Abstract
Anemia is a common but often underdiagnosed and undertreated geriatric syndrome in hospitalized older patients. In this retrospective multicenter study, we aimed at characterizing the prevalence, risk factors, diagnostic and treatment approach to anemia in older patients admitted to acute care hospitals, focusing on differences between nephrology and geriatrics units. Prevalence and risk factors for anemia, diagnostic inertia (lack of iron, vitamin B12, and folate status assessment), replacement inertia (omitted treatment with iron, vitamin B12 or folic acid), and erythropoiesis-stimulating agents (ESA) inertia were explored. 1963 patients aged 82.7 (6.8) years were included in the study; 66.7% of the study population had anemia; among anemic patients, diagnostic inertia and replacement inertia were common with rates of 22–31% and 50–87%, respectively; omitted treatment with ESA affected 67.2% of patients and was more prevalent in geriatric units. In most cases, patients with ESA inertia were not routinely screened for iron tests. COPD, cancer, eGFR 45–60 ml/min were associated with increased tendency to ESA inertia. In conclusion, anemia had a high prevalence in older patients discharged from acute care units, but it is often underdiagnosed and undertreated.
Similar content being viewed by others
Introduction
Anemia is one of the most common diseases in older individuals, as its prevalence increases with age1 and is often underdiagnosed and undertreated, due to lack of specific symptoms in the older individual2. Aging-associated decline in serum hemoglobin concentration as well as increased prevalence of nutritional deficiencies and chronic diseases are the main pathophysiological factors contributing to the onset of anemia in older individuals1,3. The burden of anemia widely varies across distinct settings of care4,5, with mean prevalences ranging from 12% in community-dwelling individuals to up to 45% among hospitalized older patients4. Despite its relatively high prevalence, anemia cannot be considered a normal consequence of aging because it is associated with higher morbidity and mortality. Indeed, in the Cardiovascular Health Study, participants in the lowest hemoglobin quintile were characterized by increased mortality, even if not classified as anemic according to the WHO criteria6. Moreover, anemia has been associated with an array of clinical conditions such as depression, cognitive decline, decreased functional ability, increased risk of falls, longer hospital stays and decreased life expectancy1. Despite its clinical importance and impact on morbidity and mortality, diagnosis and treatment of anemia in the elderly is often suboptimal7.
Risk factors for anemia in older patients include age, comorbidities (i.e. diabetes mellitus, congestive heart failure [CHF], chronic obstructive pulmonary disease [COPD], and chronic kidney disease, CKD), inflammation, and malnutrition. Iron, folate, and/or vitamin B12 deficiencies are responsible for nearly one third of anemia cases in older patients; blood loss from gastrointestinal malignancies is the primary cause of iron deficiency in the elderly8; however, routine blood tests for serum iron, ferritin and transferrin saturation have poor screening sensitivity for capturing iron deficiency in older patients 9,10. Indeed, both age and concomitant inflammatory diseases tend to increase serum ferritin concentration above the reference level and may mask the presence of underlying anemic states11. Despite adaptation of transferrin saturation (TSAT) cut-offs has been proposed to increase sensitivity in diagnosing functional iron deficiency, it is not always implemented in routinary clinical practice8. Together with nutritional deficiencies and chronic inflammation, CKD is another major cause of anemia among older patients, accounting for up to one third of cases8. According to the KEEP study, the prevalence of CKD in people 65 years and older was approximately 44% and peaked in those over age 80; the great majority (77%) belonged to CKD stage 3 and only 5% to stage 412; recent analyses from the SCOPE study reported a CKD prevalence ranging from 39 to 66% depending on the equations used to estimate kidney function13.
Decrease in endogenous erythropoietin (EPO) production, absolute and/or functional iron deficiency, and inflammation with increased hepcidin levels14 are the main mechanisms of anemia in CKD. For this reason, iron supplementation and erythropoiesis-stimulating agents (ESAs) are the mainstay of treatment in CKD-related anemia15. The remaining portion of older patients with are categorized as having unexplained anemia of aging (UAA), which is in part related to bone marrow abnormalities, including myelodysplastic syndrome16.
In any case, independent of underlying causes, appropriate assessment and management of anemia is often suboptimal2, and previous evidence hasraised this concern in community-dwelling cohorts17,18. Furthermore, in a study conducted by Rampotas et al.19 in a cohort of over 800 patients with anemia admitted to acute care hospitals, among the 19% of patients who received inappropriate blood transfusions, over 50% did not have an aetiologic diagnosis of anemia before discharge. However, to date no study has investigated the quality of diagnostic and therapeutic approach to anemia in hospitalized older patients admitted to distinct medical specialties. Considering the increasing burden of multimorbidity in geriatrics units and of CKD-related anemia in nephrology units, the clinical and socioeconomic burden of anemia on hospitals is expected to increase, and both geriatricians and nephrologists are dealing with increasing rates of anemic patients.
Therefore, in this multicenter cross-sectional study we aimed at investigating the prevalence, risk factors, and quality of diagnostic and therapeutic approach to anemia in hospitalized older patients, focusing on differences between geriatric and nephrological practices.
