Abstract
Although a sequential work-up for deep vein thrombosis has reached agreement worldwide, the mysterious nature of DVT following fractures brings challenges to early diagnosis and intervention. The objective of the present study was to develop and validate a nomogram for predicting preoperative DVT risk in patients with thoracolumbar fractures using readily available clinical data. Of the 1350 patients, 930 were randomly assigned to the training cohort. A prediction model was established and visualized as a nomogram based on eight predictors related to preoperative DVT. The performance of the model was tested by the receiver operating characteristic curve, Hosmer–Lemeshow test, calibration curve, and decision curve analysis. We further verified the model in the validation cohort. The AUCs of the prediction model were 0.876 and 0.853 in training and validation cohorts, respectively. The Hosmer–Lemeshow test demonstrated good fitness in the training set (X2 = 5.913, P = 0.749) and the validation set (X2 = 9.460, P = 0.396). Calibration and decision curve analyses performed well in training and validation sets. In short, we developed a prediction model for preoperative DVT risk in patients with thoracolumbar fractures and verified its accuracy and clinical utility.
Similar content being viewed by others
Introduction
Deep vein thrombosis (DVT) and pulmonary embolism (PE), pivotal concerns in the domain of inpatient morbidity and mortality, present a pronounced challenge in the context of traumatic injuries. Among the pantheon of traumatic afflictions, thoracolumbar fractures stand out due to their prevalence and the consequent risk of DVT, which has been documented to afflict between 4.3 and 14.5% of patients in the preoperative phase, notwithstanding the implementation of prophylactic measures1,2. The extant guidelines advocate for a multifaceted diagnostic approach entailing the assessment of clinical pretest probability, D-dimer assays, and imaging examinations3. This scheme can exclude DVT in about one-third of ambulatory outpatients in both the primary and hospital care setting4,5. Nevertheless, this paradigm exhibits diminished efficacy in individuals with an elevated predisposition to DVT or those with inherently high D-dimer levels, encompassing, but not limited to, elderly patients, pregnant women, and subjects undergoing treatment for major traumas or fractures6,7.
The literature reveals a plethora of DVT risk assessment models deployed in clinical settings; however, subsequent investigations frequently cast doubt on their predictive accuracy, particularly in hospitalized cohorts. The Caprini risk assessment model8, despite its comprehensive nature, necessitates labor-intensive and costly laboratory evaluations (such as positive Factor V Leiden, prothrombin G20210A mutation, and elevated lupus anticoagulant) that may not be universally accessible, thereby potentially undermining the model's evaluative capacity. Moreover, its complexity could preclude rapid application in clinical scenarios. The Wells score, while established for the outpatient demographic, engenders debate regarding its prognostic utility within inpatient environments, attributed mainly to its symptomatic evaluation focus which might neglect the incidence of asymptomatic DVT in the lower extremities9,10. It is noteworthy that the prevailing scoring systems have not been tailored specifically to trauma patients, much less to individuals predisposed to DVT after spinal fractures. Although the Risk Assessment Profile was conceived to forecast DVT risk among hospitalized trauma patients11, it is hampered by the delayed availability of some requisite variables, which might only be ascertainable post-discharge, thereby detracting from its effectiveness in guiding early duplex ultrasound surveillance in patients poised for spinal surgery12.
In this study, we aim to create a novel model for predicting the risk of lower extremity DVT in patients with thoracolumbar fractures when waiting for surgery. This initiative aspires to harness readily accessible demographic data and routine clinical parameters shortly after admission, aiming to ameliorate the diagnostic precision and fortify the management strategies for DVT in high-risk individuals.
