Abstract
Globally from abiotic stresses, salt stress is the major stress that limits crop production. One of them is wheat that has been utilized by more than 1/3 of the world population as staple food due to its nutritive value. Biochar is an activated carbon that can ameliorate the negative impacts on plants under saline conditions. The present study was conducted to examine the ameliorative impact of “Biochar application” to Triticum aestivum L. plant grown under salinity stress and evaluated on the basis of various growth, yield, physiological, biochemical attributes. Preliminary experiment was done to select the Triticum aestivum L. varieties with 90% germination rate for further experiment. The selected varieties, FSD08 and PUNJAB-11 of wheat were treated with two levels of sodium chloride (0 mM and 120 mM). Two varieties of wheat included FSD08 and PUNJAB-11 were treated with two levels of sodium chloride (0 mM and 120 mM). To address the impact of salt stress two levels of biochar 0% and 5% was used as exogenous application. A three way completely randomized experimentation was done in 24 pots of two wheat varieties with three replicates. The results demonstrated that salt stress affected growth, physiological attributes, yield and inorganic mineral ions (Ca2+ and K+) in roots and shoots parameters of wheat negatively while biochar overall improved the performance of plant. SOD, CAT, APX and POD activities enhanced during salt stress as the plant self-defense mechanism against salinity to minimize the damaging effect. Salt stress also significantly increased the membrane permeability, and levels of H2O2, MDA, Cl and Na ions. Biochar treatment nullified negative impacts of NaCl and improved the plant growth and yield significantly. Hence, biochar amendment can be suggested as suitable supplement for sustainable crop production under salinization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Amongst cereal the widely and major crop is wheat all over the world belong to Poaceae family that imparts about 50% and 30% of trade and production of the world grain respectively1. Worldwide, amongst grain the utmost favorable grain is wheat belong to Poaceae family use as staple food by more than 1/3 population because of having affordable price and being nutritious crop2. Wheat is an abundant source of protein, carbohydrates, vitamins, and extra calories for human as a balanced diet compared to other cereal crop3. Wheat (Triticum aestivum L.) is the chief natural concentrated source of, fatty acids, minerals, tocopherols and phytosterols for human consumption as well4. More than one-third of world population use wheat as staple food crop.
About 800 million hectares all around the world, which is equal to almost 6% of the total land area of globe, are salt affected land. Presently, salinity influences about 20% of areas completely agricultural land and in another report, 7% of the land area has been affected by salinity. Water used for irrigation purpose and most of agricultural soils contain salt5,6,7. Soil and irrigation water both decide the amount and type of salts present in soil. When the root zone has a high enough amount of salt, soil becomes saline which prevents optimum yield and growth. In another study reported salt stress decline growth of plant and production of crop in cultivated areas worldwide3. Salt stress effect the soil health such as Physiochemical properties of soils are declined largely by effect the ecological functions of soils (like nitrification, denitrification, respiration and residue decomposition) when K+ replace from Na+ and Mg2+ both. The ecological functions are disturb due to influence of salt stress on microbial enzymatic activity and community of soil8.
Following rice and maize, wheat global production is rank third, which provides 35% of the world’s total food grain9. Its global production comprises around 700 million tons from 200 million hectares. In Pakistan, land under wheat crop cultivation about 9.18 million hectares with the wheat production about 24.47 million tons per annum10,11. It is grown nearly all around the world and as a wheat produce Pakistan stands 3rd in Asia and 11th in the world2. According to its sowing seasons, wheat divided into winter and spring wheat. Around 80% of the worlds is winter wheat which is sown in autumn when temperatures are between 0 and 5 °C due to seedlings requiring a period during the vegetative phase. In South Asian countries or North Africa, spring wheat is cultivated in spring and harvested during late summer or fall12.
The global population uses about 85% for calories and 82% for protein is relied on wheat. Salt stress is one of the critical and foremost abiotic stresses of environment to limit soil and productivity of crops in some regions (arid and semi-arid) of the world. Soil salinization arises due to natural sources and some human-mediated activities (increased urbanization and deforestation)3. Salinity increased because of low precipitation, poor drainage, poor irrigation and high evaporation level in salinization areas9. Agricultural production is pressurized by intensity of environmental stress and day-by-day increasing population. Under saline conditions, plants face two primary stresses: ionic and osmotic stress. These stresses are caused by a reduction in soil water potential and increased absorption of salts, including sodium and calcium. Saline soil conditions typically lead to nutritional disorders that adversely affect plant growth, ultimately resulting in crop yield losses13. Osmotic stress develops when the salt level increases around the roots in soil solution and affects plants immediately leading to cell expansion, lateral bud development and inhibition of water uptake. Ionic stress progresses slowly as compared to osmotic stress. Ionic stress effect the plant when level of accumulate toxic ions beyond the plant specific threshold and results in chlorosis, enhanced necrosis, mortality of leaf, photosynthesis and activity of cellular metabolism reduced14.
Various investigations reported compared to other environmental factors, salinity cause the decline in crop production and development. Khan et al.15 recorded negative impact of in soil on growth of plant, photosynthesis, biosynthesis of chlorophyll, protein synthesis and disturbance of plant water status. The toxic effect of salt stress also disturbs the other metabolic processes and ion imbalance of plants due to elevated Na+ ions, and oxidative stress (e.g., H2O2 and O2•- contents). Salinity stress causes both short and long-term modifications in plants. This stress can eventually lead to the damage of crucial biomolecules through elevating the production of ROS16. As the level of salt stress increases the germination rate, rate of photosynthesis, dry weight of root, length, fresh and dry weights of plant, lipids and energy production of wheat become reduces3. The wheat crop acquired significance is because of high commercial and nutritive value. Agricultural production is pressurized by intensity of environmental stress and day-by-day increasing population. Salinity has an impact on the productivity of wheat, a significant cereal crop cultivated on over 9.0 million hectares of land in Pakistan17. Around 70% of the staple food diminishes due to soil salinization, which affected about 925 million people with extreme hunger. Salinity has declined the potential of soil for crop productivity in Pakistan. Among abiotic environmental stress, salt stress is one of the most critical stresses which limits the wheat production in Pakistan especially Balochistan. Salts present in cultivated soil affect the crop yield above permissible levels10.
Soil salinization can be reduced by using different approaches such as the application of organic matter. As organic matter, Biochar is a carbon rich charcoal-like material which is produced from any kind of organic waste i.e., poultry manure, animal, or crop residue. Biochar produced by exothermal process under absence or low oxygen at high temperature (pyrolysis)17. Biochar could improve plant growth as well. Biochar can promote plant growth under salinity in two mechanisms. Direct mechanism of action under application of biochar involves growth promotion by providing minerals nutrients (Mg, Ca, K, S and P) to plant. Although, indirect mechanism of action promotes plant growth by enhancing soil physiochemical and biological properties18. The soil physiochemical properties include water-holding capacity, pH, CEC (cation exchange capacity), soil structure and surface area19,20. Biochar amendment is significant to improve biological processes of plants under natural conditions such as improved germination percentage in several plants. Biochar is most recalcitrant because of high aromaticity and biochar properties (ash content, pH, nutrients contents and total carbon) are dependent upon the temperature of pyrolysis and the matter used for production of biochar. When the temperature of biochar pyrolysis is variable from low to high, pH (alkaline, acidic) of biochar is affected. Alkaline biochar has high pH, alkalinity (CaCO3), and EC, which can increase EC and pH of soil. Different studies reported that biochar with pH 7.0 (neutral) enhanced more electrical conductivity (EC) and pH of soil relative to acidic biochar21.
Biochar amendment has capability to increase fertility of soil, sequestration of carbon, pollutants (organic and inorganic) immobilization and bio-energy production. Biochar reduces greenhouse gases emission and improve crop productivity5. All of these biochar characters have received significant attention and are used in agriculture day by day. Biochar is very helpful in reducing salinity and enhancing uptake of potassium in plants due to having high potential of Na+ adsorption8,13,22. Biochar amendment can enhance to utilization, immobilization or holding by its surface properties and availability of mineral nutrients (Fe, K, Mn, Cu, Zn and P) to plant5,13,17. Biochar amendment alters the antioxidant enzyme activities by suppressing the oxidative stress and promote production of phytohormones in result enhance tolerance in plants during salt stress8. The objective of our study is to examine the possible regulatory role of biochar in wheat and the biochemical, morphological, and physiological characters in wheat under salt stress, to identify the biochar-controlled salt stress tolerance mechanism in wheat.
