Abstract
Recent studies have reported that methylation of the brain-derived neurotrophic factor (BDNF) gene promoter is associated with major depressive disorder (MDD). This study aimed to investigate the association between cortical thickness and methylation of BDNF promoters as well as serum BDNF levels in MDD. The participants consisted of 65 patients with recurrent MDD and 65 age- and gender-matched healthy controls. Methylation of BDNF promoters and cortical thickness were compared between the groups. The right medial orbitofrontal, right lingual, right lateral occipital, left lateral orbitofrontal, left pars triangularis, and left lingual cortices were thinner in patients with MDD than in healthy controls. Among the MDD group, right pericalcarine, right medical orbitofrontal, right rostral middle frontal, right postcentral, right inferior temporal, right cuneus, right precuneus, left frontal pole, left superior frontal, left superior temporal, left rostral middle frontal and left lingual cortices had inverse correlations with methylation of BDNF promoters. Higher levels of BDNF promoter methylation may be closely associated with the reduced cortical thickness among patients with MDD. Serum BDNF levels were significantly lower in MDD, and showed an inverse relationship with BDNF methylation only in healthy controls. Particularly the prefrontal and occipital cortices seem to indicate key regions in which BDNF methylation has a significant effect on structure.
Similar content being viewed by others
Introduction
A growing body of literature has suggested that brain-derived neurotrophic factor (BDNF) is closely associated with MDD. BDNF plays an important role in the survival and maintenance of cortical neurons and dendrites, as well as in synaptic plasticity1. Since impaired neural plasticity and neurogenesis are key mechanisms in the pathophysiology of MDD, numerous studies have reported that peripheral levels of BDNF and genetic polymorphisms of the BDNF gene are associated with the development and clinical course of MDD2,3.
Recent studies have increasingly focused on the possible role of BDNF methylation in MDD. Depression generally results from complicated interactions between genetic vulnerability and environmental stressors. With enough stress, such as early-life adversity, chromatin structure is altered without changes in the DNA sequence4. Methylation of CpG islands in the promoter region, which inhibits gene transcription, may be among the most common types of epigenetic mechanisms occurring in the major mental illnesses5.
In a rat study, Bdnf methylation in the hippocampus was associated with depressive-like behavior6. In human studies, BDNF methylation in DNA extracted from peripheral blood cells has been widely used as a surrogate for measuring central BDNF methylation levels. Several studies have reported that BDNF methylation measured in peripheral blood cells could be a possible diagnostic biomarker for depression7. Peripheral blood cell BDNF methylation could be altered by psychosocial stress8. D’Addario et al. suggested that patients with MDD had lower BDNF gene expression and higher BDNF methylation compared to healthy controls9.
If BDNF methylation influences the development and clinical course of MDD, the effects of this methylation may be reflected in brain structures. Methylation would inhibit expression of the BDNF gene, which in turn inhibits neurogenesis in cortex; because BDNF regulates neuronal survival, growth, immigration, axonal pruning, and dendritic growth10. Indeed, epigenetic controls such as DNA methylation are considered a major mechanism by which neural plasticity is altered in response to various environmental stimuli in the mature neural system11. Consequently, the altered neural plasticity might result in macroscopic structural changes in the cortex, which in turn result in mood dysregulation12.
Previously, numerous studies used voxel-based morphometry (VBM) for measuring cortical volume in MDD13. However, VBM measures gray matter volume, which consists of both surface area and cortical thickness. The cortical surface area and thickness have distinct cytoarchitectural and ontogenetic origins, and neurons within the cerebral cortex are organized into ontogenetic columns that run perpendicular to the surface of the brain14. Thus, cortical thickness reflects neural cells within a column that share a common ontogenetic origin15, and thereby more reliably measures changes in neural plasticity. Previously, we reported that the thickness of the prefrontal cortices were significantly decreased in patients with first-episode MDD compared to healthy controls. A study in rats suggested that Bdnf methylation resulted in decreased Bdnf mRNA expression in the prefrontal region16. However, despite the critical role of BDNF in determining cortical thickness, to the best of our knowledge, no studies have investigated the relationship between BDNF methylation and cortical thickness in patients with MDD.
In this study, we investigated differences in BDNF promoter methylation and cortical thickness between patients with recurrent MDD and healthy controls. We hypothesized that patients with MDD would have higher BDNF promoter methylation as well as thinner cortices than healthy controls. Since abnormal epigenetic regulation of the BDNF gene would result in neurodegenerative changes and consequently decreased cortical thickness in patients with MDD, the relationship between the thinner cortices and BDNF promoter methylation in patients with MDD would likely have an inverse correlation. Additionally, since increased BDNF methylation might reduce bioavailability of serum BDNF levels, there is a possibility that BDNF methylation and serum BDNF levels are inversely correlated. However, to the best of our knowledge, only a recent study with only 11 patients with MDD reported a possible association between serum BDNF levels and BDNF methylations17. This study reported serum BDNF levels and BDNF methylation to be inversely correlated with total methylation rates, whereas there was no association with each CpG site. Thus, we measured serum BDNF levels together with BDNF methylation to investigate more comprehensively, the correlations between each factors.