Materials and methods
General information
This study used the prospectively collected data derived from a database of Surgical Site Infection in Orthopedic Surgery (SSIOS) approved by the ethics committee of the Third Hospital of Hebei Medical University. We identified eligible patients with thoracolumbar fractures who were admitted and surgically treated from January 2017 to April 2020 in our institution, a Level I Trauma Center, a teaching and orthopedic specialty hospital in Hebei Province. The inclusion criteria were (1) age ≥ 18 years, (2) definitive diagnosis of thoracolumbar fracture, (3) color Doppler imaging performed within seven days of admission, and (4) information integrity of demographic and routine clinical test data. The exclusion criteria were (1) patients admitted to ICU within 24 h after injury, and the length of ICU stay ≥ 48 h; (2) old fracture (≥ 21 days from injury to surgery), (3) pregnancy or current use of oral contraceptives, (4) active cancer, (5) preexisting thrombotic disease with ongoing treatment, or (6) inherited thrombophilic disorders. A total of 1350 patients were included in our study (Fig. 1). The follow-up period began from admission to either diagnosis of DVT or surgery. For precise predictions and to avoid overfitting, the sample size calculation should combine the number of events, the total number of participants, the outcome proportion, and the expected predictive performance of the model13. The tailored sample size requirements for this research interest should be at least 826 in the training cohort, and therefore our sample size is adequate for model construction. All the participants in this study signed the informed consent for possible research purposes. This study complied with the principles of the Helsinki Declaration and followed the guideline of Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD).
Assessment of spinal cord injury (SCI)
Shortly after admission, the severity of traumatic SCI was evaluated by orthopaedic surgeons and was graded by the American Spinal Injury Association (ASIA) Impairment Scale. In this study, ASIA grade was grouped into grades A-B and grades C-E with or without motor function below neurologic level.
Standardized management and diagnosis of DVT
The patient's bilateral lower extremities were examined for DVT through duplex ultrasonography (DUS) within the first 24 h of admission, every 3–7 days and when DVT was suspected. Failure to fully compress the lumen of the veins with the ultrasound probe, absence of respiratory vibration in the venous segment above the knee and insufficient flow augmentation to the calf or foot are confirmatory of DVT. The scanning area covered all deep veins of both lower extremities, and intermuscular vein thrombosis was not regarded as a positive result in this study. Venography was performed if a definite diagnosis could not be made through DUS. Based on screening results, patients were divided into a DVT group or a non-DVT group. Prophylactic low-molecular-weight heparin (LMWH) was initiated within the first 24 h after admission if the bleeding risk was effectively managed. Intermittent pneumonic compression (IPC) was routinely used as indicated. Once DVT was confirmed, therapeutic doses of LMWN or interventional treatment were prescribed until symptoms were resolved.
Data collection
We selected factors that promote the formation of thrombus from literature reports as well as clinical judgments. The demographic information and comorbidities from the enrolled patients were as follows: sex, age, body mass index (BMI), hypertension, diabetes mellitus, cerebrovascular disease, heart disease, current smoking, alcohol consumption, time from injury to surgery (or diagnosis of DVT) (days), fracture site, the number of fractured vertebrae, chest injury, craniocerebral trauma, fracture of the upper limb, fracture of the lower extremity (from the pelvis to foot), and ASIA (Grade A/B). Chest injury includes but is not limited to multiple rib fractures, sternal fractures, pulmonary contusion and/or laceration, hemothorax and/or pneumothorax. Craniocerebral trauma was determined through imaging features. Laboratory biomarkers were collected based on routine fasting blood examinations after admission, including total protein (TP), albumin (ALB), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), very low-density lipoprotein (VLDL), serum sodium concentration (Na+), white blood cell (WBC) count, red blood cell (RBC) count, hemoglobin (HGB), platelet (PLT), platelet distribution width (PDW), prothrombin time (PT), activated partial thromboplastin time (APTT), thrombin time (TT), fibrinogen (FIB), antithrombin III (AT III), and D-dimer level. Patients’ overall physical fitness before surgery was evaluated using the American Society of Anesthesiologists (ASA) classification. All clinical and laboratory data were obtained within the first 24 h of admission. The worst value was adopted if multiple examinations for the same indicator were conducted within the first 24 h.
Statistical analysis
Plasma D-dimer is a well-established screening parameter for DVT, while the reference value for provoked DVT varies widely in different circumstances. Therefore, we employed Youden-index to obtain an optimal cut-off value of D-Dimer for DVT diagnosis.
