Abstract
Anthropogenic activities have impacted marine ecosystems at extraordinary scales. Biogenic reef ecosystems built by the European flat oyster (Ostrea edulis) typically declined before scientific monitoring. The past form and extent of these habitats thus remains unknown, with such information potentially providing valuable perspectives for current management and policy. Collating >1,600 records published over 350 years, we created a map of historical oyster reef presence at the resolution of 10 km2 across its biogeographic range, including documenting abundant reef habitats along the coasts of France, Denmark, Ireland and the United Kingdom. Spatial extent data were available from just 26% of locations yet totalled >1.7 million hectares (median reef size = 29.9 ha, range 0.01–1,536,000 ha), with 190 associated macrofauna species from 13 phyla described. Our analysis demonstrates that oyster reefs were once a dominant three-dimensional feature of European coastlines, with their loss pointing to a fundamental restructuring and ‘flattening’ of coastal and shallow-shelf seafloors. This unique empirical record demonstrates the highly degraded nature of European seas and provides key baseline context for international restoration commitments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Main
Destructive fishing activities, pollution and reclamation have resulted in large-scale marine and coastal habitat degradation and loss globally1. European seas are among the most impacted marine environments2, and there is common agreement on the urgency to conserve and restore habitats to support and recover key ecological functions3,4. However, without an understanding of the full extent of ecological changes resulting from human influence, the setting of policy goals can be impeded or contested5.
Assessments of human impact are commonly restricted by the short time span of modern scientific data, which is typically limited to recent decades2. In contrast, activities such as fishing and coastal harvesting have occurred for centuries to millennia1,6. The early, intense and geographically broad exploitation of marine resources in Europe presents a critical challenge for the identification of ecological baselines and requires substantially deeper time perspectives than those available from scientific monitoring data7. Yet there remains a lack of integration of historical perspectives into management and policy due to challenges such as resolving differences in spatial resolution of historical versus modern data, issues with data certainty, small sample sizes and a patchy historical record, among others7.
Despite advances in our understanding of historical dynamics in marine environments, studies focusing on historical changes across a species’ biogeographic range remain limited. The European flat oyster (Ostrea edulis, Linneaus, 1758 ‘flat oyster’ hereafter) is a benthic habitat-forming species that was once of economic and cultural importance across Europe. This led to its representation in numerous historical sources published in multiple countries8. Interrogation of the historical record for this species thus presents a unique opportunity to understand the historical distribution and characteristics of a biogenic marine habitat, one that is functionally extinct due to human activity, across its full biogeographic range, and subsequently acts as a signal of the scale of change in shallow European shelf seas over the course of centuries.
Seabed habitat-forming species are particularly vulnerable to widespread and persistent human impacts such as trawling and dredging1,2. Marine biogenic habitats are formed by assemblages of sessile benthic organisms, which create emergent physical structures distinct from the surrounding seabed9. These habitats are formed by a range of taxa, including bivalves, annelids, corals, sea grasses and macroalgae10,11,12,13,14. They support multiple ecosystem services, high levels of biodiversity and influence ecosystem functioning by creating a complex, three-dimensional surface that other species adhere to, shelter within or feed upon15,16. Their vulnerability to human-induced pressures means many have deteriorated in quality, declined in extent or vertical relief, or been rendered functionally extinct by fishing, coastal development, eutrophication and pollution, disease and the effects of climate change17,18,19,20.
Many species of oyster (for example, Ostrea, Crassostrea, Saccostrea spp.) create biogenic habitat through gregarious settlement. But centuries of degradation and loss of oyster habitat globally mean that examples of undisturbed reefs are rare21. Thus, our knowledge of the characteristics of oyster reefs (for example, extent, vertical relief, density of oysters, species composition) is variable across genera and locations, and is mostly derived from locations where extant, remnant reefs exist or have been actively restored22.
The flat oyster is a habitat-building oyster native to European seas23. Flat oyster exploitation has occurred for thousands of years, with shell remains preserved in kitchen midden deposits from the Mesolithic period24. Until the early twentieth century, European flat oysters were sufficiently abundant to support a major commercial fishery across multiple European countries; however, overexploitation led to the widespread removal and decline of oyster reefs, with population collapse exacerbated by decreasing water quality, sedimentation and the introduction in the 1970s of the disease-causing haplosporidian, Bonamia ostreae and the protozoan Marteilia refringens8,25. We know of the species’ widespread decline21, but not where habitat once existed, the form of the habitat (for example, density, areal extent) before exploitation, or its importance for associated communities.
Today, there is a growing impetus to conserve and restore marine ecosystems at scale26, furthered by policies such as the Habitats Directive of the EU, the UN Decade on Ecosystem Restoration, the EU’s Nature Restoration Law and, in the case of the European flat oyster, its recognition by OSPAR as a ‘Threatened or declining species’27. Developing a robust and evidenced historical baseline, both in terms of extent and condition of flat oyster habitats, is of critical importance for guiding restoration efforts and for informing policy relating to the conservation of this formerly widespread species5. While there are several modern examples of flat oysters of multiple size classes clustering to form small clumps28,29,30,31, the majority of known European flat oyster populations are found at average densities of <1 individual m−2 (for example, refs. 32,33,34).
Here we collate information from the historical documentary evidence to establish a uniquely resolved, ecosystem-wide, robust historical baseline for flat oyster reefs. Specifically, we identify evidence for (1) the historical range and locations of flat oyster reefs, (2) size or extent and (3) characteristics of these reefs and their associated communities in European seas.
Results
Documentary evidence was sourced from popular books, scholarly papers, government reports, customs accounts, oyster licensing records, travelogues, naturalists’ accounts, newspaper articles, nautical charts and scientific surveys. Records included reports of oyster fisheries and habitat presence recorded >2,000 years before present until the 1970s35,36.