On the other hand, recent studies have reported that first-episode patients with MDD might have increased volume in the cortical18 and hippocampal19 regions compared to healthy controls. Initial inflammatory processes are thought to play a neuroprotective function and may temporarily increase cortical volumes20, although the exact mechanisms should be further studied. Therefore, to reliably examine the possible effects of BDNF promoter methylation on neurodegenerative changes as represented by cortical thickness, we recruited only patients with recurrent MDD.
Results
Sociodemographic, clinical, and genetic data
A total of 130 subjects were recruited. They consisted of 65 patients with recurrent MDD and 65 healthy controls. Sociodemographic and clinical data for the subjects are presented in Table 1. There were no significant differences in age and gender between the two groups. Patients with recurrent MDD had significantly fewer years of education, less employment, and a higher family history of MDD compared to healthy controls. Sixteen out of 65 (24.6%) patients with MDD had more than three previous depressive episodes. Genotyping of BDNF for one healthy control failed. The distribution of polymorphisms in rs6265 was fit to HWE for each group.
Patients with MDD had significantly higher rates of methylation at CpG2 and CpG4 than healthy controls. There were no significant differences in total intracranial volume (TIV) between patients with recurrent MDD and healthy controls. There were no association between duration of illness and BDNF promoter methylation at CpG1 (r = −0.190, p = 0.136), CpG2 (r = 0.020, p = 0.879), CpG3 (r = −0.132, p = 0.302), and CpG4 (r = 0.019, p = 0.884).
With regard to the comparison of BDNF promoter methylation between medication-naïve and on-medication patients with MDD, there were no differences (Supplementary Table 1).
In the 33 patients with MDD who took antidepressants, more than half of patients (17 out of 33, 51.5%) took antipsychotics. The most frequently prescribed antidepressants and atypical antipsychotics were escitalopram and quetiapine, respectively.
Comparisons of cortical thickness between patients with MDD and healthy controls
Patients with MDD had significantly thinner right medial orbitofrontal, right lingual, right lateral occipital, left lateral orbitofrontal, left pars triangularis, and left lingual cortices as compared to healthy controls (Table 2).
Correlations between severity of depression and cortical thickness
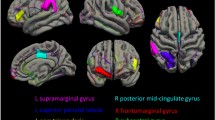
The 17-item Hamilton Rating Scale for Depression (HRSD) scores, which represent severity of depression showed positive correlation with left supramarginal cortex and negative correlation with left lingual cortex among MDD group (Supplementary Table 2).
Correlations between BDNF promoter methylation and cortical thickness
BDNF promoter methylation at CpG2 and CpG4, which were higher in patients with MDD than healthy controls, were used for the correlation analysis.
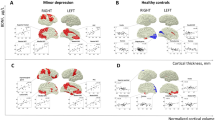
Among MDD group, BDNF promoter methylation at CpG2 had inverse correlations with thickness in right pericalcarine, right inferior temporal, right medical orbitofrontal, right rostral middle frontal, left superior frontal, left superior temporal, and left lingual, and right rostral middle frontal cortices. BDNF promoter methylation at CpG4 had also inverse correlations with thickness in right rostral middle frontal, right medial orbitofrontal, right cuneus, right precuneus, right postcentral, left lingual, left superior frontal, left superior temporal, and left frontal pole cortices (Table 3, Figs 1 and 2). There were no cortical regions having positive correlations with BDNF promoter methylation.
The inflated maps illustrate brain regions with Monte-Carlo permutation test-adjusted family-wise error (p < 0.05). The top four brain maps show correlations between BDNF methylation at CpG2 and cortical thickness in the left and right hemisphere, respectively. The bottom four brain maps show correlations between BDNF methylation at CpG4 and cortical thickness in the left and right hemisphere, respectively. The colored areas represent maximum z scores in each cluster.
As noted in the Table 3, several of the cortical regions thinned in patients with MDD were also inversely correlated with BDNF promoter methylation. Right medial orbitofrontal and left lingual cortices were inversely correlated with BDNF promoter methylation both at CpG2 and CpG4.
Among medicated MDD group, BDNF promoter methylation at CpG4 had inverse correlations with thickness in right inferior temporal, right pericalcarine, left rostral middle frontal, and left lingual cortices (Supplementary Table 4).
There were no correlations between BDNF promoter methylation at any CpG sites and cortical thickness among healthy controls (Supplementary Figures 1 and 2).
There were no BDNF promoter methylation and genotypes on cortical thickness at any CpG sites among MDD and healthy controls, respectively.
Correlations between serum BDNF levels and cortical thickness
There were no association between serum BDNF levels and cortical thickness both in MDD and healthy controls, respectively.
Correlations between BDNF promoter methylation and depressive symptoms
There were no correlations between BDNF promoter methylation and HRSD scores both in the healthy controls and MDD, respectively (Supplementary Table 5).
Correlations between BDNF promoter methylation and serum BDNF levels
Among healthy controls, BDNF promoter methylation at CpG2 (r = −0.264, p = 0.038) and CpG3 (r = −0.433, p < 0.001) had inverse correlations with serum BDNF levels (Supplementary Table 6). There were no association between BDNF promoter methylation and serum BDNF levels at CpG1 and CpG4.
There were no association between BDNF promoter methylation and serum BDNF levels at any CpG sites among patients with MDD.
