Abstract
Estimating glomerular filtration (eGFR) after Continuous Renal Replacement Therapy (CRRT) is important to guide drug dosing and to assess the need to re-initiate CRRT. Standard eGFR equations cannot be applied as these patients neither have steady-state serum creatinine concentration nor average muscle mass. In this study we evaluate the combination of dynamic renal function with CT-scan based correction for aberrant muscle mass to estimate renal function immediately after CRRT cessation. We prospectively included 31 patients admitted to an academic intensive care unit (ICU) with a total of 37 CRRT cessations and measured serum creatinine before cessation (T1), directly (T2) and 5 h (T3) after cessation and the following two days when eGFR stabilized (T4, T5). We used the dynamic creatinine clearance calculation (D3C) equation to calculate eGFR (D3CGFR) and creatinine clearance (D3Ccreat) between T2-T3. D3Ccreat was corrected for aberrant muscle mass when a CT-scan was available using the CRAFT equation. We compared D3CGFR to stabilized CKD-EPI at T5 and D3CCreat to 4-h urinary creatinine clearance (4-h uCrCl) between T2-T3. We retrospectively validated these results in a larger retrospective cohort (NICE database; 1856 patients, 2064 cessations). The D3CGFR was comparable to observed stabilized CKD-EPI at T5 in the prospective cohort (MPE = − 1.6 ml/min/1.73 m2, p30 = 76%) and in the retrospective NICE-database (MPE = 3.2 ml/min/1.73 m2, p30 = 80%). In the prospective cohort, the D3CCreat had poor accuracy compared to 4-h uCrCl (MPE = 17 ml/min/1.73 m2, p30 = 24%). In a subset of patients (n = 13) where CT-scans were available, combination of CRAFT and D3CCreat improved bias and accuracy (MPE = 8 ml/min/1.73 m2, RMSE = 18 ml/min/1.73 m2) versus D3CCreat alone (MPE = 18 ml/min/1.73 m2, RMSE = 32 ml/min/1.73 m2). The D3CGFR improves assessment of eGFR in ICU patients immediately after CRRT cessation. Although the D3CCreat had poor association with underlying creatinine clearance, inclusion of CT derived biometric parameters in the dynamic renal function algorithm further improved the performance, stressing the role of muscle mass integration into renal function equations in critically ill patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Critically ill patients at the Intensive Care Unit (ICU) often require renal replacement therapy1,2. Although many studies have focused on the optimal timing of CRRT initiation, the period after cessation of CRRT has been understudied. Immediately after cessation of CRRT, assessment of renal clearance is important not only to assess renal function and the potential need for reinitiating CRRT, but also to optimize the dosing of drugs that are cleared renally3.
After cessation of CRRT, conventional equations for estimating glomerular filtration rate (eGFR) cannot be used because these patients often do not have stable serum creatinine and suffer from muscle wasting4,5. Most eGFR equations are based on the clearance of creatinine and surrogate markers of body composition6. Creatinine is an endogenous marker of filtration and is released in a constant rate from muscles and mainly removed from the circulation by glomerular filtration and to a lesser extent by tubular secretion7. Under stable conditions, the creatinine clearance and therefore the GFR can be estimated by dividing the creatinine excretion measured in the urine by the serum creatinine concentration. To circumvent urine collection, the Cockroft-Gault equation was developed that was able to estimate creatinine clearance from the serum creatinine concentration by estimating creatinine production over time based on biometric parameters like age, weight and sex8. As creatinine is cleared not only by glomerular filtration but also by tubular secretion, new equations, such as CKD-EPI, have been developed to estimate GFR from serum creatinine concentration and biometric parameters such as age and sex6. The use of these creatinine based eGFR equations requires both a stable serum creatinine concentration and a patient with an average body composition for age and sex. When creatinine is not in steady-state, estimating equations (such as the CKD-EPI and Cockroft-Gault) overestimate GFR when creatinine is rising and underestimate GFR when creatinine is falling9.
