Abstract
Previous studies have revealed that thyroid hormone (TH) levels are associated with the risk of diabetic peripheral neuropathy (DPN) in euthyroid patients with type 2 diabetes mellitus (T2DM). However, the relationship between TH sensitivity, a complementary method for assessing thyroid function, and DPN remains unclear. This study aimed to investigate the correlation between DPN and TH sensitivity in euthyroid patients with T2DM. Exactly 708 euthyroid adults with T2DM were retrospectively enrolled. The FT3/FT4 ratio was used to estimate peripheral TH sensitivity. Central TH sensitivity was assessed using the Thyrotroph T4 Resistance Index (TT4RI), Thyroid-Stimulating Hormone Index (TSHI), Thyroid Feedback Quantile-based Index (TFQI), and Parametric TFQI (PTFQI). DPN was assessed using neurologic symptoms, signs, and nerve conduction velocity tests. The relationship between DPN and TH sensitivity was examined using logistic regression analysis. We observed that an elevated FT3/FT4 ratio was associated with DPN (OR = 1.36, 95%CI: 1.13–1.63, p = 0.0012). For each standard deviation (SD) increase in the TT4RI, TSHI, TFQI, and PTFQI, the OR of DPN was 0.80 (95%CI: 0.68–0.94, p = 0.0078), 0.72 (95%CI: 0.60–0.86, p = 0.0002), 0.69 (95%CI: 0.58–0.83, p < 0.0001), and 0.69 (95%CI: 0.58–0.82, p < 0.0001), respectively. These results suggested that reduced central and peripheral TH sensitivity is associated with a decreased risk of developing DPN.
Similar content being viewed by others
Introduction
Based on the World Health Organization (WHO) standards, the prevalence of diabetes mellitus has reached as high as 12.4% in China, with type 2 diabetes mellitus (T2DM) accounting for over 90% of the cases1. As diabetes progresses, many patients develop complications, which are the leading cause of disability and death. Diabetic peripheral neuropathy (DPN), a condition characterized by peripheral nerve injury and dysfunction caused by prolonged hyperglycemia, is a common complication of T2DM, affecting approximately 30% of patients with the condition2. It mainly presents as symmetrical sensory abnormalities in the lower limbs, including burning and pins-and-needles sensations, which can lead to diabetic foot and may require amputation in severe cases3. Managing DPN involves optimizing glycemic control and addressing various risk factors such as hypertension and dyslipidemia. Still, there is currently no disease-modifying treatment for DPN4. Therefore, identifying new risk factors for DPN is important for improving prevention and treatment strategies.
Thyroid hormones (THs) are crucial in regulating glucose and lipid metabolism. Earlier studies have identified specific correlations between TH levels and diabetes and its complications. Previous studies revealed that in euthyroid patients with T2DM, serum-free triiodothyronine (FT3) and free thyroxine (FT4) levels were negatively correlated with the prevalence of DPN, and thyroid-stimulating hormone (TSH) levels were positively correlated5,6. Some studies also suggested that elevated FT4 levels increase the risk of developing DPN and that there is no correlation between TSH and DPN occurrence7.
As TH levels alone do not stably reflect thyroid function, TH sensitivity can be used as a complementary index to assess it. The FT3/FT4 ratio can be used to assess peripheral TH sensitivity. Moreover, indices that respond to central TH sensitivity include thyrotroph T4 resistance index (TT4RI), thyroid-stimulating hormone index (TSHI), thyroid feedback quantile-based index (TFQI), and parametric TFQI (PTFQI).
Previous research has reported that TH sensitivity is strongly associated with T2DM, diabetic nephropathy (DN), and diabetic retinopathy (DR)7,8,9. However, there is only one study on peripheral TH sensitivity and DPN, and there is a lack of studies on the correlation between central TH sensitivity and DPN10. Therefore, our study aimed to assess the correlation between TH sensitivity and DPN in euthyroid patients with T2DM.
Method
Participants
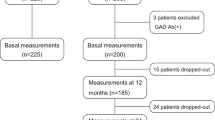
Exactly 1136 adults with T2DM who were enrolled in the MMC program at the First Hospital of Jilin University from May 2017 to April 2019 were selected. Based on the 1999 WHO diagnostic criteria, all participants were diagnosed with T2DM11. The exclusion criteria were as follows: (1) patients with acute diabetic complications; (2) individuals with thyroid diseases and use of thyroid medications; individuals with pituitary, parathyroid, or adrenal gland dysfunction; and individuals taking corticosteroids; (3) FT3, FT4, and TSH levels outside the normal range; (4) those with missing data on thyroid function; (5) patients with peripheral neuropathy caused by other reasons, such as cervical and lumbar spondylosis, Guillain–Barre syndrome, stroke, cerebral hemorrhage, vitamin B12 deficiency, and severe arteriovenous vascular disease; (6) patients with other serious illnesses, such as malignant tumors, acute infections, severe hepatic and renal insufficiency, and severe mental and psychological illnesses. Ultimately, 708 patients were included in this study (Fig. 1).
