Abstract
Observational studies have suggested the potential benefits of several medications for Parkinson’s disease (PD) and their potential for repurposing. However, the conclusions drawn from these studies are not entirely consistent. To address this inconsistency, we used the two-sample Mendelian randomization (MR) method to explore the putative causal relationships between 23 medications and the risk and progression of PD. We applied inverse-variance weighted meta-analysis (IVW) to combine MR estimates. Additionally, sensitivity analyses were conducted to evaluate the robustness of the results. Our genetic evidence suggests that thyroid preparations and calcium channel blockers reduce the risk of PD, and salicylic acid and derivatives slow the progression of PD motor symptoms. Additionally, genetic evidence also suggests that four medications were associated with PD risk or progression, but the sensitivity analysis revealed that three of the medications may have interference caused by reverse causality. Our findings suggest that there are weak causal relationships between several medications and the risk or progression of PD. Though further replication studies are needed to verify these findings, these new insights may help in understanding the etiology of the disease, generate new clues related to drug discovery, and quantify the risk of future drug intake.
Similar content being viewed by others
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disease, and it is characterized by the fastest growing prevalence, mortality, and disability-adjusted life expectancy in the neurological disease category1. An estimated 6.1 million individuals globally had a PD diagnosis in 2016, which is 2.4 times higher than in 19902,3. The course of PD is considered irreversible. Its typical pathophysiology involves the death of dopaminergic neurons and the accumulation of Lewy bodies in the cytoplasm of neurons, which is mainly manifested clinically as resting tremor, bradykinesia, rigidity, and postural gait disorders4. In addition to the above typical features, some accompanying symptoms can also affect the quality of life of patients with PD and lead to faster disease progression, including mild cognitive impairment and autonomic dysfunction (such as urinary dysfunction, constipation, and blood pressure dysregulation), among others5. It is now widely believed that in addition to traditional therapies, modulation of non-dopaminergic pathways can lead to other treatment options6.
Over the past two decades, there have been significant advancements in the development of new medications for the treatment of PD, with several medications aimed at improving symptoms have come to market5. However, to date, no drug has been discovered that can truly slow down or stop the progression of PD. This means that, due to the lack of effective disease-modifying therapies, once diagnosed, patients with PD ultimately experience unbearable disability and premature death7. Consequently, before a cure is found, neuroprotective therapies that slow or halt disease progression and prevent the accumulation of disability remain a top priority in drug development4,7. The discovery and development of new medications for PD constitute a long, expensive, and risky process. Given the number of failures of various novel interventions in disease-modifying clinical trials, as well as increasing costs and lengthy drug development processes, attention is shifting to the use of existing compounds approved for other indications as novel therapies for PD. This approach, known as drug repurposing, has identified some medications with potential benefits for treating PD and provided a potentially faster route to drug discovery8.
Some basic research and clinical trials have attempted to develop potentially therapeutic medications for PD using drug repurposing strategies. Notably, some attempts have been successful, such as the United States Food and Drug Administration (U.S. FDA) approval of five repurposed medications for PD, including ropinirole hydrochloride and rasagiline, which were used as antihypertensive medications; amantadine and memantine, which were initially used as antiviral medications; and pimavanserin, which was originally used as an antipsychotic drug9,10,11,12. These medications have demonstrated their efficacy in improving motor or non-motor symptoms in patients with PD. Furthermore, other medications that are considered promising and are currently being tested include the glucagon-like peptide-1 receptor agonists exenatide and lixisenatide, which are commonly used to treat diabetes13,14; deferiprone for transfusion-dependent thalassemia15; inosine, a urate precursor and potential antioxidant16; the tyrosine kinase inhibitor nilotinib17; the dihydropyridine antihypertensive drug isradipine18; the antiepileptic drug levetiracetam19; the bronchodilator ambroxol20; and several non-steroidal anti-inflammatory medications, antibiotics, and others21,22. Despite the high quality of these studies, they mainly employ randomized controlled trials (RCTs). RCTs are the most widely accepted standard method to determine causal relationships associated with drug treatments; however, they are expensive and may not always be feasible.
Over the past few decades, methods utilizing genetic information have been developed to overcome the limitations of RCTs and to explain the confounding factors stemming from both genetics and environment in observational studies. MR is one such method that uses genetic variations associated with exposure as instrumental variables to study causal relationships with outcomes, and vice versa23. Conceptually, MR is similar to RCTs; here, the randomization occurs at meiosis, making MR an important strategy to strengthen causal inference when RCTs are impractical or unethical24. The present study aimed to (i) investigate the causal relationship between 23 medications and the risk and progression of PD, and (ii) elucidate the causal direction of this relationship.
Materials and methods
Study design
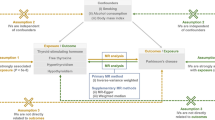
This study conducted an MR study to investigate the putative causal effects between 23 medications and the risk and progression of PD (Fig. 1). MR is grounded on three key assumptions: (I) the genetic variants chosen as instrumental variables exhibiting solid associations with exposure; (II) the genetic variants are independent of confounding factors; and (III) the genetic variants influence the outcome exclusively through exposure25. Ethical approval from an institutional review board was unnecessary, as all the analyses in this study were based on publicly available summary-level genome-wide association study (GWAS) data.
