Abstract
The correlation between diabetes and coronary artery disease (CAD) is well established. Insulin resistance (IR) is considered a primary contributor to elevated CAD risk in diabetic individuals. The triglyceride-glucose (TyG) index serves as a straightforward surrogate marker for insulin resistance. However, few studies have explored their correlations with myocardial infarction and CAD severity. Therefore, our study aimed to investigate the association between the TyG index and the occurrence of myocardial infarction, as well as the severity of coronary artery disease. We conducted a retrospective study involving 3865 consecutive patients who underwent coronary angiography at the First Affiliated Hospital of Zhejiang University, School of Medicine. Of these, 1724 patients were diagnosed with coronary artery disease. Demographic, biochemical, clinical, and angiographic data were gathered. A robust correlation exists between the TyG index and CAD subtypes, suggesting its potential as an independent clinical diagnostic marker. Moreover, the TyG index exhibited a significant positive correlation with disease severity, as assessed by the Gensini score. Elevated TyG index was associated with an increased predisposition to severe CAD, as indicated by the Gensini score, and myocardial infarction, even after adjusting for well-established cardiovascular risk factors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Coronary artery disease (CAD) represents the leading cause of mortality among adults worldwide. In 2021, 9.44 million deaths were attributed to CAD globally1. Large prospective observational studies have reported that type 2 diabetes doubles the risk of CAD events following adjustment for other risk factors2. Mendelian randomization analyses support a causal role for diabetes and its associated high glucose levels in CAD and suggest that long-term glucose lowering may reduce CAD events3. It is now widely accepted that diabetes mellitus is a major risk factor for CAD. High fasting glucose closely correlates with the burden of prediabetes, diabetes, and obesity worldwide. In 2021, elevated fasting plasma glucose was associated with 2.30 million cardiovascular deaths and 5.4 million deaths overall1.
Insulin resistance (IR) is a key contributor to increased cardiovascular disease (CVD) risk in both diabetic and non-diabetic patients4,5,6. IR plays a crucial role in the emergence and development of prediabetes, diabetes, and other metabolic syndromes, as well as CAD; its mechanism may involve damage to the vascular bed7,8. Accumulating evidence suggests that the concomitant presence of insulin resistance is an independent predictor of worse CAD outcomes9,10,11,12,13. The strong association between insulin resistance and adverse cardiovascular outcomes in both nondiabetic and diabetic individuals has been summarized in several meta-analyses14,15,16. The gold standard measurement method for IR is the hyperinsulinemic-euglycemic (HIEG) clamp protocol17. However, this protocol is laborious, time-consuming, and costly, making it impractical in clinical practice18.
The triglyceride-glucose (TyG) index, calculated as ln (fasting triglyceride [mg/dL] × fasting glucose [mg/dL]/2), is a synthetic parameter of fasting glucose in healthy individuals and serves as a simple surrogate indicator of insulin resistance. Plenty of evidence has shown a predictive role for the TyG index regarding CAD and its association with adverse clinical outcomes among patients with CAD19,20,21,22.
However, only a few studies have investigated the associations of the TyG index with myocardial infarction and the severity of coronary artery disease. Therefore, in this study, we investigated a cohort of individuals undergoing diagnostic or interventional coronary angiography to determine the association of the TyG index with angiographically confirmed CAD, the extent of atherosclerosis in CAD patients, and the occurrence of myocardial infarction (MI) among CAD cases.
Subjects and methods
Subjects
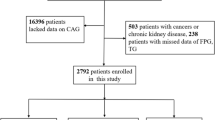
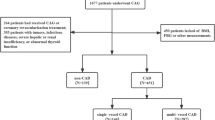
This study enrolled male patients hospitalized in the Department of Cardiology, the First Affiliated Hospital of Zhejiang University School of Medicine, from January 1, 2012, to December 31, 2015. It included patients with confirmed coronary heart disease identified by coronary angiography, comprising 335 cases of acute myocardial infarction, 551 patients with unstable angina, and 1205 patients with stable angina. Additionally, 1774 patients with no apparent stenosis on coronary angiography were included for analysis. All participants were Chinese adults aged 18 years and above. The Research Ethics Committee of the First Affiliated Hospital of Zhejiang University School of Medicine granted approval for the study.