We used SPSS 26.0 (IBM Corp, Armonk, New York, US) for statistical analysis. Kolmogorov–Smirnov test was performed to check the normality of the continuous variable's distribution. Continuous variables, if distributed normally, were expressed as mean ± standard deviation (SD), and differences between patients with or without DVT were compared using Student’s t-test; otherwise, presented as the median and inter-quartile range (IQR) and tested by Mann Whitney U test. Categorical variables were presented as numbers and percentages and were analyzed by Chi-square test. Using the Backwards LR method, a multivariate logistic regression model was established by incorporating the risk factors with significant univariate association (p < 0.05). The selected predictors in the final model were substituted into R software for Windows (Version 3.6.5) for further analysis, and the "rms" package was used to construct the nomogram. The area under the receiver operating characteristic curve (AUC) was calculated to quantify the discrimination ability of the nomogram. A high AUC indicated good classification performance. The Hosmer–Lemeshow test and calibration curve were used to assess the agreement between estimated and observed proportions, in which the higher the consistency, the better the predicted curve would correspond with the actual curve. Decision curve analysis (DCA) was performed to evaluate the clinical usefulness of the model by calculating the net benefit across a range of threshold probabilities. Finally, we externally verified the validation cohort using the above model evaluation index. P < 0.05 was considered statistically significant.
Results
Patients’ baseline data
In total, 1350 patients with thoracolumbar fractures were included in this study, consisting of 1003 males and 347 females, with an average age of 45.5 years (range, 18–79). Among them, 81 (6%) patients underwent preoperative DVT in the lower extremities. Before analysis, the data were split randomly into training and validation cohorts in a 7:3 ratio, i.e. 930 and 420 cases, respectively. The baseline levels were well-balanced between the two cohorts, as shown in Table 1. Among the training cohort, 698 were males, and 232 were females. According to diagnostic criteria, the incidence of preoperative DVT was 5.7% (53/930) in the training cohort. As for the fracture sites, 636 (68.4%) sustained lumbar fractures, accounting for the most significant proportion, followed by thoracic fractures (241 cases) and thoracolumbar fractures (53 cases). Regarding the number of involved vertebrae, the fracture at one vertebra dominated this set by 85.7%, and the more fracture sites, the smaller the proportion.
Univariate and multivariate analysis
According to the AUC analysis, the optimal cut-off value of D-dimer for this population was 1.78 mg/L. After the univariate analysis, 17 potential variables remained as significant predictors of DVT, including the time from injury to surgery, number of fractured vertebrae, chest injury, craniocerebral trauma, lower extremity fractures, ASIA (Grade A/B), TP, ALB, HDL-C, VLDL-C, Na+, WBC, HGB, PLT, PDW, D-dimer, and ASA classification (Table 1). Multivariate logistic regression analysis indicated eight variables had a significant association with DVT, including the time from injury to surgery (OR, 1.16; 95% CI 1.08–1.24; P < 0.001), number of fractured vertebrae (OR, 1.52; 95% CI 0.89–2.57; P < 0.122), chest injury (OR, 2.06; 95% CI 1.03–4.14; P < 0.042), lower extremity fractures (OR, 2.11; 95% CI 1.07–4.18; P < 0.032), ASIA (Grade A/B) (OR, 3.09; 95% CI 1.29–7.38; P < 0.011), TP (< 60 g/L) (OR, 2.04; 95% CI 1.06–3.92; P < 0.033), VLDL-C (> 0.78 mmol/L) (OR, 2.50; 95% CI 1.19–5.26; P < 0.015), and D-dimer (> 1.78 mg/L) (OR, 2.84; 95% CI 1.41–5.71; P < 0.004) (Table 2). In nomogram formulation, these variables had a minimum Akaike's information criterion (AIC) of 318.04.
