Abstract
The anatomical features spanning from the aortic arch to the proximal carotid artery and the associated cardiovascular risks might significantly influence the development of right carotid plaque. Our research aimed to compare these anatomical and risk factors between individuals with no carotid plaque and those with moderate right-side carotid plaque within a Korean cohort. We conducted a retrospective, cross-sectional analysis involving 413 participants, categorized into a normal group (n = 339) and a right moderate carotid plaque group (defined as > 50% stenosis based on NASCET criteria) (n = 74). We collected data on cardiovascular risk factors and conducted laboratory tests. A 3D model of the carotid artery was constructed using cranio-cervical computed tomography angiography (CTA) data through semi-automated software. Measurements taken on this 3D model included the common carotid artery (CCA), internal carotid artery (ICA), external carotid artery (ECA), and carotid artery bifurcation (CAB) in terms of maximal vascular diameter, sectional area, angles of carotid bifurcation and ICA, and carotid tortuosity. When compared with the normal group, individuals in the right moderate carotid plaque group exhibited smaller angles at the carotid bifurcation, larger CCA diameter and sectional area (p < 0.01), advanced age, and a higher incidence of hypertension, diabetes, and stroke history (p < 0.05), along with reduced glomerular filtration rate (GFR) (p < 0.001). Multivariate analysis revealed that the sectional area of the bifurcation, calcification of the aortic bulb, and GFR were independently associated with the presence of right moderate carotid plaque (p < 0.01). Statistical analyses disclosed significant differences in both clinical risk factors and geometric changes in the region extending from the aortic arch to the proximal carotid artery among subjects with right moderate carotid plaque when compared to those without.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
The connection between atherosclerotic conditions in large arteries, such as the carotid artery, and stroke is crucial, as arterial plaques are responsible for nearly 25% of stroke occurrence1. The bifurcation of the carotid artery (CAB) emerges as the most prevalent location for plaque development2, 3. Moreover, the internal carotid artery’s (ICA) lateral wall, specifically at a bifurcation, is the favored site for plaque accumulation, largely due to the reduced wall shear stress, which facilitates plaque formation4, 5. These findings underscore the significance of hemodynamic factors in the genesis and advancement of atherosclerotic plaques. Additionally, an asymmetry in the prevalence of carotid plaques is noticed, despite both carotid sides being subjected to identical genetic, environmental, and risk factors6, 7. Regarding anatomical attributes, it has been demonstrated that the diameter, cross-sectional area, and flow ratio of the carotid artery exhibit substantial individual variations6, 8. The phenomena of carotid tortuosity and dilation are also more observed among the aged population9. Consequently, the anatomical and geometrical parameters of the carotid artery could potentially be linked to the pathogenesis of carotid plaques. Nonetheless, the majority of research on carotid geometry has primarily concentrated on comparisons between young and old patients without atherosclerosis, with even fewer studies examining the effect of risk factors in individuals8. Hence, we postulated that anatomical and geometric indices of the carotid artery, alongside risk factors for atherosclerotic disease, might exhibit a connection with the incidence of carotid plaque. Our research explored the potential association between the geometry of the right carotid artery, imaging outcomes, laboratory results, and the presence of moderate carotid plaques on the right side.
Materials and methods
Study population
The protocols for this study were adhered to ethical standards and complied with the guidelines of our university’s institutional review board. From January 2020 through December 2022, a total of 843 subjects, complete with clinical and laboratory data, underwent computed tomography angiography (CTA) from the aortic arch to intracranial vessels for neurovascular disease assessment. Out of these, 249 subjects were excluded due to the incomplete examination of the carotid artery caused by contrast interference from the subclavian or jugular vein. Additionally, 181 subjects were excluded for having less than 50% carotid stenosis plaque (n = 98) or a history of relevant vascular conditions: previous carotid interventions like stent insertion or surgery (n = 31), complete occlusion of the proximal ICA (n = 11), significant plaque in the right CCA (n = 8), carotid artery torsion due to chronic pulmonary tuberculosis or prior thoracic surgery (n = 18), past surgery of the ascending aorta (n = 6), or ICA collapse owing to Moyamoya disease or intracranial atherosclerosis (n = 9). Ultimately, 339 subjects devoid of carotid plaques and 74 subjects with moderate right carotid plaque (> 50% stenosis, according to the North American Symptomatic Carotid Endarterectomy Trial - NASCET criteria10) were included in this study.
