Abstract
This study evaluated the therapeutic efficacy and underlying mechanisms of crisaborole combined with vitamin D in the treatment of allergic contact dermatitis. While crisaborole, a phosphodiesterase 4 inhibitor, and vitamin D analogs are commonly used in the treatment of atopic dermatitis, their combined therapeutic potential in allergic contact dermatitis (ACD) remains unexplored. Given their anti-inflammatory properties, we hypothesized that the combination of crisaborole and vitamin D could offer superior efficacy in mitigating the symptoms and underlying mechanisms of allergic contact dermatitis. In vitro, HaCaT cells stimulated with tumor necrosis factor-α and interferon-γ were treated with a combination of crisaborole and vitamin D, followed by cytokine expression analysis. In vivo, male C57BL/6 mice were divided into five groups and treated accordingly: blank control, dinitrochlorobenzene-induced model, crisaborole alone, vitamin D alone, and a combination of crisaborole and vitamin D. On day 14, dorsal skin and ear thickness were measured, followed by comprehensive pathological evaluations. In vivo and in vitro experiments showed that the expression levels of inflammatory factors were significantly lower in the DNCB + VD + Cri group than in the DNCB group. Histological analyses revealed that, compared with the DNCB group, the combined treatment group significantly reduced epidermal hyperkeratosis, improved epidermal transdermal water loss, decreased dermatitis scores, and diminished mast cell infiltration. Moreover, it lowered the expression levels of IL-6, IL-4, TNF-α, iNOS, IL-17, CC chemokine ligand 2 (CCL2), and CC chemokine receptor 2 (CCR2). CCL2 recognizes CCR2 and stimulates inflammatory cells, enhancing the inflammatory response. Increased CCL2 expression correlates with heightened inflammation and dendritic cell infiltration in ACD, while downregulation of CCL2 attenuates inflammation. Thus, the combined use of crisaborole and vitamin D demonstrates superior therapeutic efficacy over monotherapy in a mouse model of ACD. The combination of vitamin D and crisaborole significantly reduces inflammation and epidermal hyperkeratosis in a mouse model of allergic contact dermatitis, demonstrating superior therapeutic efficacy compared to either treatment alone. This suggests that the combined therapy could be a promising approach for the prevention and treatment of allergic contact dermatitis.
Similar content being viewed by others
Introduction
Allergic contact dermatitis (ACD) is the most prevalent inflammatory skin disorder encountered in occupational dermatology. Patients may present with varying degrees of erythema, edema, scabbing, and severe itching1,2. Medical workers, food handlers, and construction workers have a particularly high risk of developing ACD because of their frequent and prolonged exposure to allergens3,4. ACD constitutes a type IV delayed hypersensitivity reaction driven by the activation of allergen-specific T cells5. Allergic reactions can be divided into sensitization and induction phases. During the sensitization phase, exposure to an allergen (e.g., through the skin) triggers an innate immune response. The allergen activates CD8 + and CD4 + cells, which proliferate and differentiate into cytotoxic T lymphocytes (Tc) and helper T lymphocytes (Th), respectively6,7. In the induction phase, a sensitized body is re-exposed to the allergen, causing the previously activated T cells to proliferate and release inflammatory cytokines (e.g., tumor necrosis factor [TNF]-α, interferon [IFN]-γ, and interleukins [ILs]) at sites of inflammation. This cascade promotes the recruitment of additional cytotoxic T lymphocytes and innate immune cells, amplifying the allergic reaction and resulting in the onset of ACD8,9. Previous studies have shown that the binding of CC chemokine ligand 2 (CCL2) to CC chemokine receptor 2 (CCR2) activates and recruits monocytes and T lymphocytes to sites of inflammation, thus inducing a strong inflammatory response10,11. This pathway is closely associated with the occurrence and progression of psoriasis vulgaris, and it may play an important role in the pathogenesis of ACD12,13.
Currently, drug treatment for ACD mainly involves antihistamines and glucocorticoids; however, their efficacies are limited and many side effects have been reported14,15. Crisaborole ointment, a topical phosphodiesterase type-4 (PDE4) inhibitor, increases intracellular levels of cyclic adenosine monophosphate (cAMP); this effect suppresses the activities of inflammation-related cells and inhibits the production of inflammatory mediators, reducing the likelihood of adverse reactions16,17. Vitamin D has a potential role in the treatment of ACD; it can improve aspects of skin barrier function in dermatitis model mice, enhancing the binding and defensive capabilities of epidermal cells in a manner that protects against external allergens and reduces inflammation18,19. Vitamin D also regulates the growth and differentiation of skin cells, while inhibiting inflammatory cytokine production19,20. The regulatory effects of vitamin D help to suppress anaphylactic reactions, thereby mitigating hypersensitivity to sensitizing substances in of ACD patients21,22.