Results
Prevalence of anemia
Overall, the study population consisted of 1,903 patients aged 82.7 (7.8) years, with 51.9% men and a mean 7.3 (3.2) medications (Table 1).
Anemia had an overall prevalence of 66.7% of the study population and was slightly more common in Nephrology Units compared to Geriatric Units (75.6% vs 63.3%, p < 0.001). Patients with anemia were older and more commonly men; furthermore, they had a higher prevalence of low iron tests, hypoalbuminemia and higher neutrophil-to-lymphocyte (NLR) values compared to those without anemia; they also had atrial fibrillation, type 2 diabetes, COPD, and cancer, as well as a lower mean eGFR value; finally, anemic patients took more medications, with higher prevalence of both antianemic and potentially anemizing ones. Characteristics of anemic patients stratified by admission unit are reported in Supplementary Table 1. In summary, geriatric patients with anemia were older, with a higher prevalence of COPD, cardiovascular and cerebrovascular diseases, and a lower prevalence of type 2 diabetes mellitus compared to nephrology ones (p < 0.001). Moreover, patients admitted to Nephrology Units had a higher number of medications, a higher prescription of iron, vitamin B12, and folic acid. Prescription patterns slightly differed across admission units, partly in line with the median lower eGFR values in anemic patients discharged from nephrology units. Indeed, vitamin K antagonists and antiplatelet medications were more commonly prescribed in nephrology patients with anemia, while vitamin K antagonists had a higher prescription rate in geriatric units (Supplementary Table 1).
Characteristics of type and severity of anemia across admission units and CKD stages
Differences in severity and type of anemia in the overall study population and in the two settings are displayed in Fig. 1.
Almost two thirds of anemic patients were characterized by a mild reduction of hemoglobin concentration with no significant differences across admission units; moderate anemia was slightly above 30% in all units, while severe anemia had a relatively low prevalence; among types of anemia, normocytic normochromic and mixed forms were the most common ones, followed by macrocytic and microcytic hypochromic forms. No significant differences emerged among patients admitted to Geriatric and Nephrology units.
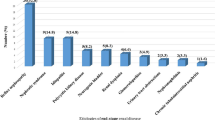
Stratification by CKD stages showed that prevalence of anemia increases with declining eGFR in the overall study population, ranging from 53.4% to 57.0% to 71.5% to 83.9% to 83.5% for patients with eGFR ≥ 60, 45–59.9, 30–44.9, and < 30, respectively (p < 0.001). The distribution of severity and type of anemia across eGFR categories and admission units is represented in Fig. 2.
Decline of eGFR was associated with a graded increase in prevalence of moderate-severe anemia in overall study population and in both settings; more specifically, the most significant increase in the rate of moderate-severe anemia was observed for eGFR below 45 ml/min/1.73 m2 in both settings; no significant changes of types of anemia across eGFR stages were observed.
Diagnostic inertia in all anemic patients and in those with indication to ESA treatment
Assessment of iron status, vitamin B12 and folic acid levels was performed infrequently in patients with anemia (Table 2).
Of all patients with anemia, only 30.6%, 23.4%, and 22.1% had iron studies, vitamin B12 and folic acid levels available, respectively. Patients admitted to Nephrology Units presented significantly higher rates of iron study availability (37.8 vs 27.8% %, p < 0.001) and lower rates of vitamin B12 (16.3% vs 26.7%, p < 0.001), and folic acid assessment (15.6% vs 25.2%, p < 0.001). In general, among patients with anemia who underwent iron level assessment, more than a half resulted to have low iron tests, with higher prevalence in Nephrology Units compared to Geriatric Units (60.5% vs 55.3%, p < 0.001), and an increasing trend in patients with eGFR < 30 ml/min/1.73 m2; conversely, deficiencies of folic acid and vitamin B12 were less common.
Diagnostic inertia was also explored in patients with renal and hemoglobin criteria for ESA use (Table 3).
Overall, more than a half of patients satisfying these criteria were not routinely screened for iron status. Logistic regression analyses showing factors associated with lack of iron status assessment in bivariate and multivariate models are reported in Supplementary Table 2. Factors associated with diagnostic inertia related to iron status were different across admission units (Supplementary Table 3); in this regard, among geriatric patients, CHF and prescription of DOACs resulted to be positively associated with diagnostic inertia, while number of drugs was negatively associated with the outcome; as expected, iron treatment was negatively associated with the outcome in overall study population and nephrology patients.
Replacement inertia
Replacement inertia was relatively common in the study population. Indeed, iron replacement inertia was frequently encountered among anemic patients with low iron levels, reaching the prevalence of 81.4% of the overall study population, 84.2% and 77.0% of patients hospitalized in geriatric and nephrology units, respectively (p = 0.244). Among anemic patients with low serum folate, folate replacement inertia was found in 87.5% of all patients, ranging from 90.9% and 77.8% of geriatric and nephrology units respectively (p = 0.151). Finally, a half of the 12 patients with anemia and low vitamin B12 status were not treated with vitamin B12 supplements.
No factor was found to be associated with iron or replacement inertia in the overall study population (Supplementary Table 4).