Materials and methods
Growth condition and treatment
Collection of seeds
Two largely growing varieties of wheat FSD-08 and PUNJAB-11 in Pakistan were obtained from Ayub Agricultural Research Institute (AARI) Faisalabad used for this experiment. Experimental research and field studies on plants (either cultivated or wild), including the collection of plant material, complied with relevant institutional, national, and international guidelines and legislation.
Workplace for experiment
The experiment was conducted to study the ameliorating role of biochar to the toxic effects of salts in Triticum aestivum L. Plants were grown in the botanical garden, research area of Department of Botany, University of Education, Township Campus Lahore.
Experimental design
The experiment was conducted to study the impact of biochar by foliar application to address salinity stress in wheat varieties FSD-08 and Punjab-11. The experimental set up was 3 ways completely randomized design (CRD) using 2 varieties, and two levels of salt stress and biochar each, with three replicates and a total of 24 pots which filled with sand medium. The size of pot was 10 × 12 inches. Levels of salt and biochar for treatment are given in Table S1. The duration of the experiment spanned around/6 months, from planting in the first week of November to harvest in April.
Sowing and seedling
Seed was sown in 1st week of November 2021 in prewashed sand. Before sowing, sand was filled in a pot having a hole of 1 inch and hole covered with cotton fabric. 12 seeds were sown in each pot in 1/2-inch depth of 6 kg sand. Seeds were germinated within 2 weeks and seedling thinning done after 3 weeks of germination. Each pot carried four uniform and healthy seedlings while rest was eradicated.
Salt and biochar treatment
Two treatments of salt (0 mM, 120 mM) applied after 39 days of germination in Hoagland solution by gradual increase in salt concentration (from 50 and 100 mM) until reached to final concentration of NaCl 120 mM (Table S2; Fig. S1). Level of Biochar treatment (5%) was sprayed on the two sets of plants by foliar application manually.
Sampling
After 1 week of treatment, a single plant of moderate size uprooted as sample from each of 24 pots having total four plants. Then uprooted plant samples were washed carefully and removed the extra water with soft fabric. Each sample of roots was collected by a sharp edge blade.
Growth parameters
By using weighing balance and scale, the Fresh weight of shoots (g plant), Fresh weight of roots (g plant), Root length (cm), Shoot length (cm), Dry weight of shoot (g), Dry weight of Root (g), Total leaf area/ plant (cm2) parameters were measured.
Physiological attributes
Chlorophyll and carotenoid contents
Content of chlorophyll a, b and carotenoid were determined by following Arnon et al.23 method. The small pieces of 0.5 g leaves sample from each of 24 pots homogenized with 10 mL of 80% acetone. The extract of samples kept for overnight at 4° C in fridge. After 24 h, the absorbance read at wavelengths 480, 645 and 663 nm of extract in UV/VIS spectrophotometer.
From recorded results calculated the content of chl. a and b by using formula as follows:
whereas OD663 is Optical Density at wavelength 663 nm, OD645= Optical Density at wavelength 645 nm, V = Volume of the extract (mL), W = Fresh leaf tissue weight (g).
Carotenoid content calculated by following Lichtenphaler in 1987 using following formula:
OD480= Optical Density of extract at 480 nm wavelength.
Relative Water Content (RWC)
By following Jones and Turner24, RWC measured by using uniform leaf size from replicate and leaves were weighed. Leaves were saturated with distilled water after recording the weight of fresh leaves (g) for 3 h at room temperature in dark. Then turgid weight was recorded. Then leaf samples placed in oven at 80 °C for overnight and measured the dry weight of leaf samples.
Percentage of RWC calculated by a formula as follows:
Hydrogen peroxide determination
According to Velikova et al.25, the level of Hydrogen peroxide in plant tissues quantified by using 500 mg fresh leaves from each replicate. 500 mg Fresh leaf tissues grounded in pre- chilled pestle and mortar with 5 mL of trichloroacetic acid (0.1% TCA). This homogenate transferred to falcon tubes and centrifuged at 12,000 g for 15 min at 4o C. The supernatant was separate from residue in a test tube. 500 µL of the supernatant added to potassium phosphate buffer of neutral pH (500 µL) and 1 M of KI (1 mL). Read absorbance of this mixture at 390 nm using UV/VIS spectrophotometer.
Stability of membrane
Relative membrane permeability (RMP)
Permeability of membrane was determined according to Yang et al.26 protocol. The youngest leaves are of uniform size and fully enlarged excised from the plants of each pot and cut into small pieces. The pieces of leaves placed in test tubes having distilled water (20 mL). These tubes were vortex for few seconds (about 5 s) then determined the initial, electric conductivity of this solution called EC0. After recording the EC0 test tubes placed for 24 h at 4 oC and then examined/ estimated EC1. Then autoclaved same samples having leaves for 20 min at 120 degrees Celsius to determine EC2. Relative membrane permeability (RMP) percentage was calculated by using formula as follows:
Malondialdehyde (MDA)
Lipid peroxidation estimated by using protocol by Cakmak and Horst27 with some modifications in terms of the concentration of the thiobarbituric acid-reactive substances (TBARS). 0.5 g Fresh leaf from each pot was homogenize at 4 °C in 3 mL of 1.0% trichloroacetic acid (TCA). The conical tubes containing that homogenate centrifuged at 2000 g for 15 min. 0.5% Thiobarbituric acid was prepared in 20% trichloroacetic acid (TCA). 0.5 mL of obtained supernatant added to 3 mL thiobarbituric acid (TBA) prepared solution in a test tube. These treated samples were incubated in a shaking water bath at 95 °C for 50 min. The test tubes containing reaction samples placed in an ice water bath in order to end reaction by cooling. After termination of reaction, these reaction samples were centrifuged at 10,000 × g for 10 min. Read the absorbance at wavelength 532 nm of supernatant using UV/VIS spectrophotometer. From all the recorded reading at absorbance 532 nm, subtracted the value at 600 nm for nonspecific absorption. By using the absorption coefficient 155 mmol-1 cm-1 TBARS concentration was calculated.
Biochemical parameters
Leaf proline
Proline content estimated according to Bates et al.28 protocol. Fresh leaf (500 mg) sample was homogenized in 3% of sulfo-salicylic acid (10 mL). Then homogenate was filter by using Whatman filter paper. Acid ninhydrin was prepared by 1.25 g of ninhydrin dissolved into glacial acetic acid (30 mL) and 6 M of O-phosphoric acid (20 mL). The 2 mL of homogenate filtrate was mixed with glacial acetic acid (2 mL) and ninhydrin acid (2 mL) in a glass test tube. This reaction solution was incubating for 60 min at 100 °C in an oven. The solution mixture reaction was terminated in an ice bath by cooling. The mixture reaction was mixed after adding 4 mL toluene for 1–2 min by passing a continuous stream of air. The chromophore (having toluene) extracted from the aqueous phase. Read absorbance at 520 nm against toluene (blank) by using UV/VIS spectrophotometer.
Extract for protein and antioxidant determination
0.5 g Fresh leaves were homogenized in 10 mL of cooled 50 mM potassium phosphate buffer (pH 7.8). The homogenate was centrifuge for 20 min at 6000 × g and 4 °C temperature. The supernatant separated from residue and kept in deep freezer for antioxidant activities determination.
Total soluble protein content
Protein content in the extract was determined by the following method of Bradford29. Bradford solution was prepared as follows: 0.1 g Coomassie Brilliant Blue dissolved in 95% ethanol (50 mL volume of ethanol used). Then added 100 mL of O-phosphoric acid (85%) and made volume up to 1 L with distilled water. 0.1 mL of prepared extract was added to 5 mL Bradford solution and then content was vortexed. Read absorbance at specific wavelength (595 nm) by UV/VIS spectrophotometer. Standard curve of bovine serum prepared by diluted stock solution of bovine serum albumin as 100 mg, 200 mg, 300 mg, 400 mg, and 500 mg from stock solution of 7.5 mg per 15 mL. This curve was used for protein determination in unknown samples.
Antioxidant activities
Activity of peroxidase (POD) and catalase (CAT)
Activities of CAT and POD enzymes were estimated by following Chance and Maehly30 method with minor alterations. The 3 mL CAT reaction mixture included 1000 µL of 50 mM potassium phosphate buffer (pH 7.0), 5.9 mM hydrogen peroxide (1.9 mL), and enzyme extract (100 µL) same as prepared in TSP (Total Soluble Protein). Remember that enzyme extract added at the end when cuvette placed in UV/VIS spectrophotometer. Read the frequent variations in reaction solution absorbance at 240 nm in every 30 s within a total time of 150 s.
