Abstract
Background
The commercial utilization of genetically modified soybeans has yielded substantial economic advantages. Nevertheless, the genetic drift towards wild soybeans is one of the main ecological risks that needs to be addressed. Previous experiments demonstrated the absence of fitness cost or florescence overlap in hybrid offspring resulting from the crossbreeding of transgenic soybean GTS40-3-2 and Zhengzhou wild soybeans. In this study, hybrid progeny was systematically crossed with wild soybeans to establish a backcross progeny system. This system was employed to evaluate the ecological risk associated with the backcross progeny of transgenic and wild soybeans.
Results
The findings indicated that the offspring from the backcross exhibited glyphosate tolerance. Furthermore, the expression of foreign proteins in the backcross offspring was notably lower than in the transgenic soybean, and there was no significant difference when compared to the hybrid progeny. Parameters such as germination rate, aboveground biomass, pods per plant, full seeds per plant, and 100-grain weight exhibited no significant differences between the negative and positive lines of the backcross progenies, and no fitness cost was identified in comparison to wild soybeans. These results underscore the potential for foreign genes to propagate within other wild soybeans, which requires continuous attention.
Conclusions
The widespread adoption of genetically modified soybeans has undeniably led to substantial economic gains. However, the research findings emphasize the critical importance of addressing the ecological risks posed by genetic drift towards wild soybeans. The backcross progeny system established in this study indicates that the potential for foreign gene dissemination to wild soybean populations warrants continued attention and mitigation strategies.
Similar content being viewed by others
Background
Soybeans play a crucial role in both human nutrition and various economic sectors, such as edible oil and feed protein production. Over the past five years, China has consistently maintained an annual soybean demand of approximately 110 million tons, primarily met through imports. In 2021, China’s soybean production yielded 16.4 million tons, while imports soared to a substantial 96.518 million tons [1], accounting for approximately 75% of the global soybean trade volume [2]. Genetically modified (GM) soybeans have demonstrated advantages in terms of yield and price [3], influencing China’s soybean industry. The commercialisation of herbicide-resistant GM soybeans in China represents a pivotal development. Currently, several GM soybean varieties, with independent intellectual property rights in China, have obtained production safety certifications and are poised for commercial cultivation [4].
The introduction of the epsps gene into soybeans confers resistance to glyphosate on the transgenic soybeans [5]. The biosafety of GM soybeans, especially noteworthy is the concern regarding the potential transfer of foreign genes from GM crops to their wild counterparts through pollen-mediated gene drift and the potential ecological risks involved, requires thorough consideration before GM soybean commercialisation [6,7,8]. The transfer of the epsps gene from genetically modified canola (Brassica napus L.) to wild turnip (Brassica rapa L.) confers a significant selective advantage for glyphosate resistance to the turnip in Argentina. The presence and spread of these glyphosate-resistant turnips in agricultural fields raise the potential for them to develop into “superweeds” [9].
Wild soybeans exhibit valuable traits, such as stress resistance, high reproductive capability, and protein content, making their genetic resources essential for soybean breeding [10,11,12]. China is an important birthplace for wild soybeans and is rich in wild soybean germplasm resources [13, 14]. In natural field conditions, foreign genes, such as epsps can drift to wild soybeans. Although the farthest recorded drift distance was 10 m and the drift rate was less than 1/10,000, the resultant hybrid progeny remained fertile [15]. Despite the relatively low outcrossing rate, the extensive distribution of both cultivated and wild soybeans in China, along with their overlapping regions and synchronous flowering periods, presents a high risk of foreign gene introgression into wild soybeans through gene flow, followed by further backcrossing with wild soybeans.
Studies have been conducted on the ecological risks associated with hybrid offspring resulting from the interbreeding of transgenic and wild soybeans. The findings revealed that while the fitness of F1 hybrid offspring from the crossbreeding of transgenic and wild soybeans in various regions was lower than that of wild soybeans, these hybrid progenies were still capable of seed production. [12, 16]. In five additional instances, the outcomes demonstrated that the fitness of the hybrid F1 generation did not significantly deviate from that of wild soybeans in the absence of glyphosate. Furthermore, in certain fitness parameters, it was notably higher, suggesting that wild soybeans expressing the epsps gene might exhibit increased invasiveness than its wild soybean parents [16,17,18,19,20]. While risk assessments of the selfing progeny of hybrid descendants from genetically modified soybeans and wild soybeans are available, there are no risk assessment results for the backcross progeny of hybrids with wild soybeans.