ESA inertia
Prevalence and risk factors for therapeutic ESA inertia among patients with ESA indication are reported in Supplementary Table 5. ESA inertia was quite common in the study population (n = 219/326, 67.2%) with significantly higher rates in geriatric patients (173/203, 85.2% vs 46/123, 37.4%, p < 0.001). In most cases, patients with ESA inertia were not routinely screened for iron tests (71.5%), especially in Geriatric units (75.4 vs 65.0%); in this subgroup of patients with ESA inertia and missed iron assessment, only a few were treated with intravenous/oral iron supplementation (10.8% in all units, 11.4% in geriatric units, 7.7% in nephrology units). Analyses of factors associated with ESA inertia in logistic regression bivariate and multivariate models are reported in Table 4.
In the overall study population, COPD and increasing eGFR were associated with increased risk of ESA inertia in fully-adjusted models, while male sex, vitamin B12/folate treatment, and admission to Nephrology Units were negatively associated with the outcome in the study population. Stratified analyses by admission unit showed that eGFR and cancer were positively associated with ESA inertia among geriatric patients, while eGFR only was positively associated with the outcome in nephrology units.
To capture the complex non-linear relationship between eGFR, and ESA inertia, we fitted restricted cubic splines in the whole population, geriatric and nephrology units (Fig. 3).
Restricted cubic spline showing the association between eGFR (as measured through BIS equation) and the odds ratio of ESA inertia in patients with ESA indication (Hb < 10 g/dl, eGFR < 60 ml/min, and either normal-high iron status or low iron status with concomitant iron treatment), in the overall study population and in geriatrics and nephrology units separately. Odds ratios with 95% confidence intervals represent the risk of association compared to the median value of the eGFR distribution in the three study populations.
Overall, a trend of increased ESA inertia was present for less advanced CKD stages with specific cut-off of increased risk at eGFR > 37 ml/min/1.73 m2, significantly higher in nephrology vs geriatric patients (43 vs 40 ml/min/1.73 m2). Logistic regression analyses using eGFR categorical variable > 45 ml/min/1.73 m2 instead of continuous eGFR confirmed this association, with eGFR 45–60 ml/min/1.73 m2 being the most consistent factor associated with ESA inertia across all settings in fully-adjusted models (OR 2.01, 1.51–3.01).
Discussion
In this retrospective cohort study, we found a high prevalence of anemia and of diagnostic and therapeutic inertia with selected differences between Geriatrics and Nephrology Units. Prevalence of anemia was around 66.7% in the overall study population, with higher rates in nephrology units (75.6%) compared to geriatric units (63.3%); lower median eGFR values in nephrology units compared with geriatric units can partly explain the observed differences. Further differentiation showed that most anemias were mild in severity and more commonly of normochromic normocytic and mixed types. The present findings were consistent with those of previous studies reporting a prevalence ranging from 40–50% in community-dwelling cohorts20 and up to 70–80% in older patients admitted to hospital21,22,23. As regards the severity and type of anemia, previous studies in out-of-hospital settings have already shown that mild anemias are considerably burdening older individuals, as they are often underdiagnosed and undertreated despite their relatively high frequency; indeed, more than one-tenth of individuals older than 65 are affected by anemia of mild degree, and this prevalence tends to exceed the 50% of the population older than 80 years, while more severe forms are less frequently detected20,24. Furthermore, while isolated iron-deficiency is recognized as the leading cause of anemia worldwide, and is common in older individuals due to malnutrition, decreased iron absorption, and gastrointestinal losses, microcytosis becomes poorly reliable in older patients, and ferritin is difficult to interpret since its levels tend to increase with age and inflammatory diseases25,26; consequently, classical ferritin thresholds for younger patients need to be adapted in advanced age1; in this regard, determination of serum level of soluble transferrin receptor (sTfR) and sTfR/ferritin index may help detecting iron deficiency in older patients with high ferritin values3,27,28 as sTfR mediates the iron release to erythroid precursors and is less influenced by total inflammation3,29.
It is not surprising that older patients are more likely to experience normochromic normocytic anemias and mixed forms. Indeed, studies conducted in large cohorts of community-dwelling older patients from the National Health and Nutrition Examination Survey (NHANES) showed that anemia of chronic inflammatory diseases and unexplained forms represent over 50% of causes, while pure iron deficiency is detected in less than one-third of patients20,24; similar data were found in studies conducted in hospitalized older patients, that were however monocentric or focused on CKD-related anemia only21,22,30.
Another relevant finding of the present study is that both diagnostic and therapeutic inertia were very commonly encountered in the hospital settings. Diagnostic inertia mainly refers to missed assessment of iron status (and, to a lesser extent, of vitamin B12 and folic acid levels) in patients with anemia; in this study, iron study, vitamin B12 and folic acid levels were checked only in approximately one-third of the study population, with slightly more frequent assessment in Nephrology Units; in patients with performed iron tests, an iron deficiency was detected in over 50% of cases, but it was infrequently corrected with appropriate iron supplementation. These results confirm previous findings in non-hospitalized populations18 and in primary care patients with CKD7. In this regard, Xu et al. recently reported that iron status assessment is performed in less than 30% of patients with anemia and CKD stages 3–5 in the primary care setting7. Similarly, in a recent study on CKD patients and Hb < 10 g/dL, iron status was assessed with rates ranging from 25 to 47%31. This means that a substantial proportion of patients with anemia and CKD who could potentially benefit from iron supplementation are not being thoroughly examined and managed.