To analyze the data, this study used the TwoSampleMR package (version 0.5.6) and MendelianRandomization package (version 0.8.0) in R software (version 4.2.2).
Data sources
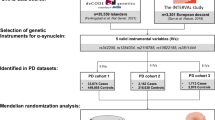
Summary-level GWAS data for 23 medications were derived from a meta-analysis of GWASs involving participants from the UK Biobank26. Specifically, Wu, Y. et al. estimated the genetic correlations between 23 medications approximately 320,000 individuals from the UK Biobank through a GWAS26. Approximately 54% of the UK Biobank study participants were female, with an average (SD) age of 56.5 (8.1) years at the first assessment visit. Based on self-reports from the original GWAS participants, only regular medications taken weekly, monthly, or every 3 months were included. Medication information was obtained through nurse-conducted interviews (Supplementary Table 1).
Summary-level data related to PD risk and progression from three large GWAS meta-analyses focused on PD diagnosis, age at onset (AAO), Unified Parkinson’s Disease Rating Scale, motor subsection (UPDRS3), Montreal Cognitive Assessment (MoCA), and Mini-Mental State Examination (MMSE)27,28,29. Specifically, we obtained genetic data on PD risk from the latest GWAS meta-analysis of 16 cohorts from the International Parkinson’s Disease Genomics Consortium (IPDGC) and 23andMe, which included 33,674 cases and 449,056 controls27. The identification of patients with PD was based on commonly used outpatient criteria and the UK Brain Bank criteria, with modifications for those with a family history of PD. Each included study employed statistical models with specific covariates to account for their particularities, thereby generating consistent summary statistics. Generally, age, gender, and population stratification were included as covariates. We extracted association estimates from the largest GWAS of AAO of PD conducted by IPDGC and 23andMe, which involved 28,568 PD cases28. The definition of AAO was based on patients’ self-report of the first motor symptoms of PD; if specific information was lacking, age at diagnosis was used instead. Data on disease progression or severity was obtained from a large GWAS of clinical biomarkers (UPDRS3/MoCA/MMSE) in 12 longitudinal PD cohorts (N = 4093)29. Higher UPDRS3 scores indicate poorer motor ability, whereas higher MMSE and MoCA scores indicate better cognitive ability. Due to data access limitations, this study did not include GWAS statistical summaries of the 23andMe dataset (Supplementary Table 1).
Additionally, summary statistics data from the IEU Open GWAS Project were incorporated to explore potential confounding factors for known drug-related diseases (Supplementary Table 1).
Selection of genetic instruments and data harmonization
This study extracted independent genetic instrument variants (IVs) from summary statistics of GWASs using several criteria. First, P-values were used with a genome-wide significance threshold (p < 5 × 10−8). When the number of single-nucleotide polymorphisms (SNPs) meeting genome-wide significance was limited, a more relaxed threshold (p < 5 × 10−6) was used, which was previously employed in MR studies. Second, clustering analysis was conducted with an r2 of 0.001 and a window size of 10,000 kb. Third, only IVs with an F-statistic > 10 were retained, as they were considered to be associated strongly with the phenotype. Fourth, the exposure and outcome GWAS datasets were harmonized to ensure that genetic variant association estimates corresponded to the exact effects of the alleles. Lastly, SNPs that were closely related to the outcome (p < 5 × 10−8) were removed to fulfill the third assumption (Supplementary Table 2–3).
MR analysis
IVW random-effects regression was employed as the primary MR method in the study to estimate the potential causal relationship between the 23 medications and the risk and progression of PD. Furthermore, other MR analysis methods were employed to ensure the robustness of the results and assess potential horizontal pleiotropy. The MR-Egger method detected horizontal pleiotropy through its intercept test and corrected the pleiotropic. The weighted median method, which relies on valid SNPs contributing to more than 50% of the weight, provided effective MR estimates. Moreover, the MR-PRESSO method was also utilized to detect and correct possible outliers, and the MR-PRESSO global test allowed the evaluation of horizontal pleiotropy arising from heterogeneity between SNP estimates. This study considered P-values less than 0.0004 (0.05/115, Bonferroni method) statistically significant, and meanwhile P < 0.05 was regarded as nominally significant30,31.
In addition, a series of sensitivity analyses were conducted to assess the robustness of the causal estimates. First, Cochran’s Q statistic was used to evaluate heterogeneity among variable-specific causal effect estimates. The MR-Egger intercept test was conducted to examine directional pleiotropy, with departure from zero (P < 0.05) indicating the presence of directional pleiotropy32. A leave-one-out analysis was also performed to assess the influence of individual variants on the associations observed. A funnel plot was used to assess the probable presence of directional pleiotropy. Finally, this study employed the Steiger directional test to determine the direction of causality between exposure and outcome endpoints. Additionally, for significant estimates (PIVW < 0.05), We further conducted bidirectional MR analysis to confirm the robustness of the results33,34.
We also checked these SNPs in PhenoScanner (www.phenoscanner.medschl.cam.ac.uk) to determine whether they were associated with potential risk factors, including neurotoxins (such as rotenone or paraquat), smoking, coffee consumption, anti-inflammatory drug use, and high plasma urea4. We excluded SNPs associated with these potential confounding factors at the whole-genome level and subsequently repeated the MR analysis to satisfy the second MR hypothesis35,36. This study also calculated statistical power using an online tool at http://cnsgenomics.com/shiny/mRnd/37.