Peroxidase (POD) activity
The POD reaction solution (2 mL) comprised 700 µL of 50 mM phosphate buffer (pH 5.0), 600 µL of 20 mM guaiacol, and 600 µL of 40 mM H2O2 and 100 µL enzyme extract. Observe the Changes in the absorbance at 470 nm within time limit of 150 s in steps of 30 s thorough UV/VIS spectrophotometer.
Activity of ascorbate peroxidase (APX)
The ascorbate peroxidase activity was estimated by using Nakano and Asada31 protocol with minor alterations. Ascorbate peroxidase reaction solution (3 mL) contained 50 mM phosphate buffer having pH 7.8 (2.7 mL), 0.1 mL of 7.5 mM ascorbic acid and 100 µL of 300 mM hydrogen peroxide, 100 µL of enzyme extract. This reaction solution run in UV/VIS spectrophotometer against blank contained 100 µL of distilled water instead of enzyme extract. The absorbance read at 290 nm with interval of 30 s in a time limit of 0–60 s.
Activity of superoxide dismutase (SOD)
Superoxide dismutase activity was determined by the following method of Giannopolitis and Ries32 with some minor alterations. SOD activity estimated by potential of SOD antioxidant to inhibit photoreduction of nitroblue tetrazolium (NBT). The concentration of enzyme that inhibited (50%) NBT photoreduction described single unit of SOD activity. 3 mL of reaction solution contained 2.725 mL of solution of 75 µM NBT, 20 µM riboflavin, 130 M methionine, 100 µM EDTA. Then added 250 µL of dH2O2 and 25 µL of enzyme extract to made volume 3 mL. All reaction mixture with extract placed under light (60W) for 20 min. Two controls were prepared such as dark control and light control. Both controls contained 2.750 ml reaction solution and 0.25 mL dH2O2. Dark control placed in dark and light blank placed at 60 W for 20 min. This reaction solution placed under fluorescent light at 60 W for 20 min. Read solution absorbance at 560 nm by a UV–Visible spectrophotometer.
Total phenolic
Total phenolic content quantified by following Kamath et al.33 with minor modifications. Leaves samples from each replicate dried overnight at 80 oC in oven. 500 mg dry grounded leaf sample were homogenized (for 1 min) in 10 mL of acetone (80%). Then homogenate of sample was centrifuge at 3000 g for 15 min and supernatant of each sample taken to dryness. Then added 10 mL of methanol in solid residue and mixed vigorously for 1–2 min. After 2 min, the methanol liquid layer separated from residue. The amount of total phenolic in each sample was determined by using Folin-Ciocalteu procedure. The reaction mixture in test tubes contained 0.8 mL of sodium carbonate (7.5%), 1.0 mL of Folin-Ciocalteu’s reagent and 2 mL of sample. The tubes were mixed and let stand for 30 min. After 30 min, absorbance read by UV/VIS spectrophotometer at 765 nm. Phenolic content determined as gallic acid equivalents (GAE) (mg) per dry material (g).
Inorganic digestion
Plant samples placed overnight at 70 °C in oven. After dried digested the sample (0.1 g) of each replicate by following method of Wolf34. 0.1 g ground material kept in 2 mL H2SO4 for 24 h in a test tube. Then samples heated on hot plate in digestion flask and meanwhile added hydrogen peroxide until digestion mixture become colorless. After digestion filtered the mixture and filtrate volume with distilled up to 50 mL.
Determination of cations (Na+, K+, Ca2+)
Ca2+, K+ and Na+ cations measured from the digested mixture by using Flame Photometer (Sherwood Model 360).
Determination of Cl-
Chloride ions were determined by using Mohr’s titration method. Titration was done by using silver nitrate as titrant in order to estimate the amount of Cl ions in a solution. Chloride and silvers ions react in molar ratio 1:1.
Pipette out 5 mL of sample of chloride solution in a conical flask. Then added few drops of 0.1 N chromate (potassium di-chromate) indicator which give the color to the sample solution. Titrated this solution against 0.1 M silver nitrate that was filled in the burette. The changes in color of sample solution occurred when titrated it against silver nitrate until the solution became colorless. Noted the initial and final reading of silver nitrate. The changes in color of sample solution during titration demonstrated that equivalence point had reached where the concentration of Ag ions was equal to concentration of Cl ions.
Yield parameters
Yield obtained from mature plant after 5 months of germination and stored to obtain seeds. Spike number/ plant, Grains number/spike, Spike length (cm) and Weight of 100 seeds in gram parameters recorded in excel sheet (Fig. S2).
Statistical analysis
Applied ANOVA on all parameters and LSD to compare all means at 5% level of significance by using Cohort software, 1988–2004 (CoStat statics program of version 6.303).
Research ethics
Experimental research and field studies on plants (either cultivated or wild), including the collection of plant material, complied with relevant institutional, national, and international guidelines and legislation. A prior approval was undertaken from Offices of Research, Innovation and Commercialization, University of Education, Lahore, Pakistan.
Results
Effect of biochar on growth parameters of wheat under salt stress
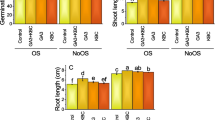
All growth parameters of FSD-08 and PUNJAB-11 are affected by 120 mM salt stress in control, but biochar treatment ameliorate the effect of salinity and plant growth much better. The Mean values of all the growth parameters with SE (Standard Error) represented as bar graphs. Plants root fresh weight and dry weight decreased with increasing in salt stress relative to control. On the other hand, at a significant level biochar caused to increase in root fresh and dry weight relative to control under conditions of saline and non-saline. Graph showed level (120 mM) of NaCl reduced root fresh and dry weight in both varieties, but the major effect is on PUNJAB-11. As interaction of biochar and salt stress, the biochar caused to mitigate salinity impact on root fresh and dry weight significantly (P < 0.001) (Fig. 1). In Contrast to control, reduced root length observed under salt stress treatment. This reduction was significant (P < 0.05). Significantly (P < 0.001), NaCl treatment reduced root length in both varieties of wheat. Relative to control biochar application enhanced root length at P < 0.05 significant level. The impact of NaCl and biochar amendment showed significant results on both varieties of wheat (P < 0.001) (Table 1, Fig. 1).
Compared to control, biochar usage increased and NaCl application decreased shoot dry and fresh weight. As in graph at 5% biochar, shoot dry and fresh weight increased in FSD-08 and PUNJAB-11 as compared to control. Biochar usage caused an increase in shoot dry and fresh weight under 120 mM NaCl treatment relative to control. While the salt stress induced in the reduction in the shoot weight. Result showed significant (P < 0.001) difference in both varieties of wheat in term of shoot length. Salt stress and biochar caused to decrease and increase shoot length respectively as compared to control. Shoot length in FSD-08 and PUNJAB- 11 increased under biochar application as compared to non- biochar. Biochar amendments ameliorate the effect of salinity and result in increased shoot length as compared to only salt stress. Significantly (P < 0.1), the combination of biochar and NaCl treatment had positive effect on both varieties. Biochar increased the shoot length of wheat varieties in presence of salt as compared to control (Table 2).
Result showed that both varieties of wheat FSD-08 and PUNJAB-11 significantly differ at P < 0.001. Overall, the highest number of leaves observed in FSD-08 as compared to PUNJAB-11. Salt stress caused maximum reduction in PUNJAB-11 to 27% as relative to control at P < 0.001%. While the application of biochar showed significantly increase in number of leaves in both varieties (P < 0.001). Under biochar treatment, varieties behaved differently in term of number of leaves at P < 0.05. The number of leaves increased in both varieties as compared to control (Fig. 1). The leaf area of both varieties of wheat differs from each other. Addition of NaCl decreased the leaf area in FSD-08 but showed opposite effect in PUNJAB-11 as compared to control. Hence, 120 mM of salt stress had a positive effect on PUNJAB-11 in terms of leaf area. The combined interaction of NaCl and biochar on variety PUNJAB-11 and FSD-08 showed opposite effect. In FSD-08, this interaction enhanced the area of leaf as compared to all other interactions. However, PUNJAB-11 showed the opposite result (Fig. 1).
