Abstract
Oral nutritional supplementation (ONS) is recommended for malnourished hemodialysis patients when their nutritional intake remains inadequate to meet energy and protein requirements. Patients were randomized into two groups: the intradialytic ONS supplements (INTRA-ONS) group (N = 16) and the interdialytic ONS supplements (INTER-ONS) group (N = 16) for a duration of 12 weeks. Malnutrition inflammation score (MIS) and serum albumin levels were assessed. The total MIS decreased significantly in patients from both the INTRA-ONS group (− 6.13, 95% CI − 8.29 to − 3.96) and the INTER-ONS group (− 3.50, 95% CI − 5.56 to − 1.35). A significant difference in the change of MIS was observed between the two groups (− 3.06, 95% CI − 5.94 to − 0.17). No significant differences were observed between the groups concerning serum albumin levels, dietary intake, anthropometric measurements, or body weight. Intradialytic ONS demonstrates similar benefits on nutritional biomarkers but improves the MIS among malnourished ESRD patients compared to interdialytic ONS.
Trial registration Thai Clinical Trials Registry (TCTR) identification number is TCTR20220322007: 16/09/2021.
Similar content being viewed by others
Introduction
A systematic review of compliance with oral nutritional supplements (ONS) among outpatients found that the overall mean compliance is good, especially with higher energy–density ONS; however, the level of cooperation varied, ranging from 30 to 100%1. Consuming less than the targeted amount of nutrients, particularly protein, can lead to malnutrition, disability, and mortality in dialysis patients2. One study indicated that 66.4% of hemodialysis patients consumed fewer than three main meals, and 59.2% reported skipping the same number of snacks on dialysis days3. Malnutrition is particularly pronounced in dialysis patients, where processes such as catabolism, inflammation, and protein loss are heightened4. Consequently, intradialytic meal supplements show that patients meet the targets for treatment-related increases in protein and energy needs5. A Consensus Statement from the International Society of Renal Nutrition and Metabolism suggests that ONS during hemodialysis may be an effective strategy to improve nutritional status, with limited reports of intradialytic complications6.
Data comparing protein metabolism during hemodialysis between intradialytic ONS and intradialytic parenteral nutrition (IDPN), along with a control group, have been examined. Both the Intradialytic ONS and IDPN groups experienced significantly reduced protein loss during hemodialysis compared to the control group. Notably, anabolism increased during hemodialysis in both the intradialytic ONS and IDPN groups, but this effect persisted post-dialysis only in the intradialytic ONS group7. Additionally, the effectiveness of intradialytic ONS in malnourished patients showed increased pre-albumin and albumin levels without complications arising from intradialytic ONS8. However, some studies have indicated that it might increase the risk of intradialytic hypotension9,10,11. Most of the data utilized originated from descriptive studies, and larger randomized trials are needed12.
To date, limited studies have compared the efficacy of ONS between intra-dialysis (INTRA-ONS group) and alternate-day dialysis (INTER-ONS) among malnourished end-stage kidney disease (ESKD) patients undergoing maintenance hemodialysis. The objective of this study is to compare these data and investigate potential side effects of oral dietary supplements during hemodialysis. Findings from this research will provide valuable insights into the treatment of malnutrition among ESKD patients undergoing hemodialysis.
Material and methods
Study population
Patients undergoing maintenance hemodialysis from September 2021 to March 2022 were included in the study. The inclusion criteria were age > 18 years, patients with ESKD on regular hemodialysis treatment for 4 h, three times weekly for ≥ 6 months, with adequate dialysis as indicated by a single pool Kt/V > 1.2 and a malnutrition inflammation score (MIS) ≥ 6. Exclusion criteria encompassed advanced liver disease, active malignancy, intradialytic hypotension, gut obstruction, gastrointestinal absorption issues, pregnancy or lactation, and recent hospitalization or major surgery within the last 3 months. Discontinuation criteria included unwillingness to continue the study, intolerable side effects, or allergies. The sample size was calculated based on the therapeutic effects of ONS during hemodialysis on changes in serum albumin, as reported in the study by Kayser et al.8 A 5% type I error rate and a 10% type II error rate were used in the calculation. Approximately 32 participants were involved in the research, providing a test power of 80%.