Because known drug-related diseases can increase drug use, relief after treatment may mediate the association between medications and PD-related phenotypes. Thus, further exploration of the relationships among these five phenotypes is necessary. The Multivariable MR (MVMR) method was employed, which estimates the causal relationships between each risk factor and the outcome using multiple genetic variants associated with various measured risk factors38. Given that the MVMR model is suitable for exploring the association between known drug-related diseases, drug usage, and PD-related phenotypes simultaneously and acts as a mediator by establishing drug-related diseases, this study designed MVMR analyses to correct known drug-related phenotypes, which had been reported in other literature39,40. Specifically, this study combined SNPs related to drug usage with known drug-related diseases from relevant GWAS. To ensure data quality, duplicated and correlated SNPs (within 10,000 base pairs; R2 ≥ 0.001) were excluded, and SNP effects and corresponding standard errors were extracted from exposure and outcome GWAS.
Results
Association of genetically predicted 23 medications with Parkinson’s disease
The main MR analysis utilizing IVW estimates indicates potential causal relationships between several medications and PD-derived phenotypes (shown in Table 1, Supplementary Tables 4–8, and Fig. 2). Specifically, genetically predicted thyroid preparations were associated with a nominally significant association with a decreased risk of PD (IVW: OR = 0.95; 95% CI 0.91, 1.00; P = 0.032) (Fig. 3). Salicylic acid and derivatives have shown a nominally significant association with improvement in of PD motor symptoms (as indicated by the UPDRS3 score). (IVW: β = − 0.42; 95% CI − 0.78, − 0.06; P = 0.023) (Supplementary Fig. 1). Furthermore, calcium channel blockers tended to reduce the risk of PD (IVW: OR = 0.92; 95% CI 0.85, 1.00; P = 0.055) (Supplementary Fig. 2). Sensitivity analyses were conducted, excluding SNPs with issues of potential pleiotropy (rs3184504 for serum urate, rs215619 for smoking, and rs2471960 for paracetamol) and showed that the estimated effect of thyroid preparations remained consistent with initial findings (IVW: OR = 0.94; 95% CI 0.90, 0.99; P = 0.020) (Supplementary Table 9–10). Similarly, after removing SNPs such as (rs3184504 for serum urate and smoking, and rs7412 for aspirin), the estimated effect of calcium channel blockers on reducing PD risk remained essentially consistent with the original study results (IVW: OR = 0.92; 95% CI 0.85, 1.00; P = 0.046). No SNPs relating to confounding factors were identified for salicylic acid and derivatives. MR-Egger intercept analysis did not reveal any horizontal pleiotropy, and Cochran’s Q test did not detect any heterogeneity effects. The directionality check by the Steiger analysis did not indicate a significant causal relationship. Furthermore, because the MR analysis was removed and repeated after detecting outliers in the MR-PRESSO analysis, no potentially instrumental outliers were detected for SNPs included in the final analysis. Moreover, no SNPs with high influence were identified using leave-one-out analysis. Finally, no evidence of reverse causality was detected by MR Steiger test.
IVW estimates from 23 medications on PD phenotypes. The color of each block represents the IVW-derived P-values for each MR analysis, examining the association between medications and PD phenotypes. Red indicates a positive association, and blue indicates a negative association. PD refers to Parkinson’s disease, AAO stands for age at onset, UPDRS3 stands for Unified Parkinson’s Disease Rating Scale part III, MMSE stands for Mini-Mental State Examination, and MoCA stands for Montreal Cognitive Assessment.
MR analysis results for thyroid preparation use and its impact on PD risk. (A) Scatter plot illustrating potential effects of SNPs on Thyroid preparation use and PD risk using IVW, MR-Egger, and weighted median methods. The slope of fitted lines represents the estimated MR effect per method. At the same time, the 95% confidence interval (CI) for the impact of size on thyroid preparation use and PD risk is shown as vertical and horizontal lines, respectively. (B) Funnel plot for thyroid preparation use, showing estimation using the inverse of the standard error of causal estimate with each SNP as a tool. The vertical line depicts the estimated causal effect obtained using IVW and MR-Egger methods. (C) Forest plot demonstrating the impact of each SNP in the MR analysis. (D) Forest plot presenting the leave-one-out sensitivity analysis results, where each SNP in the instrument is iteratively removed to check the result’s stability.
In addition, some medications have been found to potentially increase the risk of PD or accelerate its progression (Table 1, Supplementary Table 4–8, Supplementary Fig. 3–6). These medications included antidepressants (IVW: OR = 1.26; 95% CI 1.04, 1.52; P = 0.018; PD risk), diuretics (IVW: β = 0.15; 95% CI 0.01, 0.29; P = 0.040; UPDRS3), antimigraine preparations (IVW: β = 0.15; 95% CI 0.02, 0.29; P = 0.027; UPDRS3), and drugs affecting bone structure and mineralization (IVW: β = − 1.22; 95% CI − 2.25, − 0.18; P = 0.021; UPDRS3). The analyses in this study detected no heterogeneity, pleiotropy, or outliers for the SNPs included in the analysis using the aforementioned sensitivity testing methods. However, Steiger’s analysis found that the effects of diuretics and drugs on bone structure and mineralization may be affected by reverse causality interference.
