Abstract
Background
Antibiotic-resistant Salmonella is one of the main public health concerns in the world. Isolation of Salmonella in abattoirs has been considered the core source of infection in the community from meat. Still, there is limited information on the contamination rate of cattle carcasses.
Objective
This study aimed to document the occurrence and antimicrobial susceptibility profile of Salmonella species recovered from cattle carcass and abattoir personnel at Dessie, municipality abattoir, Northeast Ethiopia:
Methods
A total of 336 carcass swabs of abdomen, neck, and hind limb from cattle carcasses and 24 stool samples were collected from abattoir personnel using a systematic sampling method from February to April 2019. The collected samples were transported using Cary-Blair transport media and cultivated on Selenite cysteine F-broth, Brilliant green agar, and Xylose-lysine deoxycholate agar plates to isolate Salmonella species. Gram stain, colony morphology, and biochemical tests were performed to identify the isolated bacteria. An antimicrobial susceptibility test for Salmonella was performed using the Kirby-Bauer Disc Diffusion method. Descriptive statistics; both bivariable and multivariable logistic regression analysis was performed using SPSS version 25 software. P-value < 0.05 at 95% CI was considered statistically significant.
Results
The prevalence of salmonella species was 8%(27/336) from all samples.‘The prevalence of Salmonella isolates in cattle carcass and abattoir personnel was 8%(25/312) and 8.3%(2/24) respectively. The antimicrobial test showed that Salmonella species were 100% resistant to ampicillin, 59.3% to trimethoprim-sulfamethoxazole, 59.3% to tetracycline, and 55.6% to amoxicillin/clavulanate. From the total antimicrobial tested bacteria, 81.5%(22/27) were resistant to three and above classes of antibiotics (drug classes). Unwashed knives, carcasses, and hands of butchers during slaughtering were significantly associated (p < 0.05) with Salmonella found in carcasses.
Conclusions
Salmonella isolation rates from cattle carcasses were high, with the bacteria showing notable resistance to most tested antibiotics. Poor hygiene practices, unsanitized equipment, and unhygienic beef processing were contributing factors.
Similar content being viewed by others
Introduction
Globally, foodborne diseases have become a big concern. According to a World Health Organization (WHO) report from 2010, there were 600 million foodborne illnesses and 420,000 deaths as a result of consuming unsafe food [1]. Every year, Salmonella causes approximately 93.8 million human gastroenteritis infections and 155, 000 deaths [2]. According to a Centers for Disease Control and Prevention (CDC) analysis, Salmonella bacteria cause about 1.35 million infections, 26,500 hospitalizations, and 420 deaths in the United States every year [3].
Non-typhoidal Salmonella (NTS) is one of the common foodborne pathogens that originate from cattle, sheep, and pigs [4, 5]. Salmonella enterica and Salmonella bongori are the predominant species of Salmonella isolated from food sources of meat [4]. In most parts of the world, Salmonella enteritidis and Salmonella typhimurium transmitted from animals to humans [6]. Humans can be infected with Salmonella from animal sources, environmental exposure, and ingestion of contaminated foodstuffs [7]. Depending on the strain of the pathogen, the severity of the disease caused by Salmonella varies from asymptomatic carriage to severe life-threatening conditions [8]. The diseases were gastrointestinal disorders and severe infections, such as bloodstream, and extraintestinal diseases like meningitis, septic arthritis, osteomyelitis, cholangitis, and pneumonia [9].
In most parts of Africa, a high proportion of NTS infection occurs: 88% in eastern Africa, 97% in southern Africa, and 87% in western and central Africa, while only 1% in northern Africa [10]. In Ethiopia, the prevalence of NTS in humans ranges from 6.2 to 13.63% [2, 11, 12]. Also, the isolation rate of Salmonella food from animal sources ranged from 1.3 to 13.3% [11, 13,14,15,16].
Factors that led to the contamination of carcasses meat by Salmonella were poor hygiene practices, slaughtering processes, and food preparation of animal products [17,18,19]. Additionally, knives, cloths, carts, boxes, surfaces, and other equipment increase contamination by Salmonella. These microorganisms begin to grow and spoil the meat if the environment is favorable for their development. Asymptomatic food handlers or personnel that have an active stage of the disease play a significant role in transmitting infection [14, 20].
Nowadays, Drug-resistant pathogens are a global public health concern and Salmonella is one of the microorganisms in which some resistant serotypes have emerged, affecting the food chain [21]. In Ethiopia, the antibiotic resistance level of Salmonella food from animals emerged high [22]. The rise of multidrug-resistant (MDR) Salmonella strains against commonly prescribed antimicrobials poses public health concerns in both veterinary and human medicine sectors [14, 23]. Widespread use of first-line drugs has contributed to the proliferation of MDR isolates, exacerbating this imminent issue [14]. Moreover, Ethiopia’s prevalent consumption of raw meat fosters an environment conducive to community-wide infection development [24].
Thus, this study aimed to isolate and determine the antimicrobial susceptibility profile, and identify associated risk factors of Salmonella species recovered from cattle carcasses and abattoir personnel at Dessie, Municipality Abattoir, Northeast Ethiopia.