According to our previous experiments, the hybrid F2 generation of epsps transgenic soybean GTS40-3-2 and Zhengzhou wild soybean had no fitness cost or overlapping florescence [20] when compared to wild soybean. Additionally, these hybrid progenies could successfully backcross with wild soybeans, thereby enables the ongoing dissemination of foreign genes throughout the wild soybean population. To assess the ecological risks linked to the ongoing drift of hybrid progeny to wild soybean, we conducted crossbreeding between hybrid progeny possessing a foreign gene background and wild soybeans. This established a backcross progeny system, serving as a simulation for the transfer of foreign genes from transgenic soybeans to wild soybeans. We assessed the expression of foreign proteins, herbicide resistance, and the fitness of parents and their respective backcross progeny. Our study aims to shed light on the evolutionary potential of foreign genes after their introduction to wild soybeans and explore the potential long-term ecological risks they may pose.
Field trials on fitness are more representative of real-world conditions than greenhouse experiments. However, according to Chinese regulations [21], such experiments cannot be conducted in the field. Due to significant environmental differences, results obtained from field trials conducted abroad cannot represent the risk situation in China. Additionally, because wild soybean seeds are small, numerous, and exhibit shattering and dormancy, conducting this experiment in the field could lead to seeds from backcross progeny containing foreign genes being lost in the soil and persisting for a long time, resulting in the escape of foreign genes. Although the results of greenhouse experiments cannot fully represent the actual risks in field environments, all experimental materials in this study were grown under the same conditions. Therefore, the results obtained are of significant reference value for ecological risk assessment.
Materials and methods
Materials
In this investigation, the Roundup Ready (RR) soybean (GTS40-3-2, labeled GM) and Zhengzhou wild soybean (Labeled Wild) were employed. The GM soybean was generously supplied and preserved by the weeds research laboratory of Nanjing Agricultural University (Nanjing, China). This GM soybean expresses the synthetic CP4-EPSPS gene, providing resistance to glyphosate herbicide, and has obtained approval in numerous countries globally [22].
The wild soybean seeds used in this study were harvested by the staff of our research team in 2015 from the Yellow River Dam in Zhengzhou City, Henan Province, China (113°39’20’’, 34°54’36’’). According to our study, The Zhengzhou wild soybean does not express the EPSPS protein and, consequently, does not exhibit resistance to glyphosate. The wild soybean is stored in the Key Laboratory on Biodiversity and Biosafety of Nanjing Institute of Environmental Sciences.
The F1 generationas (Labeled F1) seeds were obtained through artificial pollination, using the GM soybean as the pollen donor (male parent) and the Zhengzhou wild soybean as the seed recipient (female parent) in 2017. In 2018, both the F1 generation and Zhengzhou wild soybeans were cultivated within a greenhouse setting. First-generation backcross progeny (Labeled BC1F1) seeds were obtained through artificial pollination, using the hybrid F1 generation as the pollen donor (male parent) and the Zhengzhou wild soybean as the seed recipient (female parent). BC1F1 soybeans were cultivated within a greenhouse in 2019. BC1F1 leaves were assessed using an epsps test strip (ENVIROLOGIX, QuickStix Kit for CP4 EPSPS), and BC1F1 plants with the epsps gene engage in self-pollination to acquire the succeeding generation of seeds (Labeled BC1F2). The negative and positive grouping of BC1F1 and BC1F2 was determined by the detection results of the epsps test strips during the trifoliate stage of soybean.
In 2020, GM, Wild, F1, BC1F1 and BC1F2 soybeans were planted in identical soil after germination rate test and under uniform management conditions, followed by a comprehensive examination of their growth and reproductive indices.
Test site
The experiment took place in a controlled greenhouse at the Nanjing Institute of Environmental Science, MEE, China, ensuring isolated conditions. All experimental materials were cultivated under uniform soil and management conditions.
