Abstract
Background
Maternal protein restriction during rat pregnancy is known to impact upon fetal development, growth and risk of disease in later life. It is of interest to understand how protein undernutrition influences the normal maternal adaptation to pregnancy. Here we investigated the mechanisms regulating renal haemodynamics and plasma volume during pregnancy, in the context of both normal and reduced plasma volume expansion. The study focused on expression of renal angiotensin receptors (ATR) and vasopressin-related aquaporins (AQP), hypothesising that an alteration in the balance of these proteins would be associated with pregnancy per se and with compromised plasma volume expansion in rats fed a low-protein diet.
Methods
Female Wistar rats were mated and fed a control (18% casein) or low-protein (9% casein) diet during pregnancy. Animals were anaesthetised on days 5, 10, 15 and 20 of gestation (n = 8/group/time-point) for determination of plasma volume using Evans Blue dye, prior to euthanasia and collection of tissues. Expression of the ATR subtypes and AQP2, 3 and 4 were assessed in maternal kidneys by PCR and western blotting. 24 non-pregnant Wistar rats underwent the same procedure at defined points of the oestrous cycle.
Results
As expected, pregnancy was associated with an increase in blood volume and haemodilution impacted upon red blood cell counts and haemoglobin concentrations. Expression of angiotensin II receptors and aquaporins 2, 3 and 4 was stable across all stages of the oestrus cycle. Interesting patterns of intra-renal protein expression were observed in response to pregnancy, including a significant down-regulation of AQP2. In contrast to previous literature and despite an apparent delay in blood volume expansion in low-protein fed rats, blood volume did not differ significantly between groups of pregnant animals. However, a significant down-regulation of AT2R protein expression was observed in low-protein fed animals alongside a decrease in creatinine clearance.
Conclusion
Regulatory systems involved in the pregnancy-induced plasma volume expansion are susceptible to the effects of maternal protein restriction.
Similar content being viewed by others
Background
Human pregnancy is associated with a 30-50% increase in plasma volume, beginning early in the first trimester and peaking at around 32 weeks of gestation [1]. Coupled with an expansion of red blood cell mass, this leads to an increase in blood volume [2]. Failure to expand circulating volume is associated with intrauterine growth restriction and hypertensive complications of pregnancy [1, 3, 4]. A similar profile of plasma volume expansion occurs in rodents during pregnancy [5], providing a useful model with which to examine the molecular and physiological mechanisms of volume expansion and the impact of modifiable environmental factors.
The events leading to plasma volume expansion are not fully understood, but evidence suggests that it is triggered by a fall in systemic vascular tone [6]. This is thought to be followed by compensatory activation of volume-restoring mechanisms, including activation of the renin-angiotensin-aldosterone (RAAS) and arginine-vasopressin (AVP) systems [7, 8], leading to renal sodium and water retention. The plasma volume expansion is associated with a substantial increase in effective renal plasma flow (ERPF) and glomerular filtration rate (GFR) from as early as the sixth week of human pregnancy [9, 10]. The initial trigger for this series of events remains poorly understood. Activity of the ovaries or corpus luteum is thought to be responsible for the initial peripheral vasodilation, as similar changes are observed in pseudopregnant rats [11] and in women in the luteal phase of the menstrual cycle [12]. A role for the feto-placental compartment has also been suggested, based on fetal reduction experiments in rodents [13] and the greater volume expansion observed in human twin pregnancies [14].
This study aimed to use a rat model of protein restriction to investigate the molecular mechanisms underlying the alterations in renal haemodynamics and plasma volume during pregnancy and to determine the sensitivity of such mechanisms to dietary insult. Low-protein diets are known to decrease urine concentrating ability in humans and rats [15], and are suggested to attenuate plasma volume expansion during pregnancy in rats [16, 17]. This is of major interest as offspring from low-protein fed rats develop a range of metabolic disorders in later life, including hypertension, insulin resistance and dyslipidaemia [18, 19]. Similar phenotypes have been observed in response to a range of other maternal dietary manipulations. The mechanisms through which such programming of disease risk occurs are not fully understood [20] and the contributions of maternal physiological responses and placental functions have been largely overlooked.
It is possible that inadequate plasma volume expansion may be one mechanism by which maternal diet impacts on the critical developmental processes related to postnatal metabolic disease. Compromised haemodilution and renal adaptation may adversely impact upon placental perfusion and hence the transfer of nutrients to the developing fetus. Inadequate nutrient supply or endocrine signalling may have irreversible effects upon organ development and therefore reduce functional capacity in later life [20]. The current study focused on renal expression of the angiotensin receptors (ATR) and the vasopressin-related aquaporins (AQP) during the oestrous cycle and pregnancy. It has been suggested that activation of arterial baroreceptors in response to peripheral vasodilation leads to nonosmotic AVP release and activation of the RAAS, over-riding the suppression of AVP release which would normally be observed in a hypo-osmotic state and thus contributing to water and sodium retention during pregnancy [21]. It has also been shown that the reduction in urine concentrating ability observed in low-protein fed rats is associated with a decrease in AQP2 protein in the inner medullary tip [15]. We therefore hypothesised that an alteration in the balance of ATR subtypes and the expression of renal AQPs would be observed in the pregnant versus non-pregnant state, and would result in a reduced plasma volume expansion in low-protein fed rats.