Thus far, there has been limited research concerning the use of drug combinations to treat ACD. In this study, we explored the efficacies of three drug treatments—crisaborole, vitamin D and crisaborole combined with vitamin D—for ACD through in vivo and in vitro experiments. By evaluating changes in expression patterns within the CCL2 and CCR2 signaling pathways, we sought to preliminarily identify the underlying mechanisms of action, thereby providing a theoretical basis for future efforts to prevent and treat ACD.
Materials and methods
Cytotoxicity assay
HaCaT cells (Wuhan Bode Bioengineering Co., Ltd., Wuhan, China) were cultured in 96-well plates and subjected to cell viability analysis using a CCK8 assay kit, in accordance with the manufacturer’s instructions(zoman,Beijing,china). Briefly, the cells were treated with various concentrations of vitamin D, crisaborole, and vitamin D + crisaborole (vitamin D: 10–7 to 10–5 mol/mL; crisaborole: 5–20 μg/mL; vitamin D + crisaborole: vitamin D: 10–7 to 10–5 mol/mL + crisaborole: 5–20 μg/mL). The CCK8 solution was added and cells were incubated at 37 °C for 48 h. After removal of the supernatant, the absorbance at 490 nm was measured using a VersaMax 340 microplate reader (Molecular Devices, San Jose, CA, USA).
Cell culture and treatments
HaCaT cellswere cultured in a humidified incubator at 37 °C with 5% CO2 using HaCaT cell-specific medium containing 10% fetal bovine serum and 1% penicillin–streptomycin antibiotic. HaCaT cells were seeded in 96-well plates at a density of 1.5 × 104 cells/well; they were treated with vitamin D (10–5 mol/mL), crisaborole (5 μg/mL), or vitamin D combined with crisaborole (10–5 + 5 μg/mL) for 1 h, followed by IFN-γ (10 ng/mL) and TNF-α (10 ng/mL) as inflammatory inducers for 24 h. Subsequently, the expression of relevant mRNA was detected.
Animals experiment
Animal experiments were approved by the “Ethics Committee of North China University of Science and Technology on Laboratory Animal Care” and the study was carried out in compliance with the ARRIVE guidelines along with the relevant guidelines and regulations present in the manuscript. Forty 8-week-old male C57BL/6 mice (mean weight, 18 g) were obtained from Weitonglihua Experimental Animal Technology Co., Ltd. (Beijing, China). Upon arrival, the mice were acclimated for 1 week before experiments. The mice were housed in a controlled environment with temperatures of 22 ± 8 °C and given free access food and water throughout the study. To induce contact dermatitis, mice (n = 40) were anesthetized with isoflurane (2% in oxygen) and their hair was shaved off. After recovery from anesthesia, the mice were randomly divided into five groups (eight mice per group). On the first day of the experiment, control mice received an equal amount of acetone-olive oil solution applied to both ears and the back, while all mice in the DNCB group were sensitized with a 1% DNCB solution on the ears and back. From days 6 to 14, control mice received an equal amount of acetone-olive oil solution applied to both ears and the back, while the DNCB group established allergic contact dermatitis (ACD) with a 0.5% DNCB solution on the ears and back. Meanwhile, on days 6 to 14, mice in the DNCB + Cri group received topical application of crisaborole (Pharmacia and Upjohn Company LLC, 2%) ointment on both ears and the back, while those in the DNCB + VD group received oral administration of vitamin D (0.025 mg/kg). The DNCB + VD + Cri group received topical application of crisaborole ointment followed by oral administration of vitamin D. The control group, DNCB group, and DNCB + Cri group received oral gavage of an equal volume of saline solution. The above procedures were performed once a day, and were spaced 12 h apart from DNCB application (Fig. 1). Following treatment completion, all animals were anesthetized with isoflurane (2% oxygen), blood samples were collected, and then animals were euthanized using CO2 for sample collection.
Ear thickness measurement
From day 1 to day 14 of the experiment, ear thickness measurements were taken daily at the same position on the mice using a digital caliper, and the measurements were recorded.
Trans epidermal water loss (TEWL)
Throughout the first to the 14th day of the experiment, the Gpskin instrument was employed daily to assess the skin condition of each group of mice, with TEWL measurements recorded using Gpskin software. To ensure experimental precision, each mouse underwent three measurements, and the average value was calculated.
Enzyme-linked immunosorbent assay (ELISA)
Serum levels of cytokines (e.g., TNF-α, IL-17, iNOS, IL-6, IL-4, and thymic stromal lymphopoietin [TSLP]) were quantified by ELISA kits (Ruixinbio, quanzhou, china) .