Expression of exogenous protein and glyphosate resistance
Selected seeds from diverse hybrid generations of transgenic soybeans and Zhengzhou wild soybeans were chosen for experimentation. Once the first true leaf had developed, the seeds underwent spraying with a glyphosate herbicide (1230 g a.i./hm2). The count of surviving plants was documented one week after the treatment.
A test strip for epsps was employed to identify soybean plants that withstood the herbicide application. Five plants with positive test strips were randomly selected from each material for the molecular detection of foreign genes. The PCR-specific primers and annealing temperatures of the exogenous epsps gene and endogenous actin reference gene are shown in Table 1.
Every leaf sample of surviving soybean was gathered, and a single complete leaf was obtained from the uppermost part of each soybean plant. The CP4-epsps ELISA protein levels in the leaves of different soybean materials were assessed using a CP4-epsps ELISA protein kit (Environ Logix) following the manufacturer’s instructions. Subsequent to the test, absorbance was promptly measured at a wavelength of 450 nm using an enzyme labeling instrument (IBM 2000). A purified epsps served as a quantitative standard protein, and the epsps protein content in the leaves was computed based on the standard curve and sample dilution factors.
Determination of biological characteristics of soybean
Germination rate, transplanting, and cultivation management of soybean seeds
GM, Zhengzhou wild soybeans, and various hybrid offspring, with each material randomly selecting 150 seeds. Each set comprised of 50 seeds for replication purposes. A single seed was placed in a well of a 12-well cell culture plate (Corning, New York, USA), two layers of filter paper were laid in each well and 400 µL of distilled water was added. Each seed was individually numbered. The cell culture plate was then placed in an artificial climate chamber (KBF720, Binder, Germany) with the following culture conditions: 25 °C, 24 h of darkness, and a relative humidity of 55%. The culture filter paper was replaced every two days, and distilled water was added as needed.Transplanted the sprouted seeds into the soil every 24 h, and after one week, recorded the germination rate. Seeds that did not germinate were manually broken the skin with a knife to expedite the germination process.
Germinated seeds were transplanted into cultivation pots (730 × 560 × 230 mm). The plant culture medium consisted of a 1:1 mixture of farmland soil and nutrient soil (organic matter content 2.5-5.0%). A bamboo pole (diameter 1.5 cm, height 2.3 m) served as a climbing support. During the experiment, all treated materials were processed according to the conventional soybean cultivation and management mode, and all weeds in the culture basin were removed by an artificial method. No fertilisers, chemical herbicides, or growth regulators were employed, except during normal watering.
At the trifoliate stage, the soybean leaves were collected and subjected to epsps testing using an epsps test strip (ENVIROLOGIX, QuickStix Kit for CP4 EPSPS). Based on the results, BC1F1 and BC1F2 cells were divided into negative (No epsps detected) and positive groups (epsps detected), respectively.
Aboveground biomass
After the soybeans reached full maturity, 30 soybean plants were chosen randomly and allocated into three groups for the assessment of aboveground biomass. The soybeans were severed near the soil surface, left to air-dry until reaching a constant weight, and then weighed using PB602-N METTLER TOLEDO.
Soybean seed indicators
After the appearance of black pods in the processed plants, individual plants were covered with nylon mesh bags to prevent seed splashing. The count of pods per plant, the number of filled grains per plant, and the hundred-grain weight were documented (100 seeds were chosen randomly from each plant, with measurements conducted on 30 plants for each material).
Calculation method for total fitness
Relative fitness values were calculated by comparing various fitness components (aboveground biomass, number of pods per plant, number of filled grains per plant, and hundred-grain weight) of the offspring to the corresponding fitness components of wild soybean, with wild soybean as the reference ‘1’. The total fitness value was the average of the relative fitness values of each fitness component.
Statistical analysis
The data are expressed as mean ± standard deviation (mean ± SD). To assess variations in plant performance among different groups, a one-way analysis of variance (ANOVA) was conducted. Tukey’s multiple comparison test was subsequently employed to ascertain the significance of differences between the groups. Statistical significance was set at P < 0.05. All statistical analyses were carried out using SPSS 20.0 software (SPSS Inc.).