Discussion
In this study, we investigated the association between BDNF promoter methylation and cortical thickness among patients with recurrent MDD. Patients with recurrent MDD had thinner right medial orbitofrontal, right rostral middle frontal, right superior temporal, right middle temporal, and right lingual cortices. In the left hemisphere, the MDD group had thinner lateral orbitofrontal, pars triangularis, precuneus, lingual, and lateral occipital cortices. There was no cortical region in which healthy controls had thinner cortex compared to the patients with recurrent MDD. Regarding BDNF promoter methylation, as expected, patients with MDD had higher methylation levels than healthy controls.
As briefly mentioned in the Introduction section, peripheral BDNF promoter has been increasingly used in the field of epigenetics of major psychiatric disorders. Recent studies reported that peripheral molecular BDNF, such as methylation, closely reflect central activity of the BDNF in brain regions closely related with mood regulation. In a postmortem study conducted on bipolar patients, BDNF promoter methylations in the peripheral tissues and quadriceps tissues, were positively associated with those in the hippocampus of patients with bipolar disorder21. The authors of this study argued that the results supported the usefulness of peripheral BDNF methylation as a surrogate for central BDNF methylation. In a rat study, Bdnf methylation resulted in decreased Bdnf mRNA expression in the prefrontal region16. The results suggest that peripheral BDNF promoter methylation could reflect molecular abnormalities in the CNS, particularly in areas where a lot of BDNF receptors are present, such as the hippocampus and prefrontal cortex. There have been increasing evidences more directly supporting the association between peripheral BDNF methylation and depression. Both BDNF methylation and gene expression of peripheral blood mononuclear cells were higher in patients MDD as compared to healthy controls9. Additionally, higher BDNF methylation exerted negative influence on the depressive severity during 1-year follow up period22.
One of the most interesting findings is that BDNF promoter methylation and cortical thickness had inverse correlation among patients with MDD, but not healthy controls. In particular, as described in the Results section, prefrontal and occipital cortices which were found to be thinner in patients with MDD also had inverse correlations with BDNF promoter methylation. Additionally, the prefrontal and occipital cortices had more frequent and significant associations with BDNF promoter methylation than other cortical areas. Right medial orbitofrontal and left lingual cortices were thinned in patients with MDD and also had inverse correlations with BDNF promoter methylation at CpG2 or CpG4, which were significantly higher in patients with MDD as compared to healthy controls.
Our results could be interpreted in several perspectives. First, there is a possibility that abnormalities in the frontal and occipital cortices might occur together, perhaps coupled with a neural pathway. In studies using diffuse tensor imaging (DTI), fractional anisotropy (FA) in the inferior fronto-occipital fasciculus was decreased in subpopulations of MDD such as early-life adversity23. A meta-analysis also reported that the inferior fronto-occipital fasciculus was one of the main tracts involved in MDD24. As BDNF is a neurotrophic factor involving neuronal survival, migration, growth, synaptogenesis, and neuroplasticity25,26, it is plausible to suggest that genetics of BDNF, such as polymorphisms and methylation, might have influence on the disruption of the fronto-occipital white matter tract. A DTI study revealed that healthy adults with BDNF val/val polymorphisms had decreased FA in the prefrontal and occipital pathway27.
Second, decreased cortical thickness and inverse relationship with BDNF methylation of the prefontal and occipital regions, could be considered as an individual anatomical region. The prefrontal cortex is one of the most widely investigated regions for major psychiatric disorders including MDD. The prefrontal cortex regulates emotion and executive functions, and numerous studies have suggested that prefrontal dysfunction is associated with depressive mood, lack of motivation, and executive dysfunction, all of which belong to the essential features of MDD28,29,30. Hence, many neuroimaging studies on MDD have focused on, or reported results regarding the prefrontal cortex31. The possible role of dysfunctional BDNF activity in the prefrontal cortex in depression may be mediated by the BDNF-neurotrophin receptor tyrosine kinase 2 signaling pathway, which contributes to the molecular vulnerability in depression32.
However, the association between the occipital cortex and MDD has recently received much attention. A previous study has suggested a thinner occipital cortex to be an endophenotype for MDD33. Subsequent studies have reported that abnormalities in the occipital lobe might be closely associated with recurrent and treatment-refractory depression34,35. We have revealed in our previous study, that lingual gyrus volume was associated with treatment response in MDD34. In this study, patients who were non-responsive to antidepressants had a smaller gray matter volume in the lingual gyrus as compared to antidepressant-responsive patients with MDD. Our results are also in accordance with major findings of recent studies. Maller et al. reported that occipital bending, which refers to a phenomenon in which the occipital lobe wraps around other brain regions, is found more frequently in treatment-refractory MDD patients than in healthy controls35. Whereas the bending of this brain region was viewed primarily from the perspective of brain asymmetry, the authors suggested that underlying neurotrophic mechanisms such as altered neuronal pruning should be considered.