Histological staining
Ear tissues were stained with hematoxylin and eosin for morphological examination and measurement of the epidermal cross-sectional area. After sample collection, tissue specimens were fixed with 4% buffered neutral paraformaldehyde. The specimens were subjected to routine histological processing and embedded in paraffin. Next, they were sectioned at a thickness of 5 µm, stained with hematoxylin and eosin, and used to measure epidermal thickness. Mast cell infiltration was examined by toluidine blue staining.
Immunohistochemical (IHC) staining
Immunohistochemistry was conducted to assess the expression patterns of caspase-1, inducible nitric oxide synthase (iNOS), TNF-α, IL-1β, CCL2, and CCR2. Briefly, paraffin-embedded sections were deparaffinized, rehydrated, and subjected to antigen retrieval using 0.05% trypsin at 37 °C for 30 min. Endogenous peroxidases were inactivated by incubation with 0.3% H2O2 at room temperature for 15 min. Next, the sections were incubated overnight at 4 °C with primary antibodies against caspase-1 (1:200, AF5418, Affinity, Jiangsu, China), iNOS (1:200,AF0199, Affinity, Jiangsu, China), TNF-α (1:200, AF7014, Affinity, Jiangsu, China), IL-1β (1:200, GTX74034, GeneTex, Southern California, America), CCL2 (1:200, BF0556, Affinity, Jiangsu, China), and CCR2 (1:200; DF2711, Affinity, Jiangsu, China). Subsequently, all sections were counterstained with hematoxylin, dehydrated, cleared with xylene, and mounted for assessment by microscopy. Target protein expression levels were quantified as the mean intensity of optical density within the region of interest (ROI), determined via 400 × magnification of mouse ear tissue. Image-Pro Plus software (Media Cybernetics, Inc., Rockville, MD, USA) was used to calculate the positive staining intensity within the ROI, defined as the sum of integrated optical density (IOD), and the area of the ROI was measured. The mean intensity of optical density (in units of IOD/mm2) was defined as the sum of IOD divided by the tissue area within the ROI.
Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells and skin tissues; RT-qPCR was conducted to measure the mRNA expression levels of IL-6, IL-4, TNF-α, iNOS, IL-17A, CCL2, and CCR2. Briefly, skin tissues were ground into powder with liquid nitrogen, and total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The RNA was reverse transcribed to cDNA using the SYBR Premium Ex Taq™ II kit (Takara, Shiga, Japan). RT-qPCR assays were performed on the FTC-3000 real-time PCR system (Funglyn Inc., Richmond Hill, ON, Canada). The mRNA Ct values for each gene were normalized to the housekeeping gene GAPDH and expressed as relative increases or decreases compared with the control group. For details, see Supplementary materials.
Western blotting analysis
Western blotting was used to analyze the expression levels of CCL2 and CCR2 proteins. Tissues were homogenized using a mortar and pestle, then lysed with radioimmunoprecipitation assay lysis buffer; protein concentrations were measured using a bicinchoninic acid protein assay kit. Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) was performed using a precast gel kit. Protein samples (15 µg each) were separated by 10% SDS-PAGE and transferred to polyvinylidene fluoride membranes. The membranes were blocked with 5% skim milk at room temperature for 1 h, then incubated overnight at 4 °C with primary antibodies against CCL2, CCR2, and actin. Next, the membranes were washed with Tris-buffered saline plus Tween (TBST) and incubated at room temperature for 1 h with a goat anti-rabbit secondary antibody. Detection was performed with an enhanced chemiluminescence kit, and the ChemiDoc MP chemiluminescence imaging system was used to acquire images. The grayscale values of target bands were analyzed using ImageJ software; the ratios of grayscale values for target bands to the internal reference bands represented the protein expression levels. The experiment was repeated three times and mean values were calculated.
Statistical analysis
Statistical analyses were conducted using one-way analysis of variance, followed by Bonferroni correction, in GraphPad Prism software (version 9.3.0; Dotmatics, Inc., San Diego, CA, USA). Data are presented as the mean ± standard deviation from at least three independent experiments. P-values < 0.05 were considered statistically significant.
Results
Relative mRNA expression in HaCaT cells
Compared with the control group, the mRNA expression levels of IL-17A, IL-6, IL-4, TNF-α, CCL2, and CCR2 were significantly higher in the in TNF-α/IFN-γ-stimulated group. Compared with the TNF-α/IFN-γ-stimulated group, the mRNA expression levels of IL-17A, IL-6, and IL-4 were significantly lower in the vitamin D group; the mRNA expression levels of IL-17A, IL-6, IL-4, TNF-α, CCL2, and CCR2 were significantly lower in the crisaborole group. The mRNA expression levels of IL-4, IL-6, IL-17A, CCL2, CCR2, and TNF-α were significantly lower in the vitamin D + crisaborole group than in the vitamin D group. The mRNA expression levels of CCR2 and TNF-α were significantly lower in the vitamin D + crisaborole group than in the crisaborole group (Fig. 2).