Flat oyster habitat distribution
Two hundred and twenty-five sources provided 1,667 records of oyster fisheries, presence or habitat, published between 1524 and 2022 (Supplementary Fig. 1 and Supplementary Information). These were mapped to 1,196 locations across Europe and North Africa where fishable quantities of flat oysters and/or oyster biogenic reef habitats were historically described. This translated to oyster presence being assigned to 713 10 km2 grid cells, with 85% (n = 606 grid cells) assigned a high confidence that biogenic reefs were once present (that is, where sources indicated the presence of oysters was high enough to support a towed-gear fishery with substantial landings, or bank/reef features were mentioned; Fig. 1 and Supplementary Fig. 2). High confidence of past oyster reef presence was assigned to broad swathes of the coastlines of the United Kingdom, France, Ireland, Denmark, Spain, Germany and the Netherlands, for which 205, 109, 75, 38, 37, 30 and 27 grid cells within 12 nautical miles of the coast were recorded as high confidence, respectively (Fig. 1a–c). High confidence of historical reef presence was also assigned to sections of the coastline around Italy (22 grid cells), most notably the Northern Adriatic (Fig. 1e). Substantial areas of reef habitat were historically reported in the southern North Sea and the English Channel8, although their locations were not able to be defined within a 10 km2 area and hence were marked as low locational certainty (Fig. 1b and Table 1). The large area of contiguous oyster habitat shown in the Southern North Sea (Fig. 1a,b in blue) probably reflects several very extensive oyster reef systems (Tables 1 and 2). Historical documentary records were not found for parts of the southern and central Mediterranean or the Baltic Sea.
Oysters were reported in fishable quantities at depths spanning the intertidal zone to >80 m (n records reporting depth = 103). The deepest locations reported were in the English Channel (84 m) and the Atlantic coast of Morocco (85 m)35. Fishable quantities of oysters were reported at depths >40 m in the southern North Sea, the English Channel, the Irish Sea and occasionally inshore locations such as Belfast Lough35. Quantities of oysters were reported in the intertidal or shallow subtidal zone in Northern Ireland (Strangford Lough), the Republic of Ireland (Sligo River), Wales (Mumbles), Scotland (Kirkcudbright) and the northern coast of France (Cancale)35 (Supplementary Fig. 3a and Table 1).
Areal extent
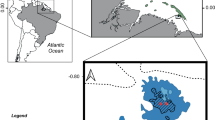
Habitat extent (area or length) was reported in 52 sources published between 1715 and 1910. Despite finding only 317 quantitative descriptions of habitat extent, the area assigned high confidence of reef presence totalled 1,758,077 hectares. Descriptions of areal extent of individual reefs ranged from 0.01 ha to 1,536,000 ha (median = 29.9 ha) and included locations along the coasts of the United Kingdom, Ireland, France, Germany and Denmark, the Netherlands, the northern coast of Spain and the north-east coast of Italy, as well as the southern North Sea and the English Channel (Fig. 2). The largest of these were reported from the southern North Sea/German Bight region, at 1.5 million ha, with substantial extents also described around the Channel Islands, southeast coast of England, south coast of Wales, and the east and west coasts of Ireland. Highly resolved oyster habitats were sourced from the coasts of France and the Wadden Sea (Fig. 2 and Supplementary Fig. 3b). The length of described reefs ranged from 0.02 to 320 km (median = 4.0 km, n = 45 locations; Supplementary Fig. 4).
a, Recorded extent (hectares, orange circles) of oyster reefs. The crosses indicate records of reefs of unknown areal extent. b, The coast of France, from which data were predominantly extracted from charts published in ref. 68. c, The Wadden Sea, with oyster reef extents extracted from ref. 66. Circle sizes remain consistent across panels. Base map source, ref. 82.
Reef form
Descriptions of reef height and structure typically referred to exploited reef habitats, with few historical descriptions of unexploited reefs found. As recently as 2008, the remains of flat oyster reefs at heights of up to 7 m were described in the Black Sea37. Within the historical literature, descriptions of reef form, although limited, exist for multiple locations (Table 2). Historical sources described ‘clumps’ of oysters in exploited areas, vertical reef formations, or an observed increase in seabed depth as reefs were removed by dredges and bottom trawlers35. When newly discovered oyster locations were described in historical accounts, catches and catch rates indicative of high densities of oysters were recorded (Table 2).
Associated species
A total of 190 species associated with oyster reef habitats were recorded across 13 phyla, representing 7 trophic guilds (Supplementary Table 1 and Fig. 3). The distribution of species differed significantly across trophic guilds (Kruskal–Wallis H(6) = 17.718, P = 0.007) and phylum classifications (H(9) = 19.494, P = 0.021). The trophic guilds were dominated by active suspension feeders (n species = 68, 36% of species observed), carnivores (n spp. = 45, 24%) and omnivores (n spp. = 36, 19%). Significantly more species were observed within the suspension feeding trophic guild (dominated by cnidarian, molluscan and bryozoan species), than most of the other phyla groupings (parasitic H = 31.45, P = 0.001; herbivorous H = 26.6, P = 0.001; filter feeders H = 20.3, P = 0.01; omnivores H = 17.1, P = 0.04). Across the 13 phyla, Arthropoda (n spp. = 47, 25%), Mollusca (n spp. = 39, 20%) and Cnidaria (n spp. = 26, 13%) contributed almost two-thirds of the species observed. The Arthropoda included species across six trophic guilds, the majority from the subphylum Crustacea. The Mollusca contained 39 species from five trophic guilds and included other suspension feeding bivalves. The 26 Cnidaria species were mostly suspension feeders. Apex predators were also observed, including thornback ray (Raja clavata), common stingray (Dasyatis pastinaca), short-snouted seahorse (Hippocampus hippocampus) and the now critically endangered European sturgeon (Acipenser sp.) (see Supplementary Table 1 for further IUCN Species Redlist Classifications and population trends of assessed species).
Number of species historically observed in association with historical oyster reef habitats in Northern Europe, assigned to phyla and trophic guild. Sources are provided in Supplementary Information.
Discussion
The results presented here provide presumably the first highly resolved, comprehensive overview of the spatial distribution, areal extent and habitat structure of a benthic marine ecosystem in Europe, before its widespread functional extinction. Centuries of economic, popular and scientific interest in the European flat oyster have produced a record that is probably unique in terms of the longevity and diversity of written sources dedicated to a marine species38,39. Despite this extensive historical record, the past distribution of the European flat oyster, its habitat structure and contributions to ecosystem functioning remain unknown and contested29. In collating an ecosystem-wide picture, this study indicates a substantial and, until now, largely unquantified scale of physical structural transformation in European seabeds before the twentieth century, with corresponding implications for the articulation of conservation and restoration goals.