Measurement and outcomes
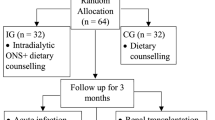
After screening, patients received standard treatment and dietary counseling from a dietitian. All patients underwent an assessment by a dietitian to ensure they understood self-care principles. Patients were randomly assigned to receive a 370 kcal supplement (80 g) of Once Dialyze (Thai Otsuka Pharmaceutical Co., Ltd., Thailand) during each hemodialysis session, three times per week (INTRA-ONS group), or on days without hemodialysis, three times per week (INTER-ONS group), for 12 weeks, as depicted in Fig. 1. The 80 g supplement is dissolved in 190 mL of water and then adjusted to a final volume of 250 mL before administration. The components of the Once Dialyze supplement, including proteins, carbohydrates with fiber, fats, electrolytes, and micronutrients, are shown in Table 1. Random allocation software was used for randomization with a block size of four.
The primary outcome assessed was the change in midweek predialysis serum albumin concentrations at 12 weeks between the INTRA-ONS and INTER-ONS groups. Secondary outcomes included changes in the MIS, dietary protein and energy intake, and body mass index (BMI) between the two groups.
Data collected before and after the study included relevant information on end-stage kidney disease (ESKD), diagnostic criteria, complications, other underlying diseases, comorbidities, medication history, and physical examination parameters such as height, weight, blood pressure, and BMI. The MIS was employed to evaluate the nutritional status of patients undergoing hemodialysis. The MIS assesses various components of nutritional and inflammatory status in patients, including changes in dry weight, dietary intake, gastrointestinal symptoms, functional capacity, fat storage, muscle wasting, co-morbidities, BMI, serum albumin levels, and total iron binding capacity (TIBC). Each component provides critical insights into the patient’s overall health and nutritional needs, particularly in those undergoing hemodialysis. The MIS comprises six anthropometric and laboratory values, each categorized into four severity levels, ranging from 0 (normal) to 3 (severely abnormal), with a total potential score of 3013.
Patients underwent data collection every 4 weeks, and follow-up blood tests were conducted both before and at the end of the study. These tests assessed fasting plasma glucose, serum electrolytes, calcium, phosphate, serum albumin, hemoglobin, blood urea nitrogen, creatinine, single-pool Kt/V, urea reduction ratio (URR), and normalized protein catabolic rate (normalized PCR). Dietary recalls for both dialysis and non-dialysis days were reviewed by a registered dietitian before and after the study period and analyzed for nutrient composition using the standard national food database program (Inmucal, Version 3.2). Adherence to ONS was also assessed through patient self-reports and dietary recalls conducted by a registered dietitian. During each hemodialysis visit, potential side effects were evaluated, and any abnormal symptoms were recorded in a side effects log form.
Ethical considerations
The study adhered to the Declaration of Helsinki (1964). It was registered at the Thai Clinical Trials Registry (TCTR20220322007, dated 16 September 2021). The study protocol received approval from the local Ethics Committee, and written informed consent was obtained from all eligible participants.
Statistical analysis
The intention-to-treat study was analyzed. Categorical variables were described as frequencies, while continuous variables were presented as mean ± standard deviation (SD) if normally distributed. Differences between groups were assessed using independent samples t-tests or Mann–Whitney U tests, and chi-squared tests or Fisher’s exact tests were used for continuous and categorical variables, respectively, as appropriate. Differences within groups were evaluated using paired t-tests. Results were reported as the difference in mean change with a 95% confidence interval (95% CI). A two-sided P-value of 0.05 was considered statistically significant for all analyses. Data analysis was conducted using SPSS for Windows, Version 12 (SPSS, Chicago, IL, USA).