HaCaT cells were treated with various concentrations of vitamin D, crisaborole, and a combination of vitamin D and crisaborole for 24 h. Inflammatory cytokine expression was measured in TNF-α/IFN-γ-stimulated HaCaT cells. Abbreviations: VD = vitamin D, Cri = crisaborole ointment. *P < 0.05, **P < 0.01, #P < 0.05, ##P < 0.01.
Macroscopic pathological changes
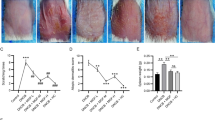
On day 6 post-modeling, ear tissue swelling and TEWL rates were significantly greater in the DNCB group than in the control group; moreover, the DNCB group exhibited ACD-like lesions with erythema, swelling, exudation, scratching, and skin thickening (Fig. 3A,B). Swelling and transdermal water loss rates were significantly lower in the vitamin D, crisaborole, and vitamin D + crisaborole groups on day 14 post-modeling compared with the DNCB group. Improvements in ACD lesions were greater in the vitamin D + crisaborole group than in either single monotherapy group. The SCORing Atopic Dermatitis (SCORAD) index indicated significant improvements of ACD in the vitamin D, crisaborole, and vitamin D + crisaborole groups. The vitamin D + crisaborole group exhibited the largest difference compared with the DNCB group (Fig. 3C–E).
Effects of vitamin D, crisaborole ointment, and the combination of vitamin D and crisaborole ointment on DNCB-induced ACD-like skin lesions are shown. (A) Photographs of mouse ears in each group on day 14. (B) Skin condition on the back of the mice. (C) Ear thickness measurements taken 14 days after DNCB application. Changes in physiological parameters in DNCB-stimulated C57BL/6 mouse skin following treatments with vitamin D, crisaborole ointment, and the combination of vitamin D and crisaborole ointment, including (D) trans-epidermal water loss (TEWL) and (E) SCORAD index. (F) Serum levels of TSLP, iNOS, TNF-α, IL-4, IL-17, and IL-6 in mice, analyzed by ELISA. Abbreviations: ACD: allergic contact dermatitis, DNCB: 1-chloro-2,4-dinitrobenzene, VD vitamin D, Cri crisaborole ointment. *P < 0.05, **P < 0.01, #P < 0.05, ##P < 0.01.
ELISA analysis
Compared to the control group, mice in the DNCB group exhibited significantly elevated serum levels of TSLP, iNOS, TNF-α, IL-4, IL-17, and IL-6. Vitamin D treatment alone markedly suppressed the expression levels of these factors, except for IL-6. Similarly, treatment with crisaborole also reduced serum levels of TSLP, iNOS, TNF-α, IL-4, IL-6, and IL-17. The combined use of vitamin D and crisaborole demonstrated a more pronounced suppression of these factors, surpassing the effects observed with either intervention alone. Importantly, in the vitamin D + crisaborole group, serum levels of TSLP, TNF-α, and IL-17 did not differ significantly from those in the control group (Fig. 3F).
Histological analysis
Hematoxylin and eosin staining showed that DNCB treatment induced epidermal hyperkeratosis in mouse ear tissues compared with the control group. The vitamin D + crisaborole treatment improved epidermal hyperkeratosis; there was no statistically significant difference in epidermal thickness between the vitamin D + crisaborole group and the control group (Fig. 4A). Toluidine blue staining showed that tissue infiltration by mast cells was significantly greater in the DNCB group than in the control group. Compared with the DNCB group, the crisaborole and vitamin D + crisaborole treatments significantly inhibited the infiltration of mast cells into skin lesions; the vitamin D + crisaborole treatment had a stronger effect. There was no statistically significant difference in the infiltration of mast cells into skin lesions between the vitamin D + crisaborole group and the control group (Fig. 4B).
Combined treatment ameliorates skin inflammation in DNCB-stimulated C57BL/6 mice. (A) Histopathological changes induced by DNCB were evaluated using hematoxylin and eosin staining, and the ear epidermal thickness of mice in each group was quantitatively analyzed. (B) Ear tissues were stained with toluidine blue, and the number of infiltrating mast cells in each group was quantified. Red arrows indicate mast cells. Abbreviations: E: Epidermal thickness, DNCB: 1-chloro-2,4-dinitrobenzene, VD: vitamin D, Cri: crisaborole ointment. Thin bar = 100 μm, thick bar = 200 μm. *P < 0.05, **P < 0.01, #P < 0.05, ##P < 0.01.