CTA imaging protocol
CTA was executed using a dual-source CT system (SOMATOM Definition Flash; Siemens Healthcare, Erlangen, Germany). An initial non-contrast CT scan covered the region from the foramen magnum to the vertex. CTA followed, extending from the ascending aorta to the intracranial arteries, utilizing a contrast agent (Ultravist; Schering, Berlin, Germany) injected at a 4 ml/sec rate, succeeded by a 30 ml saline flush. The imaging parameters were established as follows: for non-contrast CT, 120 kV and 280–320 mA, with 5.0 mm slice reconstruction; for CTA, 100–120 kV and 260–300 mA, with 1.0 mm slice reconstruction and 5 mm maximum intensity projection (MIP) reconstructions 1 mm increments.
3D reconstruction of carotid artery
Digital Imaging and Communications in Medicine (DICOM) files of cranio-cervical CT angiography (Fig. 1A) were imported into semi-automated software (Materialise Mimics v25.0; Materialise NV, Leuven, Belgium, https://www.materialise.com) for arterial tree segmentation. CTA data processing included thresholding, region growing, and a split mask function, consistent with prior studies, to ensure optimal visualization11. A 3-dimensional geometric surface model of vascular reconstruction extended from the aortic arch to brachiocephalic artery, right common carotid artery, CAB, ICA, and external carotid artery (ECA) (Fig. 1B). Geometric parameters were fine-tuned for an “optimal resolution” that precisely captured flow dynamics within CAB’s curves and branches. Following this, the surface was smoothed to reduce noise and simplify central line computation (Fig. 1B).
(A) Cranio-cervical CTA was used to segment arteries from the aortic arch to the intracranial arteries. (B) The semi-automated software MIMICS was used to regenerate 3D reconstructions and central lines of the arterial tree. (C) Measurements of diameter and sectional area were taken at the carotid bifurcation, ECA, ICA, and CCA at a 2 cm-point from the carotid bifurcation. ICA internal carotid artery, CCA common carotid artery, ECA external carotid artery, CTA computed tomography angiography.
Measurement of carotid artery geometry
The geometry and anatomy of the right carotid were evaluated using a 3D reconstructed vessel model from CTA data. Central lines, tailored to the vascular lumen extending from the CCA to the ICA and ECA, facilitated locating the maximal sphere radius centers within each vessel (Fig. 1B). Utilizing these spheres’ radii and central line paths, the nominal bifurcation plane and its origins were determined, allowing the vessel’s subdivision into its three branches. Using the central lines, diameters, and sectional areas of the ECA, ICA, and CCA were measured at a 2-cm distance from CAB (Fig. 1C).
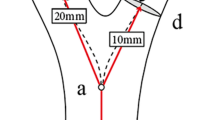
The carotid bifurcation angle was identified by the angle between ICA and ECA vector projections on the carotid bifurcation plane (Fig. 2). The ICA angle correlated with the angle formed by CCA and ICA vectors on this plane. The tortuosity, from the brachiocephalic artery to the bifurcation and from CCA to carotid bifurcation, was quantified as the length ratio of the actual vessel path to the distance between vessel endpoints12 (Fig. 2). Tortuosity index (TI) for each segment was calculated using MIMICS.
Other study variables
Diabetes mellitus was classified based on either a fasting glucose concentration ≥ 7.0 mmol/L, non-fasting glucose concentration ≥ 11.1 mmol/L, diabetes management medication use, or self-reported diabetes. Hypertension identification criteria were a systolic blood pressure ≥ 140 mm Hg, a diastolic blood pressure ≥ 90 mm Hg, or the usage of antihypertensive drugs. Dyslipidemia was determined by total cholesterol levels ≥ 5.0 mmol/L in patients with cardiovascular history and ≥ 6.0 mmol/L in those without. Smoking status was specifically attributed to current smokers to distinguish them from former or non-smokers. Glomerular filtration rate (GFR) estimation involved age, sex, ethnicity, and serum creatinine concentration, using the Modification of Diet in Renal Disease (MDRD) Study Eq. 13 .