Transformation of European seafloors
Historically, flat oysters formed complex three-dimensional, biogenic reef habitats that could span extents of >10 ha, and which supported a diverse associated community (Figs. 1–3 and Table 1). Although wild populations of flat oysters persist in some limited locations today, the biogenic habitat that once formed has almost entirely disappeared33,34,40,41. Extant flat oyster populations are universally described as patchy, with small areas of higher densities sometimes found within a broader landscape of sparsely distributed oysters31,33,42,43. In contrast, past descriptions identify oysters often clustered together, as highly abundant over extensive areas, and living and dead individuals forming raised three-dimensional seabed structures at sizes and scales not observed today36 (Tables 1 and 2). That no known wild native oyster reefs remain at a scale of >1 ha thus signals an unprecedented loss of emergent biogenic structure in European seas, with potentially analogous losses for marine biodiversity41.
Oyster habitat historically supported a high taxonomic diversity of associated species (Fig. 3). This diversity was characterized by multiple trophic levels that likely enhanced ecosystem functioning44 (Fig. 3), such as nutrient cycling through bentho-pelagic coupling, secondary production and the increased transfer of energy across trophic levels45. Given the large reported extent of the historical habitat, it is likely that these biogenic reefs played a vital role in supporting European coastal seas trophic webs, driving bentho-pelagic coupling of seston-derived nutrients and creating complex habitats that provided refuge or food sources for benthic and pelagic fish populations. This is supported by findings from extant remnant oyster reefs in other biogeographic regions, showing that the reef structure and rich biota associated with oyster habitat support consumers at higher trophic levels46 and function as nursery grounds, therefore enhancing fisheries production (for example, Eastern USA and northern Gulf of Mexico47). Although such inference is beyond the scope of our data, we show that commercially targeted species were historically recorded at oyster reefs (for example, Homarus gammarus, Cancer pagurus, Pleuronectes platessa). As oyster reefs typically increase the structural complexity of the underlying substrate, their presence also supported the persistence of a community whose composition differed from that of surrounding habitats, probably contributing to a higher beta-diversity across the wider system, as observed in other systems48,49. The complex, three-dimensional structure of reefs and biodeposition of organic matter is also likely to have influenced local hydrodynamic-regime and sedimentation processes50. The impacts of these extensive reef structures upon nearshore ecosystem functioning remain unknown, but evidence from extant and restored systems within and outside of Europe suggests that reef presence can enhance carbon burial and accumulation rates51, reduce erosion and improve water quality52.
Evidence of reef-forming habitat was particularly extensive for the southern North Sea, Irish Sea, English Channel and surrounding coastlines, and the western coasts of the Adriatic and Black Seas (Fig. 1). We found no descriptions of oyster reef habitat in the Baltic Sea, with shell remains in the region potentially linked to trade and/or failed efforts to transplant oysters53. It is less clear whether our findings represent a fair reflection of the distribution and form of oyster reefs in the Mediterranean. Low confidence of reef-forming habitat in this region could be reflective of sources remaining hidden, earlier losses of oyster habitat driven by exploitation (for example, ref. 54), changing hydrographic flows or sedimentation rates (for example, ref. 55), or different environmental conditions for growth meaning that reef habitats are less likely to form towards the edge of their range. The situation is made even more complex by the historically uncertain nomenclature of the Ostrea species complex in the Mediterranean56. Nevertheless, the available descriptions indicate that the occurrence of Ostreidae spp., with the notable exception of reef descriptions in the northern Adriatic, were largely patchy in their distribution and associated with rocky habitats throughout the Mediterranean36.
Despite the striking magnitude of oyster reefs described historically in this study, the historical accounts commonly describe oyster populations ‘after’ the commencement of wide-scale exploitation57,58. The majority of written records used in this study were published during the nineteenth century (Supplementary Fig. 1), but occasional accounts before this period described localized declines or losses of oyster habitat as a result of fishing pressure (for example, refs. 59,60). Moreover, harvested flat oyster shell remains dating from the Mesolithic period have been found in archaeological deposits across Atlantic Europe, demonstrating that coastal flat oyster populations have been exploited for thousands of years61,62. Written historical descriptions of these reefs thus cannot be considered pristine. While the findings in this study cannot represent an unexploited ecosystem, the evidence still affords robust insights into the past ecological importance and extent of oyster reef habitats across the species’ historical distribution.
Drivers of change
Historical sources are increasingly used to reveal the scale and drivers of ecological changes through time1,7. Proposed drivers of change were commonly observed in written records, including reports of the rapid loss of reef habitat when heavily exploited (Table 2). Overexploitation was mentioned in some earlier written records as being responsible for the decline and loss of oyster reefs (for example, refs. 63,64), but the frequency and geographic breadth of records proposing overexploitation as the primary cause of decline expanded rapidly in the nineteenth century (for example, refs. 57,65,66,67,68), despite attempts to bolster declining populations via translocation and culture69. Glimpses of wider environmental changes and their impacts upon oyster reef persistence are also observed. These include reports of oyster mortalities under very cold winter conditions, which frequently affected shallow-water oyster layings70, and the influence of changing hydrographic regimes, such as the nineteenth century expansion of flat oysters into the western Limfjord after storm-induced hydrodynamic and salinity changes71. While disease was reported as having serious impacts on the persistence of oyster populations from the twentieth century onwards (for example, ref. 72), disease was infrequently mentioned in earlier documents73. The almost complete removal of oyster habitat from European coastal waters started by widespread fishing and mechanical extraction, was thus compounded by a cascade of degradation, with pollution, introduced species, disease and climate change contributing to further declines from the late 1800s.
Policy applications
The restoration of biodiversity is of increasing policy interest at local to international scales4,74,75. In practice, relatively small patches of higher oyster density (a few m2 in extent) are often defined as oyster habitat in conservation advice, or larger areas of very low oyster density (that is, 0.5–2.0 oyster m-2) defined as habitat for fishery management on a national level43. Such definitions reflect the current rather than historical status of this habitat. These remaining patches of oyster habitat are of high conservation value given their rarity and the gains in local biodiversity fostered by their presence76,77. Recognition of the value of these remnant habitats is important to ensure existing protections are not removed as baselines are reconsidered. However, policies that rely on remnant populations alone to define habitat extent and form, or to articulate restoration goals, risk underestimating the past importance and influence of oyster habitat on seabed complexity, biodiversity and species-associated behaviours at an ecosystem scale31,78. In addition to directly supporting restoration science (for example, ref. 79), a historical evidence base for native oyster is of considerable importance for encouraging the reconsideration of policy decisions based on a notably shifted environmental baseline75. Our findings demonstrate that restoring even a fraction of these past habitats requires both ambitious policy agreements and a step-change in our understanding of the long-term nature of human-induced ecosystem degradation and the scales of historical loss in marine ecosystems.