Demographic and clinical data, such as age, BMI, blood pressure, coronary angiographic findings, and prevalent or incident myocardial infarction (MI), were extracted from hospital records. Venous blood samples were obtained from all subjects following an overnight fast of at least 8 h. The clinical chemistry department of the First Affiliated Hospital of Zhejiang University conducted measurements of total cholesterol (TC) level, low-density lipoprotein cholesterol (LDLc) level, high-density lipoprotein cholesterol (HDLc) level, triglycerides level, fasting blood glucose (FBG), and uric acid, along with other biochemical test results. The Triglyceride-Glucose (TyG) index was computed as ln (fasting triglyceride [mg/dL] × fasting glucose [mg/dL]/2).
Experienced interventional cardiologists performed coronary angiography. Coronary artery disease (CAD) was defined as ≥ 50% diameter stenosis in any of the major epicardial coronary arteries, as assessed by quantitative coronary angiography23. Disease severity was determined using the Gensini score. The scoring criteria were as follows: diseased vessels were categorized into left main (LM), left anterior descending (LAD), left circumflex (LCX), and right coronary artery (RCA). The degree of coronary stenosis was scored as follows: ≤ 25%, 1 point; 26–50%, 2 points; 51–75%, 4 points; 76–90%, 8 points; 91–99%, 16 points; and 100%, 32 points. Each coronary artery score was multiplied by the corresponding coefficient: the score of the left main branch was multiplied by 5, the score of the proximal part of the LAD and LCX branches was multiplied by 2.5, the score of the middle part of the LAD branch was multiplied by 1.5, and the score of the second diagonal branch was multiplied by 0.5. The scores of the remaining lesions were multiplied by 1.0, and the cumulative score of the aforementioned vessels yielded the Gensini score24. MI was diagnosed according to the World Health Organization criteria.
Statistical analysis
Data analysis was conducted using SPSS software, version 21.0 (SPSS Inc., Chicago, IL). The T-test and analysis of variance (ANOVA) were employed to discern differences in age, systolic blood pressure, diastolic blood pressure, total cholesterol (TC) level, low-density lipoprotein cholesterol (LDLc) level, high-density lipoprotein cholesterol (HDLc) level, and triglycerides level between study groups. Logistic regression analyses were executed to investigate the relationship between the TyG index and the severity of CAD cases, with or without adjustments for age, TC, and LDLc. Additionally, logistic regression was used to examine the association between the TyG index and MI among CAD cases, with or without adjustments for sex, systolic blood pressure, diastolic blood pressure, and triglyceride levels. All reported p-values are two-sided.
Results
Demographic and clinical characteristics of study subjects
In assessing baseline characteristics, differences emerged in the distribution of various blood test indicators among CAD subtypes, prompting adjustments for these factors in subsequent analyses. Notably, several well-established risk factors were significantly associated with CAD subgroups, including age, hypertension, glucose, uric acid, HDL-c, and LDL-c levels. Myocardial infarction patients exhibited higher glucose and CRP levels, as well as LDL-c levels, compared to non-CAD patients and those with stable CAD, while lower uric acid and HDL-c levels were observed in these patients. Additionally, significant differences in the distribution of Gensini Score and NYHA classification among CAD subtypes indicated varying disease severity. These demographic and clinical characteristics are summarized in Table 1.
Correlation of TyG Index with Known CAD risk factors
The TyG index correlates with various cardiometabolic risk factors, including obesity, insulin resistance, dyslipidemia, and hypertension. In our investigation, we conducted a correlation analysis between the TyG index and coronary heart disease alongside several risk factors. Our analysis revealed a correlation between the TyG index and multiple known risk factors for coronary heart disease, such as age, blood lipids, uric acid, smoking, and blood pressure (Supplementary Table 1).
TyG index is of great importance in discriminating subtypes of CAD
Our analysis revealed that the TyG index was significantly higher in patients with myocardial infarction (MI) and stable angina (SA) compared to normal subjects. However, no significant difference was observed in patients with unstable angina (UA) (Fig. 1). This suggests a potential differential relationship between the TyG index and various CAD subtypes, highlighting its potential utility as a clinical diagnostic marker.
