Abstract
Glioblastoma (GBM) is the most common malignant primary brain cancer that, despite recent advances in the understanding of its pathogenesis, remains incurable. GBM contains a subpopulation of cells with stem cell-like properties called cancer stem cells (CSCs). Several studies have demonstrated that CSCs are resistant to conventional chemotherapy and radiation thus representing important targets for novel anti-cancer therapies. Proton sensing receptors expressed by CSCs could represent important factors involved in the adaptation of tumours to the extracellular environment. Accordingly, the expression of acid-sensing ion channels (ASICs), proton-gated sodium channels mainly expressed in the neurons of peripheral (PNS) and central nervous system (CNS), has been demonstrated in several tumours and linked to an increase in cell migration and proliferation. In this paper we report that the ASIC3 isoform, usually absent in the CNS and present in the PNS, is enriched in human GBM CSCs while poorly expressed in the healthy human brain. We propose here a novel therapeutic strategy based on the pharmacological activation of ASIC3, which induces a significant GBM CSCs damage while being non-toxic for neurons. This approach might offer a promising and appealing new translational pathway for the treatment of glioblastoma.
Similar content being viewed by others
Introduction
Cancer is a relevant public health problem with increasing morbidity and mortality and is a serious threat to human health. In 2020, there were an estimated 19.3 million new cases of cancer worldwide1. Glioblastoma (GBM) is the most common malignant brain tumor and accounts for more than 50% of all gliomas2. Ninety-five percent of all GBMs are primary (arising de novo), whereas 5% arise from less malignant precursor gliomas. GBM is characterized by high proliferation and infiltration proficiency, and despite recent advances, it remains incurable, with a poor prognosis and a median survival time of approximately 15 months3. GBM complexity is reflected by its cellular and molecular heterogeneity; thus, GBM can be classified into the proneural (PN), classical (CL) and mesenchymal (MES) subtypes depending on its aggressiveness4. One of the most infamous traits of GBM is its resistance to conventional therapies and the absence of an effective antitumor strategy. Despite all these efforts, the survival of patients after one year from diagnosis remains low5. Solid tumours, including GBM, are characterized by a high metabolic rate and limited blood supply. In this context, tumor cells switch to aerobic glycolysis, generating considerable amounts of energy to support their rapid growth. This results in the continuous generation of metabolic acids, producing acidosis in tumor tissues6. The tumor pH ranges from 6.0 to 6.9, with a mean of approximately 6.8, and acidosis has been linked to tumor invasion and metastasis7,8. While acidity is deleterious to normal cells, long time coevolution of tumor cells with the host has enabled them to be more adaptable to the acidic microenvironment9. Accumulating evidence indicates that the acidity of the tumor microenvironment is associated with the stemness phenotype10. Accordingly, many tumours contain a small subpopulation of cells with stem cell-like properties called cancer stem cells (CSCs)11. CSCs are able to self-renew, differentiate and induce tumours when transplanted into an animal host, and several studies have demonstrated their resistance to conventional chemotherapy and radiation, possibly contributing to tumor growth and therapy resistance, thus representing important target cells for novel anti-cancer therapies12. Given their central role in tumor proliferation and survival, they are also generally considered the most prognostic predictive cancer models13,14,15. In this scenario, proton-sensing receptors expressed by CSCs could represent important factors involved in the adaptation of tumours to the environment. Increasing evidence has demonstrated that proton-sensing ion channels play important roles in relevant tumor physiological aspects, such as cell proliferation, migration and differentiation, thus representing putative drug targets in cancer therapy16. Among proton-sensing receptors, acid-sensing ion channels (ASICs), also known as amiloride-sensitive cation channels, neuronal (ACCN1-4) or brain sodium channels (BNaCs), are receiving attention as potential players in tumor biology because they are specifically activated by protons17,18. According to the literature, the expression of ASIC channels in different tumours correlates with an increase in tumor survival, proliferation and migration19,20, although the exact role of these channels has not been fully elucidated. Interestingly, their expression and activity have been observed in GBM CSCs lines21. ASICs are voltage-independent, amiloride-sensitive, proton-gated ion channels belong to the NaC/DEG channel superfamily, which includes epithelial Na+ channels (ENaC) and degenerins (DEG), encoded by 4 genes (ASIC1-4) with two splice variants (a and b) for ASIC1 and ASIC222,23. In the human brain, neurons preferentially express ASIC1a24,25. ASIC3 is involved in sensory system-mediating pain sensation and therefore represents the main ASIC isoform detectable in the peripheral nervous system (PNS)26,27. ASIC3 can detect pH changes as small as 0.2 units, being thus the most sensitive ASIC isoform26. ASIC channels have been reported to play a potential role in several pathologies including cancer28. In recent years, the role of ASIC1 has emerged in different tumours29,30,31,32. ASIC3 has also been linked to cancer, in particular to pancreatic cancer 31, and its expression was detected in gliomas32 and, more recently, in GBM CSCs lines21. In cancer cells, ion channel properties have been demonstrated to be profoundly altered, suggesting that their regulation is a key aspect involved in the pathological evolution of these cells 33. The potential involvement of ion channels in tumours has been linked mainly to their intracellular signalling mechanism and to their ability to modulate membrane potential 16,26, a key physiological factor also in non-excitable cells 34. Accordingly, the membrane potential has been demonstrated to play a role in the cell cycle, cell migration and cell metabolism, where alterations in the membrane potential cause cell death by impairing nutrient transport35. These consolidated evidences strongly support the idea that perturbing the membrane potential with a specific ion-channel modulator could represent a putative strategy for drug treatment aimed at controlling tumor cell growth and survival. Taking advantage from the recent identification of non-proton ligand modulators for ASIC3, we investigated the targeting of ASIC3 as a potential new strategy to control GBM CSCs growth. Agmatine and Arcaine were identified as ASIC3 activators36, and 4-chlorophenylguanidine was demonstrated to be an ASIC3 positive allosteric modulator37, as were Guanabenz and Sephin138. 2-Guanidine-4-methylquinazoline (GMQ) has been shown to cause persistent ASIC3 channel activation even at physiological pH38,39 while being almost inactive on the ASIC1a isoform40. In humans, there are no published data regarding the protein expression of ASIC3 in the brain, while mRNA expression studies confirmed the low level of expression of ASIC3 compared to ASIC141. In the human brain, ASIC1a was demonstrated to be the main ASIC isoform both at the mRNA and protein levels24,41,42,43. By targeting ASIC3 with specific agonists, we expect to chronically depolarize the GBM cell membrane, thus possibly affecting cell growth and the cell cycle and inducing cell damage in GBM CSCs with minimal effects on other cells of the brain44. This pharmacological approach entails specific targeting of ASIC3-expressing GBM cells, with an expected minor effect on physiological CNS cells that marginally express ASIC3.
Methods
Cell culture and treatments
GBM culture
GBM specimens were collected from patients with histologic diagnosis of primary GBM (WHO grade 4 glioma) after informed consent was obtained from all subjects and/or their legal guardian(s). The experimentation has been performed following the ethical standards of Declaration of Helsinki and in accordance with the protocol 01-CSC07 approved by the institutional ethical review board of San Raffaele Scientific Institute to R.G. Proneural (PN), mesenchymal (MES) and classical (CL) GBM CSCs were established at the Neural Stem Cell Biology Unit, San Raffaele Scientific Institute, Milan, Italy, and previously validated in45 and46. The cells were cultured under the conditions of the NeuroSphere Assay (NSA)13. Briefly, GBM CSCs were cultured at 37 °C in Neurocult medium (NeuroCult NS-A Proliferation Kit, human) (Cat: 5751; Voden, Monza, Italy) supplemented with hEGF (20 ng/µL; Cat: SRP3027-500UG; Sigma‒Aldrich, Burlington, VT, USA), hFGF (10 ng/µL; Cat: PHG0026; Life Technologies Italia, Monza, Italy) and heparin (0.0002%; Cat: 7908; Voden, Monza, Italy). Neurospheres were routinely subcultured by mechanical dissociation and re-plated under the same in vitro conditions. All the cultures were routinely tested for mycoplasma contamination. Studies in a normal microenvironment (NME) or a tumor microenvironment (TME) were conducted in DMEM:F12 medium (Cat: C4022.0500; Genaxxon Bioscience, Ulm, Germany). The medium was supplemented with 15 mM HEPES (Cat: ECM0180D; Euroclone, Milan, Italy), 1% GlutaMAX (Cat: 35,050,061; Gibco, NY, USA), 1% penicillin/streptomycin (Cat: P0781-100ML; Sigma‒Aldrich, Burlington, VT, USA), 1% N-2 supplement (Cat: 17502-048; Gibco, NY, USA), 20 μg/mL insulin (Cat: I9278-5ML; Sigma‒Aldrich, Burlington, VT, USA), 20 ng/µL hEGF, 10 ng/µL hFGF and 0.0002% heparin. In the NME experiments, the medium was supplemented with 50 mM glucose, and the cells were incubated in 20% O2 at pH 7.4; in the TME experiments, the medium was supplemented with 5 mM glucose, and the cells were incubated in 5% O2 at pH 6.5.