Development and validation of a predictive nomogram for DVT
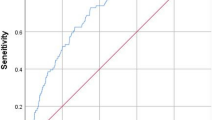
The model was constructed by incorporating these eight predictors into a panel and presented as a nomogram (Fig. 2). We further tested the performance of the nomogram and verified the model in the validation cohort. The AUCs of the predictive model in the training cohort and validation cohort were 0.876 (95% CI 0.831–0.921) and 0.853 (95% CI 0.776–0.930), respectively, manifesting a solid discriminatory ability (Fig. 3). The Hosmer–Lemeshow test demonstrated good fitness in the training set (X2 = 5.913, p = 0.749) and the validation set (X2 = 9.460, p = 0.396). Meanwhile, the calibration curves showed that the predicted risks were close to actual proportions, suggesting this model was well-calibrated (Fig. 4). Additionally, DCA suggested that using a risk prediction model could provide a positive net benefit when the threshold probability was 2–53%, as shown in Fig. 5. Good consistency between training and validation cohorts was obtained regarding distinguishability, calibration ability, and clinical utility.
Comparison of the decision curve analyses (DCA) of the nomogram in the training cohort (a) and the validation cohort (b). The X-axis represents the high-risk threshold and the Y-axis represents the net benefit. The red line represented the net benefit of the nomogram at different threshold probabilities. The area between the “All negative” (black line) and “All positive” (gray line) in the DCA curve indicated the clinical utility of the model.
Discussion
To our best knowledge, this is the first study to develop and validate a prediction tool for preoperative DVT in thoracolumbar fractures, integrating demographic factors, comorbidities, traumatic characteristics, and blood biomarkers. The incidence of DVT was 6% in a total of 1350 patients, with several significant variables selected through an appropriate method. Based on these eight user-friendly and readily available clinical features, the nomogram demonstrated relatively robust discrimination ability and good calibration power. It could be widely used for DVT prediction in this population. Furthermore, more importantly, the results of derivation and validation groups reached a good agreement with this model.
Our study revealed that a prolonged interval between injury to surgery was significantly associated with preoperative DVT (7 days vs. 4 days) following thoracolumbar fractures. The multivariate results suggested that each day of delay to surgery/DUS could increase 16% of the risk of DVT. Similarly, the adverse effect of surgical delays was well documented on subsequent DVT in fractures involving the lower extremities, hip, pelvis and acetabulum, and spine14,15,16,17. The cause of the delayed DUS/surgery could be one or more of the following. Firstly, our institution receives a considerable proportion of referrals from lower levels of facilities. For those patients, timely DVT screening and anticoagulant therapy seem challenging to implement before admission. Secondly, the diagnostic approach to suspected DVT consists of a sequential work-up combining thrombosis risk scoring, D-dimer testing, and DUS. Awaiting a scheduled DUS often extends patients’ bedridden time and postpones their mobilization, putting them at significant DVT risk. Thirdly, as a level one trauma center, the backlog of patients, especially those with critical trauma, also contributes to the delay of DUS/surgery. Hence, each surgical division should consider the accrual of cases and start determining a prioritization order for high-risk patients. However, the issue of allocating resources across competing surgical specialties is challenging to address. Excepting for the timely commencement of thromboprophylaxis immediately after patients' admission, using a successful prediction model can promote early identification of predictive DVT on a case-by-case basis. Our finding aligns with the concept of fast-track surgery, which aims at early ambulation, discharge, and enhanced recovery and has exhibited excellent efficacy and safety18.
As a well-established screening parameter, plasma D-dimer is extensively used to exclude DVT and PE events in all-cause patients. However, orthopaedic surgeons and nurses often perceive that D-dimer levels are ‘always’ abnormal (higher than normal range) in their patients. Consequently, DUS or venography as a sole test is frequently used to establish or rule out DVT in trauma patients. In these cases, the diagnostic value of D-dimer is somehow limited, and meanwhile, a preset reference is inappropriate to predict the probability of DVT provoked by acute trauma. Accordingly, growing evidence confirms the necessity of raising the D-dimer threshold used in diagnosing DVT and PE according to prediction probability, age, and specific populations19,20. For our study group, we modified the threshold to 1.78 mg/L and identified that the on-admission D-dimer lower than 1.78 was independently associated with a 2.84-fold elevated risk of preoperative DVT. Although the effect of D-dimer on preoperative dynamic monitoring of DVT has not been determined, we considered the adjusted cut-off value conducive to increasing the diagnostic specificity and improving the predictive value of DVT in patients following thoracolumbar fractures.