Physical examination and laboratory measurements
A retrospective analysis of the clinical information of 708 patients was conducted. Information regarding the weight, height, body mass index (BMI), sex, age, blood pressure, waist circumference (WC), whether the patients smoked or drank alcohol, duration of T2DM, complications of T2DM, use of lipid-lowering agents, use of antihypertensive medications, and use of glucose-lowering medications were recorded in detail.
Venous blood was obtained from all participants the following morning after fasting for more than 8 h. The measured biochemical parameters comprised the triglyceride glucose (TyG) index and the triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), glycosylated hemoglobin (HbA1c), fasting blood glucose (FBG), FT4, FT3, and TSH levels.
The normal reference ranges were 0.27–4.2 mIU/L for TSH, 12–22 pmol/L for FT4, and 3.1–6.8 pmol/L for FT3. Dyslipidemia was defined as meeting any of the following criteria: (1) LDL-C ≥ 3.4 mmol/L; (2) TC ≥ 5.2 mmol/L; (3) HDL-C < 1.04 mmol/L; (4) TG ≥ 1.7 mmol/L; and (5) the use of lipid-lowering drugs. Hypertension was defined as a prior diagnosis of hypertension, current use of antihypertensive drugs, or elevated blood pressure. The formula for the TyG index was: Ln[fasting triglycerides (mg/dl) × fasting glucose (mg/dl)/2]12.
The formulas for the TH sensitivity index included the following:
-
(1)
FT3/FT4 = FT3 (pmol/L)/FT4 (pmol/L), which represents the efficiency of converting FT4 to FT3, reflecting peripheral sensitivity to THs. Elevated ratios reflect increased peripheral TH sensitivity13.
-
(2)
TT4RI = FT4 (pmol/L) × TSH (mIU/L), which relates TSH to FT4 without introducing an additional coefficient. Higher TT4RI values indicate lower central TH sensitivity14.
-
(3)
TSHI = lnTSH (mIU/L) + 0.1345 × FT4 (pmol/L), which reflects the feedback inhibition of FT4 for TSH. Higher TSHI values reflect lower central TH sensitivity15.
-
(4)
TFQI = cdfFT4 − (1 − cdfTSH). The index varies within the range of -1 to 1, with a negative value suggesting increased central TH sensitivity and a positive value suggesting decreased central TH sensitivity.
-
(5)
PTFQI has the same scope and interpretation as TFQI. It can be applied to different populations. In this study, we used the Chinese-referenced PTFQI9,16.
Definition of DPN
Neurological symptoms, signs, and nerve conduction velocities were used to diagnose DPN. The Toronto Clinical Scoring System (TCSS) was used to assess neurological signs and symptoms17. The patients were asked if they had symptoms such as numbness, pins and needles, weakness, and unsteady gait. Knee and ankle reflexes were examined. Pain, touch, temperature, vibration, and position sensations were evaluated. The TCSS score ranged from 0 to 19, with scores of 0–5 indicating no DPN and scores above 6 indicating DPN.
Nerve conduction velocity (NCV) was detected using electromyography. The patients were awake and relaxed, ensuring the local skin temperature was > 31℃. Electrical stimulation was applied to the patients, and the motor and sensory NCV, terminal latency, and wave amplitude were recorded from the ulnar, median, common peroneal, and tibial nerves. The NCV decline threshold was determined based on the Chinese NCV reference standard18.
Statistical analysis
The associations between FT3/FT4, PTFQI, TFQI, TSHI, TT4RI, and DPN were investigated using multivariable regression models. Model 1 was adjusted for sex and age. Model 2 was additionally adjusted for BMI, FPG, HbA1c, TyG index, smoking status, drinking status, diabetes duration, hypertension, dyslipidemia, and use of antihyperglycemic, antihypertensive, and lipid-lowering agents. Stratified analyses were conducted based on BMI, age, HbA1c, and diabetes course. We further fitted the data using smoothing and generalized additive models to investigate the potential non-linear relationship.
Statistical analyses were conducted using the R package and Empower Stats (http://www.Rproject.org and http://www.empowerstats.com). The level of statistical significance was set at P < 0.05 (two-sided).