Mouse cortical neuron culture
Primary neuronal cell cultures were established from C57BL/6J E17.5 embryonic cerebral cortices (Charles River) according to our published protocol47. Mice were maintained under pathogen-free conditions at San Raffaele Hospital mouse facility (Milan, Italy). All efforts were made to minimize animal suffering and to reduce the number of mice used in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC) and with the ARRIVE guidelines. The Ethics Review Committee approved experimental protocols according to guidelines from the Italian Ministry of Health and from the Institutional Animal Care and Use Committee of the San Raffaele Scientific Institute (protocol number 821/2021-PR). Briefly, we dissected forebrains in cold HBSS supplemented with 0.6% glucose and 5 mM HEPES at pH 7.4 to obtain cerebral cortices. Cortices were mechanically dissociated into single-cell suspensions and resuspended in Neurobasal culture medium, 5 mM HEPES (pH 7.4), 0.6% glucose and 1 × B27. 3 × 10^4 cells/well were plated in 96-well plates previously coated with 0.2 mg/mL polylysine (Cat: P2636-1G) and 10 μg/mL laminin (Cat: L2020-1MG) (Merck, Darmstadt, Germany) and primed with Dulbecco’s modified Eagle’s medium (DMEM) (Cat: 11960044; Thermo Fisher, Waltham, MA, USA) supplemented with 20% FBS (Cat: ECS0180L; Euroclone, Milano, Italy). The cells were maintained in a humidified 5% CO2 air environment at 37 °C. The neurons were left to mature for 10 days and, upon visual inspection, were subjected to 2D single-cell assays.
Pharmacological treatments
The cells were treated with GMQ hydrochloride (Cat: SML0874-50MG; Sigma-Aldrich, Burlington, VT, USA), APETx2 (Cat: STA-160; Alomone Labs, Jerusalem, Israel) or temozolomide (TMZ) (Cat: T2577; Sigma Aldrich, Burlington, VT, USA). All the treatments lasted for 96 h, except for FACS analysis, where the GMQ treatment lasted for 24 h. The treatments were applied 24 h after cell plating and repeated after 48 h. GMQ was dissolved in DMSO (0.3% as the highest concentration). APETx2 was diluted in water and administered either alone or in combination with 3 µM GMQ. TMZ was diluted in water.
GBM CSC survival assays
Cells were seeded at 2 × 103 per well in black 96-well plates with a transparent flat bottom (Cat: GR655090; Euroclone, Milan, Italy) previously treated with a 1:100 matrigel solution (Cat: 354230; Corning Tewksbury, MA, USA) for at least 30 min at 37 °C. After treatment, the cells were washed twice with Krebs–Ringer solution [NaCl (0.13 M), KCl (5 mM), NaH2PO4 (1.2 mM), MgSO4 (1.2 mM), CaCl2 (2 mM), HEPES (25 mM), and glucose (6 mM); all reagents were purchased from Sigma-Aldrich]. Cells were then incubated for 15 min with 1:5.000 Hoechst (Cat: H3570; Thermo Fisher Scientific, Waltham, MA, USA) diluted in Krebs–Ringer solution. The total number of cell nuclei was measured with a Thermo Scientific™ ArrayScan™ XTI High Content Analysis (HCA) Reader (Thermo Fisher Scientific, Waltham, MA, USA). Using 20 × magnification, ten fields per well in the plate were acquired, and six wells per condition were used. For the analysis, HCS Studio software was used (Thermo Fisher Scientific, Waltham, MA, USA). All the Hoechst-positive nuclei in each field were quantified with the software. Total cell nuclei were quantified and normalized to the mean value of the controls. For pharmacological experiments in 3D, single MES1305 cells were seeded at 5 × 103 per well in black 96-well plates with transparent flat bottoms. At the end of the experiment, 100 µL of medium was removed, and then, the neurospheres were incubated with 1:5.000 Hoechst diluted in PBS for 15 min. The neurospheres were imaged with a Thermo Scientific™ ArrayScan™ XTI High Content Analysis (HCA) Reader at 5 × magnification. Four fields per well and six wells per condition were used. The analysis was performed by Fiji-ImageJ 2.15.1 software (https://imagej.net/software/fiji). First, a threshold was applied, and then, only neurospheres larger than 900 µm2 were considered. The total neurosphere area/field value was calculated by multiplying the total neurosphere number/field by the average area/field.
Extreme limiting dilution analysis (ELDA)
MES 0315 cells were seeded at 2 × 105 cells per well in a 6-well plate and subsequently treated with both 10 µM and 30 µM GMQ hydrochloride (Cat: SML0874-50MG; Sigma-Aldrich, Burlington, VT, USA). After 96 h of treatment, the neurospheres were collected, centrifuged at 100×g for 10 min, dissociated and then seeded on a transparent 96-well plate at different cell densities (1 cell/well-3 cell/well-6 cell/well-12 cell/well-25 cell/well). After 10 days of incubation at 37 °C, neurospheres with a diameter equal to or greater than 20 µm were imaged and counted with a phase-contrast microscope at 10× magnification. The obtained data were subsequently analysed by ELDA software (http://bioinf.wehi.edu.au/software/elda/).
Metabolic assay
Cells were seeded at 2 × 103 per well in black 96-well plates with clear bottoms (Cat: GR655090; Euroclone, Milan, Italy) previously treated with a 1:100 matrigel solution (Cat: 354230; Corning Tewksbury, MA, USA). Metabolic activity was measured using the Cell Counting Kit-8 (CCK8) Metabolic Assay Kit following the manufacturer’s protocol (Cat: 96992-500TESTS-F; Sigma‒Aldrich, Burlington, VT, USA). Briefly, CCK-8 solution (10 µL) was added to the cell medium (100 µL) in each well at the end of the treatment. After a 2 h incubation at 37 °C, the absorbance of the samples was measured at 450 nm using a Multiskan Sky Plate reader (Thermo Fisher Scientific, Waltham, MA, USA). Six wells per condition were used, and the CCK-8 absorbance of each sample was normalized to the total number of cells. The total cell number in the same samples was estimated by Hoechst staining.
Cell death assay
Cells were seeded at 2 × 103 per well in black 96-well plates with a transparent flat bottom (Cat: GR655090; Euroclone, Milan, Italy) previously treated with a 1:100 matrigel solution (Cat: 354230; Corning Tewksbury, MA, USA) for at least 30 min at 37 °C. At the end of the pharmacological treatments, the cells were washed twice with Krebs–Ringer solution [NaCl (0.13 M), KCl (5 mM), NaH2PO4 (1.2 mM), MgSO4 (1.2 mM), CaCl2 (2 mM), HEPES (25 mM), and glucose (6 mM); all the reagents were purchased from Sigma-Aldrich] and incubated for 15 min with 1:5000 Hoechst (Cat: H3570; Thermo Fisher Scientific, Waltham, MA, USA) and 1:10,000 Sytox-orange (Cat: S11368; Invitrogen, Waltham, MA, USA). Both dyes were diluted in Krebs–Ringer solution. A Thermo Scientific™ ArrayScan™ XTI High Content Analysis (HCA) Reader (Thermo Fisher Scientific, Waltham, MA, USA) was used to acquire cell images at 20× magnification; ten fields per well were acquired, and six wells per condition were used. For the analysis, HCS Studio software was used (Thermo Fisher Scientific, Waltham, MA, USA). An intensity threshold was used to discriminate between SYTOX-negative and SYTOX-orange-positive cells.
Western blot assay
The cells were mechanically detached and centrifuged at 8000 rpm for 10 min. The cells were incubated for 10 min on ice in 50 µL of RIPA buffer [Tris–HCl (25 mM), NaCl (150 mM), EDTA (2 mM), SDS (0.1%), sodium deoxycholate (1%), and Triton-100X (1%)]. The lysates were centrifuged for 15 min at 13,000 rpm at 4 °C. The proteins were quantified using a BCA assay kit (Thermo Fisher Scientific, Waltham, MA, USA). A healthy human brain lysate sample was purchased from Novus (Cat: NB820-59177; Novus Biologicals, Centennial, CO, USA). Prior to Western blot (WB) analysis, the samples were thawed, mixed with 4× loading buffer (250 mM Tris–HCl, 20% SDS, 50% glycerol, 4% β-mercaptoethanol, 0.4% bromophenol blue) and heated at 95 °C for 10 min. 16 μg of proteins were loaded into a 10% polyacrylamide gel for electrophoresis and run at 80 V for 3 h. The proteins were subsequently transferred to a nitrocellulose membrane (Euroclone, Milan, Italy) using a standard transfer apparatus (Bio-Rad, Hercules, CA, USA) with an electrical field of 50 V for 2 h. The blotted membranes were blocked with 5% BSA in TBS-Tween for 45 min at RT. Subsequently, the membranes were probed overnight at 4 °C with primary antibodies against ASIC1a (1:500; Cat: 27235-1-AP; Proteintech, Manchester, UK), CD133 (1:500; Cat: NB120-16518; Novus, Saint Louis, MO, USA), actin (1:120.000; Cat: A5441-100UL; Sigma-Aldrich, Burlington, VT, USA) and ASIC3 (1:500; Cat: ab190638; Abcam, Cambridge, UK or Cat: ASC-018; Alomone Labs, Jerusalem, Israel). Please note that the first ASIC3 antibody was used for most of the Western blots, while the second, given its reactivity to mouse and rat proteins, was also used for blots with mouse neuron samples. Afterwards, the membranes were incubated with HRP-linked secondary antibodies diluted in TBS-Tween with 1:5000 5% BSA for 1 h at RT [anti-rabbit (Cat: 7074S) and anti-mouse (Cat: 7076S); Cell Signalling, Denvers, MA, USA]. After incubation with the secondary antibodies, the membranes were treated with ECL Prime Western Blotting Detection reagent (Cat: GEHRPN2232; Euroclone, Milan, Italy). All the incubation steps were performed according to the manufacturer's instructions. Blot images were acquired using a ChemiDoc MP imaging system (Bio-Rad, Hercules, CA, USA). Different portions of the blot were cut at the appropriate molecular weight region in order to perform multiple staining and the actin counterstain used for quantification on the same blot. The intensities of the protein bands were analysed via the free software Fiji ImageJ. In the figures, the different portions belonging to the same blot were separated by white lines while black lines are used to separate independent blots presented in the same figure.