Given the lack of long-term records for broader benthic ecosystems, our data further serve as a rare opportunity to visualize the fundamental restructuring of coastal and shallow-shelf seafloors resulting from centuries of human impact. The expansive historical documentary record for the flat oyster provides a unique empirical record that acts as a broader signal of the highly degraded current status of European seas. Studies such as this are critical for understanding the present-day degraded status of habitat-forming species in marine coastal waters and provide key context to global sustainable development goals and recent international commitments to restore the seas. In addition to informing restoration targets, our findings present a sobering reminder of the scale of work to be achieved if we are to restore even a small fraction of what has been lost from our seas.
Methods
Team development
Initial collaborators were identified via self-selected membership of the Native Oyster Restoration Alliance, a pan-European network of researchers and practitioners specializing in historical ecology, oyster biology, ecology, conservation and restoration26,29. Calls for collaboration were also advertised at related workshops and conferences. Additional collaborators were approached individually when a specific knowledge gap was identified during the data collation phase. These experts were identified by the lead authors through targeted literature searches or by asking in-country researchers already known to the group.
Sources and search terms
National library and museum collections were searched for references to historical oyster habitat and fisheries, including government records, nautical charts, naturalists’ accounts, fishery reports, customs accounts, popular media and scientific journals. In addition to ‘oyster’ and ‘Ostrea edulis’, search terms included regional and local name variations, such as ‘flat oyster’, ‘native oyster’, ‘mud oyster’, ‘edible oyster’, ‘Pandores’ (Scotland), ‘huîtres plates’, ‘belons’ and ‘huîtrière’ (France), ‘østers’ (Denmark), ‘Auster’ (Germany), ‘zeeuwse platte’, ‘zeeuwse bolle’ (Belgium/the Netherlands), ‘ostra plana’ (Spain) and ‘ostrica piatta’ (Italy). Mapping the locations of past oyster habitat as data were submitted enabled the identification of gaps and precipitated further targeted searches. While archaeological records provide extensive and useful information about pre-industrial fisheries, it is challenging to reconstruct historical habitat extent or habitat characteristics, which were the focus of this study, hence data collection primarily focused on the written record.
Biogenic oyster habitat is referred to as both ‘reefs’ and ‘beds’ across Europe, while much historical literature referred to high densities of oysters as a ‘bed’ or ‘bank’. For consistency, we used the term ‘reef’ as analogous to oyster biogenic habitat and ‘beds’ or ‘banks’, which we collectively defined (sensu European Habitats Directive Appendix I) as ‘a biogenic hard bottom that arises from the seafloor and originates from dead or living oysters and associated species, which supply habitats for epibiotic species’.
Data extraction
Locations of oyster fisheries or oyster reefs were extracted from historical written sources. The locations of described fisheries and reef habitats were estimated from descriptions or identified from charts/mapped areas and assigned to 10 km2 grid cells. In instances where historical place names were no longer in use or where nautical locations (for example, names of fishing grounds) were mentioned, we identified locations by cross-checking with historical nautical charts or maps. Contextual descriptions within historical sources were also used to identify the likely area referred to, which was then cross-checked using historical charts. For reefs marked on nautical charts or mapped in more recent publications, areas were traced using the polygon tool in ArcGIS and the centroids of each polygon were converted into point data (latitude and longitude). In written descriptions, oyster grounds could be named after the local town and/or a cursory description of the location provided, for example, the number of miles from shore. In other cases, oyster presence might be described as occurring within a harbour or bay. As such, 10 km2 was deemed a reasonable level of precision for most locations, although some occurrences could be reasonably identified to a higher resolution. Locations where oysters were reported within the intertidal zone or shoreline were noted. ‘Shore’ was assigned when oysters were mentioned as present at very shallow depths (for example, descriptions included people ‘wading’ for oysters or otherwise picking them by hand), but it was unclear whether this included the intertidal zone. Descriptions of oyster reefs that were far larger than 10 km2 were allocated a grid point within the estimated central part of the range, and the relevant additional number of grids (related to the described size) was highlighted but identified as low confidence in location to emphasize the likely but uncertain location of this reported extent of habitat.
The extent (length or area) and depth of described oyster reef habitats were extracted from written records and nautical charts, with a mean value assigned if a range of measurements was described. Reef extents were differentiated from each other using the descriptive locational data, and where overlap was considered likely (that is, descriptions of the location of a reef were vague, such as occurred for records describing the vast extent of oyster reef habitat in the southern North Sea), suspected duplicates were removed. When using nautical charts, because some of the polygon boundaries were difficult to differentiate, areas of oysters were considered independent reefs if separated by more than 200 m.
Descriptions of habitat characteristics were also recorded, such as the depth at which oyster habitat was found, extent, habitat structure and associated species. While flat oysters form biogenic habitat in suitable environmental conditions, they also occur singly. Historical sources were commonly concerned with recording oyster extraction rather than describing the characteristics of the habitat directly, with exploited habitats commonly termed ‘beds’ or ‘banks’. For regions where descriptions of oyster reefs existed and where dredge or trawl gear were primarily used to exploit oysters, we interpreted the presence of fisheries with notable landings as high confidence that oyster reefs were once present in an area. Although today’s dredge oyster fisheries will exploit oyster populations at low densities (for example, refs. 78), historical dredge fisheries often reported high catch rates when encountering newly discovered oyster grounds35. Conversely, in regions where written descriptions of reefs were not found and/or where fisheries indicated extraction by diving and handpicking, as opposed to extracting high volumes by dredge, low confidence of reef habitat was assumed.
Survey data that identified the presence of an individual or very low numbers of oysters were excluded, as were archaeological or museum records where the abundance or original location of past oysters was unclear. Locations (for example, oyster ponds) that were clearly created for oyster culture were discarded. Records were also excluded if it was deemed likely that the species of oyster referred to was not O. edulis. Non-native species of oysters were introduced as flat oyster abundance declined; for example, the Portuguese oyster (Crassostrea angulata, also known as Magallana angulata) was introduced along the French Atlantic coast from 1860 and spread rapidly80, and was also cultivated in British waters during the nineteenth century81. Historical records that differentiated between oyster species (for example, refs. 68), or that clearly referred to flat oysters were thus preferentially sourced. Written historical records were used wherever possible, but where such records could not be identified and contemporary or material records were available, these were consulted in place of written descriptions.