Results
From a screening of a total of 48 patients undergoing regular hemodialysis three times weekly, 16 subjects were excluded. The 32 included patients were randomly divided into two groups, and all were 100% adherent to the ONS supplement. Baseline patient characteristics and laboratory tests categorized by treatment status are summarized in Table 2. Overall, the serum albumin concentration did not differ between the two groups: 3.20 ± 0.23 g/dL in the INTRA-ONS group and 3.27 ± 0.43 g/dL in the INTER-ONS group (P = 0.570). The MIS score was also similar in both groups: 12.94 ± 4.46 in the INTRA-ONS group versus 11.13 ± 4.21 in the INTER-ONS group (P = 0.247). The baseline characteristics of the two groups did not differ concerning age, gender, comorbid diseases, BMI, dietary protein and energy intake, and adequacy of dialysis.
Change of nutritional parameters during the study
The mean change in serum albumin from baseline was 0.23 (95% CI 0.04–0.43, P = 0.024) in the INTRA-ONS group and 0.19 (95% CI 0.07–0.31, P = 0.003) in the INTER-ONS group. At 12 weeks, no significant mean difference in serum albumin levels (0.04, 95% CI − 0.18 to 0.26) was found between the two groups, as shown in Fig. 2 and Table 3. Similarly, there were no significant differences in blood urea nitrogen, creatinine, daily calorie intake, dialysis protein intake, normalized PCR, body weight and BMI between the two groups, as shown in Table 3.
Interestingly, the malnutrition score using MIS significantly decreased in both the INTRA-ONS group [− 6.13 (95% CI − 8.29 to − 3.96), P < 0.001] and the INTER-ONS group [− 3.5 (95% CI − 5.56 to − 1.35), P = 0.003], as shown in Table 3. At 12 weeks, changes in the MIS significantly differed between the two groups [− 3.06 (95% CI − 5.94 to − 0.17), P = 0.038] (Fig. 2).
Adverse events after treatment
After 12 weeks, changes in blood glucose, pre- and post-dialysis blood pressure, and adequacy of dialysis did not significantly differ between the two groups (Table 3). Serum potassium and phosphate levels also showed no significant changes between the groups. No serious adverse events were observed, including severe abdominal pain, diarrhea, or serious electrolyte disturbances, in either group during the study period. Two patients experienced intradialytic hypotension, one patient experienced nausea, and one patient experienced diarrhea during ONS administration during hemodialysis. Meanwhile, one patient experienced intradialytic hypotension, two patients experienced nausea, and one patient experienced vomiting during ONS administration on non-dialysis days. None of the patients were forced to drop out of the study due to adverse effects from the treatment.
Discussion
The first randomized controlled trial investigated the efficacy of ONS supplement during hemodialysis versus on days without hemodialysis in ESKD patients undergoing hemodialysis with malnutrition. At 12 weeks of treatment, there was no significant difference in nutrition biomarkers, including serum albumin, dietary protein, and energy intake, blood urea nitrogen, and body composition between the INTRA-ONS and INTER-ONS groups, except for a significantly greater reduction in MIS in the INTRA-ONS group. No significant adverse events were observed between the two groups.
Current guideline has documented the importance of inadequate dietary protein and energy intake in ESKD patients undergoing hemodialysis14. Inadequate dietary intake contributes to decreased anabolism, and the dialysis procedure further exacerbates the negative nitrogen and energy balance due to amino acid losses into the dialysate15. Unfortunately, dietary counseling is not always successful in the ESKD population16. Meta-analysis has confirmed that nutrient supplementation during or after hemodialysis offers several benefits to patients, including increased energy, protein, and micronutrient intake, improved muscle strength, quality of life, and prevention and treatment of malnutrition17. Flexibility in administration allows ONS to be provided in various forms and timings for personalized care based on dietary preferences. Our study demonstrated some positive benefits of ONS during or after hemodialysis on nutritional status, including serum albumin, body weight, BMI and dietary nutrient intake.