Statement of ethical approval
The Ethics Committee of the First Hospital of Jilin University approved the study protocol. The study was performed in strict accordance with the Declaration of Helsinki, and all methods followed the relevant guidelines. All participants signed informed consent forms.
Results
Baseline characteristics and comparisons between the DPN and non-DPN groups
The study included 708 patients with T2DM (505 men, 203 women), comprising 407 with DPN and 301 without DPN. Table 1 details their general conditions and baseline characteristics. There was no difference in the prevalence of hypertension, dyslipidemia, and cardiovascular diseases between the two groups (all p > 0.05). The BMI, WC, FBG levels, TyG index, HbA1c levels, TSH levels, FT3 levels, and the number of smokers, drinkers, and users of anti-diabetic agents showed no significant differences among the two groups (all p > 0.05). The DPN group was generally older, had a longer duration of diabetes, and was more likely to have DR and DN. Additionally, the DPN group had lower FT4 levels, TT4RI, TSHI, and PTFQI levels, and a higher FT3/FT4 ratio (all p < 0.05).
Correlation between TH sensitivity and the risk of DPN
We used multivariate logistic regression models to analyze the correlation between TH sensitivity and DPN. The findings are presented in Table 2. After adjusting for sex, age, diabetes duration, BMI, smoking, drinking, dyslipidemia, fasting blood glucose, hypertension, HbA1c, TyG index, and anti-diabetic agents, we observed an association between elevated FT4 levels and reduced risk of DPN (OR = 0.72, 95%CI: 0.61–0.87, p = 0.0004). Moreover, an increased FT3/FT4 ratio was associated with DPN. Per each SD increase in the FT3/FT4 ratio, the odds ratio for DPN was 1.36 (95%CI: 1.13–1.63, p = 0.0012). Patients in the highest FT3/FT4 quartile had a 2.36 times greater risk of DPN than patients in the lowest quartile (OR = 2.36, 95%CI: 1.41–3.95, p = 0.0011).
Higher levels of TT4RI, TSHI, TFQI, and PTFQI were related to a decreased prevalence of DPN. The ORs for DPN were 0.80 (95%CI: 0.68–0.94, p = 0.0078), 0.72 (95%CI: 0.60–0.86, p = 0.0002), 0.69 (95%CI: 0.58–0.83, p < 0.0001), and 0.69 (95%CI: 0.58–0.82, p < 0.0001) for each SD increase in TT4RI, TSHI, TFQI, and PTFQI, respectively. As the quartiles of TT4RI, TSHI, TFQI, and PTFQI increased, the OR of DPN exhibited a declining trend (all p for tend < 0.05). In addition, smoothed curve fitting revealed a linear positive relationship between the FT3/FT4 ratio and DPN, a linear negative relationship between FT4, TT4RI, TFQI, PTFQ1, and DPN, and a non-linear negative correlation between TSHI and DPN (Fig. 2).
Smooth curve fitting for the TH sensitivity index and DPN. Sex, age, diabetes duration, body mass index, smoking, drinking, hypertension, dyslipidemia, fasting blood glucose, HbA1c, TyG index, and anti-diabetic agents were adjusted. The red dotted lines indicate a smooth curve fit between DPN and the TH sensitivity index. The upper and lower blue dotted lines indicate the range of 95% CI.
Subgroup analysis
We conducted subgroup analyses to evaluate the correlation between TH sensitivity and DPN across different populations stratified by age, BMI, HbA1c, and diabetes duration. In the stratified analysis of the correlation between TT4RI, TSHI, TFQI, PTFQI, and DPN, no p for interaction was statistically significant, suggesting that these correlations were stable across different age, BMI, HbA1c, and diabetes duration groups (all p for interaction > 0.05) (Supplement Figs. 1–4). However, diabetes duration affected the correlation between the FT3/FT4 ratio and DPN (p for interaction > 0.05). In patients with diabetes duration < 60 months, each SD increase in the FT3/FT4 ratio was associated with a 77% higher risk of developing DPN. Nonetheless, for those with diabetes duration ≥ 60 months, the relationship was not statistically significant (Fig. 3).
Discussion
In this cross-sectional study, we investigated the correlation between TH sensitivity and DPN in euthyroid patients with T2DM in China. We observed that increased TT4RI, TSHI, TTFQ, and PTFQI, which reflected decreased central TH sensitivity, were related to a reduced risk of developing DPN. In addition, a higher FT3/FT4 ratio, which reflected increased peripheral TH sensitivity, was correlated with an increased prevalence of DPN. To our knowledge, our research is the first to identify a correlation between central TH sensitivity and DPN, offering novel perspectives on the importance of thyroid homeostasis in DPN.