Levels of confidence that historical sources were referring to biogenic oyster reef habitats, as opposed to scattered oysters, and confidence of location accuracy were assigned on the basis of the following criteria:
High confidence of reef habitat, high location certainty (HH): record of habitat, for example, a bed or bank of oysters, or record of an active fishery using towed gears with notable landings and no recorded active intervention, thus indicating an initial high abundance of oysters. We are confident of the location to within 10 km, for example, oyster presence within a bay or harbour.
High confidence of reef habitat, low location certainty (HL): we were confident a habitat existed, but the location is uncertain to >10 km, for example, named open-water locations without positioning detail.
Low confidence of reef habitat, high location certainty (LH): we know oysters were fished but the descriptions (or corresponding descriptions) do not provide evidence that the species formed biogenic reefs in this location, for example, individuals were described as attached to rocks. We are confident of the location to within 10 km.
Low confidence of reef habitat, low location certainty (LL): we know oysters were fished, but the descriptions (or corresponding descriptions) do not provide evidence that the species formed biogenic reefs in this location. The location is uncertain to >10 km.
Data visualization
Digitizing and spatial visualization were completed using QGIS software v.3.24 (QGIS Development Team). European coastline boundaries were derived from the European Environment Agency’s open-source Europe coastline shapefile, and European country boundaries were derived from the open-source Eurostat shapefile titled Countries 202082. In cases of historical jurisdictional changes (for example, changes to national borders), present-day nation boundaries and waters were applied. The Coordinate Reference System (CRS) used is ETRS89-extended/LAEA Europe. The locations of major seas and sea basins as described in the manuscript are shown in Supplementary Fig. 5.
Associated biodiversity
Species associated with oyster reef habitat were extracted from 12 sources published over a period of 150 years and which predominantly focused on the coasts of Germany, Denmark, Britain and Sweden (Supplementary Table 1)35. Species identified were corrected to currently accepted species names as listed in the world register of marine species (WoRMS; https://www.marinespecies.org/) and taxonomic classification was assigned to each species from kingdom to genus levels (including phylum, subphylum, class and order where applicable). IUCN Redlist Classifications and population trends were listed for assessed species (https://www.iucnredlist.org). Each species was assigned a trophic guild using published descriptions listed in WoRMs or the Marine Life Information Network (MarLIN; https://www.marlin.ac.uk/) databases. The trophic guilds combined types of feeding and trophic level (Supplementary Table 2) to enable both the ecological and trophic functions to be resolved. Statistical analysis was performed using IBM SPSS Statistics v.27. Kolmogorov–Smirnov and Shapiro–Wilk tests of normality were used before non-parametric (independent-samples Kruskal–Wallis) tests to assess distribution of species across phyla and trophic guilds.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data that support the findings of this study are available in figshare at https://doi.org/10.6084/m9.figshare.c.6884167.v1 (ref. 35). The world register of marine species (WoRMS; https://www.marinespecies.org/) and Marine Life Information Network (MarLIN; https://www.marlin.ac.uk/) databases are publicly available. For the purpose of open access, the author has applied a Creative Commons Attribution (CC BY) licence to any author-accepted manuscript version arising from this submission.
References
Lotze, H. K. et al. Depletion, degradation, and recovery potential of estuaries and coastal Seas. Science 312, 1806–1809 (2006).
Airoldi, L. & Beck, M. W. Loss, status and trends for coastal marine habitats of Europe. Oceanogr. Mar. Biol. 45, 345–405 (2007).
Waltham, N. J. et al. UN Decade on Ecosystem Restoration 2021–2030—what chance for success in restoring coastal ecosystems? Front. Mar. Sci. https://doi.org/10.3389/fmars.2020.00071 (2020).
The UN Decade on Ecosystem Restoration 2021–2030 UNEP/FAO Factsheet (United Nations Environment Programme and the Food and Agriculture Organisation of the United Nations, 2020).
zu Ermgassen, P. S. E. et al. Forty questions of importance to the policy and practice of native oyster reef restoration in Europe. Aquat. Conserv. 30, 2038–2049 (2020).
Erlandson, J., Rick, T., Braje, T., Steinberg, A. & Vellanoweth, R. Human impacts on ancient shellfish: a 10,000 year record from San Miguel Island, California. J. Archaeol. Sci. 35, 2144–2152 (2008).
Engelhard, G. H. et al. ICES meets marine historical ecology: placing the history of fish and fisheries in current policy context. ICES J. Mar. Sci. 73, 1386–1403 (2015).
Bennema, F. P., Engelhard, G. H. & Lindeboom, H. Ostrea edulis beds in the central North Sea: delineation, ecology, and restoration. ICES J. Mar. Sci. 77, 2694–2705 (2020).
Holt, T. J., Rees, E. I., Hawkins, S. J. & Seed, R. Biogenic Reefs. An Overview of Dynamic and Sensitivity Characteristics for Conservation Management of Marine SACs Vol. 9 (UK Marine SACs Project, 1998).
De Clippele, L. H. et al. Using novel acoustic and visual mapping tools to predict the small-scale spatial distribution of live biogenic reef framework in cold-water coral habitats. Coral Reefs 36, 255–268 (2017).
Gravina, M. F. et al. Sabellaria spinulosa (Polychaeta, Annelida) reefs in the Mediterranean Sea: habitat mapping, dynamics and associated fauna for conservation management. Estuar. Coast. Shelf Sci. 200, 248–257 (2018).
Piazzi, L. et al. Variations in coralligenous assemblages from local to biogeographic spatial scale. Mar. Environ. Res. 169, 105375 (2021).
Tamburello, L. et al. Can we preserve and restore overlooked macroalgal forests? Sci. Total Environ. 806, 150855 (2022).
Richardson, M. A., Zhang, Y., Connolly, R. M., Gillies, C. L. & McDougall, C. Some like it hot: the ecology, ecosystem benefits and restoration potential of oyster reefs in tropical waters. Front. Mar. Sci. 9, 873768 (2022).
Kazanidis, G. et al. One on top of the other: exploring the habitat cascades phenomenon in iconic biogenic marine habitats. Diversity 14, 290 (2022).
Thomsen, M. S. et al. Heterogeneity within and among co-occurring foundation species increases biodiversity. Nat. Commun. 13, 581 (2022).
De’ath, G., Fabricius, K. E., Sweatman, H. & Puotinen, M. The 27-year decline of coral cover on the Great Barrier Reef and its causes. Proc. Natl Acad. Sci. USA 109, 17995–17999 (2012).