Association between genetically predicted Parkinson’s disease and 23 medications
To further address concerns about reverse causality and validate the stability of the findings in this study, bidirectional MR analyses were performed on the results of the Forward MR analysis (PIVW < 0.05) to examine the causal effects of different PD phenotypes on drug use (Supplementary Table 11). Specifically, the MR estimates for PD and various medications were as follows: PD risk (IVW: OR = 0.97; 95% CI 0.93, 1.00; P = 0.074; thyroid preparations); PD risk (IVW: OR = 0.97; 95% CI 0.95, 1.00; P = 0.091, antidepressants); PD risk (IVW: OR = 1.00; 95% CI 0.97, 1.03; P = 0.918, calcium channel blockers); UPDRS3 (IVW: OR = 1.00; 95% CI 0.95, 1.04; P = 0.848, diuretics); UPDRS3 (IVW: OR = 0.97; 95% CI 0.92, 1.02; P = 0.248, salicylic acid and derivatives); UPDRS3 (IVW: OR = 0.99; 95% CI 0.84, 1.17; P = 0.904, antimigraine preparations). Notably, there was a significant trend in the increase in PD risk and antidepressant use.
Sensitivity and Steiger analyses did not find any evidence of heterogeneity, multiple effects, or reverse causality.
Multivariable MR
In multivariable MR analyses, after correction for known drug-related diseases, only calcium channel blockers (IVW: OR = 0.90; 95% CI 0.85, 0.96; P = 0.002, Angina pectoris) retained significance among the three potential PD-protective medications. In contrast, the remaining results showed no significant relationship between other medications and PD-related phenotypes. Among the potential PD risk medications, significant associations were observed with diuretics (IVW: β = 0.99; 95% CI 0.89, 1.08; P = 0.001, Heart failure), diuretics (IVW: β = 0.17; 95% CI 0.16, 0.18; P = 0.001, Hypertension), antimigraine preparations (IVW: β = 0.03; 95% CI 0.02, 0.03; P = 0.001, Migraine), and drugs affecting bone structure and mineralization (IVW: β = 0.02; 95% CI 0.02, 0.03; P = 0.001, Osteoporosis). Notably, the correction for osteoporosis transformed the role of drugs affecting bone structure and mineralization from a risk factor to a protective one. One possible explanation is the presence of multicollinearity issues in the study. Despite heterogeneity in some analyses, the IVW method was employed as a primary analytical approach, which was minimally affected by heterogeneity (Supplementary Table 12).
Discussion
Using data from large-scale GWASs, we applied MR analyses to test whether 23 unconventional anti-Parkinson medications were causally related to the risk of PD (PD diagnosis/AAO) and the progression of PD (UPDRS3/MMSE/MOCA). The results of the two-sample MR analysis suggest the potential protective effects of thyroid preparations, salicylic acid and derivatives, and calcium channel blockers against PD. There was a positive causal relationship between antidepressants, diuretics, antimigraine preparations, and drugs affecting bone structure and mineralization and the risk or progression of PD (Fig. 4). Furthermore, sensitivity analyses and directional tests showed that the association between drugs affecting bone structure and mineralization, antidepressants, and diuretics with PD may be influenced by reverse causality, requiring cautious interpretation of these causal relationships. Moreover, MVMR analysis revealed that potential primary diseases may drive the observed causal relationships, and the presence of these diseases can complicate the association between medications and the potential PD phenotype. This may also be one of the reasons for some epidemiological studies reaching inconsistent conclusions. However, these findings are worth further investigation.
The basic principles of medical treatment for PD have been established for decades, including medication therapy (usually levodopa preparations or combination medications) and non-pharmacological treatments (such as exercise and physical, occupational, and speech therapies)3. Approaches such as deep brain stimulation and treatment with levodopa-carbidopa enteral suspension can help individuals with medication-resistant tremors, worsening symptoms when the medication wears off, and dyskinesias3. For most patients with PD, levodopa therapy is required within 2 years after symptom onset, and it remains the most effective medication for early-stage PD. However, PD becomes more complex in advanced stages due to additional functional impairment of nondopaminergic neuronal networks41,42. Consequently, patients require higher doses of levodopa and more frequent dosing intervals every 2–3 h. With disease progression, long- and short-term responses to dopaminergic medications decrease due to disease-related cerebral pathophysiological changes43. Given the adverse reactions and limitations of existing treatment options, there is an urgent need to improve drug selection. Currently, a growing number of therapies are being developed based on hypotheses about the pathogenesis of the disease44. Researchers are dedicated to exploring new medications, such as innovative treatment approaches targeting α-synuclein, including active/passive immunotherapies and induced pluripotent stem cell technologies45,46. In particular, there have been advancements in monoclonal antibody therapy. However, recent reports from phase 2 trials have not demonstrated benefits in terms of primary or secondary outcomes47,48. Given the history of failed “neuroprotection trials,” expectations should be lowered for the approval of safe and effective disease-modifying medications in the near future. The enormous cost of developing new medications has recently led to interest in repurposing previously used medications from other fields. Drug repurposing has more advantages than traditional approaches, as it can overcome significant drug discovery challenges with novel methods49. Survey studies have shown that developing a new drug costs approximately US$1 billion, whereas drug repurposing takes 60% less time and is more cost-effective50. Furthermore, the success rate of drug repurposing is higher than traditional approaches, as the characteristics of these compounds have already been established and they have undergone various tests for toxicity and side effects51.