DPN is a common long-term condition in patients with T2DM, with complex pathophysiological mechanisms involving multiple metabolic and intracellular signaling abnormalities. Clinical and experimental evidence suggests that elevated blood glucose levels cause cellular damage, a critical factor in DPN development19. Additionally, microvascular dysfunctions, dyslipidemia, and impaired insulin signaling can trigger mitochondrial dysfunction and oxidative stress, ultimately causing cellular dysfunction and death20.
THs, as critical regulators of glucose-lipid metabolism and energy homeostasis, are closely associated with T2DM and its complications21,22,23. Our study found no relationship between FT3 and TSH levels and DPN. After adjusting for confounders, the TSH levels showed a weak inverse correlation with DPN (OR = 0.84, 95%CI: 0.7–0.99, p = 0.0401). Our findings also revealed that the FT4 levels were negatively correlated with the development of DPN. The results align with those of He et al. and Hu et al.5,6. Previous studies have reported that low FT4 levels can alter lipid composition, increasing TG, TC, and LDL-C levels while decreasing HDL-C levels. Dyslipidemia is a known pathophysiologic mechanism in DPN development24,25. First, most patients with dyslipidemia are in a state of insulin resistance and chronic inflammation, and chronic inflammation can, in turn, lead to insulin resistance. Insulin resistance inhibits glucose metabolism, leading to hyperglycemia. Hyperglycemia and dyslipidemia promote DPN26. Second, clinical evidence suggests that elevated triglyceride levels in patients with DPN can decrease nerve myelinated fiber density27. In a previous study, high-fat diet mice showed motor conduction deficits, sensory conduction deficits, and destruction of myelin sheaths28. Segmental demyelination is a crucial feature of DPN29. Schwann cells provide energy and protection to neurons and are essential for peripheral nerve survival. Palmitic acid-treated Schwann cells undergo endoplasmic reticulum stress and mitochondrial dysfunction, leading to cell death30. Third, diabetes-induced oxidative stress increases ox-LDL levels through the reactive oxygen species (ROS)-p3 MAPK signaling pathway31. Peripheral sensory neurons express oxidized LDL (ox-LDL) receptors. When ox-LDL binds to these receptors, it enters the cell and activates NADPH oxidase, generating significant quantities of ROS. These two processes lead to positive feedback in ROS formation32. The positive feedback has also been observed in a high-fat diet mouse model where the ox-LDL levels were elevated and could induce oxidative stress in neurons, leading to neuropathy33. In addition to effects on lipid metabolism, He et al. found that reduced FT4 levels accompanied reduced serum calcium levels in patients with DPN5. Calcium ion is a significant contributor to the synthesis and release of neurotransmitters. A decrease in serum Ca2+ levels leads to muscle twitching, affecting neural excitatory conduction and accelerating DPN onset5. Ca2+ homeostasis and Ca2+ signaling are directly related to neuropathology. Moreover, hyperglycemia has been reported to impair calcium signaling in axons, an essential cause of DPN. In diabetes, impaired mitochondrial function in neurons and glial cells leads to impaired mitochondrial Ca2+ buffering, further exacerbating mitochondrial dysfunction and promoting the progression of DPN34.
Previous studies have shown different findings in the association between THs and DPN, suggesting that THs alone cannot elucidate the complicated connection between thyroid function and DPN. TH sensitivity is a newly proposed index that complements the assessment of thyroid status. The ratio FT3/FT4 can reflect the peripheral conversion efficiency of THs from the perspective of deiodinase. Higher peripheral TH sensitivity reflects higher deiodinase activity, which promotes the conversion of FT4 to FT3. The negative feedback regulation of the hypothalamic-pituitary-thyroid (HPT) axis is crucial for regulating TH secretion. TT4RI, TSHI, TFQI, and PTFQI are indicators that quantify the feedback circuit for the HPT axis and reflect central sensitivity to THs. The TT4RI index was first proposed for evaluating pituitary sensitivity to THs by Yagi et al.14. In 2009, Jostel et al. introduced TSHI, which reflects the feedback inhibition of TSH by FT415. In 2019, Laclaustra et al. suggested a novel indicator called TFQI. TFQI is a continuous variable discretized to quantify the negative feedback inhibition of the pituitary gland by THs, with the benefit of avoiding the generation of extremely high values in cases where the thyroid is dysfunctional. PTFQI has the same scope and interpretation as the TFQI. It can be formulated to fit the population under study; therefore, it has greater applicability9.