Methods
Animal procedures
All experiments were carried out in accordance with the 1986 Animals (Scientific Procedures) Act. 64 virgin female Wistar rats (Harlan Ltd, UK) were mated at weights of 200-250 g. Upon confirmation of mating by the presence of a semen plug on the cage floor, rats were assigned to either a control diet (180 g casein/kg) or a low-protein diet (90 g casein/kg) as described previously [22]. Within each dietary group, creatinine clearance and plasma volume were estimated at days 5, 10, 15 and 20 of gestational age (GA, n = 7-9 in each dietary GA group) prior to euthanasia. A further group of non-pregnant virgin female Wistar rats (n = 24) were maintained on a standard laboratory chow diet (Harlan Ltd) and their stage of oestrous was determined daily by vaginal swabbing. According to microscopic evaluation of the cell types present, non-pregnant rats were classified as pro-oestrous, oestrous, met-oestrous and di-oestrous, and 6 animals were euthanased per stage of oestrous following estimation of creatinine clearance and plasma volume. Following euthanasia of pregnant and non-pregnant animals, one kidney was snap frozen and stored at -80°C for molecular analyses.
Creatinine clearance
On the day prior to euthanasia, animals were housed in metabolism cages for 24 hour collection of urine. UK Home Office restrictions did not allow animals to be housed in metabolism cages for more than 24 hours. An aliquot of urine was frozen at -20°C until analysed. Plasma and urine creatinine concentrations were determined by the Jaffé alkaline picrate method [23]. A standard curve was prepared using commercially available creatinine standards (Sigma, UK). A working reagent was created at the time of sample analysis by mixing equal volumes of three solutions: A (4.4 g sodium hydroxide, 9.5 g trisodium phosphate, 9.5 g sodium tetraborate; in 400 mls distilled water), B (4% (w/v) sodium dodecyl sulphate) and C (picric acid). 200 μl of working reagent was added to 20 μl aliquots of samples and standards on a microplate in duplicate. Following 30 minutes incubation at room temperature on an orbital shaker, the absorbance was read at 492 nm. 10 μl of 30% (v/v) acetic acid was then added to each well, the microplate incubated at room temperature for a further five minutes and the absorbance read at 492 nm. The difference between absorbance before and after acidification by acetic acid was calculated. Creatinine clearance was estimated as (urinary creatinine [μmol/l] × volume urine produce in 24 hours [mls])/(plasma creatinine [μmol/l] × 1440 [minutes]). The intra- and inter-assay coefficients of variation for plasma creatinine were 3.1% and 5.5% respectively. The intra and inter-assay coefficients of variation for urinary creatinine were 3.5% and 0.5% respectively.
Blood and plasma volume
The method for determining blood and plasma volume was based on a previously published method [24]. Under isofluorane anaesthesia, a cannula was inserted into the left iliac vein through which an initial (baseline) blood sample of 1 ml was taken for use as a plasma blank and for analyses of plasma creatinine and haematological parameters. 0.3 ml Evans Blue Dye (0.5 mg/ml) was injected via the cannula, followed by a flush with 0.5 ml saline. The dye was allowed to circulate for five minutes, after which a final blood sample was taken. The animal was then euthanased by injection of sodium pentobarbitone, with death confirmed by cervical dislocation. Blood was collected into EDTA microtubes and centrifuged at 3000 rpm for collection of plasma, which was stored at -20°C prior to analyses.
75 μl of the baseline and final plasma samples were added in duplicate to a 96 well microplate. Plates were read immediately at 620 nm (Tecan Sunrise, Magellan Software version 4.0) and the absorbance of the baseline plasma samples was subtracted from the absorbance of the final plasma samples collected 5 minutes after dye injection. Baseline plasma samples from each animal acted as a blank for that individual animal. Plasma volume was calculated as (milligrams dye injected/plasma dye concentration). The inter- and intra-assay coefficients of variation for Evans Blue dye concentrations were 10.0% and 4.3% respectively. Blood volume was calculated as (plasma volume)/(1-(0.009 × haematocrit)) using an F-cells ratio of 0.9 to account for the difference between whole body and venous haematocrit (Blair & Mickelsen 2006).
Haematology analyses
Haematological parameters were measured in an aliquot of the baseline blood sample using the Vet Medonic CA 620 (Boule Medical, Sweden) within three hours of collection from the animal.
Western blotting
Tissues were homogenised in an extraction buffer containing 50 mM Tris/HCL and 5 mM EDTA. Protein concentration was determined by the Bradford method [25] and samples adjusted to equal concentrations. Samples were diluted with an equal volume of loading buffer [4% (w/v) SDS, 125 mM Tris/HCl pH 6.8, 20% (v/v, 87%) glycerol, 0.1 M dithiothreitol] and heated at 90°C for 5 minutes before being run on SDS-polyacrylamide gels. Electrophoresis was carried out in a 10× Tris/glycine/SDS running buffer (National Diagnostics, USA). Following separation by electrophoresis, proteins were transferred to nitrocellulose membrane (GE Healthcare, UK). Blots were probed with the following anti-rat antibodies: AT1R diluted 1:500 (Santa Cruz, USA), AQP2[26], AQP3 [27] and AQP4 [27], diluted 1:5000 (affinity purified rabbit anti-rat AQP2 antibodies, was kindly provided by Dr. David Marples, University of Leeds), AT2R diluted 1:45,000 (Abcam, UK) and tubulin diluted 1:30,000 (Abcam). The AT1R antibody did not distinguish between AT1aR and AT1bR isoforms. Blots were then treated with goat anti-rabbit horseradish peroxidise linked secondary antibody (GE Healthcare). Blots treated with AQP2 and AT1R antibodies were developed using Enhanced Chemiluminescence (ECL, Biological Industries, Israel). Blots treated with AT2R and tubulin were developed using ECL Advance (GE Healthcare). Blots were exposed to Hyperfilm ECL (GE Healthcare) to visualise the protein bands, which were quantified using a Quantity-One Multi Analyst system (Bio-Rad, UK). Protein expression was normalised to tubulin expression to correct for any discrepancies in the loading of samples onto the gel.