Physiological attributes
Effect of biochar on content of Chl a, b, a/b ratio and carotenoids of wheat under salt stress
Salt stress and biochar treatment had negative and positive impact compared to control, respectively. Salt stress was mitigated by biochar treatment and showed enhance in chlorophyll a and b content compared to saline condition plant. Both varieties showed enhance in chl a and b under 5% B as relative to control. Result revealed chl a and b decreased 9.5% and 9.6% respectively in PUNJAB-11as compared to control under salt treatment. While in FSD-08 decreased in chl a to 0.64% and chl b to 1.03% due to salt stress relative to control. Biochar treatment under salt stress showed similarity in chl a and b. Chla and b enhanced as relative to salt stress plants during the interaction of variety x salt stress x biochar (Fig. 2). At P < 0.001, all interaction becomes significant as depicted in ANOVA Table 5. Significantly, both varieties differed from each other for ratio of chlorophyll a/b. Biochar enhanced the ratio of chlorophyll a/b. Biochar increased the chl. a/b ratio compared to control in both varieties of wheat. Salt stress caused to increase chl. a/b ratio in leaves of both varieties compared to control plants. The highest reduction in chl. a/b ratio was 0.11% in FSD-08 in the presence salt stress relative to control. Biochar in the presence of NaCl treatment enhanced the chl. a/b compared to salt stress. The interaction variety x salt stress x B caused to increased chl. a/b in both varieties of plant compared to salt stress. While this interaction caused a decreased chl a/b in FSD-08 compared to control (Fig. 5). All interactions were significant at P < 0.001 as in Table 3. Salt stress caused a decrease in carotenoid content relative to control conditions plant. This was significant (P < 0.05). Relative to only biochar and control conditions plant, carotenoid content decrease in both varieties caused by salt stress significant. 5% B was significant to increase in content of carotenoid compared to control (Fig. 2).
Effect of biochar treatment on H2O2, RWC, MDA and RMP of wheat under salt stress
Significantly, both varieties differed from each other to hydrogen peroxide determination (P < 0.001). Hydrogen peroxide is an ROS, which increase was significant under 120 mM NaCl as relative to plants of control condition (P < 0.05). Biochar alleviated the NaCl stress and reduced the H2O2 production compared to control conditions. Salinity increased the toxic effect on plants by producing a species of ROS such as H2O2. Hydrogen peroxide enhanced in both varieties in the presence salt stress treatment relative to control was not significant. Biochar treatment caused a minor increase in hydrogen peroxide compared to control in FSD-08. While in PUNJAB-11 biochar amendment caused to decline in H2O2 content (Fig. 2). This increased and decreased was significant at P < 0.001. Varieties of wheat significantly differ with respect to relative water content (Table 6). The water content of leaf increased as apply biochar amendment compared to control plants. Biochar increased the RWC of leaf during salinity compared to plants in saline or conditions. Salt stress effect on RWC ameliorated by biochar and increased relative water content significantly (P < 0.05) (Fig. 2).
Lipid peroxidation result was significant differ between both varieties of wheat (P < 0.05). NaCl treatment enhanced MDA compared to control, biochar, and salt stress + B plant. A significant level of this treatment was P < 0.001. Overall, the salt stress caused the highest increase in MDA content FSD-08 which was 10% (Fig. 2). Salt stress decreased permeability compared to plant while increased compared to biochar amendment plants. This increase or decrease was non-significant. Addition of biochar was significant to decrease RMP compared to control and stress plants (P < 0.001). RMP of FSD-08 declined to 39% and increased in PUNJAB-11 to 20% compared to control during stress. The interaction Salt stress x Biochar made the membrane less permeable relative to salt stress and control conditions plants. The combined treatment of B and salt stress increased RMP 12% in PUNJAB-11 and 5.9% in FSD-08 compared to only biochar treated plant. While this interaction decreased the permeable in both varieties of wheat compared to control (Table 4; Fig. 2).
Effect of Biochar treatment on leaf proline of wheat under salt stress
Salt stress caused to increase in leaf proline compared to control in both varieties. Biochar was significant to mitigate the effect of salt stress. Biochar treatment responsible to enhanced leaf proline in FSD-08 and to decline proline content in PUNJAB-11 compared to control (P < 0.001). Stress of salt was significant to enhance leaf proline in FSD-08 to 51% and PUNJAB-11 to 26% compared to control (P < 0.001). Biochar during the salt stress caused a decrease in proline level in leaf of plant compared to only salt stress plant leaf. The combined interaction of salt stress and biochar caused to increase leaf proline in both varieties compared to control. While salt stress and biochar interaction on leaf proline were responsible to increased 84% in PUNJAB-11 and 15% decreased in FSD-08 compared to only biochar treatment plant (Fig. 3).
Effect of biochar treatment on total soluble protein of wheat under salt stress
The effect of salt stress increased the total soluble protein of plants of both varieties was significant compared to control. Biochar addition was significant to decline in total soluble protein in both varieties compared to control. Salt stress significantly increased total soluble protein in FSD-08 and in PUNJAB-11 compared to control. Biochar application decreased the soluble protein content in both varieties as compared to control. The effect of salt stress was mitigated by biochar amendment in interaction of salt stress x biochar. The combined effect of salt stress and biochar caused to decrease in soluble protein content compared to control and only salt treatment plants (Fig. 3). Interaction variety x biochar and salt stress x biochar was significant at P < 0.001. While the effect of interaction variety x NaCl x B was not significant (Table 5).
Effect of biochar treatment on total phenolic of wheat under salt stress
Significantly, biochar caused a decline in total phenolic content relative to control and salt stress plants in both varieties. Salt stress was responsible for enhanced in PUNJAB-11 to 11.8% and declined in FSD-08 to 14% with respect to phenolic content relative to control. The combination of stress of salt + B caused a decrease in phenolic content compared to only salt stress plant within both varieties. While compared to control condition plants, phenolic content increased 45% in PUNJAB-11 and decreased 21% in FSD-08. Significance level of this result was P < 0.1 (Table 5).
Effect of biochar treatment on antioxidants (CAT, POD, APX and SOD) of wheat under salt stress
Significantly, Catalase activity of both varieties affected by NaCl treatment. Salt stress increased CAT activity in FSD-08 and in PUNJAB-11 CAT activity decreased relative to control significantly (P < 0.001). The combined treatment of salt and biochar caused a decline in CAT activity in PUNJAB-11 compared to control and salt treatment plants (Fig. 3). Significantly, both varieties of wheat behaved different with respect to peroxidase activity (P < 0.001). Salt stress enhanced the 12% in FSD-08 and 4.6% in PUNJAB-11 POD activity compared to control. Biochar increased the activity of POD compared to control. Significantly, biochar treatment caused enhanced POD activity compared to control within both varieties. During salt stress x biochar, POD activity declined compared to only salt stress. The interaction variety x salt stress x biochar was significant to increase POD activity 46% in FSD-08 and 4.6% in PUNJAB-11 compared to only biochar treatment plant. All interactions were significant (P < 0.05) (Table 6).
Salt stress did significantly decrease APX activity compared to control. Biochar treatment with salt stress alleviated the toxicity of salt stress by decreasing the APX activity compared to only salt stress plant. (Table 6). This treatment caused a decrease in APX activity compared to control in both varieties while salt treatment plant decreased in FSD-08 and in PUNJAB-11 (Fig. 3). FSD-08 behaved differ from PUNAB-11 with respect to SOD activity significantly (P < 0.001). Superoxide dismutase activity during biochar was significant to decrease or increase compared to control. During biochar application SOD activity was significant to decrease in FSD-08 and increase in PUNJAB-11 compared to control. Salt stress x biochar treated plant showed significant results (P < 0.1). The combination of salt and biochar treatment caused to increase SOD activity of 4.4% in FSD-08 and 38% in PUNJAB-11 compared to only biochar treated plants. During interaction, variety x salt stress x biochar decreased the SOD activity compared to control in FSD-08 (Fig. 3). All these interactions were significant (Table 6).
Effect of biochar treatment on K+, Na+, Ca2+ and Cl- ions in roots and shoots of wheat under salt stress
Roots of FSD-08 and PUNJAB-11 behaved differently related to content of potassium ions. Potassium ions in roots reduced with increasing NaCl level as relative to control. Each result was significant p<0.001. Comparison to NaCl stress and control conditions, biochar significantly encouraged the uptake of potassium (p<0.05). Salt stress caused maximum reduction in potassium ions in PUNJAB-11 to 3.6% relative to control. Biochar enhanced K+ by ameliorating the impact of NaCl stress in the combination of biochar and salt stress as compared to only salt stress and control. The maximum content of K+ ions in FSD-08 roots during combined NaCl and biochar treatment as compared to control and only biochar treated plant (Fig. 4). Significantly, shoot of Wheat varieties differed from each other to potassium ions content (P<0.001). The significant (P<0.05) decreased in potassium ions during NaCl treatment as compared to control. The content of K+ enhanced within shoots was significant during biochar amendment (P<0.001). Biochar application was significant to increase the K+ by lessen the toxic effect of salt stress relative to control (P<0.001) (Fig. 4).