Zu Ermgassen, P. S. E. et al. Historical ecology with real numbers: past and present extent and biomass of an imperilled estuarine habitat. Proc. R. Soc. B 279, 3393–3400 (2012).
Sunday, J. M. et al. Ocean acidification can mediate biodiversity shifts by changing biogenic habitat. Nat. Clim. Change 7, 81–85 (2017).
Filbee-Dexter, K. & Wernberg, T. Rise of turfs: a new battlefront for globally declining kelp forests. Bioscience 68, 64–76 (2018).
Beck, M. W. et al. Oyster reefs at risk and recommendations for conservation, restoration, and management. Bioscience 61, 107–116 (2011).
Hemraj, D. A. et al. Oyster reef restoration fails to recoup global historic ecosystem losses despite substantial biodiversity gain. Sci. Adv. 8, eabp8747 (2022).
OSPAR Commission. Background document for Ostrea edulis and Ostrea edulis beds (OSPAR, 2009).
Lewis, J. P. et al. The shellfish enigma across the Mesolithic–Neolithic transition in southern Scandinavia. Quat. Sci. Rev. 151, 315–320 (2016).
Berghahn, R. & Ruth, M. The disappearance of oysters from the Wadden Sea: a cautionary tale for no-take zones. Aquat. Conserv. 15, 91–104 (2005).
Pogoda, B. et al. The Native Oyster Restoration Alliance (NORA) and the Berlin Oyster Recommendation: bringing back a key ecosystem engineer by developing and supporting best practice in Europe. Aquat. Living Resour. 32, 13 (2019).
OSPAR Commission. Recommendation 2013/4 on Furthering the Protection and Conservation of Ostrea edulis in Region II and III of the OSPAR Maritime Area and Ostrea edulis Beds in Regions II, III and IV of the OSPAR Maritime Area (OSPAR, 2013).
Merk, V., Colsoul, B. & Pogoda, B. Return of the native: survival, growth and condition of European oysters reintroduced to German offshore waters. Aquat. Conserv. 30, 2180–2190 (2020).
Preston, J. et al. European Native Oyster Habitat Restoration Handbook (The Zoological Society of London, 2020).
Smyth, D. M. et al. Wild gregarious settlements of Ostrea edulis in a semi-enclosed sea lough: a case study for unassisted restoration. Restor. Ecol. 28, 645–654 (2020).
Pouvreau, S. et al. Current distribution of the residual flat oysters beds (Ostrea edulis) along the west coast of France. SEANOE https://doi.org/10.17882/79821 (2021).
Bergström, P., Thorngren, L., Strand, Å. & Lindegarth, M. Identifying high-density areas of oysters using species distribution modeling: lessons for conservation of the native Ostrea edulis and management of the invasive Magallana (Crassostrea) gigas in Sweden. Ecol. Evol. 11, 5522–5532 (2021).
Tully, O. & Clarke, S. The Status and Management of Oyster (Ostrea edulis) in Ireland Report No. 1649-0037 (Marine Institute Galway, 2012).
University Marine Biological Station Millport. Conservation of the Native Oyster Ostrea edulis in Scotland Scottish Natural Heritage Commissioned Report (Scottish Natural Heritage, 2007).
Thurstan, R. H. et al. Historical distribution and habitat characteristics of Ostrea edulis reefs in Europe. figshare https://doi.org/10.6084/m9.figshare.c.6884167.v1 (2024).
Thurstan, R. H. et al. Historical dataset details the distribution, extent and form of lost Ostrea edulis reef ecosystems. Preprint at EcoEvoRxiv https://doi.org/10.32942/X28C99 (2023).
Todorova, V., Micu, D. & L, K. Unique oyster reefs discovered in the Bulgarian Black Sea. C. R. Acad. Bulg. Sci. 62, 871–874 (2009).
Neild, R. The English, the French and the Oyster (Quiller Press, 1995).
Stott, R. Oyster (Reaktion Books, 2004).
Nielsen, P. & Petersen, J. K. Flat oyster fishery management during a time with fluctuating population size. Aquat. Living Resour. 32, 22 (2019).
zu Ermgassen, P. S. E. et al. European native oyster reef ecosystems are universally collapsed. Preprint at EcoEvoRxiv https://doi.org/10.32942/X2HP52 (2023).
Thorngren, L., Bergström, P., Dunér Holthuis, T. & Lindegarth, M. Assessment of the population of Ostrea edulis in Sweden: a marginal population of significance? Ecol. Evol. 9, 13877–13888 (2019).
Cameron, T. Defining Oyster Beds in the Blackwater Estuary Report No. NECR411 (Natural England, 2022).
Jabiol, J. et al. Trophic complexity enhances ecosystem functioning in an aquatic detritus-based model system. J. Anim. Ecol. 82, 1042–1051 (2013).
zu Ermgassen, P. S. E. et al. The benefits of bivalve reef restoration: a global synthesis of underrepresented species. Aquat. Conserv. 30, 2050–2065 (2020).
Yeager, L. A. & Layman, C. A. Energy flow to two abundant consumers in a subtropical oyster reef food web. Aquat. Ecol. 45, 267–277 (2011).
zu Ermgassen, P. S. E., Grabowski, J. H., Gair, J. R. & Powers, S. P. Quantifying fish and mobile invertebrate production from a threatened nursery habitat. J. Appl. Ecol. 53, 596–606 (2016).
Henry, L.-A., Davies, A. J. & Murray Roberts, J. Beta diversity of cold-water coral reef communities off western Scotland. Coral Reefs 29, 427–436 (2010).
Sea, M. A., Hillman, J. R. & Thrush, S. F. Enhancing multiple scales of seafloor biodiversity with mussel restoration. Sci. Rep. 12, 5027 (2022).
Callaway, R. Interstitial Space and Trapped Sediment Drive Benthic Communities in Artificial Shell and Rock Reefs. Front. Mar. Sci. https://doi.org/10.3389/fmars.2018.00288 (2018).
Lee, H. Z. L., Davies, I. M., Baxter, J. M., Diele, K. & Sanderson, W. G. Missing the full story: first estimates of carbon deposition rates for the European flat oyster, Ostrea edulis. Aquat. Conserv. 30, 2076–2086 (2020).