RNA extraction and real-time RT-PCR
RNA was extracted from snap-frozen kidney tissue by the TRIzol procedure (Invitrogen, UK) and subjected to DNAse treatment (Promega, UK), phenol-chloroform extraction and ethanol precipitation. RNA was reverse transcribed using Moloney murine leukemia virus (MMLV) reverse transcriptase (Promega). Real-time PCR primers and a probe were designed for AQP2 using Primer Express software (version 1.5; Applied Biosystems) from the DNA sequence GenBank Accession no. NM_012909. The primer sequences were as follows: AQP2 forward primer 5'-CCATTGGTTTCTCTGTTACCCTG-3', reverse primer 5'-CGGGCTGGCTTCATGGAG-3', probe 5'-CCACCTCCTTGGGATCTATTTCACCGG-3'. Primers were ordered from MWG Biotech, Germany. Primer and probe sequences for AT1aR, AT2R and β-actin are published elsewhere [28]. Real Time PCR was performed using a Lightcycler 480 PCR machine (Roche, UK).
Statistical analysis
Data is presented throughout as means ± standard error of the mean (SEM). Data was analysed using SPSS version 16.0. To assess the effect of pregnancy per se, an independent t-test was used to compare means between non-pregnant and pregnant control fed animals, using all data from each condition irrespective of gestational age or stage of oestrus. In the pregnancy data sets, the effects of gestational age and diet during pregnancy were assessed by two-way analysis of variance. In the figures and tables, superscript letters are used to indicate outcomes of post hoc tests (Bonferroni) applied where ANOVA showed a main effect of diet or gestational age. Posthoc tests cannot be performed on interactions of these factors and so no symbols are shown where only interactive effects were noted. The statistical significance of the main factors and the interaction between them are presented throughout. In the oestrous cycle data sets, the effect of stage of oestrous was assessed by one-way analysis of variance. A probability of <5% was considered statistically significant.
Results
Weight, haematological parameters and pregnancy outcome
At the start of the experiment the two dietary groups were of similar weight (control 240 ± 5 g, low protein 247 ± 5 g, not significantly different). There was no significant effect of a maternal low-protein diet on maternal body weight or pregnancy weight gain at any stage of gestation (Table 1). There was a small but statistically significant increase in maternal kidney weight as pregnancy progressed in both the control and low-protein fed groups (Table 1, GA: P < 0.05). Significant fluctuations in kidney weight were also observed in non-pregnant rats during the oestrous cycle (Table 2, P < 0.05). Urine output was highly variable among pregnant rats but was not significantly influenced by gestational age or diet (Table 1). Non-pregnant animals (Table 2) produced less urine than pregnant animals (P = 0.031), but urine volume was not influenced by stage of oestrus cycle.
There was no effect of maternal diet on litter size or on mean fetal or placental weight at gestational age 15 or 20. Maternal red blood cell count, haemoglobin concentration and haematocrit were all unaffected by gestational age and maternal diet (Table 1). These parameters did not vary according to stage of the oestrous cycle (Table 2). Pregnant animals exhibited significantly decreased maternal red blood cell count (6.6 ± 0.1 vs. 7.2 ± 0.1 106/mm3, P < 0.05) haemoglobin concentration (12.4 ± 0.2 vs. 13.5 ± 0.2 g/dl, P < 0.01) and haematocrit (33.7 ± 0.7 vs. 36.5 ± 0.4%, P < 0.05) in comparison to non-pregnant controls.
Blood volume and creatinine clearance during pregnancy
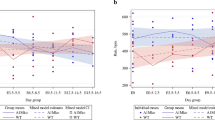
There was a significant effect of gestational age on plasma (data not shown) and blood volume (Figure 1A), with significant expansion of volume being apparent by day 15 in control animals (P = 0.036 compared to non-pregnant animals at oestrus). Although it appeared that there was a delay in the expansion of blood volume in the low-protein fed rats leading to a difference between groups on day 15 of pregnancy, there was no statistically significant interaction between gestational age and diet for either plasma or blood volume. Among the non-pregnant rats there was significant variation in blood volume across the oestrus cycle, with significantly greater volume noted at di-oestrus (Figure 1B).
Blood volume of pregnant and non-pregnant rats. Blood volume in rats fed control or low protein diet during pregnancy(A, n = 7-9) and in non-pregnant rats at different stages of the oestrus cycle (B, n = 6) Creatinine clearance (C, n = 4-8) in rats fed a control or low-protein diet during pregnancy. Data is presented as mean ± SEM and was analysed by one or two-way ANOVA, as described in the methods section. There was a significant effect of gestational age on blood volume (P < 0.05), but no effect of maternal diet. Blood volume varied with stage of oestrus (superscript letters denote a > b, P < 0.05) There was a significant interaction between gestational age and diet in their effects on creatinine clearance (P < 0.05).