Recently, we have published two new approaches that may allow the assessment of renal clearance immediately after CRRT cessation. First, creatinine clearance can be estimated using dynamic modelling in patients with an unstable serum creatinine10,11. Using this modelling approach, we developed the dynamic creatinine clearance calculation (D3C), an equation used to calculate eGFR and/or estimate creatinine clearance in cases where creatinine is not at steady-state11. In addition, we have recently shown that body composition analysis automatically derived from any computed tomography (CT) scan that includes the lumbar spine level 3 (L3) (CRAFT analysis) can be used to improve the estimates of renal function in patients with an abnormal body composition for age and sex12.
This study evaluated whether the use of D3C on its own and combined with CRAFT improves the assessment of renal function immediately after cessation of CRRT without the need for urine collection.
Methods
Patient population and data collection
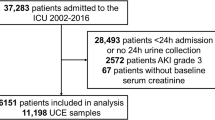
For the prospective cohort, we studied all adult patients that required CRRT in the intensive care unit of the UMC Utrecht between 10-10-2019 and 01-09-2020. Patients were excluded if they were transferred to another hospital, died during CRRT, were switched to intermittent haemodialysis or were admitted to the ICU because of a COVID-19 infection. Patients that were included and failed CRRT cessation could be included for a following iteration of CRRT cessation if there was another attempt. Therefore, some patients had multiple iterations of CRRT cessation which were all included in the analysis (Fig. 1). Patient and laboratory data were collected by care providers during treatment. After stopping CRRT, urine was collected for 4 h using an indwelling Foley catheter. Serum creatinine was collected in the morning before CRRT cessation (T1), immediately after CRRT cessation (T2), after 4 h (T3), and in the morning on the two following days (T4, T5) (Fig. 1). CT-scans that were made for clinical purposes were included if they were made within 30 days prior or after the cessation of CRRT. Pseudonymized CT-scans were extracted from the Research Imaging Architecture of the UMC Utrecht.
In the retrospective cohort, we used the National Intensive Care Evaluation (NICE) database to evaluate the course of creatinine after cessation of CRRT in a larger cohort. The NICE database is a nationwide database in the Netherlands where data from all intensive care units in the Netherlands are collected prospectively for quality control and research13. Between 01-01-2010 and 01-01-2020, we selected all patients who received CRRT and had at least one instance of successful cessation for at least 3 days. We excluded patients that were on intermittent hemodialysis and patients that did not have a rise in serum creatinine after CRRT cessation.
Calculations to correct for dynamic renal function and aberrant muscle mass
Urinary creatinine clearance was calculated by dividing the urinary creatinine excretion by the mean serum creatinine during a 4-h collection. Urinary creatinine clearance was only calculated when patients produced at least 20 ml of urine in 4 h to minimize the influence of collection errors. The development of the D3C has been described previously11. In this study, the D3C was used to calculate creatinine clearance (D3Ccreat) and model post-cessation creatinine which was used to calculate expected eGFR using the CKD-EPI equation (D3CGFR).
D3Ccreat was calculated using the following equation:
We also used the D3C to model serum creatinine after cessation of CRRT using the following equation:
In above equations, C(t) = Serum creatinine concentration at time (t), ΔC = delta creatinine concentration between two measurements, ΔT = time difference between measurements, Pcr = production of creatinine calculated using the Cockcroft-Gault equation, CrCl = Creatinine Clearance calculated using the D3Ccreat, C0 = the creatinine concentration at CRRT cessation, Cmean = the mean of two consecutive serum creatinine measurements, and Vd = the volume of distribution set at 60% bodyweight14,15,16.
The CRAFT equation has been described elsewhere12. Briefly, abdominal CT-scans covering the L3 region were used to calculate estimated creatinine production based on body composition, using an automated image-analysis algorithm that selects the L3 region, segments body compartments (muscles, and visceral and subcutaneous fat) and calculates radiomics parameters based on compartment volume and Hounsfield unit-distribution of those compartments.