We speculate that BDNF may play an important role in the association between the occipital cortex and MDD. Besides its neurotrophic role, BDNF also enhances formation and maintenance of excitatory glutamatergic and inhibitory GABAergic neuronal synapses36. When BDNF activity is disturbed, the balance between inhibitory and excitatory neurotransmission is upset. Inhibitory GABAergic neurons are primarily modulated by BDNF in particular, as BDNF acts differentially on GABAergic and glutamatergic neurons37. Whereas transcellular transfer of BDNF is crucial in the development and maturation of GABAergic neurons, it is not so correlated with dendritic development of glutamtergic neurons38,39. A recent mice study also suggested that activity-driven Bdnf influenced only GABAergic neurons, but not glutamatergic neurons. Thus, BDNF methylation might result in decreased GABAergic neurotransmission. Many studies have reported that the occipital cortex has higher GABA concentration compared to other brain regions38. Occipital GABA concentrations are reportedly decreased in patients with MDD compared to healthy controls39. The same study group also suggested that occipital GABA might be a biological marker for treatment response in MDD40. In that study, glutamate levels were decreased in the occipital cortex, suggesting that abnormal inhibitory-excitatory neurotransmission might be a neural substrate for MDD. Given the results of previous studies along with our study, we speculate that BDNF methylation might substantially damage the occipital cortex, in which GABA concentrations are the heist in the brain. However, it remains to be revealed how BDNF methylation-associated occipital cortical thinning contributes to the symptoms of depression.
It is of interest that BDNF promoter methylation showed negative correlations with serum BDNF levels only in healthy controls, but not in MDD patients. Since methylation inhibits expression of the BDNF gene, the negative correlations between methylation and serum levels of BDNF seem to be expectable. The significantly higher BDNF promoter methylation together with the dissociation between methylation and serum BDNF levels among MDD patients, might be interpreted in perspective of the loss of physiological or normal regulatory process of BDNF, which is presented in healthy controls.
The relationship between cortical thickness and severity of depression remains inconclusive. In this study, the HRSD, which represents severity of depression, showed negative correlations with left lingual cortex thickness, but positive correlations with left supramarginal cortex thickness. The negative correlation between HRSD and left lingual cortex thickness may be viewed as a representation of the association between the occipital cortex and depression, as described above. On the other hand, the positive correlation between supramarginal cortical thickness and HRSD scores suggests that one should be cautious when making the assumption that reductions in thickness will equate to reductions in depressive symptoms. Whereas a recent study reported that left supramarginal cortical thickness was increased in drug-naïve, first-episode MDD patients as compared to healthy controls41, there was no difference between MDD patients and healthy controls in our study. This raises the question on whether the positive correlation between left supramarginal cortical thickness and HRSD scores had clinical impact on MDD patients. Previous studies on brain volume and depressive symptom severity have yielded conflicting results. Among them, most studies found no correlations between depressive symptom severity and brain volume42,43,44, but some studies found positive45,46 as well as negative correlations47 with subcortical brain volume. Recent meta-analyses showed no evidence of an association between symptom severity and subcortical gray matter volumes, and it was suggested that assessing the severity of depression with HDRS sores at study inclusion could not fully characterize the severity of the entire depressive episode48. Future research could further investigate associations between cortical thickness and depression severity using a larger cohort of patients, and prospective longitudinal designs.
Lastly, there were no association between serum BDNF levels and cortical thickness among patients with MDD and healthy controls, respectively. Recent two studies reported that serum BDNF levels were differently associated with cortical thickness in schizophrenia as compared to healthy controls49,50. However, to the best of our knowledge, no studies investigated relationships between serum BDNF levels and cortical thickness in MDD, whereas one study revealed association between BDNF polymorphism and cortical thickness at a priori regions of interests (amygdala, anterior cingulate, middle frontal cortex, and orbitofrontal cortex)51. Thus, further studies are needed for comprehensive investigation for association between various BDNF measures (methylation, genotype, and peripheral levels) and cortical thickness in MDD.
Our study had several limitations. First, approximately half of the patients with recurrent MDD had taken antidepressants before the study. Similar to previous studies that included patients taking antidepressants52, BDNF polymorphism allele type was not significantly different between patients with MDD and healthy controls. There is a possibility that antidepressants might influence methylation53. Thus, the results seen in patients with recurrent MDD should be carefully interpreted. Second, this study used a cross-sectional design and not longitudinal. Thus, although we statistically demonstrated there was no association between duration of illness and BDNF methylation at CpG sites, we could not clearly confirm how early these changes in BDNF methylation and cortical thickness among patients with MDD appear. The cross-sectional design of our study may also act as a limitation in comprehensively elaborating the complex associations among BDNF methylation, serum BDNF levels, HRSD scores, and cortical thickness. Whereas BDNF methylation and cortical thickness consistently showed inverse correlations, serum BDNF levels and HRSD scores did not have significant relationships with BDNF methylation and/or cortical thickness. Serum BDNF levels are state-dependent, which are decreased predominantly during depressive periods54. Although more studies are needed to investigate whether the BDNF methylation is state- or trait –dependent, several studies have supported that BDNF methylation has no association with the severity of the current depressive episode9,22,55. One study revealed that BDNF methylation had no association with baseline severity of depression, but had an inverse relationship with 1-year follow up severity of depression22, raising a possibility that BDNF methylation would influence the longitudinal prognosis of depressive disorder, rather than the current state of depression. Third, we analyzed methylation at only four CpG sites, which is too few to determine the overall effects of these methylations. Third, we did not measure early life adversity, which has been reported to be associated with methylation of BDNF promoters among healthy controls and patients with MDD. However, the focus of our study was not to examine the lasting epigenetic effects of early life adversity, which have already been well investigated16. Rather, we aimed to investigate whether the differential levels of BDNF methylation between patients with MDD and healthy controls had any correlation with the cortical thickness of various brain regions. Lastly, since the types of antidepressants, mood stabilizers, and atypical antipsychotics were diverse, we could not control for the individual effects of antidepressants.