Immunohistochemical analysis
Immunohistochemical analysis showed that the expression levels of caspase-1, TNF-α, iNOS, IL-1β, CCL2, and CCR2 were significantly higher in the DNCB group than in the control group. The expression levels of iNOS, IL-1β, and CCR2 were slightly lower in the vitamin D group than in the DNCB group. The expression levels of TNF-α, iNOS, CCR2, IL-1β, and CCL2 were significantly lower in the crisaborole group than in the DNCB group. The expression levels of Caspase-1, TNF-α, iNOS, IL-1β, CCL2, and CCR2 were significantly lower in the vitamin D + crisaborole group than in the DNCB group and either monotherapy group. The expression levels of Caspase-1, TNF-α, iNOS, CCL2, and CCR2 did not significantly differ between the vitamin D + crisaborole group and the control group (Fig. 5A,B).
(A) Immunohistochemical staining for Caspase-1, TNF-α, iNOS, IL-1β, CCL2, and CCR2 in ear tissue of mice in each group. (B) The quantified protein levels of Caspase-1, TNF-α, iNOS, IL-1β, CCL2, and CCR2. Abbreviations: DNCB: 1-chloro-2,4-dinitrobenzene, VD vitamin D, Cri crisaborole ointment. Bar = 100 μm. *P < 0.05, **P < 0.01, #P < 0.05, ##P < 0.01.
RT-qPCR analysis
Compared with the control group, mRNA expression levels of IL-6, IL-4, TNF-α, iNOS, IL-17A, CCL2, and CCR2 in the skin were significantly increased in the DNCB group. Compared with the DNCB group, the mRNA expression levels of IL-6, IL-4, TNF-α, iNOS, IL-17A, CCL2, and CCR2 were significantly different in the crisaborole group; the mRNA expression levels of TNF-α, iNOS, IL-17A, CCL2, and CCR2 were significantly decreased in the vitamin D group; and the mRNA expression levels of IL-6, IL-4, TNF-α, iNOS, IL-17A, CCL2, and CCR2 were significantly decreased in the vitamin D + crisaborole group. Moreover, the mRNA expression levels of IL-6, IL-4, TNF-α, IL-17A, CCL2, and CCR2 were significantly lower in the vitamin D + crisaborole group than in the vitamin D group; the mRNA expression levels of CCL2 and CCR2 were significantly lower in the vitamin D + crisaborole group than in the crisaborole group (Fig. 6A).
(A) Effects of vitamin D, crisaborole ointment, and the combination of vitamin D and crisaborole ointment on the mRNA expression levels of related cytokines in the back skin of ACD mice. (B) Protein expression levels of CCL2 and CCR2 in the back skin of mice from each group. Abbreviations: DNCB: 1-chloro-2,4-dinitrobenzene, VD vitamin D, Cri crisaborole ointment. *P < 0.05, **P < 0.01, #P < 0.05, ##P < 0.01.
Protein expression levels of CCL2 and CCR2
Compared with the control group, DNCB stimulation significantly enhanced the protein expression levels of CCL2 and CCR2. Compared with the DNCB group, expression of CCL2 protein was significantly decreased in the crisaborole group. Protein expression levels of CCL2 and CCR2 were significantly lower in the vitamin D + crisaborole group than in the DNCB and crisaborole groups (Fig. 6B).
Discussion
In this study, the DNCB solution stimulation method was used to establish a chronic ACD mouse model that could evaluate three treatments for ACD: crisaborole, vitamin D, and crisaborole combined with vitamin D. Ear tissue in the model mice showed pronounced erythema, scabbing, thickening, along with significantly increased scratching frequency. Compared with crisaborole or vitamin D alone, crisaborole combined with vitamin D was more effective in terms of alleviating skin damage, erythema, and thickening among mice with DNCB-induced ACD; it also was superior in terms of reducing the expression levels of pro-inflammatory factors. These findings support the use of crisaborole and vitamin D in ACD management.
Crisaborole ointment, a topical non-steroidal PDE4 inhibitor, is used for clinical treatment of mild to severe allergic contact dermatitis23. This application is consistent with our findings that, compared with the DNCB group, mice treated with crisaborole alone exhibited less erythema, reduced epidermal thickness, and decreased desquamation, along with declines in trans-epidermal water loss and dermatitis scores. Additionally, we observed similar trends in immunohistochemical, ELISA, and RT-qPCR analyses. In vitro experiments revealed that inflammatory factors were decreased in the crisaborole group compared with the DNCB group. When a sensitized body undergoes repeated exposure to the same allergen, sites of inflammation release pro-inflammatory cytokines (e.g., TNF-α, IFNs, and ILs) that lead to the onset of ACD24,25. Crisaborole can effectively inhibit the release of these pro-inflammatory factors, thereby alleviating symptoms of dermatitis. Previous studies have identified TSLP, a cytokine produced by skin keratinocytes, as the initial irritant that triggers skin inflammation and immune responses. TSLP is highly expressed by keratinocytes in the skin lesions of atopic dermatitis patients, while TSLP is almost non-expressed in the not skin lesions26,27. ELISA revealed that TSLP expression was increased in the DNCB group and significantly decreased in the crisaborole treated group, indicating that crisaborole could reduce ACD severity.