Our study provides some new clues for drug repurposing in PD. First, numerous studies have shown a correlation between thyroid function and PD52,53. Interaction between the hypothalamic-pituitary-thyroid axis and dopaminergic system, epidemiological evidence of thyroid dysfunction with PD, and the presence of shared genes regulating PD and the dopaminergic system suggest a potential common pathophysiological basis between these two diseases52,54. In fact, thyroid hormones and the peroxisome proliferator-activated receptor (PPARα) signaling directly or indirectly act on neurons, producing neuroprotective effects55. Additionally, administration of thyroid hormones can protect dopaminergic neurons, derived from rat or human neural progenitor cells against neurotoxin-induced damage55. A possible mechanism involves inducing the differentiation and maturation of dopaminergic (DA) neurons by upregulating nuclear receptor-related 1 protein (NURR1)55. Therefore, given the neuroprotective effects of thyroid hormones, levothyroxine is a potential candidate for future PD therapy54. Second, due to the crucial role of inflammation in brain degeneration, anti-inflammatory medications are considered potential therapeutic agents that reduce central nervous system degeneration and potentially delay or prevent the onset of PD. Animal experiments have demonstrated that non-steroidal anti-inflammatory drugs (NSAIDs) and aspirin can prevent neuronal cell death56,57. Clinical evidence also suggests that non-aspirin and non-steroidal anti-inflammatory medications, especially ibuprofen, may reduce the risk of developing PD58. Moreover, recent research has found that regular use of NSAIDs may decrease the penetrance of leucine rich repeat kinase 2 (LRRK2) mutations associated with PD59. These findings provide a straightforward therapeutic approach to modify disease progression in individuals with LRRK2 variants and highlight the potential benefits of anti-inflammatory medications in PD management59,60. Our study is consistent with these findings. Nevertheless, these associations are not always consistent, and several studies have found no evidence that the use of Salicylic acid and derivatives reduces the risk of PD57,61. Given the inconsistency in studies evaluating salicylic acid and its derivatives as neuroprotective agents, further research is needed to confirm or refute this hypothesis. Such studies should consider the standard indications, contraindications, and side effects of these compound and carefully assess the timing between use and disease onset. Third, calcium homeostasis, receptor activity, and calcium-induced oxidative stress are potential factors in PD’s pathogenesis and potential targets for therapeutic interventions62. Moreover, evidence suggests that over time, the firing of substantia nigra dopamine neurons shifts from being dependent on sodium channels to being dependent on L-type calcium channels (Cav1.3) for maintaining autonomous activity63. In this regard, blocking these channels with calcium channel blockers, such as isradipine, can reverse this dependency and prevent toxin-induced damage. Furthermore, during autonomous activity, Cav1.3 channels generate mitochondria-mediated oxidative stress, which induces mitochondrial uncoupling as a protective mechanism64. Recently, selective Cav1.3 channel inhibitors have been developed as a novel approach for treating PD65.
This study has several strengths. One major advantage is the MR study design, which helps mitigate confounding and reverse causation biases. Additionally, these associations were estimated from independent data sources and combined through meta-analysis, ensuring sufficient statistical power and robustness of the findings. However, we acknowledge that this study has some limitations. First, due to the limited number of SNPs reaching genome-wide significance, the thresholding of P-values was relaxed in line with common practice. Notably, we tested the strength of the instruments and found that the F-statistics for all instruments exceeded 10, which is the traditional threshold for solid instruments. Second, the possibility that the 23 drug-related SNPs may influence PD through other causal pathways can not be fully ruled out. Third, as this study is based solely on individuals of European ancestry, caution should be exercised when extrapolating the results to other populations. Fourth, the results showed were only borderline significant, with a weak effect size. These findings did not reach the significant threshold after the Bonferroni correction. Therefore, the significance of this study lies in its suggestive and supplementary evidence. Finally, associations from MR do not provide information about temporal patterns and should be interpreted as the lifetime effects of the liability to a particular risk factor.
Conclusion
The development of neuroprotective strategies has always been challenging. Thus, drug repurposing may be a good strategy for addressing the current challenges of PD treatment. This study has provided new insights for drug repurposing from a genetic perspective, which may accelerate drug discovery and application.
Data availability
The summary statistics analyzed in the study are included in the article. Further inquiries can be directed to the corresponding author to make it accurate.
References
Feigin, V. L. et al. Global, regional, and national burden of neurological disorders during 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 16(11), 877–897. https://doi.org/10.1016/s1474-4422(17)30299-5 (2017).
Feigin, V. L. et al. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 18(5), 459–480. https://doi.org/10.1016/s1474-4422(18)30499-x (2019).
Armstrong, M. J. & Okun, M. S. Diagnosis and treatment of Parkinson disease: A review. JAMA 323, 548–560. https://doi.org/10.1001/jama.2019.22360 (2020).
Bloem, B. R., Okun, M. S. & Klein, C. Parkinson’s disease. Lancet 397, 2284–2303. https://doi.org/10.1016/s0140-6736(21)00218-x (2021).