The MIS is a tool used to assess the nutritional status of patients undergoing hemodialysis and has been shown to be a strong predictor of quality of life and mortality13,18. Therefore, appropriate therapeutic nutritional interventions are crucial for improving outcomes in hemodialysis patients19 Some studies suggest that interventions to improve nutritional status can also enhance inflammation and physical performance19,20. Each 2-unit increase in MIS is associated with a twofold greater risk of death in hemodialysis patients13. Previous studies found that intradialytic ONS combined with dietary counseling was more effective than dietary counseling alone in improving nutritional status and inflammation in chronic hemodialysis patients21,22,23,24. Our study also indicated that the effect of INTRA-ONS with a decrease in the MIS at 3 points might benefit hemodialysis patients with malnutrition compared to the INTER-ONS group.
Factors contributing to the greater reduction of MIS in the INTRA-ONS group may be related to the persistence of anabolism during and after hemodialysis. Studies have supported our finding that ONS during dialysis improves skeletal muscle protein homeostasis7 and prevents catabolism associated with dialysis25. Additionally, ONS may reduce inflammation, as ongoing inflammation can be exacerbated by declining nutritional status26. Renal-specific formulas and fat-based energy-dense supplements may effectively prevent energy deficits, improve inflammation, and dietary intake among ESKD patients on dialysis21,22,23,24. However, evidence regarding the effects of ONS during dialysis on nutritional status remains limited, necessitating further large-scale, high-quality studies.
Eating during hemodialysis can have both positive and negative effects on blood pressure. Some studies indicate that intradialytic food intake can negatively impact the postprandial blood pressure response27,28. However, other studies report no relationship between food intake and blood pressure during hemodialysis29. Several factors contribute to hemodialysis hypotension, including the reduction of circulating blood volume and sodium uptake. Our study found no significant relationship between eating during hemodialysis and blood pressure changes, and no serious adverse events were reported with ONS in either study group. However, there was an observed increase in systolic blood pressure by 10 mmHg in the INTER-ONS group. Factors that may have contributed to this finding include the consumption of ONS on non-dialysis days, which may have resulted in greater fluid intake and subsequent fluid retention, leading to increased blood volume and blood pressure. Patients in the INTER-ONS group may have had difficulty adhering to fluid restrictions on non-dialysis days when supplements were provided.
The study encountered several limitations. First, it was limited to a small, single-center design with a short follow-up period, which prevents drawing conclusions about the long-term effects of ONS during dialysis on the reduction MIS and related nutritional, metabolic, and inflammatory issues. However, the follow-up duration of up to 12 weeks was still deemed effective for observing significant changes in nutritional status and adherence to dietary plans30. Therefore, the long-term efficacy and safety of ONS during dialysis require further investigation. Second, we did not measure changes in other protein catabolism states and inflammatory biomarkers during treatment, and mechanistic associations between ONS during hemodialysis and overall nutritional changes in ESKD were not assessed.
In conclusion, ONS administration during dialysis in ESRD patients shows similar effects on overall nutritional biomarkers but produces lower MIS levels than controls after 12 weeks of treatment. Our findings suggest the therapeutic potential of ONS during hemodialysis in the ESRD population. Further study is necessary to determine the long-term effects of intradialytic ONS supplementation on the prevalence of malnutrition, hospitalization, and mortality among ESRD patients due to decreased MIS levels.
Data availability
All relevant data are within the paper.
References
Hubbard, G. P., Elia, M., Holdoway, A. & Stratton, R. J. A systematic review of compliance to oral nutritional supplements. Clin. Nutr. 31(3), 293–312. https://doi.org/10.1016/j.clnu.2011.11.020 (2012).
Lacquaniti, A. et al. Malnutrition in the elderly patient on dialysis. Ren. Fail. 31(3), 239–245. https://doi.org/10.1080/08860220802669891 (2009).
Badrasawi, M. et al. Prevalence and correlates of malnutrition among hemodialysis patients at hebron governmental hospital, Palestine: Cross-sectional study. BMC Nephrol. 22(1), 214. https://doi.org/10.1186/s12882-021-02413-y (2021).
Carrero, J. J. et al. Etiology of the protein-energy wasting syndrome in chronic kidney disease: A consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM). J. Ren. Nutr. 23(2), 77–90. https://doi.org/10.1053/j.jrn.2013.01.001 (2013).