In addition to the remarkable therapeutic effects of crisaborole regarding dermatitis, there is evidence that vitamin D-mediated immune regulation is essential for the rehabilitation of skin lesions28,29. It has been unclear whether vitamin D-mediated immunoregulation can facilitate the treatment of ACD. Typically, vitamin D deficiency is defined as a vitamin D level below 20 ng/mL, but the optimal level remains a subject of debate20,30. Through its active metabolite, 1,25(OH)2D, vitamin D directly induces transcription of the gene encoding human IL-1β, IL-1β is one of the cytokines produced by the pathogen in response to the targeted activation of the inflammatory body31. Vitamin D inhibits inflammatory factors, including TNF-α and IL-6, while protecting tissues from excessive inflammation32. Our immunohistochemical, RT-qPCR, and ELISA analyses showed that vitamin D alone could significantly reduce the expression levels of Inflammatory factors in mice, but the resulting expression levels remained greater than levels in the crisaborole group. Our in vitro experiments also showed that inflammatory factors were decreased in the vitamin D group compared with the DNCB group. Although treatment with vitamin D alone led to partial improvement, the results significantly differed from the control group.
In the mouse model, we found that crisaborole combined with vitamin D had a greater effect on ACD compared with either monotherapy. This combination leveraged the anti-inflammatory activity of crisaborole and the immunomodulatory activity of vitamin D synergistically, demonstrating enhanced efficacy in mitigating allergic contact dermatitis32. In vivo and in vitro immunohistochemical analyses also showed that the expression levels of inflammatory factors were significantly lower in the crisaborole combined with vitamin D group than in the DNCB group; these differences were significantly enhanced compared with either monotherapy group. The main role of vitamin D in adaptive immunity is to suppress or reduce local inflammatory responses; its role in innate immunity primarily comprises enhancing defensive and antibacterial functions to facilitate systemic immune homeostasis33,34. In contrast, the therapeutic effect of crisaborole ointment on ACD mainly involves upregulating intracellular cAMP and inhibiting the expression of inflammatory mediators35,36. Crisaborole and vitamin D have distinct mitigating effects on dermatitis in terms of erythema, swelling, and lesions. In the present study, the combination of crisaborole and vitamin D had robust synergistic effects, presumably because crisaborole improved local immune function and vitamin D enhanced systemic immune function; these effects alleviated the manifestations of ACD. Therefore, the combination of crisaborole and vitamin D may reduce overall inflammation and mitigate the symptoms of ACD.
The CCL2/CCR2 signaling pathway is widely expressed in the immune system and plays an important role in the skin’s inflammatory response, which involves keratinocytes as the main source of CCL237,38. There is evidence that serum CCL2 levels are significantly higher in patients with psoriasis than in normal control individuals39,40. In the present study, immunohistochemical, RT-qPCR, and western blotting analyses demonstrated that the expression levels of CCL2 and CCR2 were significantly higher in the DNCB group than in the control group, suggesting that the CCL2/CCR2 signaling pathway could be a useful therapeutic target for the treatment of ACD15. The expression levels of CCL2 and CCR2 were decreased by crisaborole and vitamin D alone. The inhibitory effect of Combined administration group on CCL2 and CCR2 was greater than the effect of either monotherapy, and there was no significant difference between the control groups. These findings suggest that crisaborole combined with vitamin D may attenuate ACD lesions, potentially involving modulation of CCL2/CCR2 signaling pathways. Further investigations, such as employing specific inhibitors of CCL2/CCR2, would be necessary to conclusively establish the regulatory effects of these treatments on the CCL2/CCR2 signaling pathway in the context of ACD.
This study demonstrated the benefits of combining crisaborole with vitamin D for the treatment of ACD in a mouse model. However, there were some limitations. Firstly, selecting a single time point (14 days) did not allow us to observe disease progression over time. Secondly, only a single dose of vitamin D was tested, and more effective dosing combinations may exist. Finally, this study focused solely on the expression of the CCL2/CCR2 signaling pathway. Although there was a significant difference in therapeutic efficacy between the combination treatment and monotherapies, the specific roles of pathway-related factors in the pathogenesis and progression of ACD remain unclear. Future studies could use transgenic mice to further explore these associations and better understand the pathological progression of ACD.