The prevalence and severity of anemia in this study showed an increasing trend with decreasing eGFR value, as observed in previous studies32,33; this finding is pathogenically related to the effects of kidney function on erythropoiesis and hemoglobin production; CKD is a risk factor for anemia development and progression from mild-moderate to severe forms30,34. Impaired iron metabolism, decreased erythropoietin production, and increased hepcidin levels concur to the pathogenesis of anemia in CKD patients14. Diagnosis of anemia in CKD raises a significant burden on individual patients, due to its negative impact on quality of life35,36, increased morbidity and mortality34,37,38, as well as a rising economic burden on health care systems due to increased costs of care39 and management complexity31. Indeed, the Chronic Kidney Disease Outcomes and Practice Patterns Study has shown that lower hemoglobin concentrations were associated with CKD progression and all-cause mortality40; similarly, hemoglobin levels less than 11 g/dl were shown to impact health-related quality of life41, while a positive association with quality of life emerged for hemoglobin levels greater than 12 g/dl40.
The impact of anemia in geriatric populations raises the need for accurate diagnosis and appropriate management; in this regard, international KDIGO guidelines recommend to frequently check iron status levels, in order to choose the best therapeutic option for the individual patient, mainly represented by intravenous/oral iron and erythropoiesis stimulating agents (ESAs); indeed, the presence of low iron stores represents a clear indication to start iron treatment, which was previously shown to decreased need of ESA injection in CKD42. Conversely, in patients with hemoglobin < 10 g/dl and normal iron stores, start of ESA injection should be considered42.
Despite this evidence, however, both replacement and ESA inertia were highly common in the present study. In patients with low iron status, inertia to iron treatment was very common in our study, reaching up the 81.7% of the overall study population, with slightly higher rates in Geriatric vs Nephrology units, and remains high also after excluding patients taking ESAs (74%, 75%, and 71%, respectively); these findings consolidate previous evidence showing that only 10% of anemic patients in general are treated with oral/IV iron7; folate and vitamin B12 inertia were also very common and characterized 87.5% and 50.0% of patients with low serum folate and vitamin B12 levels, respectively.
Furthermore, ESA inertia was also commonly encountered, with an overall prevalence of 67%, and important differences between geriatric (85%) and nephrology (37%) units; as already reported by Minutolo et al.43, inertia to ESA treatment was the second most common therapeutic inertia after iron replacement inertia; reluctance to treat anemia with ESA despite indication may be viewed in light of clinical trials demonstrating an increased risk of cardiovascular mortality and thrombotic events among patients with CKD treated with ESA44,45, that led the KDIGO consortium to consider the individualization of ESA treatment for patients under 10 g/dl of serum hemoglobin. It is not surprising that factors associated with ESA inertia included COPD and cancer, which may increase the production of red blood cells and the overall thrombotic risk, respectively. Inappropriately low ESA use was consistently observed for patients with eGFR between 45 and 60 ml/min, who generally require further assessment following discharge to define more clearly the exact kidney function, more independent of creatinine fluctuations which may occur in the hospital setting. Further research is needed in this field to adapt international guidelines to limit the risk of ESA underprescrition and misuse in older individuals, in order to decrease the prognostic impact of anemia in the geriatric population.
Strengths and limitations
This study has some strengths. First, it relies on a real-world setting population including older and multimorbid patients, with a high polypharmacy rate, that are generally excluded from prospective cohort studies or clinical trials. Additionally, this is the first study conducted in hospitalized older patients and showing a comparative analysis of the prevalence, risk factors, and diagnostic and therapeutic status of anemia in geriatric and nephrology acute care settings. On the other hand, some limitations need to be acknowledged. First, retrospective analyses were conducted on a single set of laboratory values rather than employing repeated measurements and clinical evaluations; consequently, it is not possible to ascertain whether abnormal laboratory parameters resulted from chronic or acute diseases. Second, we cannot exclude that reporting bias related to ICD coding systems has affected the differences in the distribution of diseases between patients with and without anemia; this might account for the unexpected low prevalence of COPD in patients with anemia, which contrasts with current literature. Similarly, the low prevalence of pulmonary embolism and deep vein thrombosis in the overall study population and, consequently, in patients with ESA inertia could be due to the same misreporting bias. Third, we cannot exclude that prevalence of iron, folate, and vitamin B12 assessment was underestimated due to lack of information on laboratory exams performed in the out-of-hospital setting, days or weeks before the hospitalization. Moreover, prevalence of iron deficiency may be underestimated, because of the high prevalence of comorbidities associated with increase in ferritin levels, such as CKD and cancer. However, use of age-adapted ferritin and TSAT thresholds could have blunted the risk of underestimation of iron deficiency in this population. Finally, a part of the increased rates of ESA inertia could be due to the fact that geriatricians might have sent patients to nephrology consultations in order to get ESAs prescribed.