Materials and methods
Study area and design
A cross-sectional study was conducted from February to April 2019 at the Dessie town municipality abattoir. According to the 2007 Ethiopian population and housing census, the town had a total population of 151,094 [25]. The livestock population of the area comprises 18,724 cattle [26]. However, the Dessie municipality abattoir is the only abattoir that provides a slaughter service and distributes meat products for the town and nearby kebeles. It has 20 carcass processors, two meat inspectors, four abattoir cleaners, and seven supportive and administrative workers. On average, 37 cattle were slaughtered each day and the meat product was served to the community by 89 hotels, 27 butcher shops, 38 restaurants, 64 cafeterias, and other governmental and non-governmental organizations [27].
Sample size determination
For cattle the sample size was determined using a single population proportion formula as follows: n = z2 p (1-p) / d2; where: n = The minimum required sample size; z = Standard normal distribution value at 95% CI, which is 1.96; P = the prevalence of Salmonella isolates in slaughtered cattle carcasses, beef taken from a previous study report at abattoir of Bahir Dar town which was 7.3% [18]; d = the margin of error taken as 5%.
Accordingly, the sample size was: \({\text{n}}\,{\text{ = }}\,\frac{{3.8416 \times 0.073 \times 0.927}}{{0.0025}} = 104\)
For abattoir personnel, the sample size was all abattoir workers who have contact with carcasses were taken.
Sampling technique
Cattle were selected using a systematic sampling method. On average 37 cattle were slaughtered daily. The sample was collected three days per week for 24 days within the study period. The number of samples collected each day is calculated as follows; N = total sample size to be collected which is 104; D = total number of sample collection days, which is 24 and n = number of samples to be collected each day.
Four cattle were selected each day using the identification numbers given to the animals. A total of 12 swab samples from meat, and three samples from the hind limb, abdomen, and neck region of each cattle carcass were taken. Totally, from 104 cattle a total of 312 samples were collected from the hind limb, abdomen, and neck region of each cattle to appreciate Salmonella distribution in different body regions and also to increase the isolation rate of Salmonella. The survey included all volunteer abattoir workers who had daily contact with beef. Stool samples were collected from 25 abattoir personnel.
A cattle carcass swab was collected according to the sample collection, isolation, and identification recommendations of the International Organization for Standardization (ISO) [28]. A total of 312 carcasses (one hundred-four from each cattle’s hind limb, abdomen, and neck region) were collected from 104 selected cattle from Dessie town Municipality. About 100 square centimeters of surfaces around the hind limb (medial), abdomen (lateral), and neck region were swabbed by wiping with a sterile gauze swab soaked in nearly 10 milliliters of buffered peptone water (BPW) and rubbing over each sampling site horizontally and then vertically for 30 s. Upon completion of the rubbing process, the swab was placed into the BPW used to wet the swab in a universal bottle. Then a swab sample was transported from the site of collection to the Amhara Public Health Institute, Dessie Branch, Microbiology Laboratory Department using transport medium 2 h of collection. The swab samples were analyzed immediately for the isolation of Salmonella [28].
Stool sample collection
After being clearly instructed, the abattoir personnel brought stool samples by using a sterile, clean, tight-lid sample container at the municipal. The collected stool specimens were transported by using cary - Blair transport media to Amhara Public Health Institute’s Dessie branch microbiology laboratory within two hours of collection [29]. Atotal of 25 stool samples were collected from abattoir personnel at dessie town municipal.
Isolations and identification of Salmonella
Each carcass sample was collected in four areas: the neck, brisket, flank, and rump. The area sampled in each region was 100 cm2, for a total area of 400 cm2. Swabs were transferred to a sterile plastic cup containing 10 ml of buffered peptone water, and in addition, from abattoir personnel, one [1] gram of stool was collected and transferred into nine ml of buffered peptone water and manually homogenized in a one-to-nine volume with BPW water. Homogenized carcasses and fecal samples from cattle and personnel were incubated at 37 °C for 18 h. The enrichment broths were then transferred aseptically into 10 ml of selenite cysteine and 10 ml of Rappaport-Vassiliadis soy broth and incubated for 24 h at 37 °C and 42 °C, respectively. After incubation, a loop of each culture was streaked onto Brilliant Green Agar and Xylose Lysine Deoxycholate Agar plates and incubated for 24 to 48 h at 37 °C [28].
Gram staining was used to confirm the presence of Gram-negative, rod-shaped bacteria as well as their morphology and staining characteristics. Based on ISO recommendations fermentations of carbohydrates on triple sugar iron agar, deamination of lysine iron agar, utilizations of citrate, utilizations of urea, production acid bottom, and hydrogen sulfide on TSI, productions of indole motility were biochemical tests used to identify all gram-negative and Salmonella species [28].
Antimicrobial susceptibility testing
The isolates of Salmonella were tested on Muller Hinton agar(HMEDIA), for antimicrobial drugs by disc diffusion technique [30]. Single pure colonies were transferred to five mL normal saline tubes and compared to 0.5 McFarland turbidity standards. A sterile cotton swab was dipped into the adjusted suspension, and the excess was removed by gently rotating the swab against the tubes inside the wall. The swab was evenly inoculated across the entire surface of Muller Hinton agar and the plates were allowed to air dry for 15 min. The inoculated plates were incubated at 37oC for 16–18 h after the antimicrobial discs were applied.