Evaluation of equation performance
In the prospective cohort, the reference that was used for comparison was stabilized CKD-EPI 2 days after cessation and 4 h urinary creatinine clearance. In the prospective cohort, we compared D3Ccreat and D3Ccreat corrected for muscle wasting using the CRAFT equation calculated between T2-T3 (Eq. 1) with 4-h urinary creatinine clearance using the Cockcroft-Gault as an alternative creatinine clearance. In addition, we compared expected CKD-EPI at T5 (calculated using creatinine at T2-T3 and Eqs. (1), (2)) with the observed CKD-EPI at T5 using the CKD-EPI at T3 as alternative eGFR. In the retrospective cohort, only daily creatinine measurements were available and therefore the reference that was used was stabilized CKD-EPI at T5. In the retrospective cohort, we compared expected CKD-EPI at T5 (calculated using creatinine at T1-T4 and Eqs. (1), (2)) with observed CKD-EPI at T5 using the CKD-EPI at T4 as alternative eGFR.
Statistics
We assessed bias using the mean prediction error (MPE), precision using the R2-value of the least squares regression, and accuracy using the root mean squared error (RMSE) and the number of predictions that fell within 30% (p30) and 15% (p15) of the outcome. All calculations and statistical analyses were performed using R statistics 3.5.1. P-values below 0.05 were considered significant.
Results
Characteristics of prospective and retrospective cohort
For the prospective cohort, we included 31 patients with 37 iterations of CRRT cessation (Table 1). In total, 25 patients with 28 iterations had adequate urine production immediately after CRRT cessation and could be analysed for urinary creatinine clearance. 24 patients with 25 iterations had successful cessation of CRRT for at least 2 days and could be analysed for serum creatinine course after cessation. For the retrospective cohort, we included 1856 patients with 2064 iterations of CRRT cessation, all of whom could be analysed for serum creatinine course after cessation.
In both the prospective and retrospective cohorts, most patients were male (71% and 66%, respectively). Patients in the prospective cohort were on average younger (57 vs 65 years) and had lower creatinine at cessation (110 vs 183 µmol/L). In the prospective cohort, the median number of days in the ICU before cessation of CRRT was 10 days [range 1 – 49 days].
Dynamic GFR increases accuracy calculating eGFR after CRRT cessation
First, we evaluated the association between expected and observed eGFR after CRRT cessation (Tables 2, 3, Figs. 2, 3). In the prospective cohort, the CKD-EPI at T3 (4 h after cessation) overestimated the CKD-EPI 2 days later at T5 (MPE = 14.9 ml/min/1.73m2, R2 = 0.74, p30 32%). Urine production in the 4 h immediately after CRRT cessation had a poor association with the CKD-EPI at T5 (R2 = 0.07; Supplemental Fig. 1). Calculation of the projected creatinine concentration after CRRT cessation using the D3CGFR corrected the bias and increased accuracy (MPE = -1.6 ml/min/1.73m2, R2 = 0.79, p30 = 76%).
Data of the prospective cohort. (A-B) Dot plots showing the relationship between creatinine at T5 and the creatinine at T3 (A) and D3CGFR projected creatinine at T5 (B). Solid line represents the line of identity. (C,D) Dot plots showing the relationship between CKD-EPI at T5 and the CKD-EPI at T3(C), and D3CGFR projected CKD-EPI at T5 (D). Solid line represents the line of identity (E,F) Bland–Altman plot showing difference between CKD-EPI at T3 (E), D3CGFR projected CKD-EPI at T5 (F), and CKD-EPI at T5. Solid line is the mean difference, dashed lines represent 1.96 times the standard deviation of the difference.
These findings were replicated in the retrospective NICE cohort, although the difference was less pronounced as only daily creatinine measurements were available (Table 3, Fig. 3). In this cohort, the CKD-EPI at T4 overestimated the CKD-EPI at T5 (MPE = 5.6 ml/min/1.73m2, R2 = 0.87, RMSE = 10.8, p30 = 67%). Urine production on the day before cessation had a poor association with CKD-EPI at T5 (R2 = 0.03, p < 0.001; Supplemental Fig. 1) and with urine production at T5 (R2 = 0.08, p < 0.001). The D3CGFR after CRRT cessation again reduced bias and increased accuracy (MPE = 3.2 ml/min/1.73m2, R2 = 0.88, RMSE = 10.4, p30 = 80%).