There was a significant interaction between gestational age and diet in their effects on creatinine clearance during pregnancy (Figure 1C). Whilst clearance remained relatively constant in the control animals, there was a decrease in creatinine clearance in low-protein fed animals between days 10 and 20 of gestation, leading to significantly lower clearance rates on day 20 of gestation.
Angiotensin receptor expression during pregnancy
There was no difference in the level of AT1aR mRNA expression (Figure 2A) between pregnant and non-pregnant rats. It was not possible to compare protein expression between these states due to technical reasons. As pregnancy progressed from GA5 to GA20, there was no alteration in protein or mRNA expression levels and there was no effect of dietary treatment during pregnancy (Figure 3A & 3C). Expression of AT1R protein and mRNA did not vary across the stages of the oestrus cycle (Figure 3B &3D).
Expression of angiotensin receptors and aquaporins in pregnant and non-pregnant rats. Renal expression of AT1R mRNA (A), AT2R (B & C) and AQP2 (D & E) protein and mRNA in non-pregnant (n = 23-24) and pregnant (n = 28-32) rats fed a control diet. Data are presented as mean ± SEM. Data was analysed by independent t-test. AQP2 expression was significantly decreased (P < 0.05) in pregnant versus non-pregnant controls.
Expression of renal AT 1 R. Renal expression of type 1 angiotensin receptor protein and mRNA in kidneys from rats fed a control and low-protein diet during pregnancy (A & C) and in non-pregnant rats at different stages of the oestrus cycle (B & D). Data is presented as mean ± SEM (n = 6-8 per group). Data was analysed by one or two-way ANOVA as described in the methods section. Representative western blots are shown.
The expression of AT2R mRNA and protein did not differ significantly between non-pregnant and control fed pregnant animals (Figures 2B, C) and there was no significant effect of gestational age on expression of AT2R mRNA or protein (Figure 4A & 4C). However, expression of AT2R protein was significantly decreased in response to a low-protein diet across all gestational ages (Figure 4A, Diet: P < 0.05). Expression of AT2R protein and mRNA (Figure 4B & 4D) were not significantly influenced by stage of oestrus.
Expression of renal AT 2 R. Renal expression of type 2 angiotensin receptor protein and mRNA in kidneys from rats fed a control and low-protein diet during pregnancy (A & C) and in non-pregnant rats at different stages of the oestrus cycle (B & D). Data is presented as mean ± SEM (n = 6-8 per group). Data was analysed by one or two-way ANOVA as described in the methods section. Representative western blots are shown. Protein expression of the AT2R was significantly lower in kidneys from low-protein fed rats across all gestational ages. As this was an interaction of gestational age × diet (p < 0.05), no symbols are shown.
Expression of AQP2, AQP3 and AQP4 during pregnancy
The expression of AQP2 protein was significantly decreased in pregnancy in comparison to levels at oestrous (Figure 2D, P < 0.05). This was associated with a 35% decrease in AQP2 mRNA expression overall (Figure 2E, P < 0.05). As pregnancy progressed from GA5 to GA20 there was a significant interaction between gestational age and maternal diet, which reflected an earlier down-regulation of AQP2 in low-protein fed animals in comparison to controls (Figure 5A, GA*Diet P < 0.05). There was also an interaction between gestational age and maternal diet in their effects on AQP2 mRNA expression, resulting in a difference between control and low-protein fed animals on GA5 only (Figure 5C, GA*Diet P < 0.05). The protein expression of AQP3 and AQP4 did not differ between pregnant and non-pregnant animals (data not shown) and was not affected by gestational age or maternal diet (Figure 6A & 6C). The expression of AQP2 protein and mRNA (Figure 5B & 5D) and of AQP3 and 4 proteins (Figure 6B & 6D) were similar over all stages of the oestrus cycle.
Expression of renal AQP2. Renal expression of AQP2 protein and mRNA in kidneys from rats fed a control and low-protein diet during pregnancy (A & C) and in non-pregnant rats at different stages of the oestrus cycle (B & D). Data is presented as mean ± SEM (n = 6-8 per group). Data was analysed by one or two-way ANOVA as described in the methods section. Representative western blots are shown. There was a significant interaction between gestational age and diet in their effects on AQP2 mRNA (P < 0.05) and protein (P < 0.05). Different superscripts show the effects of gestational age on AQP2 expression (b > a, P < 0.05).
Expression of renal AQP3 and AQP4. Renal expression of AQP3 (A & B) and AQP4 (C & D) proteins and mRNA in kidneys from rats fed a control and low-protein diet during pregnancy (A& C) and in non-pregnant rats at different stages of the oestrus cycle (B & D). Data is presented as mean ± SEM (n = 6-8 per group). Data was analysed by one or two-way ANOVA as described in the methods section. Representative western blots are shown.
Discussion
This study used a rat model of protein restriction to investigate the molecular mechanisms regulating renal haemodynamics and plasma volume during pregnancy and the sensitivity of these processes to dietary insult. The study focused on renal expression of the angiotensin receptors and the vasopressin-related aquaporins (AQPs), hypothesising that an alteration in the balance of these proteins would be associated with pregnancy per se and with the maternal response to protein restriction.