Immunofluorescence
GBM CSCs were plated at a density of 2.5 × 103 on 13-mm coverslips previously treated with Matrigel (1:100; Cat: 354230; Corning, Tewksbury, MA, USA). 24 h after plating, the cells were fixed with 4% PFA/4% sucrose for 15 min, washed three times with PBS and incubated with 1 mM glycine for 5 min. The cells were then incubated with BlockAid blocking solution (Cat: B10710; Invitrogen, Waltham, MA, USA) at RT for 30 min. The coverslips were incubated with either an anti-human ASIC3 (1:500, Cat: ASC-018; Alomone Labs, Jerusalem, Israel), an anti-human CD133 (1:800, Cat: D4 W4N; Cell Signaling Technology, Denvers, MA, USA) or an anti-human Ki67 (1:250, Cat: ab16667, Abcam, Cambridge, UK) primary antibody overnight at 4 °C. After three washes with PBS, the coverslips were incubated with secondary antibodies (1:600, Cat: A11037; Invitrogen, Waltham, MA, USA; 1:600, Cat: A11034; Invitrogen, Waltham, MA, USA) for 45 min at RT. To visualize the nuclei, the cells were incubated with 0.5 mg/mL 1:10,000 Hoechst (cat. no. H3570; Invitrogen, Waltham, MA, USA) in water for 10 min at RT. Coverslips were mounted with Prolong glass antifade mounting agent (Cat: P36982; Invitrogen, Waltham, MA, USA). For image acquisition, a Zeiss Axiovert 200 instrument equipped with a 60X Zeiss objective was used. Image analysis and fluorescence quantification were performed using Fiji 2.15.1 software (https://imagej.net/software/fiji ). In order to quantify positive cells, signals were estimated in control samples stained only with the secondary antibodies (not shown). A Threshold corresponding to the fluorescence value, estimated to be non- specific, was applied to the images prior to positive cells counting. Cells showing a significant residual fluorescence were considered positive.
Generation of human ASIC3 virtual models
We produced a virtual 3D model of human monomeric ASIC3 using AlphaFold48. Then, to obtain the trimeric assembly of the channel, we superimposed the ASIC3 monomeric model on each of the three monomers present in the trimeric structure of chicken ASIC1 (PDB id: 6VKT49) (r.m.s.d 1.707 Å by aligning 404 residues for each monomer). After omitting the N- and C-terminal regions of the protein, corresponding to the intermembrane segment, the trimeric model of the soluble extracellular domain of ASIC3 (amino acids E70-E432) was prepared to run a molecular dynamic simulation with the program gromacs50 using the GROMOS96 54a7 force field51. After building a parallelepiped box (11.55 × 12.22x10.15 nm3) around the protein, we filled it with 42,006 water molecules and neutralized the charge of the system by adding 41 Na+ and 20 Cl− ions. After system minimization (steepest descent algorithm; final potential energy = −2.258e + 06 kJ mol−1), we equilibrated the mixture at a constant volume and temperature (T = 300 K) for 2 ns before starting the productive run at constant pressure and temperature for 100 ns.
The analysis of the structural clusters during the MD simulation (101 different clusters were identified with r.m.s.d. cut-off of 0.14 nm) allowed identification of cluster 1, which was composed of 190/500 (38%) structures and centred at 68.2 ns, and cluster 2, which was composed of 111/500 (22%) structures and centred at 81.2 ns, as the main clusters. The central structures of the two clusters, named 682 (at 68.2 ns) and 812 (at 81.2 ns), were used as structural models for in silico docking analysis.
In silico docking
The structural models 682 and 812 were prepared by the Python Molecular Viewer52 by adding hydrogens and Kollman charges. Different putative ligands were prepared using different programs from the ChemAxon package (ChemAxon Ltd. https://www.chemaxon.com), such as MarvinSketch, to draw the molecules and cxcalc to modify them by considering the different tautomers at pH 7.4 and molconvert to generate 3D coordinates.
The first docking analysis was performed on both the charged and noncharged GMQ with the program AutoDockVina53, targeting a wide region of the trimeric model (with a volume of 78.2 Å)3, centred around Gln240). Such preliminary analysis generated 120 docking poses for charged/uncharged GMQ, most of which were located in a smaller region inside the ion channel and close to amino acids E78 and E421, known to be involved in GMQ binding39. Selecting such a region (30 × 30 × 15 Å3 centred between the Gln270 side chains of the different subunits), we ran for each channel model (i.e., structures 812 and 682) and for every ligand (GMQ, Sephyn, guanabenz, Agmatine, Arcaine, Chlorophenylguanidine, Metformin and Temozolomide in their charged and not-charged states, respectively, if present at pH 7.4) a docking analysis was performed with the program AutoDock4.254 with 80 runs of Lamarckian genetic algorithm searches (max number of evaluations 1,750,000 and population size 150).
Lentivirus generation
ASIC3-shRNA-mediated lentiviral vector generation
We generated two lentiviral vectors expressing two different shRNAs, shRNA1 (Cat: TRCN0000149157, target seq. GCTACCAGACCTTTGTT TCTT) and shRNA2 (Cat: TRCN0000 433462, target seq. TGGGAAAGTGCTACACA TTTA (Darmstadt, Germany). These compounds were selected for their predicted efficacy on the basis of their binding position on the mRNA. Virus production was carried out according to previous methods55 using CaPi methods. Briefly, HEK293T cells were grown in DMEM (Cat: 11960044; Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% foetal bovine serum (FBS) (Cat: ECS0180L; Euroclone, Milano, Italy) and 100 U mL penicillin/streptomycin (Cat: 09-757F; Lonza, Basilea, Switzerland). Cells were seeded at 8 × 106 density in a 15 cm Petri dish (Cat: 430599; Corning, Tewksbury, MA, USA). The next day, the medium was changed 2 h before transfection. Cells were transfected with lenti-ASIC3 shRNA (30 μg) in combination with VSVG (7 μg), pRRE (12.5 μg), or REV (6.25 μg) as additional viral packing plasmids per plate (packaging plasmids kindly provided by Dr. Luca Muzio, San Raffaele Scientific Institute). After 16 h of transfection, the medium was replaced with fresh, plasmid-free medium. The viral supernatant was collected after 30 h, passed through a 0.22 µm filter and centrifuged at 4 °C for 2 h at 20,000 rpm (ultracentrifuge: Optima XPN90; Ultrarotor: SW32TI 19U5686 2019). The medium was carefully removed, and the viral material was eluted by adding 150 µL of ice-cold PBS, kept at 4 °C overnight for solubilization, and finally stored at − 80 °C. To calculate the viral titre, cells were seeded at 1 × 105 in a 12-well plate and treated for 8 h with lentivirus at different dilutions (1:10–1:30–1:100). After 48 h of infection, the cells were divided into two aliquots and seeded in two identical 12-well plates. One plate was treated with 1 μg/mL puromycin (Cat: P8833; Sigma-Aldrich, Burlington, VT, USA), while the other did not receive any treatment. After 48 h of puromycin treatment, the cells were subjected to the CCK8 assay (Cat: 96992-500TESTS-F; Sigma‒Aldrich, Burlington, VT, USA) at a 1:10 ratio, as per the manufacturer’s instructions, and their absorbance was measured every 30 min at 450 nm using a Multiskan Sky Plate reader (Thermo Fisher Scientific, Waltham, MA, USA). Absorbance readings were subsequently normalized to the mean value of the untreated control. Following the manufacturer’s instructions, we used the percentage of surviving cells to obtain the multiplicity of infection (MOI) of each infected sample, in order to calculate the original viral concentration in our stock (expressed in TU/mL).
GBM CSC lentiviral infection
GBM-CSCs were seeded at 2 × 105 in a 6-well plate and infected 8 h after seeding. After 48 h of infection, the cells were treated with 1 μg/mL puromycin (Cat: P8833; Merck, Darmstadt, Germany) for 48 h to kill noninfected cells. The cells were collected, subsequently centrifuged (10 min at 100×g) and transferred to a T25 flask containing 1 μg/mL puromycin for an additional 72 h. The cells were maintained in culture in T75 flasks or plated on multiple wells for the experiments. Weekly Western Blot analysis was performed to determine the extent of ASIC3 downregulation.
Patch clamp analysis
Patch clamp recordings were performed on GBM-MES 0315 cells using a MultiClamp 700B amplifier (Molecular Devices) equipped with pCLAMP 10 software. The signals were low-pass filtered at 2 kHz and sampled at 10 kHz, and the signals were digitized using a Digidata 1550 D/A converter (Molecular Devices). Cells were seeded on Matrigel-treated coverslips and were incubated at 30–32 °C. The extracellular solution (KREBS) contained 130 mM NaCl, 10 mM HEPES, 2 mM CaCl2, 5 mM KCl, 1 mM MgCl2, and 10 mM D-glucose (pH 7.4), while the patch pipette contained 124 mM KH2PO4, 5 mM KCl, 2 mM MgCl2, 10 mM NaCl, 10 mM HEPES, 0.5 mM EGTA, 2 mM Na-ATP, and 0.2 mM Na-GTP (pH 7.25, adjusted with KOH). The solution for focal stimulation by pressure release or “puff” was KREBS adjusted to pH 7.0, in which GMQ was dissolved at a concentration of 1 mM. For the experiment in the presence of APETx2, the blocker was added both to the extracellular solution and to the puff pipette to avoid local concentration dilution. Puff stimulation was performed in voltage clamp mode by holding the cell membrane potential at −60 mV for a duration of 15 s and an interstimulus interval of 60 s. The membrane current density was obtained by normalizing the current peak to the membrane capacitance. Passive membrane properties were derived from the capacitive transients generated in response to a hyperpolarizing 50 ms test pulse of −4 mV (average of 3 responses). The input resistance was determined as ΔV/ΔI, while the time constant τ was obtained from a single exponential fit (y = y_0 + A_1 e^((−x)/t1)) to the decay of the capacitive transients. Finally, the membrane capacitance was calculated using the following equation: Cm = τ*I/∆V(1 − ∆I/I). Access resistance (Ra) was monitored continuously during the recording, and cells with a Ra > 15 MΩ or with a variation greater than 20% were excluded from analysis. All the data were analysed by using Prism 8 (GraphPad) software (https://www.graphpad.com/), and the traces were generated by Origin 2018 (Origin Lab; https://www.originlab.com/2018).