All isolates of Salmonella were tested with a total of 9 selected antibiotics discs (Oxide, UK) including amoxicillin-clavulanate (AUG) 10 µg, ciprofloxacin (CIP) 5 µg, chloramphenicol (CHL) 30 µg, kanamycin (K) 10 µg, ampicillin (AMP)10 µg, gentamicin (GM), tetracycline (TE) 30 µg, sulfamethoxazole-trimethoprim (TMP-SMX) 23.75/1.25 µg, streptomycin (S) 10 µ, amikacin (AK) 30 µg and cephalothin (CF) 30 µg. The antimicrobial agents were selected based on the CLSI 2019 guideline [30]. Finally, the inhibition zone diameters were measured to the nearest millimeter using a ruler. The result was interpreted as susceptible, intermediate, or resistant based on the recommended CLSI results in interpretive standards [30].
Quality assurance
Before data collection about 5% of the questionnaire were pre-tested. All laboratory tests were done according to the standard procedures. The media sterility was checked by incubating 5% of the prepared batch media without inoculating bacteria overnight at 35–37 °C. Throughout the study, Salmonella typhimurium (ATCC 14028) and E. coli (ATCC 25922) were used as quality control to assess the media’s ability to support bacterial growth and the quality of antibiotic discs. The quality of muller Hinton agar was checked by Enterococcus faecalis ATCC29212 [30].
Statistical analysis
To code data the data was entered into Epi-Data version 4.0.0.6 and analyzed by using SPSS version 25. To identify potential risk factors of Salmonella isolates bivariable and multivariable logistic regression analysis was performed. In bivariable analysis, variables with p-values less than 0.2 were candidates for multivariable analysis. The significance of the association between potential risk factors and the Salmonella isolates from cattle carcasses, the adjusted odds ratio at 95% confidence intervals (CI) with a P value of 0.05 was considered as statistical associations of contamination rate.
Results
Socio-demographic data of abattoir personnel
During the study period, a total of 24 meat abattoir personnel were enrolled in this study, of whom 23 (95.2%) were men. The median age was 42.5(35.3 ± 12.4) with the range of 24–58 years. About 19 (79.2%) of the abattoir personnel were assigned as a carcass processor (Table 1).
Hygiene status and sanitary condition of abattoir personnel (risk factors)
In multivariate analysis of the study subjects, carcasses not washed during slaughtering was 4.974 times more likely to have increased the risk of Salmonella isolates compared to carcass washed during slaughtering (AOR = 4.974; 95% CI, 1.076–22.994, P = 0.040). Besides, slaughtered personnel who have not washed their hands after separating intestinal content were 5.873 times increased Salmonella isolates compared to those who washed their hands after separating intestinal content (AOR = 5.873; 95% CI, 1.077–32.018, P = 0.041). All 24(100%) slaughter personnel wore garments and boots during slaughtering, and all 104(100%) cattle were inspected and their skin was not washed before slaughtering. However, there was no significant association, among other characteristics like slaughter personnel handwashing before slaughtering, washing animal carcasses after skinning, and sanitation of the slaughtering floor (p-value > 0.05) (Table 2).
Prevalence of Salmonella isolates from cattle carcass and abattoir personnel
From a total of 336 collected samples from cattle carcass and abattoir personnel, 27(8.0%) were culture positive for Salmonella isolates. We included 312 cattle carcass samples, in which 25 (8.0%) showed positive for Salmonella species, and relatively a higher growth of 10 (9.6%) of the isolates were observed from the abdomen regions. Of 27 Salmonella isolates, Salmonella arizonae 11 (40.7%), Salmonella Group A 9 (33.3%), Salmonella Typhi 6 (22.2%) and unspecified Salmonella 1 (3.7%) were identified. From 24 abattoir personnel, 2(8.3%) were positive for Salmonella isolates with species of Salmonella Typhi 1 (50%) and Salmonella Group A1 (50%) respectively, (Tables 3 and Fig. 1).
Antimicrobial susceptibility profile of Salmonella isolates
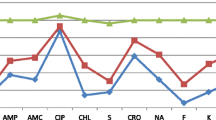
The resistance patterns of all Salmonella isolates from cattle carcass were tested against nine antimicrobial agents. In this study, the highest degree of resistance among the tested antimicrobials was observed for ampicillin 100% (25/25) followed by trimethoprim-sulfamethoxazole 56% (14/25) and tetracycline 56% (14/25) from animal source and the least resistance were observed in chloramphenicol and amikacin 5 [20] each. The two (100%) isolates from humans were resistant to ampicillin and amoxicillin-clavulanate and however, two isolates were 100% susceptible to ciprofloxacin and cephalothin (Table 4).
Multidrug resistance pattern
From the total isolates tested antibiotics classes, 27(100%) were resistant to all antibiotics. About 23 (85.2%) were resistant to two or more antibiotics classes. Multidrug resistance was detected in 22 (81.5%) of the total isolates. Multidrug resistance was detected in 20 (80%) of isolates from carcass (Table 5).