Effects of pregnancy per se
In agreement with previous literature, an expansion of plasma and blood volume was observed during pregnancy. Blood volume increased in comparison to that observed in rats at oestrous by 45% at day 5 of pregnancy and 180% at day 20. As expected, the expansion in blood volume was coupled with evidence of haemodilution, as reflected by decreased maternal haematocrit and haemoglobin concentrations
To our knowledge, this is the first study to characterise renal angiotensin receptor protein expression through the course of pregnancy in the rat. No change in AT1R or AT2R mRNA expression compared to the non-pregnant state was observed and there was no cumulative change in AT1R or AT2R expression as pregnancy progressed from day 5 to 20. Although a recent paper reported no effect of pregnancy on AT1R expression [29], the study focused on mRNA expression only. Previously Bedard et al., [30] reported down-regulation of AT1R protein expression in the pregnant rat kidney. Observations in non-pregnant animals suggest that binding to AT1R is inhibited by 17ß-oestradiol [31]. Progesterone is another endocrine factor that is known to inhibit expression of AT1R and which could have a potent influence during pregnancy [32]. However, in the current study we found that mRNA and protein expression was unchanged in pregnancy. It is important to note that pregnancy-induced plasma volume expansion occurs in the context of systemic and intra-renal vasodilation [33], and an increase in ERPF and GFR [9, 10]. This is reflected in the relative systemic pressor resistance to AngII found in pregnancy [34, 35]. Although AT1R might be envisaged as having effects upon multiple sodium and water transport systems in the nephron, the localization of this receptor is in the proximal tubule. Promotion of sodium reabsorption here is in proportion to GFR and would not impact upon blood volume. Alternatively, although systemic vascular responses to AngII are attenuated, others have shown a normal renal vascular response to AngII [36]. It is possible that site-specific up-regulation of intra-renal AT1R may therefore mediate vasoconstriction of the medullary vasculature and contribute to the blunting of the acute pressure natriuresis curve observed in pregnancy [37]. AT2R has been suggested to have a role in systemic and renal vasodilation [38] although a role for this receptor in altered renal function during pregnancy has not been fully established. However Ferreira and colleagues observed a 30 fold increase in the mRNA expression of one of the relaxin receptors (LGR7) in the renal cortex. This could result in significant renal vasodilation, allowing glomerular filtration to rise during pregnancy [38]. Relaxin has been suggested to have a major role in both renal and systemic vasodilation in pregnancy [39].
There was a significant down-regulation of the expression of AQP2 protein during pregnancy to 43% of the level observed in non-pregnant animals. AQP2 is the predominant vasopressin-sensitive water channel, cloned and located in the principal cells of the kidney collecting duct [40]. Evidence suggests that the decrease in arterial baroreceptor stretch during pregnancy leads to non-osmotic vasopressin release [21] which activates the vasopressin V2 receptors (V2Rs) and the adenylate-cyclic AMP pathway in the collecting duct, resulting in increased AQP2 gene transcription and shuttling of AQP2 water channels to the apical membranes of principal cells. Whilst translocation of AQP2 from cytosolic vesicles to the apical membrane mediates the acute response to AVP stimulation [41], long-term regulation of collecting duct water permeability is characterised by an increase in AQP2 mRNA and protein [42, 43].
The observed down-regulation of AQP2 mRNA and protein expression during pregnancy is in direct contrast to evidence published previously demonstrating a pregnancy-related up-regulation of AQP2 in renal papillae [44, 45]. Segmental differences in aquaporin protein expression in the kidney have been reported by other groups. The utilization of whole kidney samples for analyses was an important limitation of the present study. The increase in AQP2 in pregnancy reported by Ohara et al., [44] occurred in the inner medulla and may not be detectable in whole kidney studies. Furthermore, it is possible that translocation of AQP2 from the cytosol to the cell membrane may have interfered with protein extraction and skewed our analyses. It would therefore be more informative to consider the relative distributions of cytosolic and apical AQP2 in future studies and this would be best accomplished through immunohistochemical analysis.
Interestingly, in the study of Ohara et al., [44], the up-regulation of AQP2 was observed in the absence of a detectable increase in plasma AVP, but with a role for vasopressin receptor indicated by antagonist studies. Similarly, increased urinary excretion of AQP2 in human pregnancy occurred in the absence of increased circulating AVP concentrations [46]. Given the apparent dissociation between AVP and AQP2 expression in these studies and the contrasting results observed in the current study and that of Ohara et al. [44], further work investigating the regulation of AQP2 expression and shuttling is required before its involvement in the enhanced water reabsorption during pregnancy can be fully understood. AQP3 and AQP4 are both present in the basolateral plasma membrane of collecting duct principal cells [47] and represent exit pathways for water reabsorbed apically via AQP2. The protein expression of these aquaporins remained unaffected by pregnancy per se, suggesting that the key regulatory events occur at the apical plasma membrane where the abundance of AQP2 protein creates a "bottleneck" for water reabsorption.