Obeso, J. A., Monje, M. H. G. & Matarazzo, M. Major advances in Parkinson’s disease over the past two decades and future research directions. Lancet Neurol. 21, 1076–1079. https://doi.org/10.1016/s1474-4422(22)00448-3 (2022).
Chaudhuri, K. R. & Sauerbier, A. Parkinson disease Unravelling the nonmotor mysteries of Parkinson disease. Nat. Rev. Neurol. 12, 10–11. https://doi.org/10.1038/nrneurol.2015.236 (2016).
Schapira, A. H., Olanow, C. W., Greenamyre, J. T. & Bezard, E. Slowing of neurodegeneration in Parkinson’s disease and Huntington’s disease: Future therapeutic perspectives. Lancet 384, 545–555. https://doi.org/10.1016/s0140-6736(14)61010-2 (2014).
Athauda, D. & Foltynie, T. Drug repurposing in Parkinson’s disease. CNS Drugs 32, 747–761. https://doi.org/10.1007/s40263-018-0548-y (2018).
Weintraub, D. et al. Association of dopamine agonist use with impulse control disorders in Parkinson disease. Arch. Neurol. 63, 969–973. https://doi.org/10.1001/archneur.63.7.969 (2006).
Rascol, O., Fabbri, M. & Poewe, W. Amantadine in the treatment of Parkinson’s disease and other movement disorders. Lancet Neurol. 20, 1048–1056. https://doi.org/10.1016/s1474-4422(21)00249-0 (2021).
DeMaagd, G. & Philip, A. Parkinson’s disease and its management: Part 3: Nondopaminergic and nonpharmacological treatment options. P t 40, 668–679 (2015).
Hsu, W. Y., Lane, H. Y. & Lin, C. H. Medications used for cognitive enhancement in patients with schizophrenia, bipolar disorder, Alzheimer’s disease, and Parkinson’s disease. Front. Psychiatry 9, 91. https://doi.org/10.3389/fpsyt.2018.00091 (2018).
Athauda, D. et al. Exenatide once weekly versus placebo in Parkinson’s disease: A randomised, double-blind, placebo-controlled trial. Lancet 390, 1664–1675. https://doi.org/10.1016/s0140-6736(17)31585-4 (2017).
Meissner, W. G. et al. Trial of lixisenatide in early Parkinson’s disease. N. Engl. J. Med. 390, 1176–1185. https://doi.org/10.1056/NEJMoa2312323 (2024).
Devos, D. et al. Trial of deferiprone in Parkinson’s disease. N. Engl. J. Med. 387, 2045–2055. https://doi.org/10.1056/NEJMoa2209254 (2022).
Schwarzschild, M. A. et al. Effect of urate-elevating inosine on early Parkinson disease progression: The SURE-PD3 randomized clinical trial. JAMA 326, 926–939. https://doi.org/10.1001/jama.2021.10207 (2021).
Simuni, T. et al. Efficacy of nilotinib in patients with moderately advanced Parkinson disease: A randomized clinical trial. JAMA Neurol. 78, 312–320. https://doi.org/10.1001/jamaneurol.2020.4725 (2021).
Parkinson Study Group STEADY-PD III Investigators*. Isradipine versus placebo in early Parkinson disease: A randomized trial. Ann. Intern. Med. 172(9), 591–598. https://doi.org/10.7326/m19-2534 (2020).
Belete, D. et al. Association between antiepileptic drugs and incident Parkinson disease. JAMA Neurol 80, 183–187. https://doi.org/10.1001/jamaneurol.2022.4699 (2023).
Migdalska-Richards, A., Ko, W. K. D., Li, Q., Bezard, E. & Schapira, A. H. V. Oral ambroxol increases brain glucocerebrosidase activity in a nonhuman primate. Synapse 71, e21967. https://doi.org/10.1002/syn.21967 (2017).
Hernández-Parra, H. et al. Repositioning of drugs for Parkinson’s disease and pharmaceutical nanotechnology tools for their optimization. J. Nanobiotechnol. 20, 413. https://doi.org/10.1186/s12951-022-01612-5 (2022).
Elkouzi, A., Vedam-Mai, V., Eisinger, R. S. & Okun, M. S. Emerging therapies in Parkinson disease - repurposed drugs and new approaches. Nat. Rev. Neurol. 15, 204–223. https://doi.org/10.1038/s41582-019-0155-7 (2019).
Sanderson, E. et al. Mendelian randomization. Nat. Rev. Methods Primers 2, 6. https://doi.org/10.1038/s43586-021-00092-5 (2022).
Smith, G. D. & Ebrahim, S. ‘Mendelian randomization’: Can genetic epidemiology contribute to understanding environmental determinants of disease?. Int. J. Epidemiol. 32, 1–22. https://doi.org/10.1093/ije/dyg070 (2003).
Emdin, C. A., Khera, A. V. & Kathiresan, S. Mendelian randomization. JAMA 318, 1925–1926. https://doi.org/10.1001/jama.2017.17219 (2017).
Wu, Y. et al. Genome-wide association study of medication-use and associated disease in the UK Biobank. Nat. Commun. 10, 1891. https://doi.org/10.1038/s41467-019-09572-5 (2019).
Nalls, M. A. et al. Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: A meta-analysis of genome-wide association studies. Lancet Neurol. 18, 1091–1102. https://doi.org/10.1016/s1474-4422(19)30320-5 (2019).