Discussion
Abattoir contamination of cattle carcass imposes a huge impact on the occurrence of salmonellosis as well as the spread of resistant strains in the community. The overall prevalence of Salmonella from beef in this study was 8% (25/312) (95% CI: 4.8–11.2) which lies between the low prevalence rate of 0.25% (1/400) and the high rate of 75% (45/60) in different areas of the world [31, 32]. This finding revealed that there was a considerable rate of contamination of beef in the Dessie municipality abattoir, which potentially poses a risk of causing food-associated illness.
This prevalence rate was comparable to a study done in Wolayta Sodo 8% (8/100) [15], Addis Ababa 5.7% (4/70) [16], Mekelle 7.29% (7/96) [33] and Bahir Dar 7.6% (23/46) [18] and Nigeria 11% (11/100) [17]. This similarity could be due to the use of nearly similar technologies for slaughtering and beef processing, safety materials used by abattoir personnel, abattoir utensils used, and cleaning methods and agents used for cleaning [34]. However, our finding was lower than studies done in Jimma at 11.3% (22/195) [35], Gondar 35.6% (32/90) [28], Ghana at 75% (45/60) [32], South Africa at 30% (30/100) [36] and Egypt at 20% (5/25) [37] and higher than studies done in Hawassa 2.4% (6/250) [13], Addis Ababa 2.5% (4/1590) [38] the United States 4.2% (172/4,136) [39], Ireland 0.25% (1/400) [31], Germany 0.7% (29/4,170) [40] and South Korea 2.04% (1/49) [41]. This difference might be related to sampling, stress during transportation to the slaughterhouse, hygienic conditions of holding pens, carcass processing practices, abattoir facilities, employee hygiene, the sanitary condition of the abattoir, post-slaughter operations such as transportation, handling, and processing by the distributors and retailers and the laboratory methods [42].
Our study showed that the prevalence of Salmonella among abattoir personnel at the Dessie municipality was 8.3% (2/24). The current result found from abattoir personnel was in line with a study done in Addis Ababa 6.0% (18/300) [43]. However, it was higher than studies conducted in Jimma 0.9% [44], and Addis Ababa 3.4% (8/233) [45]. This prevalence indicates considerable proportion of the study participants were carriers of Salmonella with an increased probability of the transfer of infection to others through contamination of the cattle carcass. The current findings from personnel were lower than a study conducted in Jimma 18% (9/50) [35]. The possible factors that contribute to this variation might be due to the difference in environmental and personal sanitation, socioeconomic and living standards, availability of water supply, and awareness of safe food and meat handling and preparation among individuals.
In the current study, the predominant Salmonella species were Salmonella arizonae followed by Salmonella Group A (Salmonella enterica serotype Typhi). The distribution of Salmonella species among cattle had a difference over time, geographic regions, age groups, clinical manifestation, and production systems [46]. The majority of salmonellosis cases are caused by eating Salmonella enterica-infected food, which commonly infects cattle and poultry, but other animals such as domestic cats and hamsters have also been shown to be sources of infection for humans [18]. Also, animal infections by Salmonella can be caused by contact and ingestion of various reptile products and bird feces [47, 48]. In this study, the isolations of Salmonella typhi and Salmonella group A showed that the contamination from human origin was a result of poor personal hygiene during the handling and processing of the carcass. While, Salmonella arizonae contamination of cattle carcasses might be due to contamination from the slaughtering floor by bird feces, as well as from cattle contact with reptiles or ingestion of various reptile products, like snakes, and ingestion of bird feces with their feeds [49].
Antibiotic resistance in Salmonella isolates increases in both developing and developed countries. Antibiotic resistance is seen in both the veterinary and public health sectors [16, 50]. In this study, isolates from carcass samples showed relatively high resistance to antimicrobial agents such as ampicillin, amoxicillin-clavulanate, tetracycline, and TMP-SMX. All (100%) of the isolates tested were resistant to ampicillin. This high resistance of isolates for ampicillin is in line with studies conducted in Egypt 93% [51] and Jimma 100% [52]. The high rate of resistance for ampicillin in the study area might be due to its use as a broad-spectrum antimicrobial agent, its low expense, its oral administration, its frequent availability, poor drug regulation practices, the increasing rate of unprescribed utilization of antibiotics, and other factors that favor selection pressure, which increases the advantage of maintaining resistant genes in the bacteria. However, our finding contradicts a study done in Gondar [53] and South Korea [41] and in which isolates were susceptible to ampicillin. This difference might be due to restricted use of the antibiotic and technical differences.
In contrast to developed resistance, in the current study, 96.3% (26 of 27) of ciprofloxacin and 74% (20 of 27) of chloramphenicol have good antimicrobial activity against Salmonella isolates from both humans and cattle. ciprofloxacin and chloramphenicol have comparable antimicrobial activity with previous reports from animal and human isolates in Jimma [35]. Studies done in Wolayta Sodo 100% [15], Jimma 100% [52], United States 100% [39], and Ghana 100% [32] showed the resistance level of ciprofloxacin. This may be because the drug is not frequently prescribed by physicians. However, a study in Egypt found that only 63% of isolates were susceptible to ciprofloxacin [51]. This disparity could be attributed to the availability of drugs without a prescription, poor drug regulation practices, and the presence of drug-resistant bacteria in animals.