To further evaluate the association between TyG index and each CAD subtype, we explored the odds ratio of each CAD subtype under different TyG indices based on ordered multi-classification logistic regression. The results showed that in the original model, the TyG index had a significant association with MI and SA, while in the adjusted model; the TyG index had a strong association with the three CAD subtypes MI, UA and SA (Table 2). Therefore, it can be concluded that the TyG index has a strong association with all CAD subtypes and has the potential to be an independent clinical diagnostic indicator.
Recognizing hypertension and diabetes as a critical factor influencing the occurrence of CAD subtypes, we performed a stratified analysis based on hypertension and diabetes status. This analysis demonstrated that the TyG index maintained a robust correlation with each CAD subtype across different hypertensive and diabetic states (Table 3).
TyG index is associated with the severity of CAD
Based on the strong correlation between the TyG index and each CAD subtype, we speculated whether the TyG index was related to the severity of CAD (Gensini Score), and whether it was still strongly correlated in different CAD subtypes. Therefore, based on the extensive linear model, we found that the TyG index was strongly correlated with the Gensini Score of CAD (Fig. 2), and in each CAD subtype, the TyG index was still significantly positively correlated with the severity of the disease (Table 4). In addition, this correlation was consistent under different hypertension, diabetes mellitus and BMI states (Table 5). Therefore, the TyG index can effectively assess the severity of each CAD subtype.
Enhanced classification accuracy of CAD subtypes by TyG index
To assess the combined clinical discrimination effect of the TyG index and various clinical indicators, we retained 13 clinical indicators via differential analysis. Employing a greedy strategy of recursive elimination, we constructed an optimal CAD subtype prediction model based on this population. By integrating the TyG index as an additional supplementary feature variable, we observed its impact on the identification model.
Following the imputation of missing samples with the mean value, we trained a total of 300 decision trees with 200 split nodes and 2 random forest models based on the 13 original feature variables (average AUC = 0.6189). To accurately evaluate variables for discriminative performance, we randomly stratified the inner training set and cross-validation set (7:3) in each random forest model. Repeatedly training the random forest model while excluding the least important indicators, we retained 11 feature variables (average AUC = 0.6241). Upon adding the TyG index, the average AUC of cross-validation increased to 0.6253 (Table 6). In the trained model, the TyG index exhibited a high explanatory degree (Fig. 3), thereby improving the classification accuracy of CAD subtypes based on different indexes to a certain extent.
Discussion
Cardiovascular disease (CVD) stands as a leading global cause of morbidity and mortality. The identification of reliable markers for predicting and assessing CVD risk is paramount for early detection and effective management. Diabetes, a well-established risk factor for coronary artery disease (CAD), often coincides with severe diffused-three-vessel disease. Alongside elevated blood glucose, insulin resistance emerges as a prominent feature in diabetic patients. Insulin resistance has been demonstrated to foster atherosclerosis and clinically relevant late plaque progression, establishing itself as a key risk factor for CAD in diabetic cohorts. The triglyceride-glucose (TyG) index, a simple and easily calculable marker, has garnered attention as a potential tool for assessing cardiometabolic risk and predicting cardiovascular events. The widespread utilization of TyG indices as surrogate markers of insulin resistance has substantiated their applicability, yielding substantial evidence linking them to CVD risk18,19,25,26. The present study aimed to investigate the association of the triglyceride-glucose (TyG) index with angiographically confirmed coronary artery disease (CAD), the extent of atherosclerosis in CAD patients, and the occurrence of myocardial infarction (MI) among CAD cases. Our findings contribute to the growing body of literature on the utility of the TyG index as a potential clinical diagnostic index for CAD and its subtypes.