Blauwendraat, C. et al. Parkinson’s disease age at onset genome-wide association study: Defining heritability, genetic loci, and α-synuclein mechanisms. Mov. Disord. 34, 866–875. https://doi.org/10.1002/mds.27659 (2019).
Iwaki, H. et al. Genomewide association study of Parkinson’s disease clinical biomarkers in 12 longitudinal patients’ cohorts. Mov. Disord. 34, 1839–1850. https://doi.org/10.1002/mds.27845 (2019).
Zhu, Y. et al. Assessing the association between white matter lesions and Parkinson’s disease. Neurol. Sci. 44, 897–903. https://doi.org/10.1007/s10072-022-06494-x (2023).
Chen, J. et al. Gastrointestinal consequences of type 2 diabetes mellitus and impaired glycemic homeostasis: A Mendelian randomization study. Diabetes Care 46, 828–835. https://doi.org/10.2337/dc22-1385 (2023).
Bowden, J., Davey Smith, G. & Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 44, 512–525. https://doi.org/10.1093/ije/dyv080 (2015).
Hemani, G., Tilling, K. & Davey Smith, G. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet. 13, e1007081. https://doi.org/10.1371/journal.pgen.1007081 (2017).
Seyedsalehi, A. et al. Educational attainment, structural brain reserve and Alzheimer’s disease: A Mendelian randomization analysis. Brain 146, 2059–2074. https://doi.org/10.1093/brain/awac392 (2023).
Staley, J. R. et al. PhenoScanner: A database of human genotype-phenotype associations. Bioinformatics 32, 3207–3209. https://doi.org/10.1093/bioinformatics/btw373 (2016).
Li, C., Li, X., Lin, J., Cui, Y. & Shang, H. Psoriasis and progression of Parkinson’s disease: A Mendelian randomization study. J. Eur. Acad. Dermatol. Venereol. 36, 2401–2405. https://doi.org/10.1111/jdv.18459 (2022).
Brion, M. J., Shakhbazov, K. & Visscher, P. M. Calculating statistical power in Mendelian randomization studies. Int. J. Epidemiol. 42, 1497–1501. https://doi.org/10.1093/ije/dyt179 (2013).
Burgess, S. & Thompson, S. G. Multivariable Mendelian randomization: The use of pleiotropic genetic variants to estimate causal effects. Am. J. Epidemiol. 181, 251–260. https://doi.org/10.1093/aje/kwu283 (2015).
Cai, J. et al. Genetic liability for prescription opioid use and risk of cardiovascular diseases: A multivariable Mendelian randomization study. Addiction 117, 1382–1391. https://doi.org/10.1111/add.15767 (2022).
Rosoff, D. B., Smith, G. D. & Lohoff, F. W. Prescription opioid use and risk for major depressive disorder and anxiety and stress-related disorders: A multivariable Mendelian randomization analysis. JAMA Psychiatry 78, 151–160. https://doi.org/10.1001/jamapsychiatry.2020.3554 (2021).
Hayes, M. W., Fung, V. S., Kimber, T. E. & O’Sullivan, J. D. Updates and advances in the treatment of Parkinson disease. Med. J. Aust. 211, 277–283. https://doi.org/10.5694/mja2.50224 (2019).
Trenkwalder, C., Kuoppamäki, M., Vahteristo, M., Müller, T. & Ellmén, J. Increased dose of carbidopa with levodopa and entacapone improves “off” time in a randomized trial. Neurology 92, e1487–e1496. https://doi.org/10.1212/wnl.0000000000007173 (2019).
Chou, K. L. et al. The spectrum of “off” in Parkinson’s disease: What have we learned over 40 years?. Parkinsonism Relat. Disord. 51, 9–16. https://doi.org/10.1016/j.parkreldis.2018.02.001 (2018).
AlDakheel, A., Kalia, L. V. & Lang, A. E. Pathogenesis-targeted, disease-modifying therapies in Parkinson disease. Neurotherapeutics 11, 6–23. https://doi.org/10.1007/s13311-013-0218-1 (2014).
Jankovic, J. et al. Safety and tolerability of multiple ascending doses of PRX002/RG7935, an anti-α-Synuclein monoclonal antibody, in patients With Parkinson disease: A randomized clinical trial. JAMA Neurol. 75, 1206–1214. https://doi.org/10.1001/jamaneurol.2018.1487 (2018).
Barker, R. A., Studer, L., Cattaneo, E. & Takahashi, J. G-Force PD: A global initiative in coordinating stem cell-based dopamine treatments for Parkinson’s disease. NPJ Parkinsons Dis. 1, 15017. https://doi.org/10.1038/npjparkd.2015.17 (2015).
Lang, A. E. et al. Trial of Cinpanemab in early Parkinson’s disease. N. Engl. J. Med. 387, 408–420. https://doi.org/10.1056/NEJMoa2203395 (2022).
Pagano, G. et al. Trial of prasinezumab in early-stage Parkinson’s disease. N. Engl. J. Med. 387, 421–432. https://doi.org/10.1056/NEJMoa2202867 (2022).
Ashburn, T. T. & Thor, K. B. Drug repositioning: Identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 3, 673–683. https://doi.org/10.1038/nrd1468 (2004).