Conclusion
Anemia significantly burdens older individuals discharged from geriatrics and nephrology acute care units, but it its pathogenetic mechanisms are explored in a minority of patients, and undertreatment is also highly prevalent, with selected differences between Geriatrics and Nephrology Units. Considering the detrimental effects of anemia on patients’ overall functioning and quality of life and on healthcare demands and expenses, efforts should be made to increase the attention and to improve the approach to anemia.
Methods
Data collection
The present study uses data from the Italian Society of Nephrology-Italian Society of Gerontology and Geriatrics (SIN-SIGG) study46, an observational, retrospective, cross-sectional study cooperatively funded by the Italian Society of Nephrology (SIN) and the Italian Society of Gerontology and Geriatrics (SIGG); this study aimed at collecting demographic, clinical and pharmacological information in patients aged 65 years or more consecutively admitted to twenty-four geriatric and fifteen nephrology units in Italy within the first half of 2018. The study design complies with the Declaration of Helsinki and Good Clinical Practice Guidelines. The study protocol was approved by the ethics committee of Italian National Institute for Health and Science on Aging (INRCA 18,018). All patients/participants provided their written informed consent to participate in this study.
All records and hospital discharges of patients aged 65 years or more admitted to the participating units have been reviewed without any other specific inclusion criteria. In case of multiple hospitalizations for the same patient during the identified observation period, only the first hospitalization has been included. Patients undergoing dialysis, those discharged within 24 h after hospital admission or who died during hospital stay were excluded from the study.
The sample size consisted of 2,202 patients aged 65 years or more to be initially included in the study. Patients with missing data for prescribed drugs (n = 111), serum creatinine (n = 34), and serum hemoglobin concentration (n = 154) were excluded, thus leaving a final sample of 1,903 patients. Characteristics of the study population were retrospectively retrieved by each medical record in participating institutions and included: demographic data (age, sex, body mass index), number and type of diseases and medications prescribed. All diagnoses were coded according to the International Classification of Diseases ninth Edition (ICD-9 Clinical Modification) system47 and prescribed drugs at admission and discharge were assessed by the Anatomic Therapeutic Chemical (ATC) Classification System48. Polypharmacy was defined as taking 5 or more medications49.
Study variables
All patients underwent laboratory examinations during hospital stay. Anemia was defined as the presence of hemoglobin concentration < 12 g/dL in women and < 13 g/dL in men50. Anemia was further classified in relation to its severity (mild anemia: serum hemoglobin ≥ 10 g/dL; moderate anemia with hemoglobin < 10 and ≥ 8 g/dL; severe anemia with hemoglobin < 8 g/dL) and type of anemia according to mean corpuscular volume (MCV) and mean corpuscular hemoglobin concentration (MCHC) (normocytic normochromic anemia: MCV equals to 80–95 fl and MCHC equals to 32–36 g/dL; microcytic hypochromic anemia: MCV < 80 fl and MCHC < 32 g/dL; macrocytic anemia: MCV > 95 fl; other types)51.
Laboratory parameters were also used to screen for distinct etiologies of anemia in our cohort; in particular, iron status was assessed by using serum ferritin and TSAT; low iron tests were defined as having ≤ 100 ng/mL or serum ferritin < 300 with serum transferrin saturation ≤ 20% according to age-adapted definition intended to increase diagnostic sensitivity in older people52,53. Vitamin B12 deficiency was defined as a serum vitamin B12 < 150 pg/mL18, while low folic acid was defined as having a folic acid ≤ 5 ng/mL54. Hypoalbuminemia, defined as having a albumin concentration less than 3.5 g/dL, was assessed a s a surrogate marker of malnutrition55. Serum creatinine levels were used to assess renal function. The estimated glomerular filtration rate (eGFR) was calculated by using Berlin-Initiative-Study 1 (BIS1) equation56:
where SCr stands for serum creatinine. CKD stages were categorized as follows: eGFR ≥ 60, 45–59.9, 30–44.9, < 30 ml/min/1.73 m2.
We verified whether iron status, vitamin B12, and folic acid were assessed in anemic patients of both admission units, in order to capture the risk of diagnostic inertia. Therapeutic inertia was systematically assessed and defined as omitting to correct iron/B12/folate deficit (replacement inertia) or supplementing ESA when indicated (ESA inertia); indication to ESA prescription was defined as having hemoglobin < 10 g/dl, eGFR < 60 ml/min/1.73 m2 and either normal iron status or low iron status and presence of iron supplementation57,58.
Statistical analysis
Descriptive analysis was first conducted to compare patients with and without anemia regarding demographic, clinical, pharmacological, and laboratory characteristics. Continuous variables were reported as either mean and standard deviation (SD) or median and interquartile range (IQR), according to their distribution evaluated by visual inspection and Kolmogorov–Smirnov test; categorical variables were reported as number and percentage (%). The two-paired sample t-test and Mann–Whitney U test were used to compare continuous categorical variables, while the two-tailed Chi-squared Pearson’s test was used to compare categorical variables. Subgroup descriptive analyses were conducted in geriatrics and nephrology units separately, to account for differences in the prevalence and risk factors for anemia across admission units.
