populations of 9 neighborhoods (Dar-es-Salam, Djezoukouamekro, N’gattakro, Sokoura, Belle-ville, Attienkro, Kennedy, Air-France, and N’gouattanoukro) of Bouaké, especially householders, housewives, guardians of children, and all the technical staff involved in blood sample collection in the field, for their kind support and collaboration. We would also like to thank Mahamadou Barro, PhD student in mathematics, for his invaluable help in analyzing the data.
Funding
This work was supported by the French Initiative 5% and Expertise France (Grant N°12INI210).
Author information
Authors and Affiliations
Contributions
ABS, CK and FR conceived the study; ABS, DFT, SBA, and TBN conducted the field work and recorded the demographic data; YG and CAVA conducted the lab work; ABS analyzed the data and wrote the manuscript. AAK, CR and CK revised the manuscript. All the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sagna, A.B., Gebre, Y., Vera-Arias, C.A. et al. High prevalence of asymptomatic and subpatent Plasmodium falciparum infections but no histidine-rich protein 2 gene deletion in Bouaké, Côte d’Ivoire. Sci Rep 14, 19060 (2024). https://doi.org/10.1038/s41598-024-70215-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-70215-x
- Springer Nature Limited