Conclusion
In conclusion, our study demonstrates that both crisaborole and vitamin D can partially alleviate ACD, but their combination significantly enhances therapeutic efficacy. The combined treatment reduces epidermal hyperkeratosis, improves epidermal transdermal water loss, decreases dermatitis scores, and diminishes mast cell infiltration. It also lowers the expression of key inflammatory cytokines and mediators. These results suggest that the combination of crisaborole and vitamin D offers a more effective therapeutic strategy for ACD, potentially through the modulation of the CCL2/CCR2 signaling pathway. This combined approach could inform future clinical treatment strategies for managing ACD and potentially other inflammatory skin disorders.\
Data availability
Data is provided within the manuscript or supplementary information files.
References
Li, Y. & Li, L. Contact Dermatitis: Classifications and Management [J]. Clin. Rev. Allergy Immunol. 61(3), 245–281 (2021).
Patel, K. & Nixon, R. Irritant Contact Dermatitis - a Review [J]. Curr. Dermatol. Rep. 11(2), 41–51 (2022).
Slodownik, D. et al. Occupational Chronic Contact Dermatitis Successfully Treated with Dupilumab: A Case Series. Dermatology (Basel, Switzerland) 238(6), 1073–1075 (2022).
Karagounis, T. K. & Cohen, D. E. Occupational Hand Dermatitis [J]. Curr. Allergy Asthma Rep. 23(4), 201–212 (2023).
Nassau, S. & Fonacier, L. Allergic Contact Dermatitis [J]. Med. Clin. North Am. 104(1), 61–76 (2020).
Azeem, M. et al. Intricate Relationship Between Adaptive and Innate Immune System in Allergic Contact Dermatitis. Yale J. Biol. Med. 93(5), 699–709 (2020).
Kostner, L. et al. Allergic Contact Dermatitis. Immunol. Allergy Clin. North Am. 37(1), 141–152 (2017).
Brites, G. S. et al. Allergic contact dermatitis: From pathophysiology to development of new preventive strategies [J]. Pharmacol. Res. 162, 105282 (2020).
Bains, S. N., Nash, P. & Fonacier, L. Irritant Contact Dermatitis [J]. Clin. Rev. Allergy Immunol. 56(1), 99–109 (2019).
Jiang, H. et al. CCL2/CCR2 signaling elicits itch- and pain-like behavior in a murine model of allergic contact dermatitis. Brain Behav. Immun. 80, 464–473 (2019).
Shibuya, R. et al. CCL2-CCR2 Signaling in the Skin Drives Surfactant-Induced Irritant Contact Dermatitis through IL-1β-Mediated Neutrophil Accumulation. J. Investig. Dermatol. 142, 571–82.e9 (2022).
He, H. et al. Single-cell transcriptome analysis of human skin identifies novel fibroblast subpopulation and enrichment of immune subsets in atopic dermatitis. J. Allergy Clin. Immunol. 145(6), 1615–1628 (2020).
Lauerma, A. et al. New Key Players in Irritant Contact Dermatitis: Residential skin cells and neutrophils drive inflammation. J. Investig. Dermatol. 142, 509–512 (2022).
Makins, C., Sanghera, R. & Grewal, P. S. Off-Label Therapeutic Potential of Crisaborole [J]. J. Cutaneous Med. Surg. 24(3), 292–296 (2020).
Lee, H. Y. et al. Cytokines and chemokines in irritant contact dermatitis. Mediators Inflamm. 2013, 916497 (2013).
Dong, C. et al. Treatment of Skin Inflammation with Benzoxaborole Phosphodiesterase Inhibitors: Selectivity, cellular activity, and effect on cytokines associated with skin inflammation and skin architecture changes. J. Pharmacol. Exp. Ther. 358(3), 413–422 (2016).
Bissonnette, R. et al. Crisaborole and atopic dermatitis skin biomarkers: An intrapatient randomized trial. J. Allergy Clin. Immunol. 144(5), 1274–1289 (2019).
Navarro-triviño, F. J., Arias-santiago, S. & Gilaberte-calzada, Y. Vitamin D and the Skin: A Review for Dermatologists. Actas dermo-sifiliograficas 110(4), 262–272 (2019).
Piemonti, L. et al. 2000 Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J. Immunol. 164(9), 4443–4451 (1950).
Kechichian, E. & Ezzedine, K. Vitamin D and the Skin: An Update for Dermatologists. Am. J. Clin. Dermatol. 19(2), 223–235 (2018).
Abhimanyu, C. A. K. The role of UV radiation and vitamin D in the seasonality and outcomes of infectious disease. Photochem. Photobiol. Sci. 16(3), 314–338 (2017).
Bergqvist, C. & Ezzedine, K. Vitamin D and the skin: what should a dermatologist know?. Giornale italiano di dermatologia e venereologia 154(6), 669–680 (2019).
Woo, T. E. & Kuzel, P. Crisaborole 2% Ointment (Eucrisa) for Atopic Dermatitis. Skin Ther. Lett. 24(2), 4–6 (2019).