Cell cycle analysis
For cell cycle analysis, the neurospheres were treated with 10 μM or 30 μM GMQ. After 24 h of treatment, 1 × 106 cells were spun down at 100 × g for 10 min, washed twice in PBS, fixed in ice-cold 70% ethanol for 30 min and stored at −20 °C. After fixation, the cells were washed once in PBS buffer, stained with 5 μg/mL ribonuclease A (Cat: 10109142001; Roche, Basel, Switzerland) and 10 μg/mL propidium iodide (Cat: P3566; Invitrogen, Waltham, MA, USA) in PBS and incubated for 15 min in the dark at RT. Cell cycle analysis was carried out on a cytoFLEX S flow cytometer (Beckman Coulter, Brea, CA, USA) using CytExpert software. More than 10,000 events were analysed for each sample. The data were analysed by FCS Express 7 Plus using a multicycle DNA tool.
Microarray analysis
We analysed the expression level of the ACCN3 gene (ASIC3) in the publicly available Repository for Molecular Brain Neoplasia Data (TCGA datasets Affymetrix HT HG U133A) available at https://www.betastasis.com/glioma/tcga_gbm/ to investigate the expression level of the ACCN3 mRNA. The public library was accessed in September 2023.
Statistical analysis
All the data were analysed by using Prism 9 GraphPad software (https://www.graphpad.com/). The results are expressed as the mean ± SD of independent experiments. All the experiments were repeated at least three times. Student’s t test was used to evaluate the statistical significance of the differences between two samples, except in Fig. 3g, where the Mann‒Whitney nonparametric test was used. When comparing more independent samples, one-way or two-way ANOVA coupled with Bonferroni’s post-test was used. The alpha 0.05. ns = not significant; * = P value < 0.05; ** = P value < 0.01; *** = P value < 0.001; **** = P value < 0.0001.
Results
ASIC3 is highly expressed in human primary GBM cancer stem cell lines
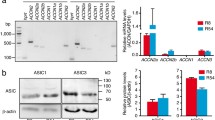
We analysed ASIC3 expression in four patient-derived proneural (PN) GBM CSC primary lines (namely, PN 0512, PN 0625, PN 0306, and PN 0125), two mesenchymal (MES) GBM CSC primary lines (namely, MES 0315 and MES 1312) and one classical (CL) GBM CSC primary line (CL 0627), as well as in two frequently used GBM MES cell lines (namely, U87 and U373). The ASIC3 isoform was detected in all tested GBM cell lines, with the MES cell primary line exhibiting the highest average expression level and the CL cell line exhibiting the lowest expression (Fig. 1a, b and Supplementary Fig. S1). In the same cell lines, the expression level of ASIC1a was high in the PN/CL CSC lines while almost absent in the MES lines (Fig. 1a, b’ and Supplementary Fig. S1). GBM CSCs express the ASIC1a isoform as demonstrated by the specific molecular weight of the ASIC1 band, which is identical to that of the bands shown by mouse cortical neurons and by the human brain lysate that are known to express only this specific isoform (Supplementary figure S2). We have additionally checked the expression of the stem cell marker CD133/ prominin1 in the same samples (Supplementary figure S3). Since no published data are available regarding the protein expression of ASIC3 in the brain, in order to further investigate its enrichment in GBM CSCs, we performed a Western Blot analysis and compared ASIC3 expression in the CSC line MES 0315, in a brain lysate from a healthy patient as well as in the biopsy of the patient originating the MES 0315 cell line. We discovered that ASIC3 was significantly enriched in MES 0315 CSCs compared with healthy human control tissue and the biopsy of patient 0315, confirming the low level of ASIC3 protein expression in the healthy human brain and its specific enrichment in GBM CSCs (Fig. 1c, c’ and Supplementary Figure S4). The low level of ASIC3 protein expression in both healthy brain and GBM patient samples was further confirmed by the low level of ASIC3 mRNA (ACCN3) expression in these tissues studied in the publicly available Repository of Molecular Brain Neoplasia Data (TCGA datasets Affymetrix HT HG U133A) (Fig. 1d,Supplementary Fig. S4 and21). We additionally checked the expression level of CD133/prominin1, a stem cell marker, in the same samples, confirming its enrichment in GBM CSCs and lack of expression in the healthy human brain and in the biopsy from patient 0315 (Fig. 1c, c’). We thus suggest that ASIC3 is enriched in GBM tumor CSCs. Since our study of ASIC3 expression in GBM CSC lines was performed in 3D cultured neurospheres, while further immunofluorescence and electrophysiological studies were carried out in 2D, we checked whether the expression of ASIC3 was similar under the two different culture conditions. Consistent with the evidence that GBM CSCs can be grown under both 2D and 3D culture conditions while maintaining their main characteristics56, we confirmed that ASIC3 expression in GBM CSCs is not altered under 2D conditions with respect to 3D cell growth conditions (Fig. 1e). We have additionally checked whether the expression of ASIC3 in GBM CSC primary cell lines was potentially altered by environmental conditions since the expression of ASIC1a and 2 isoforms has been previously described to be altered under conditions that mimic the acidic pH of the tumor niche57,58. MES 0315 cells were cultured under low pH, low oxygen and low glucose conditions, mimicking the environmental characteristics of tumors in vivo. Our results showed that the expression of ASIC3 is not altered under such environmental conditions. (Supplementary Fig. S5). We also evaluated the expression levels of ASIC3 in mouse cortical neurons, which were used as controls in pharmacological experiments. We confirmed the absence of ASIC3 in mouse CNS neurons. (Supplementary Fig. S6).
The ASIC3 isoform is enriched in glioblastoma CSCs. (a) Western blot analysis of ASIC3 and ASIC1a expression in human primary GBM-CSC lines from the three GBM subtypes (PN proneural, MES mesenchymal, CL classical). ASIC3 (n ≥ 3) (b) and ASIC1a (n = 3) (b’) protein expression quantification normalized on actin levels in the different GBM-CSC lines. (c) Western Blot analysis of CD133/prominin1 and ASIC3 expression in a healthy human brain lysate, in the GBM-MES CSCs 0315 line and in a biopsy from the same patient used to generate the CSC line 0315. (c’) ASIC3 and CD133/prominin1 protein expression quantification normalized for actin levels, in healthy human brain, GBM MES 0315 line and MES 0315 patient’s biopsy (n = 4). Statistic was performed by a two-way Anova with Bonferroni’s post-test:(****) p < 0,0001. (d) Data from publically available Repository for Molecular BRAIN Neoplasia Data (TCGA datasets Affymetrix HT HG U133A) were used to determine the relative expression of ASIC3 mRNA (ACCN3) in four human glioma subtypes (Classical, Mesenchymal, Neuronal, and Proneuronal), as well as in healthy patients. All 454 glioma patients with available mRNA were examined. (e) Western Blot analysis of ASIC3 expression in GBM-CSCs MES 0315 growth in 3D and 2D culture conditions after 24hin culture. Original blots were horizontally cropped at the appropriate MW region in order to perform multiple staining on the same blot. In the figure, portions of the same blot are separated by white lines. Black lines are used to separate independent blots presented in the same figure. Original blots are shown in supplementary figure S1 and S4. Dots represent independent experiments. Measurements were taken from distinct samples. Graphs for (b,b’,c’) were analyzed with GraphPad Prism 9, graph for (d) was obtained from https://www.betastasis.com/glioma/tcga_gbm/.
In silico modelling suggested that GMQ is a high-affinity ligand of ASIC3
We generated a virtual 3D model of the human ASIC3 monomer using AlphaFold48 (Fig. 2a), and by using the published structure of chicken ASIC1 (PDB id: 6VKT49) as a template, we reconstructed the trimeric assembly of the channel. After omitting the intermembrane portion of the trimer (maintaining amino acids from E70 to E432), we ran a molecular dynamics (MD) simulation on the trimeric model of the extracellular domain of human ASIC3 for 100 ns. The analysis of the simulation allowed the identification of two structural clusters centred at 68.2 and 81.2 ns, which were used as structural models for the in silico docking analysis (Fig. 2b). Therefore, the structural models 682 and 812 were used for an initial in silico docking analysis of charged/uncharged GMQ [ 53]. Most of the docking poses were found in a small region inside the ion channel, close to amino acids E78 and E421, known to be involved in GMQ binding39. The identified region (centered between Q270 of the three different subunits; volume 30 × 30 × 15 Å3) was explored in depth by extensive in silico docking with AutoDock4.254. We then tested several reported ASIC3 agonists, looking for any of them showing a stronger molecular interaction when compared to GMQ. Namely, we analysed Sephin 1 and guanabenz in their charged and not-charged states (Fig. 2c, d), and Agmatine, Arcaine, Chlorophenylguanidine, and Metformin, which were shown to be effective at downregulating ASIC3 in bone cancer pain (BCP) rats59, in their charged form (the only form present at physiological pH). We also tested temozolomide (charged), which represents the reference drug used in chemotherapy for GBM treatment3,11,60, as a negative control of binding model specificity. All the results are reported in Table 1. Our in silico analysis showed that, among all the tested compounds, GMQ (in both its charged and uncharged forms) had the highest predicted affinity for ASIC3 and was able to bind to both structural models 682 and 812 with dissociation constants in the high nanomolar range (KD = 810 nM, average affinity over two structural models and charged/uncharged states). Both charged and uncharged GMQ adopted a similar conformation when interacting with either model 812 or 682, binding with high affinity to a small pocket inside the ion channel. In this binding site, GMQ mainly interacted with the side chains of charged or polar amino acids, including Glu78, Gln268, Gln270, Arg374, Ala376, Glu416, and Glu421, and with the main chain carbonyl of Gln268 and Asn419 (Fig. 2c, d). All other ASIC3 agonists showed comparable affinity for ASIC3 (from 4.7 µM of Arcaine to 9.2 µM of 4-Chlorophenylguanidine), compared to a ≈ 0.8 µM GMQ affinity. Temozolomide, as expected, appeared to be the worst ligand in the series (57.2 µM). Our in silico analysis suggested that, among the tested compounds, GMQ is the ASIC3 agonist with the highest predicted affinity (Table 1).