Dynamic GFR and correction for muscle mass reduces bias calculating creatinine clearance after CRRT cessation
We compared measured creatinine clearance with the Cockroft-Gault and the D3CCreat 4 h after CRRT cessation (Table 4, Fig. 4). The Cockcroft-Gault equation overestimated the urinary 4-h creatinine clearance and was inaccurate (MPE = 43 ml/min/1.73 m2, R2 = 0.19, RMSE = 49, P30 = 16%). Although the D3CCreat reduced the bias, it was still inaccurate (MPE = 17 ml/min/1.73 m2, R2 = 0.07, RMSE = 40 ml/min/1.73 m2, p30 = 24%). Urine production in the first four hours after CRRT cessation, often used as proxy for renal function, had poor association with 4-h creatinine clearance (R2 = 0.06; Supplemental Fig. 1).
Data of the prospective cohort. (A,B) Dot plots showing the relationship between 4-h urinary creatinine clearance and Cockcroft-Gault at T3 (A) and D3Ccreat at T3 (B). Solid line represents the line of identity. (C,D) Bland–Altman plot showing difference between 4-h urinary creatinine clearance and the Cockcroft-Gault at T3 (C) and the D3Ccreat at T3 (D). Solid line is the mean difference, dashed lines represent 1.96 times the standard deviation of the difference.
We hypothesized that the inaccuracy of the D3Ccreat in assessing measured creatinine clearance could be explained, at least in part, by incorrect assumptions about creatinine production in patients with an abnormal muscle mass. We therefore combined the CRAFT equation with the D3CCreat creatinine clearance in a subset of patients where clinically obtained CT-scans were available, to correct for muscle wasting (n = 13, Supplemental Fig. 2). Median time between CT-scan and CRRT cessation 6 days (range 1–26 days). Combining the CRAFT and D3CCreat equations improved bias and accuracy (MPE = 8 ml/min/1.73 m2, RMSE = 18 ml/min/1.73 m2) compared to using the D3CCreat equation alone in the same group of patients (MPE = 18 ml/min/1.73 m2, RMSE = 32 ml/min/1.73 m2).
Discussion
This study shows that a dynamic eGFR equation (D3C) accurately models creatinine course after CRRT cessation using two serum creatinine values taken 4 h apart directly after CRRT cessation. We replicated this finding in a large nationwide retrospective cohort in the Netherlands. The estimated creatinine clearance could be further improved by correcting for muscle wasting using automatically analysed CT scan data.
Conventional creatinine based eGFR equations require a stable serum creatinine concentration. As expected, the CKD-EPI calculated directly after CRRT cessation overestimated the CKD-EPI two days later, due to the sudden loss of CRRT clearance and serum creatinine not yet reaching steady-state. The D3C corrected this bias using a simple single-compartment model with two serum creatinine values collected 4 h apart. We confirmed the results of our small proof-of-principle study in a large nationwide retrospective cohort of all the ICU’s in the Netherlands. Importantly, actual benefit of the D3C may be greater than suggested from the nationwide cohort study because in this retrospective study we could not use 2 creatinine values shortly after each other after CVVH was discontinued since only daily measurements where available. This reduced the effect of the D3C compared to the conventional CKD-EPI, as more time had elapsed since CRRT was stopped and creatinine had more time to reach steady-state.