In summary, our study provides the first evidence of an association between BDNF promoter methylation and cortical thickness in patients with recurrent MDD. Cortical thinning, particularly in the prefrontal and occipital cortices, seems to indicate key regions in which BDNF methylation has a significant effect on structure. Further studies should elucidate the neuromolecular mechanisms underlying these interactions as well as their clinical outcomes.
Methods
Participants and procedures
All subjects were aged 18 to 65 and were recruited at the Korea University Anam Hospital. All patients with recurrent MDD were required have full interepisode recovery not being met depressed episode. Some of subjects in this study were included in previous studies56. The Edinburgh Handedness Test57 was applied to all participants before imaging, and only those who were right-handed were included in this study. Among all participants, an Axis I diagnosis was determined by a board-certified psychiatrist according to the Diagnostic and Statistical Manual for Mental Disorders, fourth edition (DSM-IV)58, using the Korean version of the Structured Clinical Interview for DSM-IV Axis-I Disorders59. We contacted their close relatives in order to gather available data on social, demographic, lifestyle, personality and clinical variables, for a more reliable diagnosis of a past major depressive episode. Finally, we formulated a lifetime mood chart which we used to diagnose recurrent depression. Exclusion criteria were as follows: (1) a past history or current diagnosis of comorbid axis I or II disorders according to DSM-IV criteria, (2) an IQ score under 80, (3) a history of primary neurologic diseases, such as Parkinson’s disease or epilepsy, (4) intracranial lesions such as space-occupying lesions or cerebrovascular diseases, or (5) any contraindications for magnetic resonance imaging (MRI) such as pacemakers. Age- and gender-matched healthy controls were recruited. The healthy controls were confirmed to have no present or past history of psychiatric illnesses by board-certified psychiatrists. Depression was measured by the HRSD. At the time of enrollment, 33 out of 65 patients were on antidepressant treatment with flexible dose, whereas 32 patients were medication-naïve.
All participants gave written informed consent after a full explanation and understanding of this study. The study protocol was approved by the Institutional Review Board of Korea University Anam Hospital and was conducted in accordance with the Declaration of Helsinki.
Selection of genomic regions of the BDNF gene for methylation analysis
We used whole blood to conduct genetic analyses. We measured methylations at four CpG sites (CpG1 = −675, CpG2 = −682, CpG3 = −686, and CpG4 = −688, distance [nt] from transcription start site [+1]). The above CpG regions were selected based on a study showing that methylation of analogous regions of rat Bdnf led to decreased BDNF mRNA expression in the prefrontal cortex16. A previous study also examined those regions in humans60.
BDNF gene methylation analysis
Detailed methods for the BDNF gene methylation analysis were described in our previous study56.
MRI acquisition
Three-dimensional structural MRI scans were acquired with a 3.0 T Siemens Trio whole-body imaging system (Siemens Medical Systems, Iselin, NJ, USA), using a T1-weighted magnetization-prepared rapid gradient-echo (MP-RAGE [1900 ms repetition time, 2.6 ms echo time, 220 mm field of view, 256 × 256 matrix size, 176 coronal slices without gap, 1 × 1 × 1 mm, 3 voxels, 16° flip angle, number of excitations=1]).
MRI processing for cortical thickness
Cortical thickness was defined as the shortest distance between gray/white matter boundary and the pial surface at each point across the cortical mantle. Cortical thickness was automatically estimated using FreeSurfer (software version 5.0, http://surfer.nmr.mgh.harvard.edu). The technical details of measuring cortical thickness and TIV using FreeSurfer have been described elsewhere61. We carefully inspected all raw images at segmented and inflated stages, and we confirmed that no images had substantial defects. For further analysis, cortical maps were smoothed using a Gaussian kernel with a full width at half maximum of 10 mm.
Statistical analysis
Sociodemographic and clinical data were compared between patients with MDD and healthy controls with a chi-square test for dichotomous variables, and an independent t-test for continuous variables. BDNF promoter methylation at CpG sites where there were significant differences between MDD and healthy controls were used in the subsequent analysis for correlations between methylation and cortical thickness. Partial correlation analysis adjusting age and gender was conducted to identify association between duration of illness and BDNF promoter methylation at CpG sites. The statistical analysis was conducted using SPSS version 12.0 (SPSS Inc., Chicago, IL, US).