Statistical analysis
Statistical analyses were performed with SPSS version 23.0 (IBM Corp., Armonk, NY). Continuous variables were reported as mean ± standard deviation. The variance in risk factors, as well as geometric and anatomic indexes between subjects with normal carotid and those with moderate plaque, was evaluated using Student’s t-test. Both univariate and multivariate logistic regression models were utilized to ascertain the probability of developing moderate right side carotid plaque. A p-value less than 0.05 was considered statistically significant.
Institutional review board statement
Conducted in alignment with the principles of the Declaration of Helsinki, this investigation was sanctioned by the Institutional Review Board of Jeonbuk National Hospital (JUH).
Informed consent statement
Prior to participation in this forward-looking study, participants provided informed consent, following institutional review board approval for the MR scope of the study.
Results
Demographic data, carotid CTA imaging outcomes, and risk factors for atherosclerosis between the normal group and the right moderate carotid plaque group are compiled in Table 1. Among the demographic variables, a notable difference in age was observed between the two groups (p < 0.001). Specifically, individuals with right moderate carotid plaques were older on average (74.81 ± 1.04 years) compared to those in the normal group (67.84 ± 0.67 years). In terms of atherosclerosis risk factors, the moderate carotid plaque group exhibited significantly higher rates of hypertension (67.6% versus 48.4%), diabetes (31.1% versus 20.1%), and history of stroke (43.2% versus 27.8%) (p < 0.05). Additionally, this group showed increased instances of aortic arch calcification (44.6% versus 15%) and carotid bulb calcification (63.5% versus 2%) as detected in CT images (p < 0.001).
Table 2 illustrates the disparities in carotid geometric parameters between the groups. Notably, patients with right moderate carotid plaques had a wider CCA diameter (7.68 ± 0.11 vs. 7.37 ± 0.05 mm) and larger sectional area (46.8 ± 1.44 vs. 43.2 ± 0.53 mm2) compared to the normal group (p < 0.05). The diameter and sectional area of the carotid bifurcation in the plaque group (10.47 ± 0.24 mm and 82.8 ± 3.19 mm2, respectively) were smaller compared to the normal group (11.02 ± 0.14 mm and 90.58 ± 1.42 mm2, respectively) (P < 0.05).
In terms of laboratory observations, significant discrepancies were found in blood urea nitrogen (BUN), serum creatinine, and GFR between the two groups. Specifically, the plaque group had a reduced GFR compared to the normal group (69.46 ± 23.52 vs. 84.90 ± 22.55 mL/min) (p < 0.05) (Table 3).
Multivariable regression analysis identified aortic arch calcification (odds ratio (OR), 4.177; 95% confidence interval (CI) 1.686–10.347), carotid bulb calcification (OR, 87.199; 95% CI 31.296–238.165), right carotid bifurcation sectional area (OR, 0.966 per 1 mm2 increase; 95% CI 0.942–0.991), and GFR (OR, 0.961 per 1 ml/min increase; 95% CI 0.934–0.989) as independent predictors (p < 0.05) of right moderate carotid artery plaque (Table 4).
Discussion
Our study juxtaposed demographic, geometric, imaging, and laboratory datasets between normal individuals and those exhibiting right -side moderate carotid plaque. The findings elucidated significant disparities across various parameters among subjects with right carotid plaque.
Subjects with right moderate carotid plaques exemplified a correlation with advanced age when contrasted with the normal cohort. The ageing phenomenon contributes to escalated vessel rigidity and stiffness14, deriving from endothelial dysfunction triggered by oxidative stress and localized inflammatory processes15, 16. Moreover, the medial wall of large arteries, comprising smooth muscle cells, collagen, and elastic fibers, undergoes through age-related structural and functional changes, predisposing to plaque accrual. The degeneration-induced stiffening of elastic fibers further augments endothelial dysfunction in older demographics17. Complementary investigations have illuminated a decline in the density and continuity of circumferential and longitudinal elastin in carotid arteries, potentially facilitating passive dilatation and increased arterial wall rigidity18, 19.