Smith, R. S., Cheng, S. L. & Castorani, M. C. N. Meta-analysis of ecosystem services associated with oyster restoration. Conserv. Biol. 37, e13966 (2023).
Lõugas, L., Jürjo, I. & Russow, E. European flat oyster (Ostrea Edulis L.) in the eastern Baltic as evidence of long-distance trade in Medieval and Early Modern times. Heritage 5, 813–828 (2022).
Andrews, A. C. Oysters as a food in Greece and Rome. Class. J. 43, 299–303 (1948).
Sander, L. et al. The late Holocene demise of a sublittoral oyster bed in the North Sea. PLoS ONE 16, e0242208 (2021).
González‐Wangüemert, M., Pérez-Ruzafa, Á., Rosique, M. J. & Ortiz, A. Genetic differentiation in two cryptic species of Ostreidae, Ostrea edulis (Linnaeus, 1758) and Ostreola stentina (Payraudeau, 1826) in Mar Menor Lagoon, southwestern Mediterranean Sea. Nautilus 118, 103–111 (2004).
Royal Commission. Report of the Commissioners Appointed to Inquire into the Sea Fisheries of the United Kingdom, with Appendix and Minutes of Evidence (H.M.S.O., 1866).
Buckland, F. T. & Walpole, S. Commissioners for Sea Fisheries on the Sea Fisheries of England and Wales (H. M. Stationary Office, 1879).
Sinclair, J. The statistical accounts of Scotland (1791–1845) (William Creech of Edinburgh, Blackwoods and Sons, 1846).
Pontoppidan, E. Det første forsøg paa Norges naturlige historie: førestillende dette kongeriges luft, grund, fielde, vande, vaexter, metaller, mineralier, steen-arter, dyr, fugle, fiske og omsider indbyggernes naturel, samt saedvaner og levemaade (Trykt i de Berlingske Arvingers Bogtrykkerie, ved Ludolph Henrich Lillie, 1752).
Milner, N. in The Archaeology and Historical Ecology of Small Scale Economies 17–40 (Univ. Press of Florida, 2013).
Stiner, M. C., Bicho, N. F., Lindly, J. & Ferring, R. Mesolithic to Neolithic transitions: new results from shell-middens in the western Algarve, Portugal. Antiquity 77, 75–86 (2003).
Giovio, P. Libro de’ Pesci Romani, translation by C. Zancaruolo, 1560, 188–189. First edition in Latin was printed in Rome in 1524 and 1527, titled De piscibus marinis lacustribus fluviatilibus item de testaceis ac salsamentis liber (Venetia, 1560).
Cornide, J. Ensayo de una historia de los peces y otras producciones marinas de la costa de Galicia (Oficina de Benito Cano, 1788).
The Second Annual Report of the Commissioners of Fisheries, Ireland (Commissioners of Fisheries, 1844).
Möbius, K. A. Die Auster und die Austernwirthschaft (Wiegandy, Hempel & Parey, 1877).
Dean, B. Report on the European methods of oyster-culture. Bull. U. S. Fish. Comm. 11, 307–406 (1891).
Joubin, L. & Guérin-Ganivet, J. Cartes des gisements de coquilles comestibles des côtes de France. Ifremer https://doi.org/10.12770/c502ed30-0d7d-11de-a4b1-000086f6a603 (2009).
Bromley, C., McGonigle, C., Ashton, E. C. & Roberts, D. Bad moves: pros and cons of moving oysters – a case study of global translocations of Ostrea edulis Linnaeus, 1758 (Mollusca: Bivalvia). Ocean Coast. Manage. 122, 103–115 (2016).
Select Committee to Inquire into Reasons for Scarcity of Oysters and Effect of Measures Adopted after the Report of Royal Commission on Sea Fisheries, 1866. Report, Proceedings, Minutes Of Evidence, Appendix, Index (H. M. Stationary Office, 1876).
Huxley, T. H. Oysters and the oyster question. The English Illustrated Magazine 47–55, continued 110–121 (Central Publishing, 1883).
Elston, R. A., Farley, C. A. & Kent, M. L. Occurrence and significance of bonamiasis in European flat oysters Ostrea edulis in North America. Dis. Aquat. Org. 2, 49–54 (1986).
Giard, A. Sur une affection parasitaire de l’huitre (Ostrea edulis L.) connue sous Ie nom de maladie du pied. C. R. Seances Mem. Soc. Biol. 10, 401–403 (1894).
Report of the Conference of the Parties to the Convention on Biological Diversity on the Second Part of its Fifteenth Meeting (Convention on Biological Diversity, 2022).
Proposal for a Regulation of the European Parliament and of the Council on Nature Restoration, 22 June 2022 COM/2022/304 final (European Commission, 2022).
Guy, C., Blight, A., Smyth, D. & Roberts, D. The world is their oyster: differences in epibiota on sympatric populations of native Ostrea edulis and non-native Crassostrea gigas (Magallana gigas) oysters. J. Sea Res. 140, 52–58 (2018).
Lown, A. E., Hepburn, L. J., Heywood, J. L. & Cameron, T. C. European native oysters and associated species richness in the presence of non-native species in a southern North Sea estuary complex. Conserv. Sci. Pract. 3, e361 (2021).
Jenkin, A., Trundle, C., Owen, K., Sturgeon, S. & Naylor, H. Fal Oyster Survey (Cornwall Inshore Fisheries and Conservation Authority, 2019).
Stechele, B., Hughes, A., Degraer, S., Bossier, P. & Nevejan, N. Northern Europe’s suitability for offshore European flat oyster (Ostrea edulis) habitat restoration: a mechanistic niche modelling approach. Aquat. Conserv. 33, 696–707 (2023).
Heral, M. in Barnabe Aquaculture 342–387 (Ellis Horwood, 1989).
Philpots, J. R. Oysters and All About Them (John Richardson and Co., 1891).
European Environment Agency. EEA Coastline for Analysis (Polygon) - version 3.0, March 2017. https://sdi.eea.europa.eu/catalogue/geoss/api/records/9faa6ea1-372a-4826-a3c7-fb5b05e31c52 (2017).
Stoke, G. T. Pococke’s Tour in Ireland in 1752 (Hodges, Figgis and Co., 1891).
Harding, C. W. Inquiries concerning the propagation of American smelt and shad, and notes on the fisheries of the Wash in England. Bull. U. S. Fish. Comm. 1, 428–429 (1882).
Fishery Board for Scotland. Fifth Annual Report of the Fishery Board for Scotland being for the Year 1886 (H. M. Stationary Office, 1887).