Our findings indicated a linear positive association between FT3/FT4 and DPN. Patients with DPN had significantly lower FT4 levels, and there was no difference in FT3 levels among the two groups. Smooth curve fitting also showed that the lower the FT4 level, the higher the incidence of DPN. Therefore, we conclude that the elevated FT3/FT4 in patients with DPN was purely due to decreased levels of FT4 rather than elevated deiodinase activity. Hence, the correlation between DPN and FT3/FT4 is primarily attributed to lower FT4 levels, not peripheral TH sensitivity. In the subgroup analysis, we observed a stronger correlation between DPN and FT3/FT4 in individuals diagnosed with T2DM of less than 5 years. Several meta-analyses have shown that low FT4 levels are related to T2DM, DN, DR, and DPN, yet no study has examined the role of the diabetes duration in this process22,35,36.
Our research also found that reduced central TH sensitivity (elevated TT4RI, TSHI, TFQI, and PTFQI) was related to a reduced risk of DPN. Elevated thyroid resistance indices have been observed to be associated with many adverse metabolic factors, such as obesity, T2DM, metabolic syndrome, and even mortality related to diabetes9,37,38. Yet some studies have reported opposite results, showing that reduced central TH sensitivity is correlated with a lower risk of prediabetes and lower BMI39,40,41. There are fewer studies on central TH sensitivity and diabetic complications. According to recent research, elevated levels of TSHI, TFQI, and TT4RI have been determined to be risk factors for the onset of DR in patients with T2DM7. No studies have reported on the relationship between DPN and central TH sensitivity. Still, several avenues can explain the association we observed between central TH resistance and DPN. First, individuals with high central TH sensitivity have better pituitary responsiveness to FT4. Therefore, the level of FT4 required to achieve the same TSH level will be relatively low. Our study showed no difference in TSH levels between the two groups but revealed higher central TH sensitivity and lower FT4 levels in the DPN group. This is consistent with the theory. We have discussed in detail how a decrease in FT4 levels leads to an increased incidence of DPN, mainly affecting lipid metabolism and calcium ion homeostasis. Similarly, individuals with decreased central TH sensitivity will have elevated FT4 levels. There is evidence that elevated levels of FT4 can improve insulin sensitivity, enhance glucose utilization, and attenuate hypertriglyceridemia and metabolic syndrome42,43. It is known that disorders of glycolipid metabolism are important pathophysiological mechanisms for DPN; therefore, an increase in FT4 levels can alleviate the development of DPN to a certain extent. Second, in addition to the TH negative feedback regulation of central TSH and thyrotropin-releasing hormone (TRH) secretion, leptin can also act on the hypothalamus to promote TRH secretion. Leptin is an adipocyte secreted by adipose tissues, which can directly act on the paraventricular nucleus of the hypothalamus to promote the transcription of prepro-TRH and thus promote the secretion of TRH and indirectly stimulate the secretion of TRH in the arcuate nucleus by stimulating the production of downstream proopiomelanocortin (POMC) products44. In addition, leptin downregulates type 2 deiodinase (DIO2) activity in the pituitary gland, reducing the conversion of FT4 to FT345. In the presence of reduced central TH sensitivity, peripheral leptin levels may be compensatorily elevated, increasing TRH secretion and thereby elevating TSH and FT4 levels. Leptin also decreases pituitary DIO2 activity, increases FT4 levels, and inhibits TSH elevation. The result is that TSH levels may remain unchanged while FT4 levels rise. On the one hand, elevated leptin levels reduce fat synthesis and storage and increase insulin receptor sensitivity, thus reducing insulin resistance46. On the other hand, a recent study proposed the concept of an adipo-glial signaling axis, which identified leptin as an upstream regulator in the repair of glial (Schwann cell) damage. Schwann cell injury is followed by increased expression of its leptin receptor, and adipocyte-derived leptin acts on the membrane surface of the receptor-mediated oxidative phosphorylation and myelin autophagy to support nerve repair. This suggests that leptin has a protective effect on nerve cell injury47. In summary, decreased central TH sensitivity can lead to increased peripheral FT4 and leptin levels, which protects nerve cells and reduces the occurrence of DPN. The specific mechanism needs to be further investigated.
Our study had several strengths, including substantial sample size, assessment of five indicators of TH sensitivity with correction for multiple confounders, and stratified population analysis. Still, it had some limitations. First, the cross-sectional design prevented the establishment of a causal relationship between TH sensitivity and DPN. Second, since all patients were Chinese adults from a single center, the results may not be generalizable to all patients with T2DM in China (and globally). Third, TH sensitivity indices are supplementary indicators and cannot fully replace other thyroid function measures. Despite these limitations, our study suggests a potential relationship between DPN and TH sensitivity in euthyroid patients with T2DM. Further prospective cohort studies with larger sample sizes are needed to explore this correlation in depth.