The emergence of multiple drug-resistant Salmonella to commonly used antimicrobials has become a threat in both public health and veterinary sectors [14, 23]. In our study, Multidrug resistance was detected in 81.5% (22/27) of the total isolates. Multidrug resistance was detected in 80% (20/25) of isolates from carcasses and 100% (2/2) from personnel. The occurrence of MDR in this study was consistent with a report from Addis Ababa 83% [54] and Latvia (100%) [55] and However, it was higher than studies conducted in Asella 50% [56], South Africa 25.32% [36], and Jimma 40.3% [35] and in Mexico 28.8% [57],. The high MDR observed in this study might be due to the administration of multiple antimicrobials for infections, indiscriminate use of antimicrobials, and extensive use of a drug in farm animals [58].
Different studies show that various hygiene practices of the slaughter personnel and the sanitary conditions of the abattoir have been described to be associated with increased isolation of Salmonella from cattle carcasses [18, 59]. In our study, habit of not washing knives before slaughtering was associated with contamination of carcasses by Salmonella isolates. This finding was consistent with studies performed in Bahirdar [18], Sudan [59] and Modjo [60]. This can be explained by the fact that the unwashed knife used for slaughtering will be contaminated with Salmonella from the meat rumen and intestinal fluids as well as by the hands of carcass processors. However, this result differed from that of a Canadian study, which found no statistical difference between using a washed knife and the rate of Salmonella isolates from carcasses [61]. This variation might be due to the difference in slaughter personnel hygiene, slaughtering process, and use of different knives for different cattle and different processing steps.
In the same vein, there was an increased rate of Salmonella isolation in the carcass that was not washed during slaughtering. This was also in agreement with reports in Bahirdar [18] and Pakistan [19]. This might be feces as well as soil, adhere to an animal’s external surface and carry Salmonella into a slaughterhouse, which can serve as a source of contamination that potentially transfers to carcass surfaces during the decoding process. In addition to, fluids from eviscerated organs and intestinal content will contaminate the carcass with Salmonella during processing. So, carcass washing plays a great role in reducing the prevalence of Salmonella at the slaughterhouse [62].
We also found that the rate of Salmonella isolation was high in slaughter personnel who process the carcass without washing their hands after separating intestinal content. This result is in line with studies conducted in the Debre Zeit, Ethiopia [63], and Bahirdar [18], Sudan [59] and United Kingdom [64]. However, the United Kingdom reported that hand washing had no significant effect on the contamination of carcasses [65]. This variation might be from a difference in the use of safety materials, and environmental and utilities sanitary conditions. In the previous study done in Bahirdar [18] and Pakistan [19] and cattle slaughtered on the unsanitized floor were a risk for carcass contamination by Salmonella. However, in this study, it was not associated with carcass contamination of Salmonella. This variation might be due to the difference in the sanitary process of the floor, the use of detergent, the cleanness of the cleaning materials, and the smoothness of the floor [66].
Limitations of the study
Due to the unavailable of primers, the isolated Salmonella species were not molecularly characterized. Other limitations of this study were non-inclusions of the environmental sample.
Conclusions
The present study revealed that the rate of Salmonella isolate contamination was high in cattle carcass and that there was a considerable carriage rate of Salmonella isolates among personnel working at the Dessie municipality abattoir. High antimicrobial resistance was observed to ampicillin followed by trimethoprim-sulfamethoxazole and tetracycline. Multi-drug resistance in Salmonella isolates was detected for most antimicrobial drugs tested. Factors such as the use of un-sanitized abattoir utilities, unhygienic carcass processing, and poor personnel hygiene practices significantly increased carcass detection of Salmonella isolates. Local antibiotic policy and prescription practice should be required to decrease significant resistance for commonly used antibiotics. This highlights the need to treat potential Salmonella carriers and implement preventive measures. Improving hygiene is crucial to reduce cross-contamination from utensils, the working environment, and abattoir workers involved in slaughtering. Additionally, abattoir workers should undergo regular health checks to ensure ongoing safety.
Data availability
Data auxiliary to the conclusions of this article are within the manuscript.
Abbreviations
- BPW:
-
Buffered peptone water
- CDC:
-
Center for Disease Control
- CI:
-
Confidence interval
- CLSI:
-
Clinical and Laboratory Standard Institute
- ISO:
-
International Organization for Standardization
- MDR:
-
Multiple drug resistance
- NTS:
-
Non-typhoidal Salmonella
- SCL:
-
Selenite Cystine broth
- SIM:
-
Sulfide indole motility
- SOP:
-
Standard operating procedure
- SPSS:
-
Statistical package for social sciences
- WHO:
-
World Health Organization
- XLD:
-
Xylose lysine deoxycholate
References
WHO. World Health Organization. The global burden of foodborne diseases. 2015.
Majowicz SE, Musto J, Scallan E, Angulo FJ, Kirk M, O’brien SJ, et al. The global burden of nontyphoidal Salmonella gastroenteritis. Clin Infect Dis. 2010;50(6):882–9.
Papp JR. CDC laboratory recommendations for syphilis testing, United States, 2024. MMWR Recommendations Rep. 2024;73.
Bula-Rudas FJ, Rathore MH, Maraqa NF. Salmonella infections in childhood. Adv Pediatr. 2015;62(1):29–58.
Dallal MMS. Prevalence of Salmonella spp. in packed and unpacked red meat and chicken in south of Tehran. Jundishapur J Microbiol. 2014;7(4).