Effects of a low protein diet
In contrast to previous literature [16, 17], feeding a low-protein diet to pregnant rats did not have a statistically significant effect on plasma or blood volume expansion during pregnancy. However, a trend towards delayed volume expansion was noted at day 15 of pregnancy. The study by Rosso & Streeter [16] used a more severe protein restriction (6% versus 25% casein) than that used in the current study (9% versus 18% casein), and the latter may not have been sufficient to impact significantly on plasma volume expansion. However, a previous study using the same experimental dietary formulations as the current study did suggest a significant reduction in plasma volume expansion at day 20 of pregnancy [17]. In the current study, the relative reduction in creatinine clearance and the alterations in expression of key mediators of renal fluid homeostasis in response to a low-protein diet do provide further evidence that the regulatory systems involved in plasma volume expansion are susceptible to maternal protein restriction. However, the impact of the protein restriction was subtle and considerably less than expected. Our findings therefore suggest that maternal adaptation to pregnancy is less sensitive to protein restriction than has been previously inferred [16, 17], and that only severe undernutrition has major influence on these processes.
The expression of AT2R protein was significantly decreased in low-protein fed rats throughout pregnancy. Enhanced AT2R-mediated vascular relaxation pathways have been implicated in the systemic vasodilation of pregnancy, including increased expression and activity of endothelial AT2R [48] and involvement in the vasodilation of the uterine artery [49]. As a candidate for mediating counter-regulatory vasodilation in response to AngII in pregnancy, disruption of AT2R receptor expression may be involved in inadequate vascular adaptation to pregnancy. Interestingly, a previous study has shown vascular relaxation in response to an endothelium-dependent vasodilator to be impaired in mesenteric arteries of pregnant rats fed a 9% protein diet [50]. This study adds to this previous literature by indicating a role for intra-renal AT2R in mediating altered haemodynamic function in pregnant rats fed a low-protein diet. In addition to the alterations in AT2R expression, the down-regulation of AQP2 occurred earlier in low-protein fed rats. Given the prevailing evidence of reduced or, as observed here, delayed plasma volume expansion in low-protein-fed dams, we had hypothesised that this would occur and suggest that this signifies a reduced capacity for water reabsorption in low-protein-fed rats.
Conclusion
To conclude, this study has demonstrated that no change in expression of intrarenal AT1R or AT2R proteins occurs during pregnancy. Surprisingly, a down-regulation of AQP2 was observed and the role of this water channel during pregnancy remains unclear. A significant down-regulation of AT2R protein expression was observed in low protein fed animals alongside a relative decrease in creatinine clearance, providing evidence that regulatory systems involved in plasma volume expansion are susceptible to maternal nutrient restriction. However, the impact of protein restriction on maternal blood volume expansion was relatively minor, suggesting that adaptive responses are able to compensate for variation in nutritional status.
Abbreviations
- AMP:
-
adenosine monophosphate
- AQP:
-
aquaporin
- AT1R:
-
angiotensin II type 1 receptor
- AT2R:
-
angiotensin II type 2 receptor
- AVP:
-
arginine-vasopressin
- EDTA:
-
ethylenediaminetetraacetic acid
- EPRF:
-
effective renal plasma flow
- GA:
-
gestational age
- GFR:
-
glomerular filtration rate
- RAAS:
-
renin-angiotensin-aldosterone system.
References
Salas SP, Marshall G, Gutierrez B, Rosso P: Time course of maternal plasma volume and hormonal changes in women with preeclampsia or fetal growth restriction. Hypertension. 2006, 47: 203-208. 10.1161/01.HYP.0000200042.64517.19.
Taylor DJ, Lind T: Red-cell mass during and after normal-pregnancy. BJOG. 1979, 86: 364-370. 10.1111/j.1471-0528.1979.tb10611.x.
Duvekot JJ, Cheriex EC, Pieters FAA, Menheere P, Schouten HJA, Peeters LLH: Maternal volume homeostasis in early-pregnancy in relation to fetal growth restriction. Obstet Gynecol. 1995, 85: 361-367. 10.1016/0029-7844(94)00417-C.
Salas SP, Rosso P: Plasma volume, renal function, and hormonal levels in pregnant women with idiopathic fetal growth restriction or preeclampsia. Hypertens Pregnancy. 1998, 17: 69-79. 10.3109/10641959809072239.
Atherton JC, Dark JM, Garland HO, Morgan MRA, Pidgeon J, Soni S: Changes in water and electrolyte balance, plasma volume and composition during pregnancy in the rat. J Physiol. 1982, 330: 81-93.
Duvekot JJ, Cheriex EC, Pieters FAA, Menheere P, Peeters LLH: Early-pregnancy changes in hemodynamics and volume homeostasis are consecutive adjustments triggered by a primary fall in systemic vascular tone. Am J Obstet Gynecol. 1993, 169: 1382-1392.
Al Kadi H, Nasrat H, Pipkin FB: A prospective, longitudinal study of the renin-angiotensin system, prostacyclin and thromboxane in the first trimester of normal human pregnancy: association with birthweight. Human Reprod. 2005, 20: 3157-3162. 10.1093/humrep/dei184.
Schrier RW, Cadnapaphornchai MA, Ohara M: Water retention and aquaporins in heart failure, liver disease and pregnancy. J Royal Soc Med. 2001, 94: 265-269.
Chapman AB, Abraham WT, Zamudio S, Coffin C, Merouani A, Young D, Johnson A, Osorio F, Goldberg C, Moore LG, Dahms T, Schrier RW: Temporal relationships between hormonal and hemodynamic changes in early human pregnancy. Kid Intl. 1998, 54: 2056-2063. 10.1046/j.1523-1755.1998.00217.x.