Hanifin, J. M. et al. Type 4 phosphodiesterase inhibitors have clinical and in vitro anti-inflammatory effects in atopic dermatitis. J. investing. Dermatol. 107(1), 51–56 (1996).
Segaud, J. et al. Context-dependent function of TSLP and IL-1β in skin allergic sensitization and atopic march [J]. Nature communications 13(1), 4703 (2022).
Leyva-castillo, J. M. et al. TSLP produced by keratinocytes promotes allergen sensitization through skin and thereby triggers atopic march in mice. J. investing. Dermatol. 133(1), 154–163 (2013).
Marschall, P. et al. Dual function of Langerhans cells in skin TSLP-promoted T(FH) differentiation in mouse atopic dermatitis. J. Allergy Clin. Immunol. 147(5), 1778–1794 (2021).
Quirk, S. K. et al. Vitamin D in atopic dermatitis, chronic urticaria and allergic contact dermatitis. Expert Rev. Clin. Immunol. 12(8), 839–847 (2016).
Gorman, S. et al. Investigating the roles of regulatory T cells, mast cells and interleukin-9 in the control of skin inflammation by vitamin D. Arch. Dermatol. Res. 310(3), 221–230 (2018).
Ismailova, A. & White, J. H. Vitamin D, infections and immunity. Rev. Endoc. Metab. Disord. 23(2), 265–277 (2022).
Bikle, D. D. Vitamin D Regulation of Immune Function [J]. Curr. Osteopor. Rep. 20(3), 186–193 (2022).
Kwon B, Hong S Y, Kim E Y, et al. Effect of Cone of Pinus densiflora on DNCB-Induced Allergic Contact Dermatitis-Like Skin Lesion in Balb/c Mice [J]. Nutrients, 2021, 13(3).
Chen, X. et al. Pseudoephedrine alleviates atopic dermatitis-like inflammatory responses in vivo and in vitro. Life Sci. 258, 118139 (2020).
Fakhoury, H. M. A. et al. Vitamin D and intestinal homeostasis: Barrier, microbiota, and immune modulation. J. Steroid Biochem. Mol. Biol. 200, 105663 (2020).
Mcdowell, L. & Olin, B. Crisaborole: A Novel Nonsteroidal Topical Treatment for Atopic Dermatitis. J. Pharm. Technol. 35(4), 172–178 (2019).
Hoy, S. M. Crisaborole Ointment 2%: A Review in Mild to Moderate Atopic Dermatitis. Am. J. Clin. Dermatol. 18(6), 837–843 (2017).
Daly C, Rollins B J. Monocyte chemoattractant protein-1 (CCL2) in inflammatory disease and adaptive immunity: therapeutic opportunities and controversies. Microcirculation (New York, NY : 1994), 10(3–4): 247–57. (2003).
Qi, X., Xing, Y. & Wang, X. Blockade of CCL2/CCR2 Signaling Pathway Exerts Anti-Inflammatory Effects and Attenuates Gestational Diabetes Mellitus in a Genetic Mice Model [J]. Hormone Metab. Res. 53(1), 56–62 (2021).
Teler, J. et al. CCL2, CCL5, IL4 and IL15 Gene Polymorphisms in Women with Gestational Diabetes Mellitus. Hormone and metabolic research Hormon- und Stoffwechselforschung Hormones et metabolisme 49(1), 10–15 (2017).
Behfar, S. et al. A brief look at the role of monocyte chemoattractant protein-1 (CCL2) in the pathophysiology of psoriasis. Cytokine 110, 226–231 (2018).
Acknowledgements
We thank Ryan Chastain-Gross, Ph.D., from Liwen Bianji (Edanz) (www.liwenbianji.cn/) for editing the english text of a draft of this manuscript.
Funding
The study was funded by National Natural Science Foundation of China (81773327).
Author information
Authors and Affiliations
Contributions
Hetong Li and Huachun wang as the first author performed data analysis and wrote the manuscript. Zhengxiao Li, Xiaomei Zhao, Xiaoli Hou, Lu Chen, Lei Xing, Faming Tian contributed suggestions for manuscript revision and revised the manuscript. Zhengxiao Li, Xiaomei Zhao, Xiaoli Hou, Lu Chen, Lei Xing, Faming Tian provided advice and suggestions while we met some problems during the data analysis process. Hetong Li and Huachun wang conceived and initiated this project, provided advice on experimental design, oversaw the implementation of the statistical method, and revised or finalized the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Animal experiments were approved by the Laboratory Animal Ethical and Welfare Committee of North China University of Science and Technology.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, H., Li, H., Li, Z. et al. Crisaborole combined with vitamin D demonstrates superior therapeutic efficacy over either monotherapy in mice with allergic contact dermatitis. Sci Rep 14, 20092 (2024). https://doi.org/10.1038/s41598-024-71135-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-71135-6
- Springer Nature Limited