Sodium toxicity increased as the increasing salinity level relative to plant grown in control conditions. Biochar amendment had positive effect and decreased the sodium toxicity as compared to control. This increased and decreased by 120 mM NaCl and 5% B treatment respectively was significant (P<0.001). Punjab-11 demonstrated the highest increase in Na+ content to 9.5% compared to control during 120 mM NaCl. The interaction of B and NaCl was significant to decreased Na+ content because biochar lessens the sodium toxicity during salinity as compared to alone salt treatment. While alone biochar treatment decreases in Na+ content of both varieties of wheat compared to plants under control conditions. Biochar showed positive impact on both varieties during salinity by decreasing sodium toxicity as compared to alone NaCl treatment and control plants (Fig. 4). All interactions become significant (Table 7).
Significantly, Uptake of sodium ions by shoots of FSD-08 and PUNJAB-11 was differed from each other (P<0.05). Salt stress caused to increase Na+ content in shoots compared to controlled conditions. While biochar treatment exhibited the improve content of Na+ in shoots as compared control. These were significant at P<0.001. Under the combined treatment of B and NaCl, Na+ content reduced by biochar in both varieties as compared to control and alone NaCl treatment. Both varieties did not show similarity in results under interaction of alone biochar amendment compared to their control plants. FSD-08 shoots had high content of Na+ compared to control (Fig. 4). All these results were significant. Both varieties of wheat were not significant with respect to calcium ions content in roots. Ca2+ content in roots decreased during the treatment of 120 mM salt stress treatment compared to control plants significantly. Corresponding to only NaCl treatment and control plants, biochar treatment had significant capability to translocate highest Ca2+ content by plant roots. The effect of each single treatment of salt stress and biochar was significant (P<0.001). While in biochar and salt stress combined treatment, biochar addressed salt effect on plant and increased Ca2+ ions content compared to control and salt treatment plants. Varieties of wheat behaved non-significantly with respect to calcium ions in shoots (P<0.1). Biochar treatment enhanced translocation of Ca ions content in shoots compared to control plants. Biochar treatment showed significant results. Significantly, biochar increased the calcium ions content relative to control in the presence of NaCl treatment. Salt stress enhanced the calcium ions content in shoot to 7.4% in FSD-08 and 16.54% in PUNJAB-11 compared to control (Fig. 4). Biochar improved growth parameters, RWC, K+ and Ca2+ content in shoot and root and chlorophyll content in saline soil. Biochar increases the growth of plants by improving the supply of mineral nutrients including K and Ca because biochar has a high concentration of essential nutrients of plants (K, Ca, P, and Mg). Ca and potassium are important for the maintenance of cell membranes integrity and other metabolic processes.
Chloride ions content in roots of both varieties significantly differ from each other (P < 0.001). Salt stress toxicity increased the chloride ions content while biochar decreased the effect of salinity as decreasing chloride ions content compared to control plants. These were significant. Salinity caused enhance in chloride ions content of both varieties’ roots compared to control (Fig. 4). A significant level of this interaction was P < 0.05. Significantly, chloride ions content in both varieties’ shoots behaved differently. Biochar decreased the content of chloride ions compared to salt stressed plant. Stressed plants had non-significant high content of chloride ions compared to control and biochar treated plants. Salt stress was significant to enhance Cl- ions in both varieties of wheat compared to control. Biochar treatment caused reduction in chloride ions content of shoots in the presence of salt stress (Fig. 4). This was significant at (P < 0.001) (Table 8).
Effect of biochar treatment on yield parameters (SP/Plant, spike length, GN/Spike and weight of 100 seeds)
Spike number/ plant of wheat varieties significantly differed at P < 0.05. The significant decreased in spike number by salt stress and increased by biochar amendment (P < 0.001). Spike number of both wheat varieties increased by biochar addition under salinity. This increase to 26% was significant (P < 0.1). Spike length varieties significantly (P < 0.01) differed from each other. Significantly, salt stress (P < 0.05) was affected the spike length and biochar (P < 0.001) mitigated this effect of salinity. Biochar amendment during salt stress significantly increased the spike length in FSD-08 to 0.57% and PUNJAB-11 4.24% as compared to control (only salt stress) (Fig. 5). This interaction was significant at P < 0.1.
GN/ spike significantly affected both varieties by NaCl (P < 0.001) and treatment of biochar (P < 0.05) showed in Fig. 5. The variety x salt stress interaction was significantly reduced the grain number (P < 0.001) compared to control. Addition of biochar increased the grain number in PUNJAB-11 as compared to control and FSD-08. Whereas the highest grain number recorded in FSD-08 to 66.7% under salt stress and biochar application association compared to control showed in. The weight of 100 seeds of FSD-08 differed from the weight of PUJAB-11 seeds. This different was significant (P < 0.05). Significantly, salinity effected the seeds weight (P < 0.05) relative to non-saline. A significant improved in weight of seed by application of biochar (P < 0.001) relative to normal and saline conditions. The increased weight of seeds in wheat varieties exhibited significant during biochar treatment (P < 0.05) relative to control. The highest increased in PUNJAB-11 to 18.24% during combined effect of biochar and salt stress compared to control. This increase was significant, and results are shown in Fig. 5 and Table 9.
Discussion
Salt stress is one of the major challenges for various agricultural lands all over the world. According to earlier studies salt stress affected the early stages of growth, physiological and biochemical processes that lead to reduced cash crop harvest35,36. The present study was designed to assess the salinity stress on different parameters of wheat with biochar application. Salt stress reduced growth parameters (shoot fresh weight, root fresh weight, shoot dry weight, root dry weight, shoot length, root length and leaf area) of wheat plants while biochar improved all growth parameters as relative to the plants under control and stressed conditions. Similar results were observed by several scientists that all growth parameters were decreased under salt stress as compared to biochar treated plants18,37,38,39. The reduction in growth attributes might be due to accumulation of different osmolytes, restricted cell division, changes in activity of metabolic enzymes and metabolic pathways which play a pivotal role during salt stress40.
In present study, results revealed that chlorophyll a, b and carotenoids were decreased in both varieties (PUNJAB-11as and FSD-08) due to salt stress relative to control plants. These results were correlated with findings of Akhtar et al. (2015), which showed reduction in chlorophyll content, and this reduction in photosynthetic pigments might be due to enhanced performance of chlorophyllase enzyme and ROS production, decreased N uptake, inhibition of photosynthesis and instability of protein compilation of photosynthetic pigments41. According to present findings biochar treatment increased chlorophyll and carotenoid content in wheat plants. The association of salt and biochar treatment increased the content of chlorophyll and carotenoid compared to stressed plant as already reported in wheat, bean, tomato, summer savory plants37,38,39,42. Increase in N uptake and decrease in activity of chlorophyllase enzyme might be a possible reason of reduction in chlorophyll content18.
Present results showed that MDA, H2O2 and relative membrane permeability (RMP) was increased as the salinity level increased with respect to control plant in both varieties of wheat. Increased level of MDA and H2O2 was an indication of oxidative stress in wheat plants. Oxidative stress affects several important cellular functions of plants by causing oxidation of protein, DNA as well as RNA and by lipid peroxidation42. On other hand, biochar relieved the plant from conditions of NaCl stress and decreased the MDA and H2O2 in the leaves of both varieties. Similar results of biochar have been reported in previous studies on bean seedlings and wheat under abiotic stress38,42. Relative membrane permeability increased during salt stress in both wheat varieties compared to control plants. Although biochar usage declined the injury of cell membrane compared to stressed plant as reported in previous study on summer savory39. Relative water content decreased under salt stress and alleviated by biochar usage under stressed and non-stress condition same as in bean and tomato plant reported37,38.
Among compatible solutes, proline and soluble sugars are very important, under stress conditions, their synthesis and accumulation in the cell are a common way to grow. Proline is a vital solute for protein synthesis, metabolic activities and in immune responses under stress conditions40. The present study demonstrated proline content of leaf is highest in the stressed plant of both wheat varieties. Present results were in agreement with those obtained by Refs.43,44 in wheat. Increased level of proline after exposure to salt stress could be due to decreased activity of dehydrogenase and less oxidation45.