Our study population comprised male patients with confirmed coronary heart disease, including acute myocardial infarction (MI), unstable angina (UA), and stable angina (SA), as well as a group without apparent stenosis on coronary angiography. Notably, we observed significant differences in demographic and clinical characteristics among CAD subtypes, highlighting the heterogeneity within this patient population. Consistent with previous research, our analysis revealed a correlation between the TyG index and several established risk factors for CAD, including age, blood lipids, uric acid, smoking, and blood pressure. These findings underscore the multifactorial nature of CAD pathogenesis and the potential role of the TyG index as a comprehensive cardiometabolic risk assessment tool.
Importantly, our study demonstrated a significant increase in the TyG index among MI and SA patients compared to normal subjects, with no significant difference observed in UA patients. This differential pattern suggests a potential relationship between the TyG index and different CAD subtypes, with implications for clinical diagnosis and risk stratification. Consistent with prior research, our study, boasting a larger sample size, validated the significant association between a higher TyG index and increased CAD risk. Notably, our findings revealed a notable elevation in the TyG index among myocardial infarction (MI) and stable angina (SA) patients compared to normal subjects, with no significant difference observed in unstable angina (UA) patients. Echoing our study, data from a community-based prospective study in the Kailuan cohort underscored the association of the TyG index with a 2.08-fold increased risk of myocardial infarction when comparing individuals in the highest quartile of baseline TyG index with those in the lowest quartile26.
The absence of a significant correlation between the TyG index and disease severity in UA patients could be attributed to several factors. First, UA represents a diverse clinical condition with varying degrees of underlying pathology, which may not be directly related to insulin resistance as measured by the TyG index. Unlike acute myocardial infarction (AMI), which typically involves more extensive and stable atherosclerotic plaques, UA is often characterized by transient and less predictable plaque instability. Additionally, the variability in clinical presentation and the acute nature of UA might obscure the relationship between the TyG index and CAD severity. The inflammatory and thrombotic processes involved in UA could also play a more dominant role, overshadowing the contribution of insulin resistance. It is also possible that the sample size of UA patients in our study was insufficient to detect a significant correlation. Larger studies with a more extensive cohort of UA patients might be necessary to explore this relationship more comprehensively. It is worth mentioning that serious diseases, including acute myocardial infarction, can lead to a stress state, and the body will secrete related hormones, leading to stress-induced hyperglycemia. Moreover, no relevant research has been able to confirm how long the stress state caused by acute myocardial infarction lasts. Therefore, it is indeed difficult for us to rule out the related effects caused by the stress state for patients with acute myocardial infarction27.
Furthermore, recognizing hypertension as a pivotal factor influencing the occurrence of each CAD subtype, we conducted a stratified analysis based on hypertension incidence to examine the robust correlation between the TyG index and each CAD subtype across different hypertensive states. Our results unveiled a strong correlation between the TyG index and each CAD subtype across varied hypertensive states, with a particularly heightened correlation in hypertensive states. Given the TyG index's association with various cardiometabolic risk factors, including obesity, insulin resistance, dyslipidemia, and hypertension, it follows that our finding of a stronger correlation between the TyG index and CAD subtypes in hypertensive patients may be explained by these underlying cardiometabolic risk factors. Insulin resistance (IR) is closely linked to obesity and ectopic fat deposition, such as epicardial adipose tissue, which are known markers of CAD severity and high-risk plaque. Studies have shown that elevated BMI is associated with increased IR and greater ectopic fat accumulation, contributing to the progression of atherosclerosis. In our study, although BMI data were not stratified, future research should consider analyzing the TyG index in relation to BMI categories and ectopic fat to further elucidate these relationships. Including measures of epicardial adipose tissue could provide deeper insights into the role of ectopic fat in CAD severity.
Of particular interest is our observation of a positive correlation between the TyG index and disease severity in each CAD subtype, as assessed by the Gensini score. This finding suggests that the TyG index may serve as a useful indicator of CAD severity, facilitating risk stratification and treatment decision-making. Moreover, our study demonstrated the enhanced classification accuracy of CAD subtypes achieved by incorporating the TyG index into predictive models. By integrating the TyG index as an additional feature variable, we observed improvements in model performance, as evidenced by increased area under the curve (AUC) values in random forest analysis. This suggests that the TyG index contributes valuable discriminatory information beyond traditional clinical indicators, enhancing the precision of CAD subtype classification.