We investigated potential predictors of replacement and ESA inertia, based on previous knowledge from the literature and clinical relevance: demographic characteristics, comorbidities known to be associated with cardiovascular risk and thrombosis (hypertension, atrial fibrillation, CHF, coronary artery disease [CAD], cerebrovascular disease [CVD], diabetes mellitus, chronic obstructive pulmonary disease [COPD], diabetes mellitus, cancer), eGFR and the use of concomitant medications potentially causing anemia (antiplatelets, anticoagulants, PPI, corticosteroids)57,58,59. Assessment of risk factors associated with ESA inertia was also explored by fitting logistic regression models including covariates retrieved by literature review. Collinearity among covariates included in the multivariate models was verified by the Spearman’s rank correlation coefficient and the variance inflation factor (VIF). A p-value < 0.05 was considered statistically significant. To explore the non-linear relationships between ESA inertia and continuous predictors, such as eGFR, albumin, and NLR, we fitted restricted cubic splines with number of knots selected through Akaike’s information criteria (AIC) and according to the best model fit evaluated with Wald Chi-squared test. Statistical analysis was performed using the R version 4.6.
Data availability
The raw data supporting the conclusion of this article will be made available by the authors without undue reservation (contact name Luca Soraci, Unit of Geriatric Medicine, IRCCS INRCA, l.soraci@inrca.it).
References
Girelli, D., Marchi, G. & Camaschella, C. Anemia in the elderly. Hemasphere 2(3), e40. https://doi.org/10.1097/hs9.0000000000000040 (2018).
Stauder, R., Valent, P. & Theurl, I. Anemia at older age: Etiologies, clinical implications, and management. Blood 131(5), 505–514. https://doi.org/10.1182/blood-2017-07-746446 (2018).
Sanford, A. M. & Morley, J. E. Anemia of old age. J. Nutr., Health Aging 23(7), 602–605. https://doi.org/10.1007/s12603-019-1214-x (2019).
Gaskell, H. et al. Prevalence of anaemia in older persons: Systematic review. BMC Geriatr. 8, 1. https://doi.org/10.1186/1471-2318-8-1 (2008).
Zaninetti, C. et al. Prevalence of anemia in hospitalized internal medicine patients: Correlations with comorbidities and length of hospital stay. Eur. J. Intern. Med. 51, 11–17. https://doi.org/10.1016/j.ejim.2017.11.001 (2018).
Zakai, N. A. et al. A prospective study of anemia status, hemoglobin concentration, and mortality in an elderly cohort: the Cardiovascular Health Study. Arch. Intern. Med. 165(19), 2214–2220. https://doi.org/10.1001/archinte.165.19.2214 (2005).
Xu, Y. et al. Poor recognition and undertreatment of anemia in patients with chronic kidney disease managed in primary care. J. Intern. Med. 294(5), 628–639. https://doi.org/10.1111/joim.13702 (2023).
Katsumi, A. et al. Anemia in older adults as a geriatric syndrome: A review. Geriatr. Gerontol. Int. 21(7), 549–554. https://doi.org/10.1111/ggi.14183 (2021).
Goldhaber, G., Segal, G. & Dagan, A. Hyperferritinemia in the elderly can differentiate the bad from the worst: A retrospective cohort analysis. Med. (Baltimore) 99(31), e21419. https://doi.org/10.1097/md.0000000000021419 (2020).
Sezgin, G. et al. Clinical thresholds for diagnosing iron deficiency: comparison of functional assessment of serum ferritin to population based centiles. Sci. Rep. 10(1), 18233. https://doi.org/10.1038/s41598-020-75435-5 (2020).
Dignass, A., Farrag, K. & Stein, J. Limitations of Serum ferritin in diagnosing iron deficiency in inflammatory conditions. Int. J. Chronic Dis. 2018, 9394060. https://doi.org/10.1155/2018/9394060 (2018).
Stevens, L. A. et al. Prevalence of CKD and comorbid illness in elderly patients in the United States: Results from the kidney early evaluation program (KEEP). Am. J. Kidney Dis. 55(3 Suppl 2), S23-33. https://doi.org/10.1053/j.ajkd.2009.09.035 (2010).
Corsonello, A. et al. Clinical implications of estimating glomerular filtration rate with three different equations among older people preliminary results of the project “screening for chronic kidney disease among older people across Europe (SCOPE)”. J. Clin. Med. https://doi.org/10.3390/jcm9020294 (2020).
Portoles, J. et al. Anemia in chronic kidney disease: From pathophysiology and current treatments, to future agents. Front. Med. (Lausanne) 8, 642296. https://doi.org/10.3389/fmed.2021.642296 (2021).
Mikhail, A. et al. Renal association clinical practice guideline on anaemia of chronic kidney disease. BMC Nephrol. 18(1), 345. https://doi.org/10.1186/s12882-017-0688-1 (2017).
Guralnik, J. et al. Unexplained anemia of aging: Etiology, health consequences, and diagnostic criteria. J. Am. Geriatr. Soc. 70(3), 891–899. https://doi.org/10.1111/jgs.17565 (2022).
Tanaka, S. et al. Prevalence, treatment status, and predictors of anemia and erythropoietin hyporesponsiveness in Japanese patients with non-dialysis-dependent chronic kidney disease: a cross-sectional study. Clin. Exp. Nephrol. 26(9), 867–879. https://doi.org/10.1007/s10157-022-02227-8 (2022).