Hypoproteinemia and metabolic abnormality of lipoprotein are common risk factors for atherosclerosis and venous thrombosis in trauma patients, and their correlation with DVT has been widely studied in many works of literature15,21,22. Our results suggested that the low level of total protein (< 60 g/L) was independently associated with the occurrence of DVT (p = 0.033). However, albumin, which accounted for up to 60% of the total protein, did not show relevance with DVT when below the normal range. Similar findings were obtained by a previous study focusing on the tibial fracture population23. Therefore, we assumed that the decrease in total protein correlated with other protein types. For instance, protein C and protein S, predominantly synthesized in the liver, are vitamin K-dependent components of the natural anticoagulant system and function to maintain physiologic hemostasis in the body. A large body of evidence showed that deficiency of proteins C and S undermines their anticoagulant properties, resulting in unchecked thrombin generation and increased risk of venous thrombosis24.
Despite conflicting results in several clinical studies16,25, our finding was consistent with previous laboratory research on the association between VLDL and DVT occurrence. These studies showed that VLDL had significantly higher activity than LDL or HDL in promoting coagulation26. Furthermore, it was supported by results from an in vitro study in which VLDL promoted prothrombinase complex assembly and thrombin generation without platelets or endothelial cells27. Although the underlying molecular mechanism involved in metabolic parameters and DVT remains fully clarified, the findings suggest new directions regarding monitoring thrombosis-specific biomarkers for DVT risk assessment. Moreover, as these parameters could be improved, proactive correction of these indexes may provide clinical value to prevent thrombosis and improve prognosis.
It is widely acknowledged that the formation of DVT correlates with hypercoagulability, vascular endothelium disruption and venous stasis, known as Virchow's triad. In recent years, the prevalence of thoracolumbar fractures has been growing, resulting from the increase in industrial and traffic accidents28. Unlike low-energy spinal compression fractures, which typically occur in the elderly with osteoporosis, these high-energy fractures in accidents are generally accompanied by multiple and severe trauma. Whereas physiologic hemostasis is typically restricted to a local reaction, traumatic injuries and subsequent haemorrhage substantially upregulate the activity of plasma procoagulants, leading to an uncontrolled systemic coagulation response29. The return of venous blood in lower limbs primarily depends on the effect of the calf muscle pump. However, the impaired or absent muscle pump function would impede blood return and predispose patients to the risk of DVT. Evidence suggests DVT was a well-known complication after trauma and lower limb fractures30,31. Another study found a significant positive association between paralysis and DVT after spine surgery32. In this study, we observed that either lower limb fracture or neurologic deficit following thoracolumbar fractures was a predictor for preoperative DVT. The results of the multivariable analysis showed that ASIA (grade A/B) led to a 3.09-fold increase in the risk of preoperative DVT, and those with lower extremity fractures had a 2.11-fold elevated risk. Although these results might provide limited reference value in making proactive decisions about chemoprophylaxis, the severity of spinal cord injury and fractures in the lower limb remain of significant predictive value in preoperative DVT.
It is well known that most traumatic thoracolumbar fractures are caused by vehicle accidents, blunt force trauma, and falling from height. Accompanied by spinal fractures, many chest injuries, such as lung damage, may require emergency hospitalization. Given the substantial differences in injury severity and therapeutic regimen, we excluded those critically ill patients who were admitted to the ICU within 24 h of admission. Despite that, chest injuries in the object of this study ranged from pneumothorax to hemothorax, rib fractures to the frail chest, and pulmonary contusion to tracheobronchial injuries. The correlation between blunt chest trauma and PE has been widely discussed by scholars33,34,35. However, only a few pieces of literature have reported the relationship between DVT and chest injury, accompanied by spinal fracture or not. A multicenter prospective cohort revealed that the chest Abbreviated Injury Scale (AIS) ≥ 3 was the only significant injury severity metric predictive for DVT and the composite endpoint VTE in patients after severe injury36. Battle et al.37 and Manay et al.38 highlighted that three or more rib fractures and increasing Injury Severity Score were significantly associated with morbidity and mortality following chest trauma. Our finding showed that chest injury could independently predict the occurrence of DVT, which was consistent with the studies mentioned above. We speculated that thoracic injuries might represent the severity of the overall injury and inflammation response. The underlying mechanism might be related to the extensive cross-talk between coagulation and inflammation, whereby activation of the inflammatory cascade may amplify the coagulation process, promoting the formation of DVT39. Thus, it stands to reason that patients with thoracolumbar fractures and chest injuries should be prioritized because they are at high risk for DVT and other possible life-threatening complications. In this current study, the number of fractured vertebrae did not show a remarkable predictive value for DVT (p = 0.122), but this variable was included in the final model. It was because when adding this parameter (the number of fractured vertebrae), a minimum AIC value could be obtained, with a high goodness-of-fit and avoiding overfitting.