In the analyses of cortical thickness, a vertex-wise general linear model was used for detecting the main effects of diagnosis for cortical thickness between patients and controls. Correlations between BDNF promoter methylation and the estimated cortical thickness among MDD and healthy controls were analyzed, respectively. Based on a previous study62 and heterogeneous clinical status of patients, age, gender, intracranial volume, and severity of depression were included as covariates. Several additional analyses were also conducted to identify possible role of genetic or clinical variables. To control for possible effects of polymorphisms of BDNF genotypes, correlations between BDNF promoter methylation and cortical thickness according to genotypes (AA vs. GG) were analyzed in MDD and health controls, respectively. To control for possible effects of being treated with medication, correlations between BDNF promoter methylation and cortical thickness additionally adjusted for the presence of medication were analyzed among MDD. Correlations between clinical variables, including HRSD scores and period of medications, and cortical thickness were also analyzed. Lastly, an analysis was conducted to examine possible associations between serum BDNF levels and BDNF methylations as well as cortical thickness.
To prevent a type I error from multiple comparisons, the Monte-Carlo permutation test implemented in FreeSurfer as applied, and the statistical significance level was considered at cluster-wise probability (CWP), which is similar to alpha significance, p < 0.05.
Additional Information
How to cite this article: Na, K.-S. et al. Brain-derived neurotrophic factor promoter methylation and cortical thickness in recurrent major depressive disorder. Sci. Rep. 6, 21089; doi: 10.1038/srep21089 (2016).
References
Kramar, E. A. et al. BDNF upregulation rescues synaptic plasticity in middle-aged ovariectomized rats. Neurobiol Aging 33, 708–719, doi: 10.1016/j.neurobiolaging.2010.06.008 (2012).
Zou, Y. F. et al. Meta-analysis of BDNF Val66Met polymorphism association with treatment response in patients with major depressive disorder. Eur Neuropsychopharmacol 20, 535–544, doi: 10.1016/j.euroneuro.2009.12.005 (2010).
Sen, S., Duman, R. & Sanacora, G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol Psychiatry 64, 527–532, doi: 10.1016/j.biopsych.2008.05.005 (2008).
Nestler, E. J. Epigenetic mechanisms of depression. JAMA Psychiatry 71, 454–456, doi: 10.1001/jamapsychiatry.2013.4291 (2014).
Vialou, V., Feng, J., Robison, A. J. & Nestler, E. J. Epigenetic mechanisms of depression and antidepressant action. Annu Rev Pharmacol Toxicol 53, 59–87, doi: 10.1146/annurev-pharmtox-010611-134540 (2013).
Tsankova, N. M. et al. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci 9, 519–525, doi: 10.1038/nn1659 (2006).
Guintivano, J., Arad, M., Gould, T. D., Payne, J. L. & Kaminsky, Z. A. Antenatal prediction of postpartum depression with blood DNA methylation biomarkers. Mol Psychiatry 19, 560–567, doi: 10.1038/mp.2013.62 (2014).
Unternaehrer, E. et al. Dynamic changes in DNA methylation of stress-associated genes (OXTR, BDNF) after acute psychosocial stress. Transl Psychiatry 2, e150, doi: 10.1038/tp.2012.77 (2012).
D’Addario, C. et al. Epigenetic modulation of BDNF gene in patients with major depressive disorder. Biol Psychiatry 73, e6–7, doi: 10.1016/j.biopsych.2012.07.009 (2013).
Singh, K. K. et al. Developmental axon pruning mediated by BDNF-p75NTR-dependent axon degeneration. Nat Neurosci 11, 649–658, doi: 10.1038/nn.2114 (2008).
Borrelli, E., Nestler, E. J., Allis, C. D. & Sassone-Corsi, P. Decoding the epigenetic language of neuronal plasticity. Neuron 60, 961–974, doi: 10.1016/j.neuron.2008.10.012 (2008).
Harrison, P. J. The neuropathology of primary mood disorder. Brain 125, 1428–1449 (2002).
Bora, E., Fornito, A., Pantelis, C. & Yucel, M. Gray matter abnormalities in Major Depressive Disorder: a meta-analysis of voxel based morphometry studies. J Affect Disord 138, 9–18, doi: 10.1016/j.jad.2011.03.049 (2012).
Mountcastle, V. B. The columnar organization of the neocortex. Brain 120 (Pt 4), 701–722 (1997).
Rakic, P. Specification of cerebral cortical areas. Science 241, 170–176 (1988).
Roth, T. L., Lubin, F. D., Funk, A. J. & Sweatt, J. D. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol Psychiatry 65, 760–769, doi: 10.1016/j.biopsych.2008.11.028 (2009).
Kleimann, A. et al. BDNF serum levels and promoter methylation of BDNF exon I, IV and VI in depressed patients receiving electroconvulsive therapy. J Neural Transm (Vienna) 122, 925–928, doi: 10.1007/s00702-014-1336-6 (2015).
Qiu, L. et al. Regional increases of cortical thickness in untreated, first-episode major depressive disorder. Transl Psychiatry 4, e378, doi: 10.1038/tp.2014.18 (2014).
Han, K. M. et al. Cortical thickness, cortical and subcortical volume, and white matter integrity in patients with their first episode of major depression. J Affect Disord 155, 42–48, doi: 10.1016/j.jad.2013.10.021 (2014).
Liberto, C. M., Albrecht, P. J., Herx, L. M., Yong, V. W. & Levison, S. W. Pro-regenerative properties of cytokine-activated astrocytes. J Neurochem 89, 1092–1100, doi: 10.1111/j.1471-4159.2004.02420.x (2004).