Ferrari RG, Rosario DK, Cunha-Neto A, Mano SB, Figueiredo EE, Conte-Junior CA. Worldwide epidemiology of Salmonella serovars in animal-based foods: a meta-analysis. Appl Environ Microbiol. 2019;85(14):e00591–19.
Heredia N, García S. Animals as sources of food-borne pathogens: a review. Anim Nutr. 2018;4(3):250–5.
EFSA CDC. The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2015. EFSA J. 2017;15(2):e04694.
Terentjeva M, Avsejenko J, Streikiša M, Utināne A, Kovaļenko K, Bērziņš A. Prevalence and antimicrobial resistance of Salmonella in meat and meat products in Latvia. Ann Agric Environ Med. 2017;24(2):317–21.
Ao TT, Feasey NA, Gordon MA, Keddy KH, Angulo FJ, Crump JA. Global burden of invasive nontyphoidal Salmonella disease, 2010. Emerg Infect Dis. 2015;21(6):941.
Zewdu E, Cornelius P. Antimicrobial resistance pattern of Salmonella serotypes isolated from food items and personnel in Addis Ababa, Ethiopia. Trop Anim Health Prod. 2009;41(2):241–9.
Eguale T, Gebreyes WA, Asrat D, Alemayehu H, Gunn JS, Engidawork E. Non-typhoidal Salmonella serotypes, antimicrobial resistance and co-infection with parasites among patients with diarrhea and other gastrointestinal complaints in Addis Ababa, Ethiopia. BMC Infect Dis. 2015;15(1):497.
Kore K, Asrade B, Demissie K, Aragaw K. Characterization of Salmonella isolated from apparently healthy slaughtered cattle and retail beef in Hawassa, southern Ethiopia. Prev Vet Med. 2017;147:11–6.
Wabeto W, Abraham Y, Anjulo AA. Detection and identification of antimicrobial-resistant Salmonella in raw beef at Wolaita Sodo municipal abattoir, Southern Ethiopia. J Health Popul Nutr. 2017;36(1).
Mekuriaw E, Walelign HBB. Isolation, Identification and Drug Resistance Profile of salmonella from apparently healthy cattle slaughtered at Wolaita Sodo Municipality Abattoir, Ethiopia. J Biology Agric Healthc 2016;6(13).
Kebede A, Kemal J, Alemayehu H, Habte Mariam S. Isolation, identification, and antibiotic susceptibility testing of Salmonella from slaughtered bovines and ovines in Addis Ababa Abattoir Enterprise, Ethiopia: a cross-sectional study. International journal of bacteriology. 2016;2016.
Bata S, Karshima N, Yohanna J, Dashe M, Pam V, Ogbu K. Isolation and antibiotic sensitivity patterns of Salmonella species from raw beef and quail eggs from farms and retail outlets in Jos, Plateau State, Nigeria. J Veterinary Med Anim Health. 2016;8(4):29–34.
Muluneh G, Kibret M. Salmonella spp. and risk factors for the contamination of slaughtered cattle carcass from a slaughterhouse of Bahir Dar Town, Ethiopia. Asian Pac J Trop Disease. 2015;5(2):130–5.
Aftab M, Rahman A, Qureshi M, Akhter S, Sadique U, Sajid A, et al. Level of Salmonella in beef of slaughtered cattle at Peshawar. J Anim Plant Sci. 2012;22:24–7.
Wales A, Cook A, Davies R. Producing Salmonella-free pigs: a review focusing on interventions at weaning. Vet Rec. 2011;168(10):267–76.
Bennani H, Mateus A, Mays N, Eastmure E, Stärk KD, Häsler B. Overview of evidence of antimicrobial use and antimicrobial resistance in the food chain. Antibiotics. 2020;9(2):49.
Darwish WS, Eldaly EA, Abbasy MT, Ikenaka Y, Nakayama S, Ishizuka M. Antibiotic residues in food: the African scenario. Jpn J Vet Res. 2013;61:S13–22.
Abdi RD, Mengstie F, Beyi AF, Beyene T, Waktole H, Mammo B, et al. Determination of the sources and antimicrobial resistance patterns of Salmonella isolated from the poultry industry in Southern Ethiopia. BMC Infect Dis. 2017;17(1):352.
Andargie G, Kassu A, Moges F, Tiruneh M, Huruy K. Prevalence of bacteria and intestinal parasites among food-handlers in Gondar town, northwest Ethiopia. J Health Popul Nutr. 2008;26(4):451.
Commission PC. Summary and statistical report of the 2007 population and housing census. Population size by age and sex. 2008.
Bureau DFaED. Overall Environmental Condition and Livestock Wealth Assessment of Dessie, Annual Report. 2007:28–36.
Dessie Municipality Abattoir Organization. 2019.
ISO. Microbiology of food and animal feeding staff-horizontal method for the detection of Salmonella. 4th edition. ISO, 6579;Geneva. 2017.
WHO. A WHO network building capacity to detect, control and prevent food borne and other enteric infections from farm to table. Lab Protocol: WHO. 2010.
Wayne P. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; 29th edition. 2019.