In present findings, total soluble protein level in both varieties was increased under salt stress while decreased on exposure to biochar application. The effect of salt stress and biochar on total soluble protein of wheat varieties showed similarity with bean seedlings reported in another study37. According to present results, osmoprotectants such as total phenolic content enhanced under abiotic stress (salinity) in wheat varieties. Total phenolic content in FSD-08 decreased under salt stress compared to control which deviate from the result reported already in wheat and bean plants under an abiotic stress37,39.
Oxidative stress produced by salinity which affected the plant growth and development, due to reactive oxygen species (ROS) that caused toxicity in plant stress. In plants, ROS are scavenged by important antioxidant enzymes which are produced in plants such as SOD, POD, and CAT46. As SOD directly converts superoxide (O2• −) to hydrogen peroxide (H2O2), CAT converts H2O2 to water and oxygen, while POD directly detoxifies H2O2. Hence, detoxification of ROS takes place in plant cells and overall, a balance is maintained between ROS production and degradation for normal plant functions. However, plants failed in detoxification of ROS under stressful conditions, as a consequence, oxidative stress produced in plants46. In the present study, enzymatic antioxidants such as SOD, POD of both varieties’ wheat and CAT of FSD-08 increased to ameliorate the impact of salt stress on the plants. These findings were similar to results reported in tomatoes, bean seedlings, maize37,38,47. While CAT in PUNJAB-11 and APX in both wheat varieties was decreased under salt stress. Biochar amendment as organic conditioner decreased the enzymatic activities includes APX, POD, SOD and CAT compared to control plants. Reduced activity of SOD, POD and CAT under these conditions indicated oxidative stress has occurred due to damage of antioxidant defense system. Similar findings about results have been reported in tomato38.
Results from the present study indicated that sodium and chloride ions increased under salt stress and decreased by biochar treatment in the presence of non-saline and saline conditions. These results were inconsistent with the finding of42 in wheat. They observed Na uptake was reduced in biochar treated plants that was might be due to higher adsorption of Na by biochar which may lessen the osmotic stress on plants48. Additionally, enhanced mineral nutrients availability in the soil and improvement in soil chemical and physical properties in the presence of biochar might also play an important role in reduced Na uptake by plants49. While the potassium ions and calcium ions decreased by salinity and increased under biochar application in the presence of saline and non-saline conditions as in bean plants37.
In recent study yield parameters includes spike number/plant, spike length, grain number/spike and 100 seeds weight (g) increased as biochar applied under normal and stressed condition compared to control while these parameters were declined under stressful conditions. However, similar results were demonstrated with biochar in others research under abiotic stress amendment in wheat48,50. Field studies and research trial have shown that the application of biochar to plants under salt stress largely mitigated stress and reinforced the growth of plant directly through the release of essential micronutrients and macronutrients like N, K, P, Ca,and Zn in the soil51, that helped the plants to nullify the harmful effects of salts (Saifullah et al. 2018). Similar results were obtained from studies of Ref.52 in rice.
Salt stress is one of the major challenges for various agricultural lands all over the world. Salt stress affects the growth parameters, morphological, physiological, biochemical, yield and inorganic mineral ions. According to earlier studies salt stress affected the early stages of growth, physiological and biochemical processes that lead to reduced cash crop harvest35,36. The present study was designed to assess the salinity stress on different parameters of wheat with biochar application. Growth parameters effected by NaCl, and biochar mitigated the effect of salt stress. However, the present study showed that the biochar improved all morphological characters of wheat plants as relative to the plants under control and stressed conditions. In previous studies exhibited the all-morphological parameters decreased under stress and increased under biochar treatment compared to control except root length in PUNJAB-11, shoot length in FSD-08 and leaf area in FSD-08 increased under stress compared to control. While all the parameters decreased under stress compared to biochar treated plants37,38,39,48,50.
In earlier study displayed that due to salinity, chlorophyll content (a, b, and a/b ratio) reduced and enhanced carotenoids relative to plants of control. While 5% biochar increased the chlorophyll and carotenoid content relative to control42. In the present study result revealed that chl. a and b decreased 9.5% and 9.6% respectively in PUNJAB-11as compared to control under salt treatment. While in FSD-08 decreased in chl a to 0.64% and chl b to 1.03% due to salt stress relative to control. The association of salt and biochar treatment increased the content of chlorophyll and carotenoid compared to stressed plant as already reported in wheat, bean, tomato, summer savory plants. In present study showed that MDA and H2O2 increased as the salinity increasing with respect to control plant in both varieties of wheat. On other hand, biochar relieved the plant from conditions NaCl of stress and decreased the MDA and H2O2 in the leaves of both varieties. Similar results of biochar have been reported in previous on bean seedlings and wheat under abiotic stress37,42. Relative membrane permeability increased during salt stress in both wheat varieties compared to control and non-biochar. Although biochar usage declined the injury of cell membrane compared to stressed plant as reported in previous study on summer savory (Mehdizadeh et al. 2020). Relative water content decreased under salt stress and alleviated by biochar usage under stressed and non-stress condition same as in bean and tomato plant reported37,42.
Proline is a vital solute for protein synthesis, metabolic activities and in immune responses under stress conditions40. The present study demonstrated proline content of leaf highest in the stressed condition plant of both wheat varieties compared to control. According to Farhangi-Abriz & Torabian37 biochar treatment declined the leaf proline content under stressed condition in both varieties while under non-stressed condition declined only in PUNJAB-11. Stress of salt was significant to enhance leaf proline in FSD-08 to 51% and PUNJAB-11 to 26% compared to control (P < 0.001). In earlier studies proline content was elevated the same under saline conditions43. Biochar treatment responsible to enhanced leaf proline in FSD-08 and declined proline content in PUNJAB-11 compared to control (P < 0.001). Stress of salt was significant to enhance leaf proline in FSD-08 to 51% and PUNJAB-11 to 26% compared to control (P < 0.001). The effect of NaCl stress and biochar on total soluble protein of wheat varieties showed similarity with bean seedlings reported in another study37. The highest soluble protein content measured under stress condition and 5% biochar caused a decrease in the protein content. In saline conditions the increase in soluble sugar was due to breakdown of carbohydrates for maintenance of cell turgidity Nazarli et al.53. In recent study salt stress significant to increased total soluble protein in FSD-08 and in PUNJAB-11 compared to control. Osmo-protectants such as total phenolic content enhanced under abiotic stress (salinity) in wheat varieties. Total phenolic content in FSD-08 decreased under salt stress compared to control which deviate from the result reported already in wheat and bean plants under an abiotic stress37,39.
Oxidative stress produced by salinity which affected the plant growth and development, rose reactive oxygen species (ROS) that caused toxicity in plant stress. In this study enzymatic antioxidants such as SOD, POD of both varieties wheat and CAT of FSD-08 increased to ameliorate the impact of salt stress as the plants exposed to 120 mM NaCl relative to controlled conditions. These findings are similar to result reported in tomatoes, bean seedlings, maize37,38,47. While the CAT in PUNJAB-11 and APX in both varieties of wheat become decrease in the exposure of NaCl compared to control. Biochar amendment as organic conditioner decreased the enzymatic activities includes APX, POD, SOD and CAT compared to control plants. Biochar amendment decreased the all-antioxidant enzymatic activities by ameliorating the oxidative stress under the conjugation of B 5% and 120 mM NaCl compared to only NaCl treated plant. Similar findings about results have been reported in tomato38. In the present study, Sodium and Chloride ions increased under stress and decreased by biochar treatment in the presence of non-saline and saline conditions as shown in Table 9. While the potassium ions and calcium ions decreased by salinity and increased under biochar application in the presence of saline and non-saline conditions as in bean plants37. In recent study parameters of yield includes spike number/ plant, spike length, grain number/ spike and 100 seeds weight (g) increased as applied 5% biochar under normal and stressed condition compared to control. These parameters were declined under the 120 mM NaCl level compared to biochar treated plants under stress. These similar results also demonstrated with biochar in others research under abiotic stress amendment in wheat48,50. The gram weight of seeds in PUNJAB-11 and grain number/ spike in FSD-08 increased in the presence of salt stress relative to control. While all interaction and both varieties with respect to length of spike and grain number per spike was not significant, respectively.
Purposed mechanism of biochar can mitigate effects of salt stress
Biochar amendment has been proven effective in increasing germination percentage and seedling vigor index across various salinity levels54,55,56,57,58. The addition of biochar improves all measured vegetative features across different salt concentrations compared to unamended plants. Biochar improves soil structure, water retention, and nutrient availability, which collectively enhance plant resilience to saline conditions59. Biochar application significantly reduced sodium uptake while increasing potassium uptake, thereby improving the overall ionic balance in plants under salt stress60.