Overall, our findings support the potential utility of the TyG index as a simple and readily accessible biomarker for CAD diagnosis, risk assessment, and prognostication. Future research should aim to validate these findings in larger and more diverse populations and explore the underlying mechanisms linking the TyG index to CAD pathophysiology. Additionally, prospective studies are warranted to evaluate the longitudinal predictive value of the TyG index for CAD outcomes and its utility in guiding personalized therapeutic interventions.
Several limitations merit consideration. Our study was conducted at a single center, which may limit the generalizability of our findings to broader populations. Variations in patient demographics, clinical practices, and disease characteristics across different regions and healthcare settings could affect the applicability of our results. The retrospective nature of our study introduces inherent biases and limitations, including potential selection bias and incomplete data capture. Prospective studies are needed to confirm our findings and establish causality between the TyG index and CAD outcomes. Our study enrolled only male patients, which may restrict the generalizability of our results to female populations. Gender-specific differences in CAD pathophysiology and risk factors warrant further investigation to elucidate the relevance of the TyG index in female patients. Although our study included a sizable cohort of patients with confirmed CAD, the sample size may still be insufficient to detect small but clinically significant associations or to conduct subgroup analyses effectively. Larger studies are needed to validate our findings and explore potential interactions between the TyG index and other variables. Our analysis was cross-sectional, limiting our ability to establish temporal relationships between the TyG index and CAD subtypes. Longitudinal studies are necessary to assess the predictive value of the TyG index for CAD incidence, progression, and outcomes over time. Despite adjusting for several demographic and clinical variables, residual confounding may still exist due to unmeasured or inadequately controlled factors. Confounders such as dietary habits, physical activity levels, and medication use could influence the association between the TyG index and CAD outcomes. The TyG index was calculated based on fasting triglyceride and glucose levels, which may be subject to measurement variability and laboratory assay limitations. Standardization of measurement protocols and validation studies are needed to ensure the reliability and reproducibility of TyG index measurements. The classification of CAD subtypes (MI, UA, SA) based on angiographic findings may not fully capture the heterogeneity of CAD presentation and severity. Incorporating additional diagnostic modalities, such as imaging or biomarkers, could provide a more comprehensive assessment of CAD phenotypes. Addressing these limitations in future studies will enhance the robustness and clinical utility of the TyG index as a biomarker for CAD risk assessment and management.
Data availability
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation. Dr. Xiangyuan Pu should be contacted if someone wants to request the data from this study.
References
Vaduganathan, M. et al. The global burden of cardiovascular diseases and risk: A compass for future health. J. Am. Coll. Cardiol. 80(25), 2361–2371 (2022).
Sarwar, N. et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet 375(9733), 2215–2222 (2010).
Ross, S. et al. Mendelian randomization analysis supports the causal role of dysglycaemia and diabetes in the risk of coronary artery disease. Eur. Heart J. 36(23), 1454–1462 (2015).
Lambie, M. et al. Insulin resistance in cardiovascular disease, uremia, and peritoneal dialysis. Trends Endocrinol. Metab. 32(9), 721–730 (2021).
Howard, G. et al. Insulin sensitivity and atherosclerosis. The Insulin Resistance Atherosclerosis Study (IRAS) investigators. Circulation 93(10), 1809–1817 (1996).
Isomaa, B. et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care 24(4), 683–689 (2001).
Bornfeldt, K. E. & Tabas, I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. 14(5), 575–585 (2011).
Ormazabal, V. et al. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 17(1), 122 (2018).
Karamanakos, G. et al. Highlighting the role of obesity and insulin resistance in type 1 diabetes and its associated cardiometabolic complications. Curr. Obes. Rep. 11(3), 180–202 (2022).
Bonora, E. et al. HOMA-estimated insulin resistance is an independent predictor of cardiovascular disease in type 2 diabetic subjects: prospective data from the Verona diabetes complications study. Diabetes Care 25(7), 1135–1141 (2002).
Bonora, E. et al. Insulin resistance as estimated by homeostasis model assessment predicts incident symptomatic cardiovascular disease in caucasian subjects from the general population: the Bruneck study. Diabetes Care 30(2), 318–324 (2007).