Farrington, D. K. et al. Anemia prevalence, type, and associated risks in a cohort of 5.0 million insured patients in the United States by level of kidney function. Am. J. Kidney Dis. https://doi.org/10.1053/j.ajkd.2022.07.014 (2022).
Rampotas, A., Prodger, C. F. & Murphy, M. F. An assessment of the management of anaemia in acute care settings in the United Kingdom: The value of a collaborative approach. Transfus. Med. 31(5), 322–327. https://doi.org/10.1111/tme.12789 (2021).
Tettamanti, M. et al. Prevalence, incidence and types of mild anemia in the elderly: the “Health and Anemia” population-based study. Haematologica 95(11), 1849–1856. https://doi.org/10.3324/haematol.2010.023101 (2010).
Alsaeed, M. et al. The prevalence and impact of anemia in hospitalized older adults: A single center experience from Bahrain. J Taibah Univ Med Sci 17(4), 587–595. https://doi.org/10.1016/j.jtumed.2022.02.003 (2022).
Randi, M. L. et al. Prevalence and causes of anemia in hospitalized patients: Impact on diseases outcome. J. Clin. Med. https://doi.org/10.3390/jcm9040950 (2020).
Migone De Amicis, M. et al. Anemia in elderly hospitalized patients: Prevalence and clinical impact. Intern. Emerg. Med. 10(5), 581–586. https://doi.org/10.1007/s11739-015-1197-5 (2015).
Guralnik, J. M. et al. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood 104(8), 2263–2268. https://doi.org/10.1182/blood-2004-05-1812 (2004).
Casale, G. et al. Serum ferritin and ageing. Age Ageing 10(2), 119–122. https://doi.org/10.1093/ageing/10.2.119 (1981).
Busti, F. et al. Iron deficiency in the elderly population, revisited in the hepcidin era. Front. Pharmacol. 5, 83. https://doi.org/10.3389/fphar.2014.00083 (2014).
Joosten, E. Iron deficiency anemia in older adults: A review. Geriatr. Gerontol. Int. 18(3), 373–379. https://doi.org/10.1111/ggi.13194 (2018).
López-Sierra, M. et al. Prevalence of anaemia and evaluation of transferrin receptor (sTfR) in the diagnosis of iron deficiency in the hospitalized elderly patients: anaemia clinical studies in Chile. Anemia 2012, 646201. https://doi.org/10.1155/2012/646201 (2012).
Kang, M. et al. Soluble transferrin receptor can predict all-cause mortality regardless of anaemia and iron storage status. Sci. Rep. 12(1), 11911. https://doi.org/10.1038/s41598-022-15674-w (2022).
Minutolo, R. et al. Prevalence, incidence, and treatment of anaemia in patients with non-dialysis-dependent chronic kidney disease: Findings from a retrospective real-world study in Italy. J. Nephrol. 36(2), 347–357. https://doi.org/10.1007/s40620-022-01475-x (2023).
Guedes, M. et al. Management of anemia in nondialysis chronic kidney disease: Current recommendations real-world practice, and patient perspectives. Kidney360 1(8), 855–862 (2020).
Stauffer, M. E. & Fan, T. Prevalence of anemia in chronic kidney disease in the United States. PLoS One 9(1), e84943. https://doi.org/10.1371/journal.pone.0084943 (2014).
Wittbrodt, E. T. et al. Contemporary outcomes of anemia in US patients with chronic kidney disease. Clin. Kidney J. 15(2), 244–252 (2021).
Palaka, E. et al. The impact of CKD anaemia on patients: Incidence, risk factors, and clinical outcomes-a systematic literature review. Int. J. Nephrol. 2020, 7692376–7692376. https://doi.org/10.1155/2020/7692376 (2020).
Grandy, S. et al. Understanding patient perspectives of the impact of anemia in chronic kidney disease: A United States patient survey. J. Patient Exp. 9, 23743735221092628. https://doi.org/10.1177/23743735221092629 (2022).
van Haalen, H. et al. Impact of chronic kidney disease and anemia on health-related quality of life and work productivity: analysis of multinational real-world data. BMC Nephrol. 21(1), 88. https://doi.org/10.1186/s12882-020-01746-4 (2020).
Cai, A. et al. Association of anaemia and all-cause mortality in patients with ischaemic heart failure varies by renal function status. ESC Heart Fail 8(3), 2270–2281. https://doi.org/10.1002/ehf2.13325 (2021).
Sato, Y. et al. Anemia as a risk factor for all-cause mortality: Obscure synergic effect of chronic kidney disease. Clin. Exp. Nephrol. 22(2), 388–394. https://doi.org/10.1007/s10157-017-1468-8 (2018).
Spinowitz, B. et al. Economic and quality of life burden of anemia on patients with CKD on dialysis: A systematic review. J. Med. Econ. 22(6), 593–604. https://doi.org/10.1080/13696998.2019.1588738 (2019).
Mariani, L. et al. The CKD outcomes and practice patterns study (CKDopps): Rationale and methods. Am. J. Kidney Dis. 68(3), 402–413. https://doi.org/10.1053/j.ajkd.2016.03.414 (2016).
de Goeij, M. C. et al. Haemoglobin levels and health-related quality of life in young and elderly patients on specialized predialysis care. Nephrol. Dial. Transplant 29(7), 1391–1398. https://doi.org/10.1093/ndt/gft533 (2014).