In conclusion, our study shows that decreased central and peripheral TH sensitivity is associated with a lower prevalence of DPN. These findings can guide managing and preventing peripheral neuropathies in patients with T2DM. Nonetheless, further studies are needed to confirm the underlying mechanisms.
Data availability
The data analyzed and generated in this study are available from the corresponding author on reasonable request.
References
Tu, W. J., Xue, Y. & Nie, D. The Prevalence and Treatment of Diabetes in China From 2013 to 2018. Jama 327, 1706. https://doi.org/10.1001/jama.2022.3014 (2022).
Ziegler, D., Papanas, N., Vinik, A. I. & Shaw, J. E. Epidemiology of polyneuropathy in diabetes and prediabetes. Handb. Clin. Neurol. 126, 3–22. https://doi.org/10.1016/b978-0-444-53480-4.00001-1 (2014).
Barrell, K. & Smith, A. G. Peripheral Neuropathy. Med. Clin. North Am. 103, 383–397. https://doi.org/10.1016/j.mcna.2018.10.006 (2019).
Sloan, G., Selvarajah, D. & Tesfaye, S. Pathogenesis, diagnosis and clinical management of diabetic sensorimotor peripheral neuropathy. Nat. Rev. Endocrinol. 17, 400–420. https://doi.org/10.1038/s41574-021-00496-z (2021).
He, Q., Zeng, Z., Zhao, M., Ruan, B. & Chen, P. Association between thyroid function and diabetes peripheral neuropathy in euthyroid type 2 diabetes mellitus patients. Sci. Rep. 13, 13499. https://doi.org/10.1038/s41598-023-40752-y (2023).
Hu, Y. et al. Association of thyroid hormone levels with microvascular complications in euthyroid type 2 diabetes mellitus patients. Diabetes Metab. Syndr. Obes. 15, 2467–2477. https://doi.org/10.2147/dmso.S354872 (2022).
Zhao, X., Sun, J., Xu, X., Xin, S. & Zhang, X. The effect of Central and peripheral thyroid resistance indices on diabetic retinopathy: A study of hospitalized euthyroid patients with T2DM in China. Ann. Med. 55, 2249017. https://doi.org/10.1080/07853890.2023.2249017 (2023).
Zhao, X., Sun, J., Xin, S. & Zhang, X. Predictive Effects of FT3/FT4 on Diabetic Kidney Disease: An Exploratory Study on Hospitalized Euthyroid Patients with T2DM in China. Biomedicines https://doi.org/10.3390/biomedicines11082211 (2023).
Laclaustra, M. et al. Impaired sensitivity to thyroid hormones is associated with diabetes and metabolic syndrome. Diabetes Care 42, 303–310. https://doi.org/10.2337/dc18-1410 (2019).
Lin, J., Xiang, X., Qin, Y., Gui, J. & Wan, Q. Correlation of thyroid-related hormones with vascular complications in type 2 diabetes patients with euthyroid. Front. Endocrinol. (Lausanne) 13, 1037969. https://doi.org/10.3389/fendo.2022.1037969 (2022).
Alberti, K. G. & Zimmet, P. Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 15, 539–553 (1998).
Guerrero-Romero, F. et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J. Clin. Endocrinol. Metab. 95, 3347–3351. https://doi.org/10.1210/jc.2010-0288 (2010).
Park, S. Y. et al. Free triiodothyronine/free thyroxine ratio rather than thyrotropin is more associated with metabolic parameters in healthy euthyroid adult subjects. Clin. Endocrinol. (Oxf) 87, 87–96. https://doi.org/10.1111/cen.13345 (2017).
Yagi, H., Pohlenz, J., Hayashi, Y., Sakurai, A. & Refetoff, S. Resistance to thyroid hormone caused by two mutant thyroid hormone receptors beta, R243Q and R243W, with marked impairment of function that cannot be explained by altered in vitro 3,5,3’-triiodothyroinine binding affinity. J. Clin. Endocrinol. Metab. 82, 1608–1614. https://doi.org/10.1210/jcem.82.5.3945 (1997).
Jostel, A., Ryder, W. D. & Shalet, S. M. The use of thyroid function tests in the diagnosis of hypopituitarism: definition and evaluation of the TSH Index. Clin. Endocrinol. (Oxf) 71, 529–534. https://doi.org/10.1111/j.1365-2265.2009.03534.x (2009).