Regarding CAB/CCA diameter and sectional area, the plaque-affected group presented with diminished bifurcation and an enlarged CCA relative to the normal group. While it may appear that bifurcation parameters and carotid stenosis measurements could converge, diverse methodologies were employed to ascertain the optimal fit diameter and sectional area of the carotid artery, minimizing similar measurement acquisition. The association of smaller CAB and larger CCA with carotid stenosis intimates that the unique anatomical configuration of the carotid artery may predispose the area to flow disturbances, thereby facilitating plaque development. Jiang et al.’s study reinforced this by correlating reduced luminal expansion at carotid bifurcations with vulnerable carotid plaques20. Other research suggests that reduced bifurcation expansion correlates with flow disturbance and elevated wall shear stress21, 22, predisposing arteries to plaque formation. Nevertheless, the primary pathogenic mechanisms diverge from classic atherosclerosis pathology, necessitating advanced investigations, possibly through computational fluid dynamics or 4D flow magnetic resonance imaging (MRI), to elucidate the nexus between bifurcation geometry and plaque development.
Our analysis also highlighted a significant association between the right CCA diameter and sectional area at the 2 cm-point from the bifurcation and the presence of ipsilateral moderate plaque. The increased CCA diameter observed in the plaque group could stem from bifurcation stenosis, where heightened intraluminal pressure distal to carotid stenosis may precipitate distal CCA enlargement, as demonstrated in a preceding study23.
Furthermore, GFR levels in patients with moderate carotid plaques were markedly lower compared to normal subjects (69.46 ± 23.52 versus 84.90 ± 22.55 mL/min, p < 0.001). Despite chronic kidney disease not being recognized in the Framingham score as a cardiovascular risk factor24, reduced GFR has been substantiated to correlate with an elevated risk of cardiovascular events. The research by Tonelli et al. advocates for the inclusion of chronic kidney disease in the criteria delineating patients at heightened risk for coronary events25. Another study within a Chinese cohort with normal to mildly decreased kidney function identified a significant relationship between GFR and the 10-year risk of atherosclerotic cardiovascular disease26. In chronic kidney disease scenarios, an expedited atherosclerotic process is noted, marked by early atherosclerotic plaque formation and arterial wall thickening due to calcification27.
Following both univariate and multivariate logistic regression analyses, several variables were identified as independently correlated with the presence of right carotid moderate plaque, including aortic arch calcification, carotid bulb calcification, carotid bifurcation sectional area, and GFR. These findings align with other studies indicating a strong correlation between systemic arterial calcification (aortic valve, carotid, and peripheral artery) and low GFR28. Additional research noted a link between carotid artery distensibility coefficient or arterial wall stiffness, as assessed via B-mode ultrasound, and thoracic aorta calcification29. As elaborated upon, chronic kidney disease accelerates the atherosclerotic process through myriad mechanisms, encompassing oxidative stress, endothelial dysfunction, inflammation, toxic effluents, and albuminuria30. These pathophysiological pathways contribute to systemic arterial calcification, culminating in atherosclerosis across critical arterial systems, including coronary, carotid, intracranial, and peripheral arteries31. Given the multifaceted mechanism of plaque development, integrating geometric, imaging, and laboratory data into a predictive model for carotid plaque enhances the practical applicability and viability of our findings.
Nonetheless, our study is subject to several limitations. Firstly, the retrospective and cross-sectional design may introduce selection bias. Conducted at a tertiary hospital, our patient cohort typically manifests greater comorbidities and heightened vascular risk compared to the general population. To mitigate this limitation, an extensive sample size exceeding 1900 cases was employed in the initial data screening, aiming to diminish bias. Secondly, our cross-sectional study design inadequately captures the long-term impact of cardiovascular risk factors on carotid plaque development, thereby emphasizing the need for a prospective, longitudinal study to unravel detailed mechanisms. Thirdly, the Glagov phenomenon might introduce bias due to the exclusive reliance on CTA for lumen measurement32. While CTA facilitates relatively straightforward assessment of the carotid lumen, distinguishing arterial wall and plaque proves challenging due to similar density with surrounding tissue. Consequently, a future study amalgamating CTA with vessel wall imaging, either through ultrasound or MRI, appears imperative.