Bekheirnia MR, Schrier RW: Pathophysiology of water and sodium retention: edematous states with normal kidney function. Curr Op Pharmacol. 2006, 6: 202-207. 10.1016/j.coph.2005.09.008.
Paller MS, Gregorini G, Ferris TF: Pressor responsiveness in pseudopregnant and pregnant rats - role of maternal factors. Am J Physiol. 1989, 257: R866-R871.
Chapman AB, Zamudio S, Woodmansee W, Merouani A, Osorio F, Johnson A, Moore LG, Dahms T, Coffin C, Abraham WT, Schrier RW: Systemic and renal hemodynamic changes in the luteal phase of the menstrual cycle mimic early pregnancy. Am J Physiol. 1997, 273: F777-F782.
Van Mieghem T, van Bree R, Van Herck E, Deprest J, Verhaeghe J: Insulin-like growth factor-II regulates maternal hemodynamic adaptation to pregnancy in rats. Am J Physiol. 2009, 297: R1615-R1621.
Hytten F: Blood-volume changes in normal-pregnancy. Clin Haematol. 1985, 14: 601-612.
Sands JM, Naruse M, Jacobs JD, Wilcox JN, Klein JD: Changes in aquaporin-2 protein contribute to the urine concentrating defect in rats fed a low-protein diet. J Clin Invest. 1996, 97: 2807-2814. 10.1172/JCI118736.
Rosso P, Streeter MR: Effects of food or protein restriction on plasma-volume expansion in pregnant rats. J Nutr. 1979, 109: 1887-1892.
Welham SJM, Wheeler T, Langley-Evans SC: Maternal plasma volume expansion is modulated in early pregnancy by a low protein diet in the rat. Clin Sci. 1998, 94: M15-
McMullen S, Mostyn A: Animal models for the study of the developmental origins of health and disease. Proc Nutr Soc. 2009, 68: 306-320. 10.1017/S0029665109001396.
Langley-Evans SC, Jackson AA: Intrauterine programming of hypertension: nutrient-hormone interactions. Nutr Reviews. 1996, 54: 163-169. 10.1111/j.1753-4887.1996.tb03923.x.
Langley-Evans SC, McMullen S: Developmental origins of adult disease. Med Princ Pract. 2010, 19: 87-98. 10.1159/000273066.
Schrier RW: Water and sodium retention in edematous disorders: Role of vasopressin and aldosterone. Am J Med. 2006, 119: S47-S53. 10.1016/j.amjmed.2006.05.007.
Langley-Evans SC, Gardner DS, Jackson AA: Association of disproportionate growth of fetal rats in late gestation with raised systolic blood pressure in later life. J Reprod Fertil. 1996, 106: 307-312. 10.1530/jrf.0.1060307.
Bowers LD, Wong ET: Kinetic serum creatinine assays. 2. A critical-evaluation and review. Clin Chem. 1980, 26: 555-561.
Blair ML, Mickelsen D: Plasma protein and blood volume restitution after hemorrhage in conscious pregnant and ovarian steroid-replaced rats. Am J Physiol. 2006, 290: R425-R434.
Bradford MM: Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal Biochem. 1976, 72: 248-254. 10.1016/0003-2697(76)90527-3.
Mobasheri A, Wray S, Marples D: Distribution of AQP2 and AQP3 water channels in human tissue microarrays. J Mol Histol. 2005, 36: 1-14. 10.1007/s10735-004-2633-4.
Floyd RV, Mason SL, Proudman CJ, German AJ, Marples D, Mobasheri A: Expression and nephron segment-specific distribution of major renal aquaporins (AQP1-4) in Equus caballus, the domestic horse. Am J Physiol. 2007, 293: R492-R503.
McMullen S, Langley-Evans SC: Maternal low-protein diet in rat pregnancy programs blood pressure through sex-specific mechanisms. Am J Physiol. 2005, 288: R85-90.
Ferreira VM, Gomes TS, Reis LA, Ferreira AT, Razvickas CV, Schor N, Boim MA: Receptor-induced dilatation in the systemic and intrarenal adaptation to pregnancy in rats. Plos One. 2009, 4: e4845-10.1371/journal.pone.0004845.
Bedard S, Sicotte B, St-Louis J, Brochu M: Modulation of body fluids and angiotensin II receptors in a rat model of intra-uterine growth restriction. J Physiol. 2005, 562: 937-950. 10.1113/jphysiol.2004.064683.
Rogers JL, Mitchell AR, Maric C, Sandberg K, Myers A, Mulroney SE: Effect of sex hormones on renal estrogen and angiotensin type 1 receptors in female and male rats. Am J Physiol. 2007, 292: R794-R799.
Kalenga MK, De Gasparo M, Thomas K, De Hertogh R: Down-regulation of angiotensin AT1 receptor by progesterone in human placenta. J Clin Endocrinol Metab. 1996, 81: 998-1002. 10.1210/jc.81.3.998.
Conrad KP: Mechanisms of renal vasodilation and hyperfiltration during pregnancy. J Soc Gynecol Invest. 2004, 11: 438-448. 10.1016/j.jsgi.2004.05.002.