Generation of a virtual 3D model of the human ASIC3 channel. (a) AlphaFold model of human ASIC3 represented as cartoons colored by model quality (from low quality pLDDT = 50 in blue, to high quality pLDDT = 100 in red); inset: close up view of the extracellular domain of ASIC3, highlighting with spheres amino acids Glu78 and Glu421, known to be involved in GMQ binding39. (b) Superposition of the two structural models (depicted as cartoon) selected after MD simulation using cluster analysis: 682 (68.2 ns) as blue and 812 (81.2 ns) as cyan and extent of the region explored during in silico docking analysis. (c,d) Close-up views of the interaction between charged (yellow carbon atoms) and non-charged (orange carbons) GMQ with protein residues [sticks with carbon atoms colored in cyan (812) or blue (682)].
ASIC3 is expressed in almost all cells of GBM-MES 0315 and can be activated by GMQ.
Fluorescence staining demonstrated the expression of ASIC3 and that of the stem cell marker CD133/prominin1 in almost all the cells analysed (Fig. 3a, b). In addition, we stained the same line for Ki67, a marker used in clinic to evaluate the proliferative potential of tumor cells whose expression is also related to cancer stem cell maintenance (Fig. 3c)61. A quantitative study revealed that the % of positive cells were 96 ± 2% for ASIC3, 97 ± 2% for CD133/Prominin1 and 82 ± 3% for Ki67 (Fig. 3d and Supplementary figure S7). ASIC3 staining was observed in the GBM-MES 0315 cell line both in intracellular compartments, probably representing a fraction of the protein in trafficking, and at the cell surface (Fig. 3e). This intracellular localization of ASIC3 in tumor cells is in agreement with the findings of previous reports62. By means of single-cell electrophysiology, we further investigated ASIC3 expression and tested the ability of GMQ to activate ASIC3 in GBM-MES 0315 CSCs. Upon the application of 1 mM GMQ to GBM-MES 0315 CSCs, we observed a persistent current. (Fig. 3f) that was elicited in all cells (n = 11) with an average membrane current density of 0.59 pA/pF (Fig. 3g). These observations are in line with previous studies on different cellular models38,63, and despite their low amplitude, they are consistent with effective chronic perturbation of the membrane potential. To confirm the specific effect of GMQ on the ASIC3 channel in our cell model, we took advantage from the specific peptide blocker of ASIC3 APETx264. We performed GMQ stimulation of GBM-MES 0315 CSCs in the presence or absence of 3 µM APETx2. As shown in Fig. 3f, g, APETx2 completely ablated GMQ-induced activation. These data strongly support the expression and functionality of ASIC3 in GBM-MES 0315 CSCs.
ASIC3 is expressed in almost all CSCs and can be activated by GMQ. (a) Immunofluorescence image of GBM CSCs MES 0315 in culture stained for ASIC3 (red) and for hoechst (blue). Arrowhead points to an ASIC3 negative cell. (b) Immunofluorescence image of GBM CSCs MES 0315 in culture stained for CD133/Prominin1 (green) and for hoechst (blue). (c) Immunofluorescence image of GBM CSCs MES 0315 in culture stained for Ki67 (green) and for hoechst (blue). (d) Histogram showing the percentage of positive cells for ASIC3, CD133 and Ki67 n ≥ 3 independent experiments. The graph was made with GraphPad Prism v9 (e) High magnification image of ASIC3 (left), hoechst (middle) and double staining (right), arrowheads point to a membrane enriched ASIC3 staining. (f) Representative traces of a GMQ-induced current in GBM-MES 0315 cells in the presence or absence of APETx2 (black and red traces respectively). (g) Box plot of membrane current density upon stimulation with GMQ alone (red) or in the presence of APETx2 (black). Plot displays the mean (+), median (internal horizontal line), first and third quartiles (upper and lower box edges) and standard deviation (whiskers) of data distribution (n = 11). Dots represent individual cells. Significant difference was assessed by Mann–Whitney non-parametric test (****p < 0.0001). Scale Bar = 20 µm for (a–c) and 10 µm for (e). All data were analysed by using the Prism 8 (GraphPad) software and traces were generated by Origin 2018 (Origin Lab.).
GMQ treatment affects GBM CSC growth in vitro
We next assayed the effect of GMQ on GBM CSCs by investigating cell growth in vitro via automated single-cell analysis in 2D cultures. We first treated GBM-MES 0315 CSCs with different GMQ concentrations (30 µM, 100 µM and 300 µM) for different durations (24 h, 48 h and 96 h). Treatment with 96 h GMQ had a greater effect on reducing the cell number even at low doses (Supplementary Fig. S8). Accordingly, we treated two MES cell lines (GBM-MES 0315 and GBM-MES 1312), one PN cell line (PN 0625) and one CL cell line (CLA 0627) with increasing concentrations of GMQ. GMQ almost completely blocked GBM CSC growth in both lines, with an IC50 of 2.9 µM for MES 0315, 7 µM for the MES 1312 line, and 8 µM for PN 0625 and 11 µM for CLA 0627 (Fig. 4a). To detect any GMQ toxicity, we reasoned that neurons are the most sensitive non-tumor cells in the brain. We treated mouse cortical neurons with GMQ in a concentration-dependent manner without observing any significant reduction in the number of cells up to 30 µM (Fig. 4a). We subsequently compared the effect of GMQ treatment on GBM MES 0315 growth with that of temozolomide (TMZ), the reference drug for the treatment of GBM tumours, which has also been demonstrated to affect mainly the cancer stem cell population65,66. In contrast to GMQ, TMZ was able to reduce the cell number by approximately 70% at 30 µM and had an estimated EC50 of 7.5 µM, which is in line with the literature (Fig. 4b)60. These data demonstrated that GMQ is more potent than TMZ in controlling GBM MES 0315 growth in vitro. Representative images of GBM CSCs upon treatment with GMQ and TMZ at different concentrations are shown in Fig. 4c. The effect of GMQ on GBM CSC growth was also tested on MES-0315 cells using a 3D neurosphere assay. As shown in Fig. 5a–d, GMQ controlled both the number of neurospheres and their dimensions in a dose-dependent manner. Since neurospheres are known to originate from CSC proliferation67,68, these data confirm that the proposed strategy can specifically affect CSC growth. We additionally tested the sphere-forming potential of GBM CSCs after GMQ treatment by taking advantage of the extremely low dilution assay (ELDA)69. This method exploits the ability of single or a few seeded cancer stem cells to self-renew and form neurospheres. Our analysis was carried out using the ELDA online tool (https://bioinf.wehi.edu.au/software/elda/). Our data demonstrated that GMQ treatment impaired the self-renewal and formation of neurospheres in GBM CSCs (Fig. 5e–g), confirming its ability to control GBM CSC growth.
Effect of GMQ and temozolomide on GBM CSCs. (a) Concentration‒response effect of GMQ treatment for 96 h on the GBM CSC lines MES 1312 (n = 3), MES 0315 (n = 3), PN 0625 (n = 3), CLA 0627 (n = 3) and mouse cortical neurons (n = 3). The values represent the % of cells normalized to the concentration of the smallest drug. Neither were the neurons affected by GMQ. (b) The GBM CSC line MES 0315 was treated with GMQ (n = 3) or temozolomide (TMZ) (n = 3) for 96 h. The values represent the normalized % of cells at the lowest drug concentration. The estimated EC50s were 3.0 μM and 7.5 μM for GMQ and TMZ, respectively. (c) Representative images of MES 0315 cells grown for 96 h with increasing concentrations of GMQ or TMZ. The cells were stained with Hoechst. Scale bar = 50 µm. Measurements were taken from distinct samples. All the data were analysed with GraphPad Prism v9.
Effect of GMQ on CSC-generated neurospheres. (a) Representative images of MES 0315 cells grown in a neurosphere assay after 96 h of treatment with increasing concentrations of GMQ or under control condition. Hoechst nuclear staining Bar = 200 µM; 50 µM in the inset. Number of neurospheres per area (b), average neurosphere area (c) and total neurosphere area per field (d) in MES 0315 cells after 96 h of GMQ treatment (n = 3 independent experiments). (e) Plot of the results of the ELDA experiment (n = 3 independent experiments, 10 wells per condition). (f) Statistical analysis of the experiment shown in (e), p values are shown. (g) 1/stem cell frequency confidence intervals. The data in (b–d) were analysed with GraphPad Prism v9, and the data in (e) were analysed with the ELDA online tool (https://bioinf.wehi.edu.au/software/elda/).