Macdougall, I. C. et al. The FIND-CKD study–a randomized controlled trial of intravenous iron versus oral iron in non-dialysis chronic kidney disease patients: background and rationale. Nephrol. Dial Transplant 29(4), 843–850. https://doi.org/10.1093/ndt/gft424 (2014).
Minutolo, R. et al. Anaemia management in non-dialysis chronic kidney disease (CKD) patients: A multicentre prospective study in renal clinics. Nephrol. Dial. Transplant. 28(12), 3035–3045. https://doi.org/10.1093/ndt/gft338 (2013).
Drüeke, T. B. et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl. J. Med. 355(20), 2071–2084. https://doi.org/10.1056/NEJMoa062276 (2006).
Pfeffer, M. A. et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl. J. Med. 361(21), 2019–2032. https://doi.org/10.1056/NEJMoa0907845 (2009).
Aucella, F. et al. A focus on CKD reporting and inappropriate prescribing among older patients discharged from geriatric and nephrology units throughout Italy: A nationwide multicenter retrospective cross-sectional study. Front. Pharmacol. 13, 996042. https://doi.org/10.3389/fphar.2022.996042 (2022).
Iezzoni, L. I. Using administrative diagnostic data to assess the quality of hospital care: pitfalls and potential of ICD-9-CM. Int. J. Technol. Assess Health Care 6(2), 272–281 (1990).
Miller, G. & Britt, H. A new drug classification for computer systems: The ATC extension code. Int. J. Bio-Med. Comput. 40(2), 121–124 (1995).
Gnjidic, D. et al. Polypharmacy cutoff and outcomes: Five or more medicines were used to identify community-dwelling older men at risk of different adverse outcomes. J. Clin. Epidemiol. 65(9), 989–995. https://doi.org/10.1016/j.jclinepi.2012.02.018 (2012).
Baker, S. & DeMaeyer, E. M. Nutritional anemia: Its understanding and control with special reference to the work of the World Health Organization. Am. J. Clin. Nutr. 32(2), 368–417 (1979).
Cappellini, M. D. & Motta, I. Anemia in clinical practice-definition and classification: Does hemoglobin change with aging?. Semin. Hematol. 52(4), 261–269. https://doi.org/10.1053/j.seminhematol.2015.07.006 (2015).
Fernández-Rodríguez, A. M. et al. Diagnosis of iron deficiency in chronic renal failure. Am. J. Kidney Dis. 34(3), 508–513. https://doi.org/10.1016/s0272-6386(99)70079-x (1999).
Thomas, D. W. et al. Guideline for the laboratory diagnosis of functional iron deficiency. Br. J. Haematol. 161(5), 639–648. https://doi.org/10.1111/bjh.12311 (2013).
Clarke, R. et al. Vitamin B12 and folate deficiency in later life. Age and Ageing 33(1), 34–41. https://doi.org/10.1093/ageing/afg109 (2004).
Soeters, P. B., Wolfe, R. R. & Shenkin, A. Hypoalbuminemia: Pathogenesis and clinical significance. JPEN J. Parenter Enteral Nutr. 43(2), 181–193. https://doi.org/10.1002/jpen.1451 (2019).
Schaeffner, E. S. et al. Two novel equations to estimate kidney function in persons aged 70 years or older. Ann. Intern. Med. 157(7), 471–481. https://doi.org/10.7326/0003-4819-157-7-201210020-00003 (2012).
Inker, L. A. et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am. J. Kidney Dis. 63(5), 713–735. https://doi.org/10.1053/j.ajkd.2014.01.416 (2014).
Drüeke, T. B. & Parfrey, P. S. Summary of the KDIGO guideline on anemia and comment: Reading between the (guide)line(s). Kidney Int. 82(9), 952–960. https://doi.org/10.1038/ki.2012.270 (2012).
Del Vecchio, L. & Minutolo, R. ESA, iron therapy and new drugs: Are there new perspectives in the treatment of anaemia?. J. Clin. Med. https://doi.org/10.3390/jcm10040839 (2021).
Acknowledgements
The authors thank the Italian Society of Gerontology and Geriatrics (SIGG) and the Italian Society of Nephrology (SIN) collaborators of the participating geriatrics and nephrology units (SIN-SIGG group, supplementary information).
Funding
This work was partially supported by the Ricerca Corrente funding from the Italian Ministry of Health to IRCCS INRCA.
Author information
Authors and Affiliations
Contributions
L.S., A.D.V., A.C., F.A., and R.A.I. conceptualized and prepared the manuscript; L.S. and P.F. performed the statistical analysis; all authors reviewed the manuscript and the figures. All authors declare to have no conflict of interest to disclose.
Corresponding author
Ethics declarations
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Soraci, L., de Vincentis, A., Aucella, F. et al. Prevalence, risk factors, and treatment of anemia in hospitalized older patients across geriatric and nephrological settings in Italy. Sci Rep 14, 19721 (2024). https://doi.org/10.1038/s41598-024-70644-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-70644-8
- Springer Nature Limited