Results
Glyphosate resistance
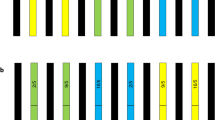
The results showed that transgenic soybeans carrying the epsps gene, as well as the hybrid and backcross offspring, survived under glyphosate selection (Table 2). The use of CP4 epsps protein test strips confirmed the presence of surviving plants. Molecular testing was conducted on five randomly selected surviving plants from each material, and the PCR results were consistent with the test strip results(Fig. 1). These findings indicate that the escape of exogenous genes to wild soybeans can confer glyphosate resistance to the hybrid and backcrossed offspring carrying the exogenous epsps gene.
Expression level of exogenous proteins
The expression of exogenous epsps protein were detected in surviving trifoliate-leafed plants following glyphosate treatment for each material (Table 3). Quantitative analysis revealed the absence of exogenous proteins in the parental wild soybean samples, whereas epsps exhibited normal expression in GM soybeans and their hybrid and backcross progenies. The expression level of epsps in GM soybeans was notably higher compared to that in the hybrid and backcross progenies. Among the surviving plants of the hybrid and backcross progenies, the epsps protein expression ranked in the following order from highest to lowest: BC1F2, BC1F1, and F1 generations, with no significant differences observed between them. These results affirm the normal expression of exogenous proteins in backcross progenies, thereby conferring glyphosate resistance.
Growth and development indicators
Germination rate
The germination rates of soybean seeds, from highest to lowest, were as follows: GM soybean, BC1F2, BC1F1, F1 generation, and wild soybean (Table 4). The germination rate of BC1F1 seeds was significantly lower than that of GM and BC1F2 soybeans (P < 0.001), but it did not differ significantly from that of F1 hybrids and wild soybeans (P > 0.05). The emergence rate of BC1F2 is between GM soybean and wild soybean, significantly lower than that of GM soybean (P < 0.05), and significantly higher than that of wild soybean and F1 hybrid (P < 0.05). This finding indicates that the offspring of backcrossing can emerge in natural ecosystems and the exogenous genes can persist in wild soybeans for an extended period.
Aboveground biomass
The aboveground biomass of GM soybean, wild soybean, and hybrid and backcross offspring under greenhouse cultivation conditions is shown in Table 5. The order of aboveground biomass, from the highest to the lowest, was as follows: GM soybean, BC1F2, wild soybean, BC1F1, and hybrid F1 offspring. While the aboveground biomass of BC1F2 surpassed that of BC1F1 and wild soybeans, these differences lacked statistical significance. Furthermore, no notable distinctions in aboveground biomass were noted between the BC1F1 and BC1F2 populations, whether negative or positive for exogenous genes. This suggests that the presence or absence of foreign genes did not have an impact on the aboveground biomass of the backcross offspring.
Number of pods per plant
Table 6 presents the number of pods per plant for various soybean materials under greenhouse cultivation conditions. The ranking of the number of pods per plant, from highest to lowest, was as follows: wild soybean, BC1F2, BC1F1, transgenic soybean, and hybrid progeny. Notably, BC1F1 exhibited a significantly higher number of pods per plant than transgenic soybean and hybrid progeny but a significantly lower number than BC1F2 and wild soybean. BC1F2, however, displayed a significantly lower number of pods per plant than wild soybean. Importantly, no significant difference was observed between the negative and positive populations of BC1F1 and BC1F2, indicating that foreign genes did not influence the number of pods per plant in the backcross progenies.
Number of full grains per plant
Table 7 shows the number of full grains per plant of soybean materials under greenhouse cultivation conditions. The ranking of the number of full grains per plant, from highest to lowest, was as follows: wild soybean, BC1F2, BC1F1, transgenic soybean, and hybrid progeny. There was no notable distinction in the number of filled grains per plant among BC1F1, transgenic soybean, and the F1 generation. However, this count was significantly lower compared to BC1F2 and wild soybeans. Additionally, the number of pods per plant in BC1F2 was significantly lower than that in wild soybeans. Notably, no significant differences were observed between the negative and positive populations of BC1F1 and BC1F2, suggesting that the presence of foreign genes did not influence the number of filled grains per plant.