Nomograms, which depend on user-friendly digital interfaces and can visualize the final prediction outcomes, have been widely used in studies of clinical predictive models40,41,42. Furthermore, our study was the first study using a nomogram to predict preoperative DVT in thoracolumbar fractures. The eight predictors included in this nomogram were obtained from readily available data within hours of admission. Nomograms can help clinicians and nurses quickly identify an accurate probability of DVT. For patients awaiting surgery, the nomogram facilitates regular monitoring of the risk of DVT, which can not only improve the clinical value of haematological tests but also reduce unnecessary imaging tests, thus improving the efficiency of the diagnostic process. The predictive model allocates one point for each variable. When assessing the risk, draw a vertical straight line from the variable value to the axis labelled “Points”. Then calculate all variables’ points. The sum of points on the bottom scale corresponding to the DVT risk for each patient can be acquired immediately.
In this study, we successfully developed a nomogram and confirmed its effectiveness via internal and external validations, which were conducted based on a large sample size of 1350. Besides, the clinical data related to the predictors were readily available immediately after admission, and the risk probability could be dynamically monitored during the preoperative period. However, we acknowledge the limitations of our research. Firstly, although the robustness of our nomogram was tested extensively, the subjects in this study were from a single center. These findings should be cautiously treated and reevaluated in the broader population when applied to different regions and countries. Secondly, this study was conducted retrospectively, so the inherited selection bias might have affected the results. Thirdly, despite the 35 variables included in this study, some potential predictors significantly associated with DVT could not be recorded, which might compromise the effectiveness of the prediction model.
Conclusion
In summary, we identified eight factors that could provide a significant predictive value of preoperative DVT in patients with thoracolumbar fractures, including the time from injury to surgery, number of fractured vertebrae, chest injury, lower extremity fractures, ASIA (Grade A/B), TP, VLDL-C, and D-Dimer. Moreover, a model composed of these predictors was converted into a visual and user-friendly nomogram. After being validated internally and externally, this nomogram demonstrated excellent calibration, discriminability, and clinical practicability. Medical care personnel can deploy this tool to calculate the probability of preoperative DVT in hospitalized patients with thoracolumbar fractures to optimize the sequential work-up of suspected DVT. Furthermore, if necessary, early intervention can be taken to prevent the progression of blood clots with catastrophic consequences.
Data availability
All the data used during the current study are available from the corresponding author on reasonable request.
References
Akeda, K. et al. Prevalence and countermeasures for venous thromboembolic diseases associated with spinal surgery: A follow-up study of an institutional protocol in 209 patients. Spine (Phila Pa 1976) 39(10), 791–797 (2014).
Wang, H. Clinical study on preoperative deep vein thrombosis in patients with thoracolumbar fractures caused by high-energy injuries. Hebei Medical University (2022).
Kearon, C. et al. Antithrombotic therapy for VTE disease: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 141(2 Suppl), e419S-e496S (2012).
Geersing, G. J. et al. Exclusion of deep vein thrombosis using the Wells rule in clinically important subgroups: individual patient data meta-analysis. BM. 348, g1340 (2014).
van Es, N. et al. Wells rule and d-dimer testing to rule out pulmonary embolism: A systematic review and individual-patient data meta-analysis. Ann. Intern. Med. 165(4), 253–261 (2016).