ASIC3 downregulation and pharmacological blockade reduce the effect of GMQ on GBM-MES cell proliferation
To confirm the ASIC3-dependent effect of GMQ on GBM CSC growth in vitro, we generated syngeneic controls with downregulated ASIC3 expression. For this purpose, we took advantage of the evidence that ASIC3 expression can be efficiently regulated by shRNAs26,70,71. GBM CSCs, and in general, any CSCs, are known to be refractory to transfection72. This finding prompted us to use a viral infection-based system for shRNA transduction. We generated two lentiviruses (LVs) encoding two independent shRNAs that map to two different positions in the 3′–5′ UTR of the ASIC3 messenger. By using two independent shRNAs and infections with low multiplicity of infection (MOI 6 and 2 for shRNA1 and shRNA2, respectively), we obtained specific syngeneic controls to study the effect of GMQ, which strongly reduced the potential off-target effects on the expression of other genes. The expression of ASIC3 in the GBM CSCs MES 0315 after lentiviral infection was evaluated by Western Blot, demonstrating a reduction of ASIC3 expression of 75 ± 13% and 65 ± 16% for shRNA1 and shRNA2, respectively, compared to that in control cells (Fig. 6a, a’ and Supplementary Figures S9a and S9b). ASIC3 downregulation resulted in a significant reduction in the efficacy of GMQ in controlling the growth of the GBM CSC line MES 0315, indicating that GMQ specifically acts through ASIC3 activation (Fig. 6b). To confirm the dependency of the GMQ effect on ASIC3, we treated the GBM-MES CSC 0315 line with the specific ASIC3-blocking toxin ApeTx264 and administered GMQ. The effect of GMQ was almost completely abolished by treatment with ApeTx2 (Fig. 6c), confirming the dependency of GMQ on GBM CSC growth on ASIC3.
ASIC3 downregulation and its pharmacological blocking reduces the effect of GMQ. (a) Blot analysis of ASIC3 expression in GBM CSCs MES 0315 cells after infection with lentiviruses carrying specific anti-ASIC3 shRNAs (shRNA1 and shRNA2). Original blots were horizontally cropped at the appropriate MW region in order to perform multiple staining on the same blot. Portions of the same blot are separated by white lines. (a’) ASIC3/Actin protein expression ratio of MES 0315 before and after infection with Lentiviruses carrying shRNA1 or shRNA2 (n = 7) and normalized on control samples. CTRL vs shRNA1: P = 0,0007; CTRL vs shRNA2 P = : 0,0007. Original blots are shown in supplementary figure S9a and S9b. (b) Effect of ASIC3 downregulation on GMQ-induced GBM CSCs growth. Plot represents the number of MES 0315 cells after 96 h of GMQ treatments (3 and 10 μM), normalized to untreated samples: control (n = 4); shRNA1 (n = 3); shRNA2 (n = 3). (****) P < 0,0001. (c) Plot represents the % of GBM CSCs MES 0315 after 96 h of 3 μM GMQ treatment, in the presence or absence of 1 μM APETx2 toxin, normalized to the untreated sample, (n = 3); Unt vs GMQ p = 0.0214; Untr vs APETX2 + GMQ p = 0.9058; APETX2 vs GMQ p = 0.0189; GMQ vs APETX2 + GMQ p = 0.0029. Unpaired T-test for (a’), two-way Anova with Bonferroni’s post-test for (b) and One-way Anova with Bonferroni’s post-test for (c). Dots represent independent experiments. All graphs were analysed with GraphPad Prism v9.
GMQ treatment induces cell metabolism, cell cycle alterations and cell death in GBM-CSCs
After 96 h of treatment with GMQ, a strong reduction in the number of GBM CSCs in culture was observed (Fig. 4a, b). These findings indicate that GMQ may affect survival, proliferation or both aspects of cell physiology. To understand the mechanisms of GMQ-induced GBM CSC loss, we measured cell death, cell cycle alterations and cell metabolism effects caused by GMQ through single-cell analysis with SYTOX-red, propidium iodide (PI) flow cytometric analysis and a CKK8 assay, respectively. The results suggested that GMQ induces cell death in a dose-dependent manner, while cell death was not detected in cultured mouse cortical neurons used as control cells (Fig. 7a). These data, as well as the evidence that GMQ does not induce any neuronal loss after 96 h of GMQ treatment (Fig. 4a), support the good safety of ASIC3-targeted pharmacological intervention in non-tumor brain cells. A dose-dependent increase in cell metabolism was observed after 96 h of treatment with GMQ at both 3 µM and 10 µM (Fig. 7b). We suggest that an additional energy request is due to chronic alterations in the membrane potential and to the consequent alterations in pump and transporter activity to restore the membrane potential. Such increased energy consumption may lead to cell suffering and consequent cell death. Our flow cytometry analysis of the cell cycle indicated that GMQ (10 µM and 30 µM) induces a small but significant alteration in the cell cycle, increasing the number of cells in the G0-G1 phase and reducing the number of cells in the S phase of the cell cycle (Fig. 7c, c’ and Supplementary Figure S10). Taken together, these results indicate that GMQ strongly affects many physiological aspects of GBM CSCs by inducing chronic membrane potential alterations, increasing cell metabolism, altering the cell cycle and inducing cell death.
GMQ induces Cell cycle and metabolism alterations as well as cell death in CSCs. (a) Single cell analysis of the % of dead cells induced by GMQ treatment, measured by Sytox staining, in MES 0315 lines and mouse cortical Neurons (n = 3). Data were normalized to untreated samples. (b) Metabolic activity of GBM-MES CSCs after GMQ treatment (3 and 10 μM, 96 h) measured by CCK8 assay. CCK8 absorbance was measured with a plate reader and normalized for the total number of cells present in the samples by Hoechst staining, to determine the metabolic activity per cell. Data were normalized over control samples. Unt. Vs GMQ 3 μM p = 0.035; Unt. Vs GMQ 10 μM p < 0.0001. (c) Cell cycle analysis after 24 h of GMQ treatment at 10 and 30 μM by propidium iodide incorporation at flow cytometer. Bars represent the average % of cells in different cell cycle phases after treatment (n = 3). Phases G0–G1: control vs 10 μM p = 0.001; Control vs 30 μM p = 0.0004. Phase S: control vs 10 μM p = 0.019; control vs 30 μM p = 0.0039. (c’) An example of PI intensity distribution of samples as in c. Statistical analysis: One-way Anova with Bonferroni’s post-test for (b,c). (ns) not significant; (*) P < 0.05; (**) P < 0.01; (***) P < 0.001; (****) P < 0.0001. Dots represent independent experiments. Measurements were taken from distinct samples. Graphs for (a–c) were analysed with GraphPad Prism v9, graph for (c’) was analysed with FCS Express 7 Plus using multicycle DNA tool.