Assessing renal function immediately after CRRT cessation is important to guide the decision whether CRRT can be safely discontinued or should be restarted1. Previous research has focused on the safety of stopping CRRT while patients are still on dialysis. Two retrospective studies found that during CRRT, a urine output of > 400-500 ml/day without diuretic therapy during CRRT was associated with successful discontinuation and lower in-hospital mortality17,18. Others have suggested a stepwise approach using a timed urine collection over 6 h to calculate creatinine clearance in patients with a urine output > 750 ml/day19. There is one previous retrospective study that evaluated an alternative dynamic eGFR equation, the KeGFR, in 52 patients and showed that a better KeGFR 24 h after CRRT cessation was associated with successful CRRT discontinuation20. We add to these observations that a dynamic eGFR equation (the D3CGFR) can be used to accurately model the creatinine course after CRRT cessation using two creatinine values taken 4 h apart. The D3CGFR might therefore be used to support the assessment of the underlying GFR as an argument in the decision to restart CRRT. Dynamic GFR evaluation should however always be used in the context of metabolic and hemodynamic parameters to guide the decision to start and or stop dialysis and should not be the sole parameter to base this decision on. Future studies may provide data on whether the D3C and CRAFT equations can actually improve quality of care and clinical outcomes in a cost-effective manner.
Our study has several limitations. First, the prospective study had a limited sample size due to its single-center design. However, the results of the prospective study were reproduced in the larger retrospective study which showed similar results. We did not compare the dynamic GFR with methods of measuring GFR such as inulin clearance or iohexol clearance which were not available. Assessment of mGFR may have improved the accuracy of the assessment of GFR, mainly since it is not affected by tubular secretion of creatinine (as is the creatinine clearance). However, measurement of urinary clearance of these markers requires adequate urine production, and measurement of plasma clearance requires estimates of the volume of distribution, which can be difficult to estimate in the edematous states which are prevalent in ICU patients. Although the D3C accurately modelled the creatinine course and CKD-EPI two days after cessation, the bias between urinary creatinine clearance and D3C was still substantial, which may be explained by muscle wasting which affects creatinine-based equations such as the D3C. Although correction for muscle wasting using clinically obtained CT-scans with the CRAFT equation reduced this bias, some disagreement remained between the 4-h creatinine clearance and the calculated creatinine clearance using the D3C and CRAFT equations. One explanation is that these equations should be specifically updated with data from ICU patients. However, it is also likely that part of the residual discrepancy is based on collection errors of urine production, as even small collection errors of 20–50 ml may significantly affect the measured creatinine clearance in patients with a limited urine production. Finally there was time between CT-scan and cessation of CRRT, which may have affected the accuracy of the CRAFT equation, since body composition may have changed.
In the future, integrated diagnostics may increasingly support physicians in their work. As clinically obtained CT-scans become increasingly available, particularly in the ICU, the CRAFT equation can automatically provide a creatinine-based estimate of renal function corrected for the patient’ s actual muscle mass. In addition, laboratory systems will also be able to detect rapid changes in serum creatinine concentrations over time and could subsequently switch from GFR estimation equations based on stable serum creatinine concentrations to those based on dynamic creatinine concentrations. Future studies may provide data on whether the D3C and CRAFT can actually improve quality of care and clinical outcome in a cost effective manner.
In conclusion, the D3C can be used to accurately model the creatinine levels after CRRT cessation without the need for urine collection. The CRAFT equation can be used to correct for muscle wasting if a CT-scan is available. These equations are promising tools for clinicians to advance decisions about drug dosing and reinitiation of CRRT immediately after cessation of CRRT.
Data availability
The data is available upon request and under specific conditions, see (in Dutch): Extractieverzoek Procedure (stichting-nice.nl).
References
Tandukar, S. & Palevsky, P. M. Continuous renal replacement therapy: Who, when, why, and how. Chest 155(3), 626–638 (2019).
Hoste, E. A. et al. Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensive Care Med. 41(8), 1411–1423 (2015).
Schetz, M. Drug dosing in continuous renal replacement therapy: General rules. Curr. Opin. Crit. Care 13(6), 645–651 (2007).
Sunder, S. et al. Estimation of renal function in the intensive care unit: The covert concepts brought to light. J. Intensive Care 2(1), 31 (2014).
Puthucheary, Z. A. et al. Acute skeletal muscle wasting in critical illness. JAMA 310(15), 1591–1600 (2013).
Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 150(9), 604–612 (2009).
Kashani, K., Rosner, M. H. & Ostermann, M. Creatinine: From physiology to clinical application. Eur. J. Intern. Med. 72, 9–14 (2020).
Levey, A. S. & Inker, L. A. Assessment of glomerular filtration rate in health and disease: A state of the art review. Clin. Pharmacol. Ther. 102(3), 405–419 (2017).
Waikar, S. S. & Bonventre, J. V. Creatinine kinetics and the definition of acute kidney injury. J. Am. Soc. Nephrol. 20(3), 672–679 (2009).
Chen, S. Retooling the creatinine clearance equation to estimate kinetic GFR when the plasma creatinine is changing acutely. J. Am. Soc. Nephrol. 24(6), 877–888 (2013).
Pieters, T. T. et al. Early estimation of renal function after transplantation to enable appropriate dosing of critical drugs: Retrospective analysis of 103 patients in a single center. Clin. Pharmacokinet. 59, 1303–11 (2020).
Pieters, T. T. et al. Deep learning body-composition analysis of clinically acquired CT-scans estimates creatinine excretion with high accuracy in patients and healthy individuals. Sci. Rep. 12(1), 9013 (2022).
van de Klundert, N., Holman, R., Dongelmans, D. A. & de Keizer, N. F. Data resource profile: The Dutch National Intensive Care Evaluation (NICE) registry of admissions to adult intensive care units. Int. J. Epidemiol. 44(6), 1850 (2015).
Jones, J. D. & Burnett, P. C. Creatinine metabolism in humans with decreased renal function: Creatinine deficit. Clin. Chem. 20(9), 1204–1212 (1974).
Chow, M. S. A method for determining the pharmacokinetics of endogenous creatinine without exogenous creatinine administration. Biopharm. Drug Dispos. 6(2), 201–208 (1985).
Coroas, A. et al. Sequential body composition analysis by bioimpedance early post-kidney transplantation. Transpl. Int. 18(5), 541–547 (2005).
Uchino, S. et al. Discontinuation of continuous renal replacement therapy: A post hoc analysis of a prospective multicenter observational study. Crit. Care Med. 37(9), 2576–2582 (2009).
Mendu, M. L. et al. A decision-making algorithm for initiation and discontinuation of RRT in severe AKI. Clin. J. Am. Soc. Nephrol. 12(2), 228–236 (2017).
Network VNARFT et al. Intensity of renal support in critically ill patients with acute kidney injury. N. Engl. J. Med. 359(1), 7–20 (2008).
Yoshida, T. et al. Kinetic estimated glomerular filtration rate as a predictor of successful continuous renal replacement therapy discontinuation. Nephrology (Carlton) https://doi.org/10.1111/nep.13396 (2018).
Acknowledgements
We thank all Dutch ICU’s that participated in providing data for the NICE-registration. We thank the NICE research and support team for help with acquisition and curation of the data for the retrospective cohort.
Funding
This authors did not receive specific funding for this study. TTP was supported by the Alexandre Suerman Stipend from the UMC Utrecht.
Author information
Authors and Affiliations
Contributions
TTP, MBR, DWL, MCV, AA, MAS, MCV, and MJD were involved in conceptualization of the study. TTP, W.B.V and W.B.V were involved in data acquirement and analysis. All authors involved in interpreting the analyses. TTP wrote the original draft. All authors reviewed and edited the final draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and the ethical guidelines of our institutions. The research protocol for was approved by the institutional review board of the UMC Utrecht, which waived the requirement for informed consent (protocol number 19–152/C). Pseudonymized data were used for this study. Data collection and handling was conducted in accordance with European Data Protection Regulation (GDPR).
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Pieters, T.T., van Dam, M.J., Sikma, M.A. et al. Estimation of renal function immediately after cessation of continuous renal replacement therapy at the ICU. Sci Rep 14, 21098 (2024). https://doi.org/10.1038/s41598-024-72069-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-72069-9
- Springer Nature Limited