Biochar amendment results in a decreased level of soluble sugar under stress conditions. Sugars play a critical role in cellular membranes and are involved in oxidative stress response by scavenging reactive oxygen species in saline environments61. The increased levels of sugars under stress conditions are attributed to the breakdown of larger carbohydrate molecules, which helps maintain cell turgidity53. This elevated sugar level under stress conditions protects various membranes and other cellular structures from the adverse effects of stress62.
Biochar also mitigates the negative impacts of stress by lowering the activity of superoxide dismutase (SOD), an enzyme that plays a defensive role under stress conditions63. Biochar-mediated reduction of MDA and H2O2 production also reduces the electrolyte leakage by making the membranes stronger64.
Chlorophyll concentration is directly related to photosynthesis, and the application of biochar has been reported to substantially increase chlorophyll synthesis44. Moreover, biochar has been found to modulate the expression of stress-responsive genes. Studies have shown that biochar amendments can upregulate the expression of genes involved in antioxidant defense, osmotic adjustment, and ion transport65. This genetic modulation helps plants better cope with the oxidative and ionic stresses associated with saline conditions. In summary, biochar serves as an effective amendment to mitigate the negative effects of salt stress in plants. By, enhancing membrane stability, nutrient uptake, chlorophyll synthesis and modulating stress-responsive genes. Overall, biochar significantly enhances plant resilience and productivity under saline conditions.
Conclusion
The findings of this research suggest that applying the biochar ameliorating the negative impact of salinity on wheat plants. Salinity caused reduction in yield (number of seeds, spike numbers per plant and weight of 100 seeds), growth (length, fresh and dry weight of stem and root, leaf number), physiological (RWC, Chlorophyll and carotenoid content) parameters and K, Ca content in wheat verities. By using biochar alleviating the adverse impact of salinity and enhanced the chlorophyll content, yield and growth parameters under salt stressed conditions. This effect was achieved by reducing antioxidant activities and osmo-protectants (leaf proline and total phenolic), MDA in biochar application. 5% Biochar is effective to enhance the yield and growth of Triticum aestivum L. under saline conditions.
Limitations of pot experiment
Plants grown in pots are kept apart from some other environmental conditions such as competition or natural interactions with other plants and soil creatures. Those factors may have an impact on plant growth and development. The findings of this study may not always be directly applicable in the field conditions, limiting the applicability and scalability of the research outcomes.
Future recommendations
Wheat is an important cereal crop on which the world population food depends. Salinization causing the agricultural land barren in results reduction in wheat/ crop production day by day. In future, biochar will improve the crop yield and productivity of wheat to meet the demands of it as staple food, which is pressurized by intensity of environmental stress and the day-by-day increasing population. Biochar amendment will be beneficial to promote wheat cultivation and improve productivity under saline conditions. So, it will be enhanced the productivity of wheat. Biochar produced from biomass so its organic form will not pollute the environment and make the land fertile.
Data availability
All relevant data is present within the article and supplementary files. Furthermore, data will be made available on reasonable request to the corresponding author of this article.
References
Poudel, P. B. & Poudel, M. R. Heat stress effects and tolerance in wheat: A review. J. Biol. Today’s World 9(3), 1–6 (2020).
Miransari, M. & Smith, D. Sustainable wheat (Triticum aestivum L.) production in saline fields: A review. Crit. Rev. Biotechnol. 39(8), 999–1014 (2019).
Desoky, E. S. M., Saad, A. M., El-Saadony, M. T., Merwad, A. R. M. & Rady, M. M. Plant growth-promoting rhizobacteria: Potential improvement in antioxidant defense system and suppression of oxidative stress for alleviating salinity stress in Triticum aestivum (L.) plants. Biocatal. Agric. Biotechnol. 30, 101878 (2020).
Boukid, F., Folloni, S., Ranieri, R. & Vittadini, E. A compendium of wheat germ: Separation, stabilization and food applications. Trends Food Sci. Technol. 78, 120–133 (2018).
Bashir, M. H. et al. Characterization and advancement of microsatellite (SSR) markers for various stresses in wheat. Int. J. Agri. Biosci. 11(2), 131–138 (2022).
Yassin, M. et al. Comparative performance of two bread wheat (Triticum aestivum L.) genotypes under salinity stress. Appl. Ecol. Environ. Res. 17(2), 5029–5041 (2019).
Zahra, N., Mahmood, S. & Raza, Z. A. Salinity stress on various physiological and biochemical attributes of two distinct maize (Zea mays L.) genotypes. J. Plant Nutr. 41(11), 1368–1380 (2018).
Yousaf, M. T. B. et al. Effect of three different types of biochars on eco-physiological response of important agroforestry tree species under salt stress. Int. J. Phytoremediat. 23(13), 1412–1422 (2021).
Ghonaim, M. M., Mohamed, H. I. & Omran, A. A. Evaluation of wheat (Triticum aestivum L.) salt stress tolerance using physiological parameters and retrotransposon-based markers. Genet. Resour. Crop Evol. 68(1), 227–242 (2021).
Mushtaq, A. et al. Influence of silicon sources and controlled release fertilizer on the growth of wheat cultivars of Balochistan under salt stress. Pak. J. Bot. 51(5), 1561–1567 (2019).
Sultana, R., Adnan, M. Y. & Ali, H. Salicylic acid seed priming modulates some biochemical parametrs to improve germination and seedling growth of salt stressed wheat (Triticum aestivum L.). Pak. J. Bot. 51(2), 385–391 (2019).
de Sousa, T., Ribeiro, M., Sabença, C. & Igrejas, G. The 10,000-year success story of wheat!. Foods 10(9), 2124 (2021).
Rezaie, N., Razzaghi, F. & Sepaskhah, A. R. Different levels of irrigation water salinity and biochar influence on faba bean yield, water productivity, and ions uptake. Commun. Soil Sci. Plant Anal. 50(5), 611–626 (2019).
Saddiq, M. S. et al. Effect of salinity stress on physiological changes in winter and spring wheat. Agronomy 11(6), 1193 (2021).
Khan, R. A., Noreen, Z., Khan, A. & Qadri, T. A. Nutri-priming as an efficient means to improve germination and growth of Mung bean (Vigna radiata L.) grown under NaCl stress. Pure Appl. Biol. (PAB) 10(1), 34–45 (2020).
Zafar, F., & Noreen, Z. Influence of foliar application of zinc sulfate on pea (Pisum sativum L.) Under saline conditions. Plantarum 5(si), (2023).
Duan, S. et al. Effects of biochar types on seed germination, growth, chlorophyll contents, grain yield, sodium, and potassium uptake by wheat (Triticum aestivum L.) under salt stress. BMC Plant Biol. 24(1), 1–14 (2024).
Akhtar, S. S., Andersen, M. N., Naveed, M., Zahir, Z. A. & Liu, F. Interactive effect of biochar and plant growth promoting bacterial endophytes on ameliorating salinity stress in maize. Funct. Plant Biol. 42, 770–781 (2015).
Alfadil, A. A. et al. Wheat straw biochar application improves the morphological, physiological, and yield attributes of maize and the physicochemical properties of soil under deficit irrigation and salinity stress. J. Plant Nutr. 44(16), 2399–2420 (2021).
Soliman, M. H. et al. Biochar and selenium nanoparticles induce water transporter genes for sustaining carbon assimilation and grain production in salt-stressed wheat. J. Plant Growth Regul. 42(3), 1522–1543 (2023).
Soothar, M. K. et al. Assessment of acidic biochar on the growth, physiology and nutrients uptake of maize (Zea mays L.) seedlings under salinity stress. Sustainability 13(6), 3150 (2021).
Agegnehu, G., Srivastava, A. K. & Bird, M. I. The role of biochar and biochar-compost in improving soil quality and crop performance: A review. Appl. Soil Ecol. 119, 156–170 (2017).
Arnon, D. I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 24(1), 1 (1949).
Jones, M. M. & Turner, N. C. Osmotic adjustment in leaves of sorghum in response to water deficits. Plant Physiol. 61(1), 122–126 (1978).
Velikova, V., Yordanov, I. & Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 151(1), 59–66 (2000).
Yang, G., Rhodes, D. & Joly, R. J. Effects of high temperature on membrane stability and chlorophyll fluorescence in glycinebetaine-deficient and glycinebetaine-containing maize lines. Funct. Plant Biol. 23(4), 437–443 (1996).