Gant NF, Daley GL, Chand S, Whalley PJ, Macdonal PC: Study of angiotensin-II pressor response throughout primigravid pregnancy. J Clin Invest. 1973, 52: 2682-2689. 10.1172/JCI107462.
Baker PN, Pipkin FB, Symonds EM: Longitudinal-study of platelet angiotensin-II binding in human-pregnancy. Clin Sci. 1992, 82: 377-381.
Masilamani S, Baylis C: The renal vasculature does not participate in the peripheral refractoriness to administered angiotensin II (AII) in the late pregnant (LP) rat. J Am Soc Nephrol. 1992, 3: 566-
Masilamani S, Hobbs GR, Baylis C: The acute pressure natriuresis response blunted and the blood pressure response reset in the normal pregnant rat. Am J Obstet Gynecol. 1998, 179: 486-491. 10.1016/S0002-9378(98)70384-9.
Ferreira VM, Gomes TS, Reis LA, Ferreira AT, Razvickas CV, Schor N, Boim MA: Receptor-induced dilatation in the systemic and intrarenal adaptation to pregnancy in rats. Plos One. 2009, 4: e4845-10.1371/journal.pone.0004845.
Debrah DO, Novak J, Matthews JE, Ramirez RJ, Shroff SG, Conrad KP: Relaxin is essential for systemic vasodilation and increased global arterial compliance during early pregnancy in conscious rats. Endocrinology. 2006, 147: 5126-5131. 10.1210/en.2006-0567.
Fushimi K, Uchida S, Hara Y, Hirata Y, Marumo F, Sasaki S: Cloning and expression of apical membrane water channel of rat-kidney collecting tubule. Nature. 1993, 361: 549-552. 10.1038/361549a0.
Nielsen S, Chou CL, Marples D, Christensen EI, Kishore BK, Knepper MA: Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels to plasma-membrane. Proc Natl Acad Sci USA. 1995, 92: 1013-1017. 10.1073/pnas.92.4.1013.
Yamamoto T, Sasaki S, Fushimi K, Kawasaki K, Yaoita E, Oota K, Hirata Y, Marumo F, Kihara I: Localization and expression of a collecting duct water channel, aquaporin, in hydrated and dehydrated rats. Exp Nephrol. 1995, 3: 193-201.
Saito T, Ishikawa SE, Sasaki S, Fujita N, Fushimi K, Okada K, Takeuchi K, Sakamoto A, Ookawara S, Kaneko T, Marumo F: Alteration in water channel AQP-2 by removal of AVP stimulation in collecting duct cells of dehydrated rats. Am J Physiol. 1997, 272: F183-F191.
Ohara M, Martin PY, Xu DL, St John J, Pattison TA, Kim JK, Schrier RW: Upregulation of aquaporin 2 water channel expression in pregnant rats. J Clin Invest. 1998, 101: 1076-1083. 10.1172/JCI649.
Abreu N, Tardin JC, Boim MA, Campos RR, Bergamaschi CT, Schor N: Hemodynamic parameters during normal and hypertensive pregnancy in rats:evaluation of renal salt and water transporters. Hypertens Pregnancy. 2008, 49-63.
Buemi M, D'Anna R, Di Pasquale G, Floccari F, Ruello A, Aloisi C, Leonardi I, Frisina N, Corica F: Urinary excretion of aquaporin-2 water channel during pregnancy. Cell Physiol Biochem. 2001, 11: 203-208. 10.1159/000047807.
Nielsen S, Frokiaer J, Marples D, Kwon TH, Agre P, Knepper MA: Aquaporins in the kidney:From molecules to medicine. Physiol Rev. 2002, 82: 205-244.
Stennett AK, Qiao XY, Falone AE, Koledova VV, Khalil RA: Increased vascular angiotensin type 2 receptor expression and NOS-mediated mechanisms of vascular relaxation in pregnant rats. Am J Physiol. 2009, 296: H745-H755.
Hannan RE, Davis EA, Widdop RE: Functional role of angiotensin II AT(2) receptor in modulation of AT(1) receptor-mediated contraction in rat uterine artery:involvement of bradykinin and nitric oxide. Br J Pharmacol. 2003, 140: 987-995. 10.1038/sj.bjp.0705484.
Koumentaki A, Anthony F, Poston L, Wheeler T: Low-protein diet impairs vascular relaxation in virgin and pregnant rats. Clin Sci. 2002, 102: 553-560. 10.1042/CS20010252.
Acknowledgements
RC was funded by a PhD studentship from the BBSRC. We would like to acknowledge Dr David Marples (University of Leeds) for donating the polyclonal antibody to rat AQP2 and the technical support of Mr Richard Plant for blood volume determination and Dr Nigel Kendall for haematology analyses. We also thank Mrs Carol Armett and Sarah Kirkland for their care of their animals.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
RC carried out the animal experiments and analyses of gene and protein expression. SLE participated in the design of the study and the drafting of the manuscript. AM contributed to the design of the study and the analyses of AQP expression. SM conceived the study, participated in its design and co-ordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Cornock, R., Langley-Evans, S.C., Mobasheri, A. et al. The impact of maternal protein restriction during rat pregnancy upon renal expression of angiotensin receptors and vasopressin-related aquaporins. Reprod Biol Endocrinol 8, 105 (2010). https://doi.org/10.1186/1477-7827-8-105
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1477-7827-8-105