Righini, M. et al. Diagnosis of pulmonary embolism during pregnancy: A multicenter prospective management outcome study. Ann. Intern. Med. 169(11), 766–773 (2018).
Park, S. et al. Incidence and factors predicting venous thromboembolism after surgical treatment of fractures below the hip. J. Orthop. Trauma 29(10), e349-354 (2015).
Caprini, J. A. Thrombosis risk assessment as a guide to quality patient care. Dis Mon. 51(2–3), 70–78 (2005).
Piazza, G., Seddighzadeh, A. & Goldhaber, S. Double trouble for 2,609 hospitalized medical patients who developed deep vein thrombosis: Prophylaxis omitted more often and pulmonary embolism more frequent. Chest 132(2), 554–561 (2007).
Silveira, P. et al. Performance of Wells score for deep vein thrombosis in the inpatient setting. JAMA Intern. Med. 175(7), 1112–1117 (2015).
Greenfield, L. et al. Posttrauma thromboembolism prophylaxis. J. Trauma 42(1), 100–103 (1997).
Kay, A. et al. The Risk Assessment Profile is suboptimal for guiding duplex ultrasound surveillance in trauma patients. Surg. Pract. Sci. 11, 100127 (2022).
Riley, R. et al. Calculating the sample size required for developing a clinical prediction model. BMJ 368, m441 (2020).
Zhao, W. et al. Incidence and risk factors of preoperative deep venous thrombosis following pelvic and acetabular fractures: A retrospective case-control study. J. Orthop. Surg. Res. 17(1), 77 (2022).
Ma, J. et al. Incidence and risk factors predicting deep venous thrombosis of lower extremity following spinal fractures. Sci. Rep. 11(1), 2441 (2021).
Cheng, X. et al. Development and validation of a predictive nomogram for preoperative deep vein thrombosis (DVT) in isolated calcaneal fracture. Sci. Rep. 12(1), 5923 (2022).
Zhao, W., Zhao, J., Liu, T., Liu, Z. & Liu, L. Incidence and risk factors of preoperative isolated calf deep venous thrombosis following hip fractures. Medicine 101(12), e29140 (2022).
Jiang, M., Liu, S., Deng, H., Liang, X. & Bo, Z. The efficacy and safety of fast track surgery (FTS) in patients after hip fracture surgery: A meta-analysis. J. Orthop. Surg. Res. 16(1), 162 (2021).
Cracknell, R. & Salim, E. Sensitivity and specificity of instrumentation lab age-adjusted D-dimer threshold values in a single hospital site: A retrospective analysis. Cureus https://doi.org/10.7759/cureus.30719 (2022).
Stals, M., Klok, F. & Huisman, M. Diagnostic management of acute pulmonary embolism in special populations. Expert Rev. Respir. Med. 14(7), 729–736 (2020).
Luo, Z. et al. Preoperative incidence and locations of deep venous thrombosis (DVT) of lower extremity following ankle fractures. Sci. Rep. 10(1), 10266 (2020).
Zhu, Y. et al. Prevalence of preoperative lower extremity deep vein thrombosis in bilateral calcaneal fractures. J. Foot Ankle Surg. 60(5), 950–955 (2021).
Guo, H. Study on relationship between biomarkers after tibial fracture and the incidence of preoperative deep vein thrombosis (DVT). Shanxi Medical University (2022).
Gierula, M. & Ahnström, J. Anticoagulant protein S-New insights on interactions and functions. J. Thromb. Haemost. JTH 18(11), 2801–2811 (2020).
Spasić, I., Ubavić, M., Šumarac, Z., Todorović, M. & Vučković, B. Influence of lipid metabolism disorders on venous thrombosis risk. J. Med. Biochem. 40(3), 245–251 (2021).
Moyer, M. et al. Plasma lipoproteins support prothrombinase and other procoagulant enzymatic complexes. Arterioscler. Thromb. Vasc. Biol. 18(3), 458–465 (1998).
Rota, S., McWilliam, N., Baglin, T. & Byrne, C. Atherogenic lipoproteins support assembly of the prothrombinase complex and thrombin generation: Modulation by oxidation and vitamin E. Blood 91(2), 508–515 (1998).