Lv, F. et al. Sensitivity to thyroid hormone and risk of components of metabolic syndrome in a Chinese euthyroid population. J. Diabetes 15, 900–910. https://doi.org/10.1111/1753-0407.13441 (2023).
Bril, V. & Perkins, B. A. Validation of the Toronto Clinical Scoring System for diabetic polyneuropathy. Diabetes Care 25, 2048–2052. https://doi.org/10.2337/diacare.25.11.2048 (2002).
Li, M. F. et al. Serum free triiodothyronine is inversely associated with diabetic peripheral neuropathy but not with carotid atherosclerotic lesions in euthyroid patients with type 2 diabetes. Diabetol. Metab. Syndr. 13, 142. https://doi.org/10.1186/s13098-021-00760-2 (2021).
Strand, N. et al. Diabetic neuropathy: Pathophysiology review. Curr. Pain Headache Rep. https://doi.org/10.1007/s11916-024-01243-5 (2024).
Albers, J. W. & Pop-Busui, R. Diabetic neuropathy: mechanisms, emerging treatments, and subtypes. Curr. Neurol. Neurosci. Rep. 14, 473. https://doi.org/10.1007/s11910-014-0473-5 (2014).
Roa Dueñas, O. H. et al. Thyroid Function and the Risk of Prediabetes and Type 2 Diabetes. J. Clin. Endocrinol. Metab. 107, 1789–1798. https://doi.org/10.1210/clinem/dgac006 (2022).
Zou, J. et al. Association between normal thyroid hormones and diabetic retinopathy in patients with type 2 diabetes. Biomed. Res. Int. 2020, 8161797. https://doi.org/10.1155/2020/8161797 (2020).
Yang, Z. et al. The Correlation between Thyroid Hormone Levels and the Kidney Disease Progression Risk in Patients with Type 2 Diabetes. Diabetes Metab. Syndr. Obes. 15, 59–67. https://doi.org/10.2147/dmso.S347862 (2022).
Jung, K. Y. et al. Association between thyroid function and lipid profiles, apolipoproteins, and high-density lipoprotein function. J. Clin. Lipidol. 11, 1347–1353. https://doi.org/10.1016/j.jacl.2017.08.015 (2017).
Pleić, N., Gunjača, I., Babić Leko, M. & Zemunik, T. Thyroid function and metabolic syndrome: A two-sample bidirectional mendelian randomization study. J. Clin. Endocrinol. Metab. 108, 3190–3200. https://doi.org/10.1210/clinem/dgad371 (2023).
Zhu, J. et al. Diabetic peripheral neuropathy: pathogenetic mechanisms and treatment. Front Endocrinol. 14, 1265372. https://doi.org/10.3389/fendo.2023.1265372 (2023).
Wiggin, T. D. et al. Elevated triglycerides correlate with progression of diabetic neuropathy. Diabetes 58, 1634–1640. https://doi.org/10.2337/db08-1771 (2009).
Yorek, M. S. et al. Effect of diet-induced obesity or type 1 or type 2 diabetes on corneal nerves and peripheral neuropathy in C57Bl/6J mice. J. Peripher. Nerv. Syst. 20, 24–31. https://doi.org/10.1111/jns.12111 (2015).
Xie, F. et al. High energy diets-induced metabolic and prediabetic painful polyneuropathy in rats. PLoS One https://doi.org/10.1371/journal.pone.0057427 (2013).
Padilla, A., Descorbeth, M., Almeyda, A. L., Payne, K. & De Leon, M. Hyperglycemia magnifies Schwann cell dysfunction and cell death triggered by PA-induced lipotoxicity. Brain Res. 1370, 64–79. https://doi.org/10.1016/j.brainres.2010.11.013 (2011).
Ishiyama, J., Taguchi, R., Yamamoto, A. & Murakami, K. Palmitic acid enhances lectin-like oxidized LDL receptor (LOX-1) expression and promotes uptake of oxidized LDL in macrophage cells. Atherosclerosis 209, 118–124. https://doi.org/10.1016/j.atherosclerosis.2009.09.004 (2010).
Honjo, T. et al. Essential role of NOXA1 in generation of reactive oxygen species induced by oxidized low-density lipoprotein in human vascular endothelial cells. Endothelium 15, 137–141. https://doi.org/10.1080/10623320802125433 (2008).
Vincent, A. M. et al. Dyslipidemia-induced neuropathy in mice: the role of oxLDL/LOX-1. Diabetes 58, 2376–2385. https://doi.org/10.2337/db09-0047 (2009).