Khen BK, Lynch OA, Carroll J, McDowell DA, Duffy G. Prevalence and characteristics of Salmonellain the Beef Chain in the Republic of Ireland. Zoonoses Public Health. 2014: n/a-n/a
Adzitey F, Nsoah JK, Teye GA. Prevalence and antibiotic susceptibility of Salmonella species isolated from beef and its related samples in Techiman Municipality of Ghana. Turkish J Agriculture-Food Sci Technol. 2015;3(8):644–50.
Teklebrhan WALT, Kibrom AS, Getachew T, Hagos HK. Salmonella and risk factors for the contamination of cattle carcass from abattoir of Mekelle City. Ethiopia Cogent Food Agric 2018; 4(1557313).
Gutema FD, Agga GE, Abdi RD, Jufare A, Duchateau L, De Zutter L, et al. Assessment of hygienic practices in beef cattle slaughterhouses and retail shops in bishoftu, Ethiopia: implications for public health. Int J Environ Res Public Health. 2021;18(5):2729.
Takele S, Woldemichael K, Gashaw M, Tassew H, Yohannes M, Abdissa A. Prevalence and drug susceptibility pattern of Salmonella isolates from apparently healthy slaughter cattle and personnel working at the Jimma municipal abattoir, South-West Ethiopia. Trop Dis Travel Med Vaccines. 2018;4(1):13.
Madoroba E, Kapeta D, Gelaw AK. Salmonella contamination, serovars and antimicrobial resistance profiles of cattle slaughtered in South Africa. Onderstepoort J Vet Res. 2016;83(1):1–8.
Hassanein R, Ali SFH, El-Malek A, Mohamed A, Mohamed MA, Elsayh KI. Detection and identification of Salmonella species in minced beef and chicken meats by using Multiplex PCR in Assiut city. Veterinary World. 2011;4(1).
Lidya KZK, Bitsu, Haile A, YitageleT, Mohammed I, Tadesse E. Prevalence and Antimicrobial Susceptibility Profile of Salmonella Serovars Isolated from Slaughtered Cattle in Addis Ababa, Ethiopia. BioMed Research International 2018;Volume 2018.
Bosilevac JM, Guerini MN, Kalchayanand N, Koohmaraie M. Prevalence and characterization of Salmonellae in Commercial Ground Beef in the United States. Appl Environ Microbiol. 2009;75(7):1892–900.
Meyer C, Thiel S, Ullrich U, Stolle A. Salmonella in raw meat and by-products from pork and beef. J Food Prot. 2010;73(10):1780–4.
Hyeon JY, Chon JW, Hwang IG, Kwak HS, Kim MS, Kim SK, et al. Prevalence, antibiotic resistance, and molecular characterization of Salmonella serovars in retail meat products. J Food Prot. 2011;74(1):161–6.
Gashe M. Assessment of Abattoir Facilities, Slaughtering Practices and Evaluation of Bacterial Load on Carcass and In-Contacts In Abattoirs of North West Amhara, Ethiopia. 2022.
Molla B, Alemayehu D, Salah W. Sources and distribution of Salmonella serotypes isolated from food animals, slaughterhouse personnel and retail meat products in Ethiopia: 1997–2002. Ethiop J Health Dev. 2003;17(1):63–70.
Tsegaye AHT, Beyene W, Jemal B. Contamination of bacteria and associated factors among food handlers working in the student cafeterias of Jimma University Main campus, Jimma. Altern Integr Med. 2015;4(1):185.
Getnet F, Gebre-selassie S, Alemayehu H, Kassa T, Kebede N. Prevalence and antimicrobial resistance of Salmonella isolated from food handlers in Addis Ababa University students ’ cafeteria, Ethiopia. Afr J Basic Appl Sci. 2014;6(6):210–6.
Hoelzer K, Switt AIM, Wiedmann M. Animal contact as a source of human non-typhoidal salmonellosis. Vet Res. 2011;42(1):34.
Evangelopoulou G, Kritas S, Govaris A, Burriel AR. Pork meat as a potential source of Salmonella enterica subsp. arizonae infection in humans. J Clin Microbiol. 2014;52(3):741–4.
Hoelzer K, Moreno Switt AI, Wiedmann M. Animal contact as a source of human non-typhoidal salmonellosis. Vet Res. 2011;42:1–28.
Djeffal S, Mamache B, Elgroud R, Hireche S, Bouaziz O. Prevalence and risk factors for Salmonella spp. contamination in broiler chicken farms and slaughterhouses in the northeast of Algeria. Veterinary World. 2018;11(8):1102.
Kemal J. A review on the public health importance of bovine salmonellosis. Veterinary Sci Technol. 2014;5(2):1.
Sallam KI, Mohammed MA, Hassan MA, Tamura T. Prevalence, molecular identification and antimicrobial resistance profile of Salmonella serovars isolated from retail beef products in Mansoura. Egypt Food Control. 2014;38:209–14.
Dabassa Anbessa, Ketema B. The prevalence and Antibiogram of Salmonella and Shigella isolated from Abattoir, Jimma Town, Southwestern Ethiopia. Int J Pharm Biol Res (IJPBR). 2012;3(4):143–8.
Ejo M, Garedew L, Alebachew Z, Worku W. Prevalence and antimicrobial resistance of Salmonella isolated from animal-origin food items in Gondar, Ethiopia. BioMed research international. 2016;2016.