Discussion
The potential of ion channels as therapeutic targets in cancer therapy is neither new nor unexplored73,74,75. The relevance of targeting ASIC channels in tumours is supported by their expression in several tumor cells, where they may represent a link between the tumor acidic environment and the specific metabolic and adaptive properties of tumor cells to the tumor niche. ASIC channel expression has been observed in several tumours and has been demonstrated to play a role in tumor progression and metastasis16,32,76. The expression of ASIC1 is positively correlated with GBM patient survival, suggesting that ASIC1 expression, and its potential chronic activation in the tumor niche, may impair tumor growth20. Accordingly, several papers have demonstrated that ASIC1 inhibition or downregulation can decrease both tumor cell proliferation and migration29,77. In contrast with this hypothesis, ASICs are known to undergo channel inactivation after a few seconds of proton stimulation, with a small non-inactivating current described only for the ASIC2 and ASIC3 subunits78. In this scenario, we focused on the ASIC3 isoform, which has received much less attention in the tumor context, particularly in brain tumours. Since no data are publicly available regarding the role of ASIC3 in GBM and considering that the ASIC3 isoform is highly sensitive to pH and has no an inactivating current, its potential role in GBM is worth to be elucidated in future works. We first checked whether the expression of ASIC3 was altered in GBM patients. Analysis of the expression level of the ACCN3 gene (ASIC3) in the publicly available Repository for Molecular Brain Neoplasia Data (TCGA datasets Affymetrix HT HG U133A) demonstrated that the level of ASIC3 mRNA expression is extremely low; nevertheless, ASIC3 has been positively correlated with patient survival21. Interestingly, GBM CSC lines have been reported to express functional ASIC3, suggesting its potential specific expression in the GBM CSCs population21. A comparison of ASIC3 protein expression in a MES GBM cell line with the biopsy of its original tumor, as well as with a healthy human control revealed a much higher level of ASIC3 expression in the CSCs sample that correlated with high expression of the stem marker CD133/prominin1, confirming previous observations. Patch clamp experiments further confirmed the expression and activity of ASIC3 channels on the plasma membrane of GBM CSCs. These observations suggest a potentially relevant role of ASIC3 in GBM through the exposure of the CSC population to an acidic environment through ASIC3 activation. However, further experiments are needed to clarify the relationship between ASIC3 and the tumor niche in GBM CSCs. We demonstrated that chronic activation of ASIC3 by GMQ treatment affects the growth of GBM-MES CSCs without significantly affecting control neurons. The effect of GMQ on the growth of neurospheres generated by GBM CSCs according to the Extremely Low Dilution Assay (ELDA) confirmed the specific detrimental effect of GMQ on the CSC population. Surprisingly, the efficacy of GMQ in controlling GBM CSC MES 0315 growth “in vitro” was greater than that of TMZ, the standard therapy for this tumor. Since the relevance of the methylation status of the MGMT promoter as prognostic for the TMZ therapeutic efficacy79, it would be of interest studying in the future the efficacy of the ASIC3 targeting strategy with respect to TMZ treatment and the MGMT methylation status. This evidence confirms that chronic ASIC3 activation is a promising new translational pathway for GBM treatment. Considering the frequency and significance of the side effects of standard chemotherapies, a new treatment based on different pharmacodynamics could be particularly useful as a novel therapy even when combined with already in clinic drugs. Downregulation of ASIC3 expression in GBM-MES CSCs by using two independent shRNAs significantly reduced the effect of GMQ, demonstrating that ASIC3 is involved in this effect. Since these two shRNAs map to a different position on the mRNA, this accounts for the specificity of the effect on ASIC3 downregulation. We have also shown that pre-treatment with a specific ASIC3 inhibitory toxin (ApeTx2)64 almost completely prevents alterations in the GMQ membrane potential and its effect on GBM CSC growth. These data confirmed that GMQ acts specifically through ASIC3 to control GBM CSC growth. This effect of GMQ could be explained by specific activation of ASIC3, which results in chronic membrane potential alteration followed by destabilization of membrane potential-related physiological processes. A cell may react to membrane potential alterations through transporters and ion channels activity modulation, possibly leading to detrimental energy consumption. In line with this hypothesis, we confirmed that GMQ treatment induced an increase in cell metabolism. Future studies will aim to define the interplay between membrane dysfunctions and increased metabolic demand. We also observed a significant alteration in the cell cycle upon GMQ treatment. This effect can be related to chronic alterations in the plasma membrane potential via a well-known relationship between these two physiological processes. In fact, it has been described that membrane potential fluctuations, due to activation of sodium and potassium channels, accompany cell cycle phases76. We have also confirmed that GMQ could induce a general cell suffering that leads to cell death. All these physiological alterations account for the complete elimination of GBM CSCs in culture after 96 h of treatment with GMQ. We herein propose a novel approach in which ASIC3 is considered a key target that induces direct damage to the GBM CSC population. This approach is innovative and, to our knowledge, opens a totally unexplored new scenario in the context of GBM treatment. This therapeutic strategy, like any pharmacological treatment already used in the clinic, can potentially induce side effects. We have reasoned that a systemic use of ASIC3 agonists may induce side effects such as pain induction up to cell suffering of peripheral neurons, since the specific expression of ASIC3 in the sensory system80,81. This could be overcome by different strategies, including the administration of a pain relief drug in combination or the combination of a blood‒brain barrier-permeable ASIC3 antagonist for peripheral competition. However, further experiments are needed to quantify the side effects of these agents and clarify potential strategies for their compensatory effects. We generated a virtual 3D model of the human ASIC3 monomer data that will be useful to provide detailed molecular insights, thereby enhancing our understanding of the binding mechanisms and functional modulation of human ASIC3 channel. Finally, a medicinal chemistry program based on the rational design (modelling) and synthesis of novel analogues of the ASIC3 activator should be carried out to select hits with higher target potency and better CNS bioavailability. These data strongly support the possibility of pharmacologically targeting ASIC3 for GBM treatment as an innovative strategy for GBM therapy.
Data availability
The authors declare that the data supporting the findings of this study are available within the article and its supplementary information. Any other associated data supporting the findings of this study are available from the corresponding author upon request. The datasets analysed during the current study are available at https://www.betastasis.com/glioma/tcga_gbm/.
References
Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Reifenberger, G., Wirsching, H.-G., Knobbe-Thomsen, C. B. & Weller, M. Advances in the molecular genetics of gliomas—Implications for classification and therapy. Nat. Rev. Clin. Oncol. 14, 434–452 (2017).
Stupp, R. et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 352, 987–996 (2005).
Hu, Y. et al. Neural network learning defines glioblastoma features to be of neural crest perivascular or radial glia lineages. Sci. Adv. 8, 6340 (2022).
Rodríguez-Camacho, A. et al. Glioblastoma treatment: State-of-the-art and future perspectives. Int. J. Mol. Sci. 23(13), 7207 (2022).
Corbet, C. & Feron, O. Tumour acidosis: From the passenger to the driver’s seat. Nat. Rev. Cancer 17(10), 577–593 (2017).
Ibrahim-Hashim, A. & Estrella, V. Acidosis and cancer: From mechanism to neutralization. Cancer Metastasis Rev. 38, 149–155 (2019).
Estrella, V. et al. Acidity generated by the tumor microenvironment drives local invasion. Cancer Res. 73, 1524–1535 (2013).
Damaghi, M., Wojtkowiak, J. W. & Gillies, R. J. pH sensing and regulation in cancer. Front. Physiol. 4, 370 (2013).
Hjelmeland, A. B. et al. Acidic stress promotes a glioma stem cell phenotype. Cell Death Differ. 18(5), 829–840 (2011).
Lathia, J. D., Mack, S. C., Mulkearns-Hubert, E. E., Valentim, C. L. L. & Rich, J. N. Cancer stem cells in glioblastoma. Genes Dev. 29, 1203–1217 (2015).
Lytle, N. K., Barber, A. G. & Reya, T. Stem cell fate in cancer growth, progression and therapy resistance. Nat. Rev. Cancer 18(11), 669–680 (2018).
Galli, R. et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 64, 7011–7021 (2004).
Reya, T., Morrison, S. J., Clarke, M. F. & Weissman, I. L. Stem cells, cancer, and cancer stem cells. Nature 414(6859), 105–111 (2001).
Piccirillo, S. G. M. et al. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature 444(7120), 761–765 (2006).
Prevarskaya, N., Skryma, R. & Shuba, Y. Ion channels and the hallmarks of cancer. Trends Mol. Med. 16, 107–121 (2010).
Liu, C., Zhu, L. L., Xu, S. G., Ji, H. L. & Li, X. M. ENaC/DEG in tumor development and progression. J. Cancer 7, 1888 (2016).
Gründer, S., Vanek, J. & Pissas, K. P. Acid-sensing ion channels and downstream signalling in cancer cells: Is there a mechanistic link?. Pflügers Arch.-Eur. J. Physiol. 4, 1–14 (2024).
Chen, B., Liu, J., Ho, T. T., Ding, X. & Mo, Y. Y. ERK-mediated NF-κB activation through ASIC1 in response to acidosis. Oncogene 512(5), e279–e279 (2016).
King, P. et al. Regulation of gliomagenesis and stemness through acid sensor ASIC1a. Int. J. Oncol. 59, 1–15 (2021).
Tian, Y. et al. Glioblastoma cancer stem cell lines express functional acid sensing ion channels ASIC1a and ASIC3. Sci. Rep. 7, 1–14 (2017).
Wemmie, J. A. et al. Acid-sensing ion channels in pain and disease. Nat. Rev. Neurosci. 14(7), 461–471 (2013).
Boscardin, E., Alijevic, O., Hummler, E., Frateschi, S. & Kellenberger, S. The function and regulation of acid-sensing ion channels (ASICs) and the epithelial Na+ channel (ENaC): IUPHAR review 19. Br. J. Pharmacol. 173, 2671–2701 (2016).
Wemmie, J. A. et al. Acid-sensing ion channel 1 is localized in brain regions with high synaptic density and contributes to fear conditioning. J. Neurosci. 23, 5496–5502 (2003).
Baron, A., Voilley, N., Lazdunski, M. & Lingueglia, E. Acid sensing ion channels in dorsal spinal cord neurons. J. Neurosci. 28, 1498–1508 (2008).
Deval, E. et al. ASIC3, a sensor of acidic and primary inflammatory pain. EMBO J. 27, 3047–3055 (2008).
Lin, Y. W. et al. Identification and characterization of a subset of mouse sensory neurons that express acid-sensing ion channel 3. Neuroscience 151, 544–557 (2008).
Zhang, Z. et al. Acid-sensing ion channel 1a modulation of apoptosis in acidosis-related diseases: Implications for therapeutic intervention. Cell Death Discov. 9(1), 330 (2023).
Wang, Y., Zhou, H., Sun, Y. & Huang, Y. Acid-sensing ion channel 1: Potential therapeutic target for tumor. Biomed. Pharmacother. 155, 113835 (2022).
Gupta, S. C. et al. Regulation of breast tumorigenesis through acid sensors. Oncogene 35(31), 4102–4111 (2016).
Zhu, S. et al. ASIC1 and ASIC3 contribute to acidity-induced EMT of pancreatic cancer through activating Ca2+/RhoA pathway. Cell Death Dis. 8(5), e2806–e2806 (2017).
Berdiev, B. K. et al. Acid-sensing ion channels in malignant gliomas. J. Biol. Chem. 278, 15023–15034 (2003).
Litan, A. & Langhans, S. A. Cancer as a channelopathy: Ion channels and pumps in tumor development and progression. Front. Cell. Neurosci. 9, 132394 (2015).
Yang, M. & Brackenbury, W. J. Membrane potential and cancer progression. Front. Physiol. 4, 185 (2013).
Niklasson, M. et al. Membrane-depolarizing channel blockers induce selective glioma cell death by impairing nutrient transport and unfolded protein/amino acid responses. Cancer Res. 77, 1741–1752 (2017).
Li, W. G., Yu, Y., Zhang, Z. D., Cao, H. & Xu, T. L. ASIC3 channels integrate agmatine and multiple inflammatory signals through the nonproton ligand sensing domain. Mol. Pain 6, 1744–8069 (2010).
Agharkar, A. S. & Gonzales, E. B. 4-Chlorophenylguanidine is an ASIC3 agonist and positive allosteric modulator. J. Pharmacol. Sci. 133, 184–186 (2017).