Saglam, N., Dogan, S., Ozcan, C. & Turkmen, I. Comparison of four different posterior screw fixation techniques for the treatment of thoracolumbar junction fractures. World Neurosurg. 123, e773–e780 (2019).
Cardenas, J. et al. Measuring thrombin generation as a tool for predicting hemostatic potential and transfusion requirements following trauma. J. Trauma Acute Care Surg. 77(6), 839–845 (2014).
Chen, F., Xiong, J. & Zhou, W. Differences in limb, age and sex of Chinese deep vein thrombosis patients. Phlebology 30(4), 242–248 (2015).
Yumoto, T. et al. Venous thromboembolism in major trauma patients: A single-center retrospective cohort study of the epidemiology and utility of D-dimer for screening. Acute Med. Surg. 4(4), 394–400 (2017).
Yoshioka, K. et al. Comparative study of the prevalence of venous thromboembolism after elective spinal surgery. Orthopedics 36(2), e223-228 (2013).
Schultz, D. et al. Incidence of asymptomatic pulmonary embolism in moderately to severely injured trauma patients. J. Trauma 56(4), 727–731 (2004) (discussion 731–723).
Coleman, J. et al. Factors associated with pulmonary embolism within 72 hours of admission after trauma: A multicenter study. J. Am. Coll. Surg. 220(4), 731–736 (2015).
Van Gent, J. et al. Pulmonary embolism without deep venous thrombosis: De novo or missed deep venous thrombosis?. J. Trauma Acute Care Surg. 76(5), 1270–1274 (2014).
Brakenridge, S. et al. Comparing clinical predictors of deep venous thrombosis versus pulmonary embolus after severe injury: A new paradigm for posttraumatic venous thromboembolism?. J. Trauma Acute Care Surg. 74(5), 1231–1237 (2013) (discussion 1237–1238).
Battle, C. et al. Risk factors that predict mortality in patients with blunt chest wall trauma: an updated systematic review and meta-analysis. Emerg. Med. J. 40(5), 369–378 (2022).
Manay, P., Satoskar, R., Karthik, V. & Prajapati, R. Studying morbidity and predicting mortality in patients with blunt chest trauma using a novel clinical score. J. Emerg. Trauma Shock 10(3), 128–133 (2017).
Foley, J. & Conway, E. Cross talk pathways between coagulation and inflammation. Circ. Res. 118(9), 1392–1408 (2016).
Bian, F., Cheng, X. & An, Y. Preoperative risk factors for postoperative blood transfusion after hip fracture surgery: Establishment of a nomogram. J. Orthop. Surg. Res. 16(1), 406 (2021).
Liu, B., Pan, J., Zong, H. & Wang, Z. The risk factors and predictive nomogram of human albumin infusion during the perioperative period of posterior lumbar interbody fusion: A study based on 2015–2020 data from a local hospital. J. Orthop. Surg. Res. 16(1), 654 (2021).
Wang, H. et al. Development and validation of a nomogram for prediction of the risk of positive hidden blood loss in the perioperative period of single-level thoracolumbar burst fracture. J. Orthop. surg. Res. 16(1), 560 (2021).
Acknowledgements
The Key Laboratory of Biomechanics of Hebei Province provided the site for querying data.
Funding
The research was supported by the Innovation Project for Graduate Students of Hebei Medical University (XCXZZS202323), Medical Scientific Research Project of Hebei Provincial Health Commission (20221170), and Clinical Nursing Research Funding 2023 (ydsy202301). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Y.Z.Z. and X.T.L. designed the study. M.T. and J.L.H. searched relevant studies and collected data on variables of interest. J.T.M. and Y.B.Z. analyzed and interpreted the data. J.T.M. wrote the manuscript. The authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ma, J., Tian, M., Zhu, Y. et al. Development and validation of a predictive model for preoperative deep vein thrombosis following traumatic thoracolumbar fractures. Sci Rep 14, 19547 (2024). https://doi.org/10.1038/s41598-024-70464-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-70464-w
- Springer Nature Limited