In summation, right moderate carotid plaque exhibits independent associations with carotid bifurcation and CCA sectional area, alongside aortic arch and carotid bulb calcification and diminished GFR in high cardiovascular risk patients. These intrinsic variables warrant consideration in evaluating carotid atherosclerosis development.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Phan, T. G., Brown, R. D. & D, W. Asymptomatic carotid stenosis. In: Noseworthy J, ed. Neurological Therapeutics: Principles and Practice (Martin Dunitz, 2003).
Malek, A. M., Alper, S. L. & Izumo, S. Hemodynamic Shear Stress and its role in Atherosclerosis. JAMA 282, 2035–2042. https://doi.org/10.1001/jama.282.21.2035 (1999).
Wasilewski, J. et al. The role of septal perforators and myocardial bridging effect in atherosclerotic plaque distribution in the coronary artery disease. Pol. J. Radiol. 80, 195–201. https://doi.org/10.12659/pjr.893227 (2015).
Gnasso, A. et al. In vivo association between low wall shear stress and plaque in subjects with asymmetrical carotid atherosclerosis. Stroke 28, 993–998. https://doi.org/10.1161/01.str.28.5.993 (1997).
Shaaban, A. M. & Duerinckx, A. J. Wall shear stress and early atherosclerosis: A review. AJR Am. J. Roentgenol. 174, 1657–1665. https://doi.org/10.2214/ajr.174.6.1741657 (2000).
Schulz, U. G. & Rothwell, P. M. Major variation in carotid bifurcation anatomy: A possible risk factor for plaque development?. Stroke 32, 2522–2529. https://doi.org/10.1161/hs1101.097391 (2001).
Koch, S., Nelson, D., Rundek, T., Mandrekar, J. & Rabinstein, A. Race-ethnic variation in carotid bifurcation geometry. J. Stroke Cerebrovasc. Diseases: Official J. Natl. Stroke Assoc. 18, 349–353. https://doi.org/10.1016/j.jstrokecerebrovasdis.2009.01.002 (2009).
Thomas, J. B. et al. Variation in the carotid bifurcation geometry of young versus older adults: Implications for geometric risk of atherosclerosis. Stroke 36, 2450–2456. https://doi.org/10.1161/01.STR.0000185679.62634.0a (2005).
Jeon, S. J., Kwak, H. S. & Chung, G. H. Widening and rotation of carotid artery with age: Geometric approach. J. Stroke Cerebrovasc. Dis. Official J. Natl. Stroke Assoc. 27, 865–870. https://doi.org/10.1016/j.jstrokecerebrovasdis.2017.10.026 (2018).
Ferguson, G. G. et al. The North American symptomatic carotid endarterectomy trial. Stroke 30, 1751–1758. https://doi.org/10.1161/01.STR.30.9.1751 (1999).
Mokin, M. et al. Semi-automated measurement of vascular tortuosity and its implications for mechanical thrombectomy performance. Neuroradiology 63, 381–389. https://doi.org/10.1007/s00234-020-02525-6 (2021).
Spencer, M. P. & Reid, J. M. Quantitation of carotid stenosis with continuous-wave (C-W) doppler ultrasound. Stroke 10, 326–330. https://doi.org/10.1161/01.str.10.3.326 (1979).
Levey, A. S. et al. Using Standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann. Intern. Med. 145, 247–254. https://doi.org/10.7326/0003-4819-145-4-200608150-00004 (2006).
Tesauro, M. et al. Arterial Ageing: From endothelial dysfunction to vascular calcification. J. Intern. Med. 281, 471–482. https://doi.org/10.1111/joim.12605 (2017).
Simioni, C. et al. Oxidative stress: Role of physical exercise and antioxidant nutraceuticals in adulthood and aging. Oncotarget 9, 17181–17198. https://doi.org/10.18632/oncotarget.24729 (2018).
Vgontzas, A. N. et al. Impaired Nighttime sleep in healthy old versus young adults is associated with elevated plasma interleukin-6 and cortisol levels: physiologic and therapeutic implications. J. Clin. Endocrinol. Metab. 88, 2087–2095. https://doi.org/10.1210/jc.2002-021176 (2003).
Jacob, M. P. Extracellular Matrix remodeling and matrix metalloproteinases in the vascular wall during aging and in pathological conditions. Biomed. Pharmacother. 57, 195–202. https://doi.org/10.1016/s0753-3322(03)00065-9 (2003).