Olsen, O. T. The Fisherman’s Practical Navigator 2nd edn (Imray & Son, 1885).
Rapport du capitaine des garde-pêches au Préfet Maritime de Brest sur la pêche des huîtres plates exercée en rade de Brest, 29 décembre 1864 Report No. CC5/175 (Archives du Service Historique de la Défense de Vincennes, 1864).
Faber, G. L. The Fisheries of the Adriatic and the Fish Thereof. A report of the Austro-Hungarian Sea-fisheries (B. Quaritch, 1883).
Marsili, F. Giornali e memorie per la ricognizione della spiaggie pontificie dell’Adriatico (Biblioteca Univ. di Bologna, 1715).
First Report of the Commissioners of Inquiry into the State of the Irish Fisheries; with the Minutes of Evidence and Appendix (Irish Fisheries, 1836).
Sea Fishing of Cardigan Bay, Past And Future (The Cambrian News and Merionethshire Standard, 15 March 1889).
Levasseur, O. Histoire de l’huître en Bretagne 58 (Coop breizh, 2006).
Acknowledgements
We acknowledge the support of the following agencies: European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (Grant Agreement No. 856488 – ERC Synergy project ‘SEACHANGE: Quantifying the impact of major cultural transitions on marine ecosystem functioning and biodiversity’) to R.H.T.; Convex Seascape Survey to R.H.T. and C.M.R.; Environment Agency to R.H.T.; Flotilla Foundation to P.S.E.z.E. and H.M.; COST Action MAF-WORLD CA20102, supported by COST (European Cooperation in Science and Technology; www.cost.eu) to R.H.T., P.S.E.z.E. and F.d.C.; the International Council for Exploitation of the Sea (ICES) Working Group on the History of Fish and Fisheries provided feedback on the work to R.H.T.; The RemediOS Project, developed with the collaboration of the Biodiversity Foundation (Spanish Ministry for Ecological Transition and Demographic Challenge), through the Pleamar Program, co-financed by the European Maritime and Fisheries Fund (EMFF) to F.d.C. and E.G.; Independent Research Fund Denmark, project ‘Living on the Edge – Risk, Resources, Resilience and Relocations in the Western Limfjord, c. 1750-1900’ to B. Poulsen; a pluri-annual agreement between two French National Institutes, Ifremer and OFB, French Office for Biodiversity (Grant Agreement No. OFB.22.0034, ‘REEFOREST: REstoring the European Flat Oyster Reefs & their Ecosystem Services on the french coasT’) to S.P.; RESTORE (FKZ 3516892001, FKZ 3519892016, FKZ 3520892013) and PROCEED (FKZ 3517685013), funded by the German Federal Agency for Nature Conservation (BfN) with funds from the German Federal Ministry for the Environment, Nature Conservation, Nuclear Safety and Consumer Protection (BMUV) to B.P. and C.P.; UKRI, Cefas and Natural England to T.C.C.; the Irish Research Council (IRC) under their COALESCE call for the ‘Food Smart Dublin project’ (Grant No. COALESCE/2019/97) to C.S.; Project PO-FEAMP (Programma Operativo - Fondo Europeo per gli Affari Marittimi e la Pesca) 2014-2020 ECOGESTOCK ‘Approccio ECOsistemico per la tutela e la GEStione delle risorse biologiche e STOCK ittici nelle acque interne’ and Project funded under the National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.4 - Call for tender No. 3138 of 16 December 2021, rectified by Decree n.3175 of 18 December 2021 of Italian Ministry of University and Research funded by the European Union – NextGenerationEU; Project code CN_00000033, Concession Decree No. 1034 of 17 June 2022 adopted by the Italian Ministry of University and Research, CUP D33C22000960007, Project title National Biodiversity Future Center (NBFC) to D.G.; the Program LIFE-Climate Change Adaptation (LIFE18 CCA/ES/001160): Adaptation to climate change through management and restoration of European estuarine ecosystems (LIFE ADAPTA BLUES) to J.A.J. and B.O. D.G. thanks M. Doneddu and E. Trainito for shared experience. We thank members of the NORA historical ecology working group and the following collaborators for data and discussion during working group meetings and conferences: A. Debney, C. Gamble, L. Darcy, I. Dummet, F. Sandford, Z. Laurence, C. Ranger, A. Kraidovskiy, A. Fischel, A. Frankic, C. Joanaz de Melo, S. Mortensen, P. Chainho, M. Albentosa, L. Bosseboeuf, C. Bromley, J. Costa, S. Cabral, J. Cano, M. Cornwell, T. Day, S. Hornborg, C. Bertolini, G. Brundu, J. M. Fariñas-Franco, H. Sas, L. de Nicolò, L. Divari, F. Kerckhof, T. Kerkhove, M. J. Rosique, U. Tenreiro, F. Volckaert, R. Whiteley. The NORA Secretariat was funded by the Federal Ministry for the Environment, Nature Conservation and Nuclear Safety (BMU) and the German Federal Agency for Nature Conservation (Bundesamt für Naturschutz, BfN) through the Federal Program for Biodiversity and the Alfred Wegener Institute Helmholtz Centre for Polar and Marine Research within the project PROCEED (FKZ 3517685013).
Author information
Authors and Affiliations
Contributions
R.H.T. and P.S.E.z.E. conceptualized the project, developed the methodology, contributed and curated data, and wrote the original draft. H.M. conducted formal analysis and wrote the original draft. J.P. contributed data, conducted formal analysis and wrote the original draft. E.C.A., F.P.B., A.B.C., J.H.B., T.C.C., F.d.C., D.W.D., C.E., T.F., E.G., O.G., R.G., D.G., M.H.-H., L.H., K.T.J., J.A.J., J.L., A.B.M.M., D.K.M., P.N., H.v.N., B.O., C.P., B. Pogoda, B. Poulsen, S.P., C.S., A.C.S., D.S., A.S. and J.A.T. contributed data, and reviewed and edited the manuscript. C.M.R. reviewed and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Sustainability thanks Dominic McAfee, Leslie Reeder-Myers and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–5, Tables 1 and 2, References and statistical source data for the supplementary figures.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Thurstan, R.H., McCormick, H., Preston, J. et al. Records reveal the vast historical extent of European oyster reef ecosystems. Nat Sustain (2024). https://doi.org/10.1038/s41893-024-01441-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41893-024-01441-4
- Springer Nature Limited