Addis ZKN, Sisay Z, Alemayehu H, Yirsaw A, Kassa T. Prevalence and antimicrobial resistance of Salmonella isolated from lactating cows and in contact humans in dairy farms of Addis Ababa: a cross sectional study. BMC Infect Dis. 2011;11(222).
Thung TY, Radu S, Mahyudin NA, Rukayadi Y, Zakaria Z, Mazlan N, et al. Prevalence, virulence genes and Antimicrobial Resistance profiles of Salmonella Serovars from Retail Beef in Selangor, Malaysia. Front Microbiol. 2017;8:2697.
Beyene T, Yibeltie H, Chebo B, Abunna F, Beyi AF, Mammo B et al. Identification and antimicrobial susceptibility profile of Salmonella isolated from selected dairy farms, abattoir and humans at Asella town. J Vet SciTechno. 2016;7(3).
Realpe Quintero M, Barba Leon J, Perez Montano JA, Pacheco Gallardo C, Gonzalez Aguilar D, Dominguez Arias RM, et al. Genetic diversity and antimicrobial resistance of Salmonella serotypes recovered throughout the beef production chain and from patients with salmonellosis. PeerJ. 2018;6:e5482.
Abdi RD, Mengstie F, Beyi AF, Beyene T, Waktole H, Mammo B, et al. Determination of the sources and antimicrobial resistance patterns of Salmonella isolated from the poultry industry in Southern Ethiopia. BMC Infect Dis. 2017;17:1–12.
Magdaa AAM, Suliman SE, Shuaib YA, Abdalla MA. Assessment of bacterial contamination of sheep carcasses at slaughterhouse in Khartoum State. Sudan J Sci Technol. 2012;13:68–72.
Teklu A, Negussie H. Assessment of risk factors and prevalence of Salmonella in slaughtered small ruminants and environment in an export abattoir, Modjo, Ethiopia. Am Eurasian J Agric Environ Sci. 2011;10:992–9.
Letellier A, Beauchamp G, Guévremont E, D’ALLAIRE S, Hurnik D, Quessy S. Risk factors at slaughter associated with presence of Salmonella on hog carcasses in Canada. J Food Prot. 2009;72(11):2326–31.
Ferede B. Isolation, identification, antimicrobial susceptibility test and public awareness of Salmonella on raw goat meat at Dire Dawa Municipal Abattoir, eastern Ethiopia. Addis Ababa University; 2014.
Sibhat B, Molla Zewde B, Zerihun A, Muckle A, Cole L, Boerlin P, et al. Salmonella serovars and antimicrobial resistance profiles in beef cattle, slaughterhouse personnel and slaughterhouse environment in Ethiopia. Zoonoses Public Health. 2011;58(2):102–9.
Burton M, Cobb E, Donachie P, Judah G, Curtis V, Schmidt W-P. The effect of handwashing with water or soap on bacterial contamination of hands. Int J Environ Res Public Health. 2011;8(1):97–104.
Whyte R, Holder J, Tinker D, Allen V, White R, Hinton M. Assessment and development of procedures and apparatus to reduce contamination of lamb carcasses during pelt removal in low-throughput abattoirs. J Food Prot. 2002;65(1):41–9.
Bedassa A. Prevalence and Antimicrobial Resistance of Salmonella enterica in cow milk and cottage cheese in major milk shades of Oromia Region. Ethiopia: Addis Ababa University; 2021.
Acknowledgements
The authors would like to acknowledge the Department of Medical Microbiology, School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar Hospital, for allowing me to conduct this work. All the study participants, APHI staff especially the Microbiology Department, and the staff of DRH are for their participation and support during the sample collection.
Funding
No external funds were obtained.
Author information
Authors and Affiliations
Contributions
Alemayehu Tadesse, Bekele Sharew, Mihret Tilahun, and Yihenew Million were involved in proposal writing and designing the study; and participated in the analysis and interpretation of data. Alemayehu Tadesse, Bekele Sharew, and Mihret Tilahun were involved in the data collection and drafting of the manuscript. All authors finalized the write-up of the manuscript. Alemayehu Tadesse, Mihret Tilahun, and Yihenew Million authors critically revised the manuscript and read and approved the final manuscript for publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical clearance and permissions were obtained from the ethical review committee University of Gondar, College of Medicine and Health Sciences, School of Biomedical and Laboratory Sciences with protocol number SMBLS 2122/2011, and before data collection, support letters were obtained from Dessie Town administration health office and Dessie abattoir administration. After clarifying the objectives of the study, abattoir personnel provided verbal and written informed consent. For animals, informed consent was obtained from the owners. All data and samples obtained are kept confidential by using codes instead of personal identifiers. Participants in the study who tested positive for Salmonella in their stool were advised to go to health institutes and consult clinicians, and they received the necessary medical treatment at the expense of the Dessie Town municipality administration.
Competing interests
The authors declare no competing interests.
Consent for publication
Non-applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tadesse, A., Sharew, B., Tilahun, M. et al. Isolation and antimicrobial susceptibility profile of Salmonella species from slaughtered cattle carcasses and abattoir personnel at Dessie, municipality Abattoir, Northeast Ethiopia. BMC Microbiol 24, 357 (2024). https://doi.org/10.1186/s12866-024-03507-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12866-024-03507-x