O’Rourke, M. F., Safar, M. E. & Nichols, W. W. Proximal aortic diameter and aortic pressure-flow relationship in systolic hypertension. Circulation. 109, e227–228. https://doi.org/10.1161/01.Cir.0000128539.55559.3d (2004).
Kamenskiy, A. V., Pipinos, I. I., Carson, J. S., MacTaggart, J. N. & Baxter, B. T. Age and disease-related geometric and structural remodeling of the carotid artery. J. Vasc. Surg. 62, 1521–1528. https://doi.org/10.1016/j.jvs.2014.10.041 (2015).
Jiang, P. et al. Association between carotid bifurcation geometry and atherosclerotic plaque vulnerability. Arterioscler. Thromb. Vasc. Biol. 40, 1383–1391. https://doi.org/10.1161/ATVBAHA.119.313830 (2020).
Lee, S. W., Antiga, L., Spence, J. D. & Steinman, D. A. Geometry of the carotid bifurcation predicts its exposure to disturbed flow. Stroke 39, 2341–2347. https://doi.org/10.1161/STROKEAHA.107.510644 (2008).
Bijari, P. B., Antiga, L., Gallo, D., Wasserman, B. A. & Steinman, D. A. Improved Prediction of disturbed flow via hemodynamically-inspired geometric variables. J. Biomech. 45, 1632–1637. https://doi.org/10.1016/j.jbiomech.2012.03.030 (2012).
Bladin, C. F. et al. The clinical value of methods to measure carotid stenosis. Int. Angiol. 15, 295–299 (1996).
D’Agostino, R. B. et al. General cardiovascular risk profile for use in primary care. Circulation 117, 743–753. https://doi.org/10.1161/CIRCULATIONAHA.107.699579 (2008).
Tonelli, M. et al. Risk of Coronary events in people with chronic kidney disease compared with those with diabetes: A population-level cohort study. Lancet 380, 807–814. https://doi.org/10.1016/S0140-6736(12)60572-8 (2012).
Chen, F., Liu, J., Han, S. & Xu, T. Association between 10-year atherosclerotic cardiovascular disease risk and estimated glomerular filtration rate in chinese people with normal to slightly reduced kidney function: A cross-sectional study. Int. J. Environ. Res. Public Health 19 (2022).
Campean, V. et al. Atherosclerosis and vascular calcification in chronic renal failure. Kidney Blood Press. Res. 28, 280–289. https://doi.org/10.1159/000090182 (2005).
Arita, Y. et al. Association of Aortic valve calcification with carotid artery lesions and peripheral artery disease in patients with chronic kidney disease: A cross-sectional study. BMC Nephrol. 21, 203. https://doi.org/10.1186/s12882-020-01864-z (2020).
Blaha, M. J. et al. Relationship of carotid distensibility and thoracic aorta calcification. Hypertension 54, 1408–1415. https://doi.org/10.1161/HYPERTENSIONAHA.109.138396 (2009).
Poznyak, A. V. et al. Atherosclerosis Specific features in chronic kidney disease (CKD). Biomedicines 10 (2022).
Li, Y. et al. Role of Macrophages in the progression and regression of vascular calcification. Front. Pharmacol. 11 (2020).
Glagov, S., Weisenberg, E., Zarins, C. K., Stankunavicius, R. & Kolettis, G. J. Compensatory enlargement of human atherosclerotic coronary arteries. N. Engl. J. Med. 316, 1371–1375. https://doi.org/10.1056/nejm198705283162204 (1987).
Funding
This study received funding from the Basic Science Research Program through the National Research Foundation of Korea (NRF), supported by the Ministry of Education (NRF-2022R1A6A3A01086735).
Author information
Authors and Affiliations
Contributions
DHAN.; manuscript—initial draft preparation, DHAN and UYL.; quantitative data analysis, HSK.; grant funding acquisition and manuscript review All authors have perused and consented to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hong An Ngo, D., Lee, U.Y. & Kwak, H.S. Geometric changes and clinical risk factors from aortic arch to proximal internal carotid artery between normal subjects and moderate right carotid plaques. Sci Rep 14, 19632 (2024). https://doi.org/10.1038/s41598-024-70653-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-70653-7
- Springer Nature Limited