Martins, V. S. et al. Can an intradialytic snack model compensate the catabolic impact of hemodialysis?. Clin. Nutr. ESPEN 42, 292–298. https://doi.org/10.1016/j.clnesp.2021.01.018 (2021).
Kistler, B. M. et al. Eating during hemodialysis treatment: A consensus statement from the international society of renal nutrition and metabolism. J. Ren. Nutr. 28(1), 4–12. https://doi.org/10.1053/j.jrn.2017.10.003 (2018).
Pupim, L. B., Majchrzak, K. M., Flakoll, P. J. & Ikizler, T. A. Intradialytic oral nutrition improves protein homeostasis in chronic hemodialysis patients with deranged nutritional status. J. Am. Soc. Nephrol. 17(11), 3149–3157. https://doi.org/10.1681/ASN.2006040413 (2006).
Caglar, K. et al. Therapeutic effects of oral nutritional supplementation during hemodialysis. Kidney Int. 62(3), 1054–1059. https://doi.org/10.1046/j.1523-1755.2002.00530.x (2002).
Zoccali, C., Mallamaci, F., Ciccarelli, M. & Maggiore, Q. Postprandial alterations in arterial pressure control during hemodialysis in uremic patients. Clin. Nephrol. 31(6), 323–326 (1989).
Agarwal, R. & Georgianos, P. Feeding during dialysis-risks and uncertainties. Nephrol. Dial. Transplant. 33(6), 917–922. https://doi.org/10.1093/ndt/gfx195 (2018).
Choi, M. S. et al. Pilot study of the effects of high-protein meals during hemodialysis on intradialytic hypotension in patients undergoing maintenance hemodialysis. J. Ren. Nutr. 29(2), 102–111. https://doi.org/10.1053/j.jrn.2018.06.002 (2019).
Kistler, B. et al. To eat or not to eat-international experiences with eating during hemodialysis treatment. J. Ren. Nutr. 24(6), 349–352. https://doi.org/10.1053/j.jrn.2014.08.003 (2014).
Rambod, M. et al. Association of Malnutrition-Inflammation Score with quality of life and mortality in hemodialysis patients: A 5-year prospective cohort study. Am. J. Kidney Dis. 53(2), 298–309. https://doi.org/10.1053/j.ajkd.2008.09.018 (2009).
Fiaccadori, E. et al. ESPEN guideline on clinical nutrition in hospitalized patients with acute or chronic kidney disease. Clin. Nutr. 40(4), 1644–1668. https://doi.org/10.1016/j.clnu.2021.01.028 (2021).
Ikizler, T. A. et al. Hemodialysis stimulates muscle and whole body protein loss and alters substrate oxidation. Am. J. Physiol. Endocrinol. Metab. 282(1), E107–E116. https://doi.org/10.1152/ajpendo.2002.282.1.E107 (2002).
Dwyer, J. T. et al. The hemodialysis pilot study: nutrition program and participant characteristics at baseline. J. Ren. Nutr. 8(1), 11–20. https://doi.org/10.1016/S1051-2276(98)90032-2 (1998).
Liu, P. J., Guo, J., Zhang, Y., Wang, F. & Yu, K. Effects of oral nutritional supplements on the nutritional status and inflammatory markers in patients on maintenance dialysis: A systematic review and meta-analysis of randomized clinical trials. Clin. Kidney J. 16(11), 2271–2288. https://doi.org/10.1093/ckj/sfad130 (2023).
Sa Martins, V. et al. Prognostic value of the malnutrition-inflammation score in hospitalization and mortality on long-term hemodialysis. J. Ren. Nutr. 32(5), 569–577. https://doi.org/10.1053/j.jrn.2021.11.002 (2022).
Alp Ikizler, T. et al. Prevention and treatment of protein energy wasting in chronic kidney disease patients: A consensus statement by the International Society of Renal Nutrition and Metabolism. Kidney Int. 84(6), 1096–1107. https://doi.org/10.1038/ki.2013.147 (2013).