Stenz, L. et al. BDNF promoter I methylation correlates between post-mortem human peripheral and brain tissues. Neurosci Res 91, 1–7, doi: 10.1016/j.neures.2014.10.003 (2015).
Kim, J. M. et al. A longitudinal study of BDNF promoter methylation and genotype with poststroke depression. J Affect Disord 149, 93–99, doi: 10.1016/j.jad.2013.01.008 (2013).
Frodl, T. et al. Effects of early-life adversity on white matter diffusivity changes in patients at risk for major depression. J Psychiatry Neurosci 37, 37–45, doi: 10.1503/jpn.110028 (2012).
Liao, Y. et al. Is depression a disconnection syndrome? Meta-analysis of diffusion tensor imaging studies in patients with MDD. J Psychiatry Neurosci 38, 49–56, doi: 10.1503/jpn.110180 (2013).
Bibel, M. & Barde, Y. A. Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev 14, 2919–2937 (2000).
Chatterjee, D. et al. Maternal isolation alters the expression of neural proteins during development: ‘Stroking’ stimulation reverses these effects. Brain Res 1158, 11–27, doi: 10.1016/j.brainres.2007.04.069 (2007).
Tost, H. et al. Effects of the BDNF Val66Met polymorphism on white matter microstructure in healthy adults. Neuropsychopharmacology 38, 525–532, doi: 10.1038/npp.2012.214 (2013).
Snyder, H. R. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychol Bull 139, 81–132, doi: 10.1037/a0028727 (2013).
Nitschke, J. B. & Mackiewicz, K. L. Prefrontal and anterior cingulate contributions to volition in depression. Int Rev Neurobiol 67, 73–94, doi: 10.1016/S0074-7742(05)67003-1 (2005).
Rogers, M. A. et al. Executive and prefrontal dysfunction in unipolar depression: a review of neuropsychological and imaging evidence. Neurosci Res 50, 1–11, doi: 10.1016/j.neures.2004.05.003 (2004).
Rive, M. M. et al. Neural correlates of dysfunctional emotion regulation in major depressive disorder. A systematic review of neuroimaging studies. Neurosci Biobehav Rev 37, 2529–2553, doi: 10.1016/j.neubiorev.2013.07.018 (2013).
Barreto, R. A., Walker, F. R., Dunkley, P. R., Day, T. A. & Smith, D. W. Fluoxetine prevents development of an early stress-related molecular signature in the rat infralimbic medial prefrontal cortex. Implications for depression? BMC Neurosci 13, 125, doi: 10.1186/1471-2202-13-125 (2012).
Peterson, B. S. et al. Cortical thinning in persons at increased familial risk for major depression. Proc Natl Acad Sci USA 106, 6273–6278, doi: 10.1073/pnas.0805311106 (2009).
Jung, J. et al. Impact of lingual gyrus volume on antidepressant response and neurocognitive functions in Major Depressive Disorder: a voxel-based morphometry study. J Affect Disord 169, 179–187, doi: 10.1016/j.jad.2014.08.018 (2014).
Maller, J. J. et al. Occipital bending in depression. Brain 137, 1830–1837, doi: 10.1093/brain/awu072 (2014).
Poo, M. M. Neurotrophins as synaptic modulators. Nat Rev Neurosci 2, 24–32, doi: 10.1038/35049004 (2001).
Kohara, K. et al. Inhibitory but not excitatory cortical neurons require presynaptic brain-derived neurotrophic factor for dendritic development, as revealed by chimera cell culture. J Neurosci 23, 6123–6131 (2003).
Bhagwagar, Z. et al. Reduction in occipital cortex gamma-aminobutyric acid concentrations in medication-free recovered unipolar depressed and bipolar subjects. Biol Psychiatry 61, 806–812, doi: 10.1016/j.biopsych.2006.08.048 (2007).
Sanacora, G. et al. Subtype-specific alterations of gamma-aminobutyric acid and glutamate in patients with major depression. Arch Gen Psychiatry 61, 705–713, doi: 10.1001/archpsyc.61.7.705 (2004).
Abdallah, C. G. et al. Decreased occipital cortical glutamate levels in response to successful cognitive-behavioral therapy and pharmacotherapy for major depressive disorder. Psychother Psychosom 83, 298–307, doi: 10.1159/000361078 (2014).
Qiu, L. et al. Characterization of major depressive disorder using a multiparametric classification approach based on high resolution structural images. J Psychiatry Neurosci 39, 78–86 (2014).
Kronenberg, G. et al. Reduced amygdala volume in newly admitted psychiatric in-patients with unipolar major depression. J Psychiatr Res 43, 1112–1117, doi: 10.1016/j.jpsychires.2009.03.007 (2009).
Hickie, I. et al. Reduced hippocampal volumes and memory loss in patients with early- and late-onset depression. Br J Psychiatry 186, 197–202, doi: 10.1192/bjp.186.3.197 (2005).
Frodl, T. et al. Reduced hippocampal volume correlates with executive dysfunctioning in major depression. J Psychiatry Neurosci 31, 316–323 (2006).
van Eijndhoven, P. et al. Amygdala volume marks the acute state in the early course of depression. Biol Psychiatry 65, 812–818, doi: 10.1016/j.biopsych.2008.10.027 (2009).