Morgan, S., Grootendorst, P., Lexchin, J., Cunningham, C. & Greyson, D. The cost of drug development: A systematic review. Health Policy 100, 4–17. https://doi.org/10.1016/j.healthpol.2010.12.002 (2011).
Polamreddy, P. & Gattu, N. The drug repurposing landscape from 2012 to 2017: Evolution, challenges, and possible solutions. Drug Discov. Today 24, 789–795. https://doi.org/10.1016/j.drudis.2018.11.022 (2019).
Xu, J. et al. Genetic correlation between thyroid hormones and Parkinson’s disease. Clin. Exp. Immunol. 208, 372–379. https://doi.org/10.1093/cei/uxac044 (2022).
Kim, J. H. et al. Association between thyroid diseases and Parkinson’s disease: A nested case-control study using a national health screening cohort. J. Parkinsons Dis. 11, 211–220. https://doi.org/10.3233/jpd-202265 (2021).
Mohammadi, S., Dolatshahi, M. & Rahmani, F. Shedding light on thyroid hormone disorders and Parkinson disease pathology: Mechanisms and risk factors. J. Endocrinol. Invest. 44, 1–13. https://doi.org/10.1007/s40618-020-01314-5 (2021).
Lee, E. H. et al. Dopamine neuron induction and the neuroprotective effects of thyroid hormone derivatives. Sci. Rep. 9, 13659. https://doi.org/10.1038/s41598-019-49876-6 (2019).
Teismann, P. & Ferger, B. Inhibition of the cyclooxygenase isoenzymes COX-1 and COX-2 provide neuroprotection in the MPTP-mouse model of Parkinson’s disease. Synapse 39, 167–174. https://doi.org/10.1002/1098-2396(200102)39:2%3c167::Aid-syn8%3e3.0.Co;2-u (2001).
Driver, J. A., Logroscino, G., Lu, L., Gaziano, J. M. & Kurth, T. Use of non-steroidal anti-inflammatory drugs and risk of Parkinson’s disease: Nested case-control study. Bmj 342, d198. https://doi.org/10.1136/bmj.d198 (2011).
Rees, K. et al. Non-steroidal anti-inflammatory drugs as disease-modifying agents for Parkinson’s disease: Evidence from observational studies. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD008454.pub2 (2011).
Fyfe, I. Aspirin and ibuprofen could lower risk of LRRK2 Parkinson disease. Nat. Rev. Neurol. 16, 460. https://doi.org/10.1038/s41582-020-0394-7 (2020).
San Luciano, M. et al. Nonsteroidal anti-inflammatory use and LRRK2 Parkinson’s disease penetrance. Mov. Disord. 35, 1755–1764. https://doi.org/10.1002/mds.28189 (2020).
Becker, C., Jick, S. S. & Meier, C. R. NSAID use and risk of Parkinson disease: A population-based case-control study. Eur. J. Neurol. 18, 1336–1342. https://doi.org/10.1111/j.1468-1331.2011.03399.x (2011).
Surmeier, D. J. Calcium, ageing, and neuronal vulnerability in Parkinson’s disease. Lancet Neurol. 6, 933–938. https://doi.org/10.1016/s1474-4422(07)70246-6 (2007).
Chan, C. S. et al. ‘Rejuvenation’ protects neurons in mouse models of Parkinson’s disease. Nature 447, 1081–1086. https://doi.org/10.1038/nature05865 (2007).
Guzman, J. N. et al. Oxidant stress evoked by pacemaking in dopaminergic neurons is attenuated by DJ-1. Nature 468, 696–700. https://doi.org/10.1038/nature09536 (2010).
Kang, S. et al. CaV1.3-selective L-type calcium channel antagonists as potential new therapeutics for Parkinson’s disease. Nat. Commun. 3, 1146. https://doi.org/10.1038/ncomms2149 (2012).
Acknowledgements
The authors thank all the relevant consortiums and investigators for the management and sharing of summary-level data.
Funding
This work was funded by the National Natural Science Foundation of China (81060096; 81560202) and the Natural Science Foundation of Hainan (821QN0979); Hainan Key Scientific Research Projects (14A110060), Key R&D Plan Projects of Hainan Province (ZDYF2021SHFZ091), Hainan Natural Science Foundation Innovative Research Team Projects (2016CXTD010), Key Educational Reform Projects of Hainan Province (Hnjg2019ZD-15), and High-level Talents of the Hainan Natural Science Foundation (820RC759). This work was Supported by the Construction Project of Hainan Province Clinical Medical Center and Science, Innovation Platform for Academicians of Hainan Province. The funding bodies had no role in the design of the study; the collection, analysis, or interpretation of the data; or the writing of the manuscript.
Author information
Authors and Affiliations
Contributions
W.Q.: Conceptualization, Writing—original draft, Formal analysis. L.F.: Writing—original draft, Formal analysis. W.X.: Writing—original draft, Formal analysis. Z.L.: Writing; review & editing-Equal. C.B.: Conceptualization, Writing—review & editing, Formal analysis. C.T.: Conceptualization, Writing—review & editing, Formal analysis. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, Q., Liu, F., Wang, X. et al. Identifying potential repurposable medications for Parkinson’s disease through Mendelian randomization analysis. Sci Rep 14, 19670 (2024). https://doi.org/10.1038/s41598-024-70758-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-70758-z
- Springer Nature Limited