Callejo, G. et al. In silico screening of GMQ-like compounds reveals guanabenz and sephin1 as new allosteric modulators of acid-sensing ion channel 3. Biochem. Pharmacol. 174, 113834 (2020).
Yu, Y. et al. A nonproton ligand sensor in the acid-sensing ion channel. Neuron 68, 61–72 (2010).
Besson, T., Lingueglia, E. & Salinas, M. Pharmacological modulation of acid-sensing ion channels 1a and 3 by amiloride and 2-guanidine-4-methylquinazoline (GMQ). Neuropharmacology 125, 429–440 (2017).
Delaunay, A. et al. Human ASIC3 channel dynamically adapts its activity to sense the extracellular pH in both acidic and alkaline directions. Proc. Natl. Acad. Sci. USA 109, 13124–13129 (2012).
Foster, V. S. et al. Acid-sensing ion channels: Expression and function in resident and infiltrating immune cells in the central nervous system. Front. Cell. Neurosci. 15, 738043 (2021).
Minghua, L. et al. Acid-sensing ion channels in acidosis-induced injury of human brain neurons. J. Cereb. Blood Flow Metab. 30, 1247–1260 (2010).
Bortner, C. D., Gómez-Angelats, M. & Cidlowski, J. A. Plasma membrane depolarization without repolarization is an early molecular event in anti-Fas-induced apoptosis. J. Biol. Chem. 276, 4304–4314 (2001).
Narayanan, A. et al. The proneural gene ASCL1 governs the transcriptional subgroup affiliation in glioblastoma stem cells by directly repressing the mesenchymal gene NDRG1. Cell Death Differ. 26(9), 1813–1831 (2019).
Di Tomaso, T. et al. Immunobiological characterization of cancer stem cells isolated from glioblastoma patients. Clin. Cancer Res. 16, 800–813 (2010).
De Ceglia, R. et al. Down-sizing of neuronal network activity and density of presynaptic terminals by pathological acidosis are efficiently prevented by diminazene aceturate. Brain Behav. Immun. 45, 263–276 (2015).
Pahari S., Sun L., & Alexov E. PKAD: A database of experimentally measured pKa values of ionizable groups in proteins. Database baz024 (2019)
Yoder, N. & Gouaux, E. The his-gly motif of acid-sensing ion channels resides in a reentrant ‘loop’ implicated in gating and ion selectivity. Elife 9, 1–18 (2020).
Abraham, M. J. et al. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1–2, 19–25 (2015).
Schmid, N. et al. Definition and testing of the GROMOS force-field versions 54A7 and 54B7. Eur. Biophys. J. 40, 843–856 (2011).
Sanner, M. F. Python: A programming language for software integration and development. J. Mol. Graph Model. 17, 57–61 (1999).
Trott, O. & Olson, A. J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 31(2), 455–461 (2010).
Morris, G. M. et al. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 30, 2785–2791 (2009).
Casella, G. et al. IL4 induces IL6-producing M2 macrophages associated to inhibition of neuroinflammation in vitro and in vivo. J. Neuroinflamm. 13, 1–10 (2016).
Rahman, M. et al. Neurosphere and adherent culture conditions are equivalent for malignant glioma stem cell lines. Anat. Cell Biol. 48, 25–35 (2015).
Cortés Franco K.D. et al. Aggressive migration in acidic pH of a glioblastoma cancer stem cell line in vitro is independent of ASIC and KCa3.1 ion channels, but involves phosphoinositide 3-kinase. Pflugers Arch. Eur. J. Physiol. 475, 405–416 (2023)
Zhou, Z. H. et al. The acid-sensing ion channel, ASIC2, promotes invasion and metastasis of colorectal cancer under acidosis by activating the calcineurin/NFAT1 axis. J. Exp. Clin. Cancer Res. 36, 1–12 (2017).
Qian, H. Y. et al. Metformin attenuates bone cancer pain by reducing TRPV1 and ASIC3 expression. Front. Pharmacol. 12, 713944 (2021).
Poon, M. T. C., Bruce, M., Simpson, J. E., Hannan, C. J. & Brennan, P. M. Temozolomide sensitivity of malignant glioma cell lines—A systematic review assessing consistencies between in vitro studies. BMC Cancer 21, 1–9 (2021).
Cidado, J. et al. Ki-67 is required for maintenance of cancer stem cells but not cell proliferation. Oncotarget 7, 6281 (2016).
Wu, Y. et al. Acid-sensing ion channels contribute to the effect of extracellular acidosis on proliferation and migration of A549 cells. Tumor Biol. 39, 12 (2017).
Alijevic, O. & Kellenberger, S. Subtype-specific modulation of acid-sensing ion channel (ASIC) function by 2-guanidine-4-methylquinazoline. J. Biol. Chem. 287, 36059–36070 (2012).
Diochot, S. et al. A new sea anemone peptide, APETx2, inhibits ASIC3, a major acid-sensitive channel in sensory neurons. EMBO J. 23, 1516–1525 (2004).
Beier, D. et al. Temozolomide preferentially depletes cancer stem cells in glioblastoma. Cancer Res. 68, 5706–5715 (2008).
Wu, Y., Franzmeier, S., Liesche-Starnecker, F. & Schlegel, J. Enhanced sensitivity to ALDH1A3-dependent ferroptosis in TMZ-resistant glioblastoma cells. Cells 12(21), 2522 (2023).
Yuan, X. et al. Isolation of cancer stem cells from adult glioblastoma multiforme. Oncogene 23, 9392–9400 (2004).
Laks, D. R. et al. Neurosphere formation is an independent predictor of clinical outcome in malignant glioma. Stem cells 27, 980–987 (2009).
Hu, Y. & Gordon, K. S. ELDA: Extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J. Immunol. Methods 347, 70–78 (2009).
Deval, E. et al. Acid-sensing ion channels in postoperative pain. J. Neurosci. 31, 6059–6066 (2011).
Wang, D. et al. Effects of hypoxia and ASIC3 on nucleus pulposus cells: From cell behavior to molecular mechanism. Biomed. Pharmacother. 117, 109061 (2019).
Walker, K. & Hjelmeland, A. Method for efficient transduction of cancer stem cells. J. Cancer Stem Cell Res. 2, 1 (2014).
Rooj, A. K. et al. Glioma-specific cation conductance regulates migration and cell cycle progression. J. Biol. Chem. 287, 4053–4065 (2012).
Kapoor, N. et al. Knockdown of ASIC1 and epithelial sodium channel subunits inhibits glioblastoma whole cell current and cell migration. J. Biol. Chem. 284, 24526–24541 (2009).
Kellenberger, S. & Schild, L. International Union of Basic and Clinical Pharmacology. XCI. Structure, function, and pharmacology of acid-sensing ion channels and the epithelial Na+ channel. Pharmacol. Rev. 67, 1–35 (2015).
Cheng, Q. et al. Novel insights into ion channels in cancer stem cells (review). Int. J. Oncol. 53, 1435–1441 (2018).
Takayasu, T., Kurisu, K., Esquenazi, Y. & Ballester, L. Y. Ion channels and their role in the pathophysiology of gliomas. Mol. Cancer Ther. 19, 1959–1969 (2020).
Pollak, J. et al. Ion channel expression patterns in glioblastoma stem cells with functional and therapeutic implications for malignancy. PLoS One 12, e0172884 (2017).
Pöhlmann, J. et al. High costs, low quality of life, reduced survival, and room for improving treatment: An analysis of burden and unmet needs in glioma. Front. Oncol. 14, 1368606 (2024).
Li, W. G. & Xu, T. L. ASIC3 channels in multimodal sensory perception. ACS Chem. Neurosci. 2, 26–37 (2011).
Dulai, J. S., Smith, E. S. J. & Rahman, T. Acid-sensing ion channel 3: An analgesic target. Channels 15, 94–127 (2021).
Acknowledgements
We thank Dr. Dey Sumit Kumar for his technical assistance in LVs production.
Author information
Authors and Affiliations
Contributions
A.B., C.D. and N.C. contributed to experimental design, performed pharmacological experiments and analysed data. C.D. performed Immunofluorescence and Cell Cycle studies. S.B. and C.DB. performed Electrophysiological experiments; A.T. designed and generated shRNAs encoding LVs; B.M. performed immunoblotting experiments; E.M. and M.M. performed in silico studies; P.S. revised the manuscript and performed in silico studies. V.B. revised the manuscript, L.M. performed neuronal cultures, helped with virus design and revised the manuscript; R.G. provided biological samples and gave assistance in GBM culture. A.M. conceived the aim, led the project, wrote the paper and provided funding to complete the research. All authors reviewed, contributed to, and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
GBM specimens used for molecular analysis and stem cell establishment were collected from patients with histologic diagnosis of primary GBM (WHO grade 4 glioma) after informed consent was obtained from all subjects and/or their legal guardian(s). The experimentation has been performed following the ethical standards of Declaration of Helsinki and in accordance with the protocol 01-CSC07 approved by the institutional ethical review board of San Raffaele Scientific Institute to R.G. Cortical neurons were prepared from C57BL6J mice that were maintained under pathogen-free conditions at San Raffaele Hospital mouse facility (Milan, Italy). Mice were used in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC) and with the ARRIVE guidelines. The Ethics Review Committee approved experimental protocols according guidelines from the Italian Ministry of Health and from the Institutional Animal Care and Use Committee of the San Raffaele Scientific Institute (protocol number 372/2021-PR).
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Balboni, A., D’Angelo, C., Collura, N. et al. Acid-sensing ion channel 3 is a new potential therapeutic target for the control of glioblastoma cancer stem cells growth. Sci Rep 14, 20421 (2024). https://doi.org/10.1038/s41598-024-71623-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-71623-9
- Springer Nature Limited