Cheng, Y. Q. et al. Brain volume alteration and the correlations with the clinical characteristics in drug-naive first-episode MDD patients: a voxel-based morphometry study. Neurosci Lett 480, 30–34, doi: 10.1016/j.neulet.2010.05.075 (2010).
Vasic, N., Walter, H., Hose, A. & Wolf, R. C. Gray matter reduction associated with psychopathology and cognitive dysfunction in unipolar depression: a voxel-based morphometry study. J Affect Disord 109, 107–116, doi: 10.1016/j.jad.2007.11.011 (2008).
Schmaal, L. et al. Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder working group. Mol Psychiatry, doi: 10.1038/mp.2015.69 (2015).
Song, X. et al. Decreased cortical thickness in drug naive first episode schizophrenia: in relation to serum levels of BDNF. J Psychiatr Res 60, 22–28, doi: 10.1016/j.jpsychires.2014.09.009 (2015).
Zugman, A. et al. Serum brain-derived neurotrophic factor and cortical thickness are differently related in patients with schizophrenia and controls. Psychiatry Res, doi: 10.1016/j.pscychresns.2015.08.009 (2015).
Legge, R. M. et al. Modulatory effects of brain-derived neurotrophic factor Val66Met polymorphism on prefrontal regions in major depressive disorder. Br J Psychiatry 206, 379–384, doi: 10.1192/bjp.bp.113.143529 (2015).
Murphy, M. L. et al. Neurotrophic tyrosine kinase polymorphism impacts white matter connections in patients with major depressive disorder. Biol Psychiatry 72, 663–670, doi: 10.1016/j.biopsych.2012.04.015 (2012).
Melas, P. A. et al. Antidepressant treatment is associated with epigenetic alterations in the promoter of P11 in a genetic model of depression. Int J Neuropsychopharmacol 15, 669–679, doi: 10.1017/S1461145711000940 (2012).
Molendijk, M. L. et al. Serum levels of brain-derived neurotrophic factor in major depressive disorder: state-trait issues, clinical features and pharmacological treatment. Mol Psychiatry 16, 1088–1095, doi: 10.1038/mp.2010.98 (2011).
Carlberg, L. et al. Brain-derived neurotrophic factor (BDNF)-epigenetic regulation in unipolar and bipolar affective disorder. J Affect Disord 168, 399–406, doi: 10.1016/j.jad.2014.07.022 (2014).
Choi, S. et al. Association of brain-derived neurotrophic factor DNA methylation and reduced white matter integrity in the anterior corona radiata in major depression. J Affect Disord 172C, 74–80, doi: 10.1016/j.jad.2014.09.042 (2014).
Oldfield, R. C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9, 97–113 (1971).
APA. Diagnostic and Statistical Manual of Mental Disorders, Fourth edition . fourth edn, (American Psychiatric Association (APA), 1994).
Hahn, O.-S. et al. Development of Korean Version of Structured Clinical Interview Schedule for DSM-IV Axis I Disorder : Interrater Reliability. Korean J Neuropsychiatr Assoc 39, 362–372 (2000).
Devlin, A. M., Brain, U., Austin, J. & Oberlander, T. F. Prenatal exposure to maternal depressed mood and the MTHFR C677T variant affect SLC6A4 methylation in infants at birth. PLoS One 5, e12201, doi: 10.1371/journal.pone.0012201 (2010).
Fischl, B. & Dale, A. M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 97, 11050–11055, doi: 10.1073/pnas.200033797 (2000).
McLaughlin, K. A. et al. Widespread Reductions in Cortical Thickness Following Severe Early-Life Deprivation: A Neurodevelopmental Pathway to Attention-Deficit/Hyperactivity Disorder. Biol Psychiatry, doi: 10.1016/j.biopsych.2013.08.016 (2013).
Acknowledgements
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (grant number NRF-2011-0023272, NRF-2014R1A1A2058864) and Korean Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (HI12C0003).
Author information
Authors and Affiliations
Contributions
K.S.N. wrote the first draft of the manuscript. B.J.H. and H.K. designed the study and managed overall process. H.K.Y., E.W. and Y.K.K. undertook data management and statistical analysis. J.K., W.S.T., H.S.C., M.S.L. and S.H.J. contributed to the concept of the study and literatures review. All authors contributed to and have approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Na, KS., Won, E., Kang, J. et al. Brain-derived neurotrophic factor promoter methylation and cortical thickness in recurrent major depressive disorder. Sci Rep 6, 21089 (2016). https://doi.org/10.1038/srep21089
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep21089
- Springer Nature Limited
This article is cited by
-
Integrative omics analysis reveals epigenomic and transcriptomic signatures underlying brain structural deficits in major depressive disorder
Translational Psychiatry (2024)
-
The Possible Role of Brain-derived Neurotrophic Factor in Epilepsy
Neurochemical Research (2024)
-
BDNF genetic variants and methylation: effects on cognition in major depressive disorder
Translational Psychiatry (2019)
-
The association between substance P and white matter integrity in medication-naive patients with major depressive disorder
Scientific Reports (2017)
-
Inter and intra-hemispheric structural imaging markers predict depression relapse after electroconvulsive therapy: a multisite study
Translational Psychiatry (2017)