Verkhratsky, A. & Fernyhough, P. Mitochondrial malfunction and Ca2+ dyshomeostasis drive neuronal pathology in diabetes. Cell Calcium 44, 112–122. https://doi.org/10.1016/j.ceca.2007.11.010 (2008).
Han, C. et al. Subclinical hypothyroidism and type 2 diabetes: A systematic review and meta-analysis. PLoS One https://doi.org/10.1371/journal.pone.0135233 (2015).
Rong, F. et al. Association between thyroid dysfunction and type 2 diabetes: A meta-analysis of prospective observational studies. BMC Med. 19, 257. https://doi.org/10.1186/s12916-021-02121-2 (2021).
Nie, X. et al. Increased serum adipocyte fatty acid-binding protein levels are associated with decreased sensitivity to thyroid hormones in the Euthyroid population. Thyroid 30, 1718–1723. https://doi.org/10.1089/thy.2020.0011 (2020).
Mehran, L. et al. Reduced sensitivity to thyroid hormone is associated with diabetes and hypertension. J. Clin. Endocrinol. Metab. 107, 167–176. https://doi.org/10.1210/clinem/dgab646 (2022).
Liu, B. et al. Sensitivity to Thyroid Hormones and Risk of Prediabetes: A Cross-Sectional Study. Front Endocrinol (Lausanne) https://doi.org/10.3389/fendo.2021.657114 (2021).
Nie, X. et al. Associations Between Thyroid Hormones and Glycated Albumin in Euthyroid and Subclinical Hypothyroid Individuals: Results of an Observational Study. Diabetes Metab. Syndr. Obes. 13, 915–923. https://doi.org/10.2147/dmso.S236698 (2020).
Zhou, Y. et al. Correlation between thyroid homeostasis and obesity in subclinical hypothyroidism: Community-based cross-sectional research. Int. J. Endocrinol. 2021, 6663553. https://doi.org/10.1155/2021/6663553 (2021).
Mehran, L., Amouzegar, A., Tohidi, M., Moayedi, M. & Azizi, F. Serum free thyroxine concentration is associated with metabolic syndrome in euthyroid subjects. Thyroid 24, 1566–1574. https://doi.org/10.1089/thy.2014.0103 (2014).
Mehran, L. et al. Variations in serum free thyroxine concentration within the reference range predicts the incidence of metabolic syndrome in non-obese adults: A cohort study. Thyroid 27, 886–893. https://doi.org/10.1089/thy.2016.0557 (2017).
Mullur, R., Liu, Y. Y. & Brent, G. A. Thyroid hormone regulation of metabolism. Physiol. Rev. 94, 355–382. https://doi.org/10.1152/physrev.00030.2013 (2014).
Fontenelle, L. C. et al. Thyroid function in human obesity: underlying mechanisms. Horm. Metab Res. 48, 787–794. https://doi.org/10.1055/s-0042-121421 (2016).
Friedman, J. The long road to leptin. J. Clin. Invest. 126, 4727–4734. https://doi.org/10.1172/jci91578 (2016).
Sundaram, V. K. et al. Adipo-glial signaling mediates metabolic adaptation in peripheral nerve regeneration. Cell Metab. 35, 2136-2152.e2139. https://doi.org/10.1016/j.cmet.2023.10.017 (2023).
Acknowledgements
We would like to thank Editage (www.editage.cn) for English language editing.
Funding
This work was supported by the First Hospital of Jilin University (2021-zl-01 and JDYYCB-2023010 to Xue Zhao; JDYYZH-2102030 to Guixia Wang), the Department of Science and Technology of Jilin Province (20210101439JC to Xue Zhao; YDZJ202202CXJD042, 20210303001SF, and 20190901006JC to Guixia Wang), and grants from the National Natural Science Foundation of China (82270850 and 81970687 to Guixia Wang).
Author information
Authors and Affiliations
Contributions
M.S. reviewed relevant literature and wrote the manuscript. L.Y. collected and analyzed data. X.Z. generated relevant charts and graphs. L.R. and X.L. reviewed and revised the manuscript. X.G. and G.W. were responsible for financial support. All the authors have read and approved the manuscript. M.S. and L.Y. contributed equally to this work and should be considered co-first authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sun, M., Yu, L., Zhao, X. et al. Correlation between thyroid hormone sensitivity and diabetic peripheral neuropathy in euthyroid patients with type 2 diabetes mellitus. Sci Rep 14, 19603 (2024). https://doi.org/10.1038/s41598-024-70673-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-70673-3
- Springer Nature Limited