Kanda, E. et al. The combination of malnutrition-inflammation and functional status limitations is associated with mortality in hemodialysis patients. Sci. Rep. 11(1), 1582. https://doi.org/10.1038/s41598-020-80716-0 (2021).
Yang, Y. et al. The effects of oral energy-dense supplements on nutritional status in nondiabetic maintenance hemodialysis patients: A randomized controlled trial. Clin. J. Am. Soc. Nephrol. 16(8), 1228–1236. https://doi.org/10.2215/CJN.16821020 (2021).
Satirapoj, B. et al. Nutritional status among peritoneal dialysis patients after oral supplement with ONCE dialyze formula. Int. J. Nephrol. Renovasc. Dis. 10, 145–151. https://doi.org/10.2147/IJNRD.S138047 (2017).
Limwannata, P., Satirapoj, B., Chotsriluecha, S., Thimachai, P. & Supasyndh, O. Effectiveness of renal-specific oral nutritional supplements compared with diet counseling in malnourished hemodialysis patients. Int. Urol. Nephrol. 53(8), 1675–1687. https://doi.org/10.1007/s11255-020-02768-5 (2021).
Tomayko, E. J., Kistler, B. M., Fitschen, P. J. & Wilund, K. R. Intradialytic protein supplementation reduces inflammation and improves physical function in maintenance hemodialysis patients. J. Ren. Nutr. 25(3), 276–283. https://doi.org/10.1053/j.jrn.2014.10.005 (2015).
Veeneman, J. M. et al. Protein intake during hemodialysis maintains a positive whole body protein balance in chronic hemodialysis patients. Am. J. Physiol. Endocrinol. Metab. 284(5), E954–E965. https://doi.org/10.1152/ajpendo.00264.2002 (2003).
Wright, S. & Weiner, D. E. Oral nutritional supplement use in dialysis patients: Full speed ahead?. Am. J. Kidney Dis. 60(4), 507–509. https://doi.org/10.1053/j.ajkd.2012.07.004 (2012).
Avci, M. & Arikan, F. The effect of food intake during hemodialysis on blood pressure: A nonrandomized experimental trial. Ther. Apher. Dial 27(4), 661–668. https://doi.org/10.1111/1744-9987.13967 (2023).
Sivalingam, M., Banerjee, A., Nevett, G. & Farrington, K. Haemodynamic effects of food intake during haemodialysis. Blood Purif. 26(2), 157–162. https://doi.org/10.1159/000114094 (2008).
Benaroia, M. & Iliescu, E. A. Oral intake during hemodialysis: Is there an association with intradialytic hypotension?. Hemodial. Int. 12(1), 62–65. https://doi.org/10.1111/j.1542-4758.2008.00242.x (2008).
Ingstad, K., Uhrenfeldt, L., Kymre, I. G., Skrubbeltrang, C. & Pedersen, P. Effectiveness of individualised nutritional care plans to reduce malnutrition during hospitalisation and up to 3 months post-discharge: A systematic scoping review. BMJ Open 10(11), e040439. https://doi.org/10.1136/bmjopen-2020-040439 (2020).
Acknowledgements
The authors wish to thank the staff in the Division of Nephrology, Department of Medicine, Phramongkutklao Hospital and College of Medicine, for their research contributions.
Author information
Authors and Affiliations
Contributions
T.A. and B.S. conceived the research idea and study design and performed data acquisition as well as data analysis/interpretation. T.A., P.T., N.N., O.S. and B.S. performed data acquisition. O.S. and B.S. supervised the work and provided mentorship. B.S. takes responsibility that this study has been reported honestly, accurately, and transparently and accepts the accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statement
The study protocol was approved by the Ethics Committee of the Institute Review Board of the Royal Thai Army Medical Department (R103h/64).
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Satirapoj, B., Apiyangkool, T., Thimachai, P. et al. Intradialytic oral nutrition effects on malnourished hemodialysis patients: a randomized trial. Sci Rep 14, 21400 (2024). https://doi.org/10.1038/s41598-024-72402-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-72402-2
- Springer Nature Limited