Hedblad, B. et al. Relation between insulin resistance and carotid intima-media thickness and stenosis in non-diabetic subjects. Results from a cross-sectional study in Malmo, Sweden. Diabetic Med. J. Br. Diabetic Assoc. 17(4), 299–307 (2000).
Golden, S. H. et al. Risk factor groupings related to insulin resistance and their synergistic effects on subclinical atherosclerosis: The atherosclerosis risk in communities study. Diabetes 51(10), 3069–3076 (2002).
Gast, K. B. et al. Insulin resistance and risk of incident cardiovascular events in adults without diabetes: Meta-analysis. PLoS ONE 7(12), e52036 (2012).
Mottillo, S. et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J. Am. Coll. Cardiol. 56(14), 1113–1132 (2010).
Sarwar, N. et al. Circulating concentrations of insulin markers and coronary heart disease: A quantitative review of 19 Western prospective studies. Eur. Heart J. 28(20), 2491–2497 (2007).
Bonora, E. et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: Studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care 23(1), 57–63 (2000).
Che, B. et al. Triglyceride-glucose index and triglyceride to high-density lipoprotein cholesterol ratio as potential cardiovascular disease risk factors: An analysis of UK biobank data. Cardiovasc. Diabetol. 22(1), 34 (2023).
Barzegar, N. et al. The impact of triglyceride-glucose index on incident cardiovascular events during 16 years of follow-up: Tehran Lipid and Glucose Study. Cardiovasc. Diabetol. 19(1), 155 (2020).
Cho, Y. K. et al. Triglyceride-glucose index predicts cardiovascular outcome in metabolically unhealthy obese population: A nationwide population-based cohort study. J. Obes. Metab. Syndr. 31(2), 178–186 (2022).
Liu, Q. et al. Triglyceride-glucose index associated with the risk of cardiovascular disease: The Kailuan study. Endocrine 75(2), 392–399 (2022).
Zhao, Q. et al. Impacts of triglyceride-glucose index on prognosis of patients with type 2 diabetes mellitus and non-ST-segment elevation acute coronary syndrome: Results from an observational cohort study in China. Cardiovasc. Diabetol. 19(1), 108 (2020).
Fihn, S. D. et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 130(19), 1749–1767 (2014).
Rampidis, G. P. et al. A guide for Gensini Score calculation. Atherosclerosis 287, 181–183 (2019).
Hong, S., Han, K. & Park, C. Y. The triglyceride glucose index is a simple and low-cost marker associated with atherosclerotic cardiovascular disease: A population-based study. BMC Med. 18(1), 361 (2020).
Tian, X. et al. Triglyceride-glucose index is associated with the risk of myocardial infarction: An 11-year prospective study in the Kailuan cohort. Cardiovasc. Diabetol. 20(1), 19 (2021).
Dungan, K. M., Braithwaite, S. S. & Preiser, J. C. Stress hyperglycaemia. Lancet 373(9677), 1798–1807 (2009).
Funding
National Natural Science Foundation of China 81600339 (X. Pu). Natural Science Foundation of Zhejiang Province LY19H020009 (X. Pu). Clinical Scientist • Elite Training Program-Most Valuable Proposal (MVP).
Author information
Authors and Affiliations
Contributions
Lujing Tang wrote the main manuscript text and performed preliminary analysis of the data. Xiaodong Xu and Jiahao Li helped collect the original data and performed preliminary data verification. Mengsha Chen helped us with the statistical analysis of the data. Xiangyuan Pu provided research ideas, designed the research plan and wrote the article. All authors reviewed the manuscript. All authors have seen the manuscript and agree with publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. No animal studies are presented in this manuscript. The studies involving human participants were reviewed and approved by research ethics committee of the First Affiliated Hospital of Zhejiang University Medical College. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Inclusion of identifiable human data
Generated Statement: Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tang, L., Xu, X., Chen, M. et al. Association of triglyceride-glucose index with severity of coronary artery disease among male patients. Sci Rep 14, 20342 (2024). https://doi.org/10.1038/s41598-024-71718-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-71718-3
- Springer Nature Limited