Cakmak, I. & Horst, W. J. Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiologia Plantarum 83(3), 463–468 (1991).
Bates, L. S., Waldren, R. P. & Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 39(1), 205–207 (1973).
Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72(1–2), 248–254 (1976).
Chance, B. & Maehly, A. Assay of Catalases and Peroxidases (Elsevier, 1955).
Nakano, Y. & Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 22(5), 867–880 (1981).
Giannopolitis, C. N. & Ries, S. K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 59(2), 309–314 (1977).
Kamath, S. D., Arunkumar, D., Avinash, N. G. & Samshuddin, S. Determination of total phenolic content and total antioxidant activity in locally consumed food stuffs in Moodbidri, Karnataka. India. Adv. Appl. Sci. Res. 6(6), 99–102 (2015).
Qasim, M. et al. Effect of drought stress on fertile tillers of wheat genotypes (Triticum aestivum l.). Int. J. Agri. Biosci. 11(3), 172–180 2022.
Khalid, W. et al. Evaluation of various double haploid maize hybrids under water deficit condition. Int. J. Agri. Biosci. 11(3), 194–198 (2022).
Ali, S. et al. Zinc fortification and alleviation of cadmium stress by application of lysine chelated zinc on different varieties of wheat and rice in cadmium stressed soil. Chemosphere 295, 133829. https://doi.org/10.1016/j.chemosphere.2022.133829 (2022).
Farhangi-Abriz, S. & Torabian, S. Antioxidant enzyme and osmotic adjustment changes in bean seedlings as affected by biochar under salt stress. Ecotoxicol. Environ. Saf. 137, 64–70 (2017).
Kul, R. et al. Biochar as an organic soil conditioner for mitigating salinity stress in tomato. Soil Sci. Plant Nutr. 67(6), 693–706 (2021).
Mehdizadeh, L., Moghaddam, M. & Lakzian, A. Amelioration of soil properties, growth and leaf mineral elements of summer savory under salt stress and biochar application in alkaline soil. Sci. Hortic. 267, 109319 (2020).
Kanwal, S. et al. Application of biochar in mitigation of negative effects of salinity stress in wheat (Triticum aestivum L.). J. Plant Nutr. 41(4), 526–538 (2018).
Heidari, M. & Golpayegani, A. Effects of water stress and inoculation with plant growth promoting rhizobacteria (PGPR) on antioxidant status and photosynthetic pigments in basil (Ocimum basilicum L.). J. Saudi Soc. Agric. Sci. 11, 57–61 (2012).
Abbas, T. et al. Effect of biochar on alleviation of cadmium toxicity in wheat (Triticum aestivum L.) grown on Cd-contaminated saline soil. Environ. Sci. Pollut. Res. 25(26), 25668–25680 (2018).
Peng, Y. et al. Ecophysiological characteristics of alfalfa seedlings in response to various mixed salt-alkaline stresses. J. Integr. Plant Biol. 50, 29–39 (2008).
Kanwal, S. et al. Application of biochar in mitigation of negative effects of salinity stress in wheat (Triticum aestivum L.). J. Plant Nutr. https://doi.org/10.1080/01904167.2017.1392568 (2017).
Ahmed, W. et al. Sustainable water use in agriculture: A review of worldwide research. Int. J. Agri. Biosci. 11(4), 246–250 (2022).
Abbasi, G. H. et al. Profiling of anti-oxidative enzymes and lipid peroxidation in leaves of salt tolerant and salt sensitive maize hybrids under NaCl and cd stress. Sains Malay. 45, 177–184 (2016).
Kim, H. S. et al. Effect of biochar on reclaimed tidal land soil properties and maize (Zea mays L.) response. Chemosphere 142, 153–159 (2016).
Akhtar, S. S., Andersen, M. N. & Liu, F. Residual effects of biochar on improving growth, physiology and yield of wheat under salt stress. Agric. Water Manag. 158, 61–68 (2015).
Lashari, M. S. et al. Effects of amendment of biochar-manure compost in conjunction with pyroligneous solution on soil quality and wheat yield of a salt-stressed cropland from Central China Great Plain. Field Crops Res. 144, 113–118 (2013).
Salim, B. B. M. Influence of biochar and seaweed extract applications on growth, yield and mineral composition of wheat (Triticum aestivum L.) under sandy soil conditions. Ann. Agric. Sci. 61(2), 257–265 (2016).
Sun, H. J., Zhang, H. C., Shi, W. M., Zhou, M. Y. & Ma, X. F. Effect of biochar on nitrogen use efficiency, grain yield and amino acid content of wheat cultivated on saline soil. Plant Soil Environ. 65, 83–89 (2019).
Huang, M., Yang, L., Qin, H. D., Jiang, L. G. & Zou, Y. B. Fertilizer nitrogen uptake by rice increased by biochar application. Biol. Fertil. Soils 50, 997–1000 (2014).
Nazarli, H., Faraji, F. & Zardashti, M. R. Effect of drought stress and polymer on osmotic adjustment and photosynthetic pigments of sunflower. Cercetari Agronomice Moldova https://doi.org/10.2478/v10298-012-0022-9 (2010).
Zakaria, M., Daniel, S., Murphy, V. & Abbott, L. K. Biochars influence seed germination and early growth of seedlings. Plant Soil 353, 273–287 (2012).
Wang, G. & Xu, Z. The effects of biochar on germination and growth of wheat in different saline-alkali soil. Asian Agric. Res. 5, 116–9 (2013).
Rajalakshmi, A. G. et al. Effect of biochar in seed germination: In-vitro study. Int. J. Biosci. Nanosci. 2, 132–136 (2015).
Kamara, A., Kamara, A., Mansaray, M. M. & Sawyerr, P. A. Effects of biochar derived from maize stover and rice straw on the germination of their seeds. Am. J. Agric. For. 2, 246–249 (2014).
Yang, A. et al. Biochar mitigates combined effects of drought and salinity stress in quinoa. Agronomy 10(6), 912 (2020).
Gul, S., Whalen, J. K., Thomas, B. W., Sachdeva, V. & Deng, H. Physico-chemical properties and microbial responses in biochar-amended soils: Mechanisms and future directions. Agric. Ecosyst. Environ. 206, 46–59 (2015).
El-Naggar, A. et al. Biochar application to low fertility soils: A review of current status, and future prospects. Geoderma 337, 536–554 (2019).
Bohnert, H. J. & Jensen, R. G. Strategies for engineering water-stress tolerance in plants. Trends Biotechnol. 14(3), 89–97 (1996).
Prado, F. E., Boero, C., Gallardo, M. R. A., & González, J. A. Effect of NaCl on growth germination and soluble sugars content in Chenopodium quinoa Willd. Seeds. (2000).
Younis, U., Athar, M., Malik, S. A., Raza Shah, M. H. & Mahmood, S. Biochar impact on physiological and biochemical attributes of Spinach (Spinacia oleracea L.) in nickel contaminated soil. Glob. J. Environ. Sci. Manag. 1(3), 245–254 (2015).
Ran, C. et al. Benefits of biochar for improving ion contents, cell membrane permeability, leaf water status and yield of rice under saline–sodic paddy field condition. J. Plant Growth Regul. 39(1), 370–377 (2020).
Qayyum, M. F. et al. Effects of co-composting of farm manure and biochar on plant growth and carbon mineralization in an alkaline soil. Environ. Sci. Pollut. Res. 24, 26060–26068 (2017).
Acknowledgements
The authors extend their appreciation to Researchers Supporting Project number (RSP-2024 R241), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
Aqsa Shehzadi and Zahra Noreen designed the study, Aqsa Shehzadi, Fizza Zafar, Madiha Rashid, Muhammad Aslam helped Aqsa Shehzadi to perform the experiments and analysis. Aqsa Shehzadi, Zahra Noreen, Fizza Zafar, Madiha Rashid, Muhammad Aslam, Sajid Fiaz and Afifa Younas helped in data analysis, software standardization and writing of original draft. Afifa Younas, Adeela Haroon, Sezai Ercisli, Kotb A. Attia, Arif Ahmed Mohammed, Salman Alamery and Zahra Noreen provided technical expertise, reviewed and revised the article and helped in funding acquisition. All authors review and edit the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shahzadi, A., Noreen, Z., Alamery, S. et al. Effects of biochar on growth and yield of Wheat (Triticum aestivum L.) under salt stress. Sci Rep 14, 20024 (2024). https://doi.org/10.1038/s41598-024-70917-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-70917-2
- Springer Nature Limited