Hundred-grain weight
Table 8 shows the weight of 100 seeds for each soybean material under greenhouse cultivation conditions. The ranking of the Hundred-grain weight, from highest to lowest, was as follows: transgenic soybean, BC1F2, BC1F1, wild soybean, and hybrid progeny. Transgenic soybeans exhibited a significantly higher 100-grain weight than other soybean materials. However, there were no significant differences observed among the other soybean materials. Additionally, there were no significant differences between the negative and positive populations of BC1F1 and BC1F2, suggesting that the foreign genes did not influence the 100-grain weight of the backcross progenies.
Relative fitness
The relative fitness of the hybrid and backcross progeny was calculated using wild soybean as the control(Fig. 2). In comparison to wild soybean, hybrid F1 generation and backcross BC1F1 generation soybeans showed fitness costs. Nonetheless, the fitness level of the BC1F2 backcross generation closely resembled that of wild soybeans, with no notable fitness differences observed between the negative and positive populations of BC1F1 and BC1F2.These results indicate that foreign genes have the potential to persist in hybrid offspring, propagate with their reproduction, and present the fundamental conditions for generating specific ecological risks.
Discussion
Based on prior research findings from our team, foreign genes exhibit normal expression in the hybrid offspring resulting from the crossbreeding of transgenic soybeans and wild soybeans. The flowering periods of the hybrid progeny and wild soybeans coincide, enabling the potential spread of foreign genes within the wild soybean population. In this study, a backcross progeny system was established by employing the hybrid F1 generation as the pollen donor (male parent) and the Zhengzhou wild soybean as the seed recipient (female parent). This system proves effective in simulating the natural environment and restoring the flow of foreign genes. In a previous study, the protein expression and fitness indices of homozygous and heterozygous wild soybeans were assessed, and no significant differences were found [20, 23]. Hence, the backcross progenies were classified into two groups: foreign protein-positive and foreign protein-negative.
Expression of foreign protein and herbicide resistance in backcross progeny
Whether exogenous transgenes that escape into cultivated crops or their wild relatives through gene flow can be expressed normally and function is a critical step in assessing the environmental risks associated with transgene escape [24, 25]. Studies indicates that exogenous proteins can be adequately expressed in the hybrid offspring resulting from the crossbreeding of transgenic crops and wild relatives. Moreover, the expression of these exogenous proteins may bestow resistance traits similar or identical to those found in the hybrid offspring of transgenic crops, potentially leading to unpredictable environmental risks. [20, 25,26,27]. In Argentina, the acquisition of exogenous CP4-epsps genes from GM rapeseed by wild turnips has made it challenging to control because of glyphosate tolerance [9]. In previous experiments, we confirmed the normal expression of exogenous proteins in hybrid offspring [20, 23]. However, it remains unclear whether the expression of exogenous genes confers glyphosate resistance to wild soybeans. Hence, this study examined herbicide tolerance in the backcrossed progeny of transgenic and wild soybeans. Additionally, it investigated the expression of exogenous proteins following the acquisition of the epsps gene, aiming to elucidate the ecological risks associated with the gene flow of epsps into wild soybeans.
In this study, it was recommended that glyphosate be sprayed at the third trifoliate stage of soybeans, and the survival rates of the soybean materials were counted one week later. After two weeks of glyphosate treatment, GM soybean and hybrid F1 showed nearly complete survival, whereas the survival rate of the backcrossed first-generation (BC1F1) was 46.7%. This survival rate aligns with the Mendelian genetics law, confirming the presence of exogenous genes (X2 = 0.667, df = 1, P = 0.414, chi-square test). Subsequently, BC2F2 was obtained through self-pollination of BC1F1 plants that tested positive in a strip test. The post-herbicide survival rate was 72.7%, which also conforms to the Mendelian genetics law regarding the proportion of exogenous genes (X2 = 0.436, df = 1, P = 0.509, chi-square test). Through exogenous protein strip tests and molecular detection, we determined that all surviving plants expressed exogenous genes. The expression levels of exogenous proteins in these surviving plants were analysed using a protein quantification detection kit. The findings indicated that the expression levels of exogenous proteins in the backcrossed progeny were notably lower than those in GM soybeans, yet there was no significant difference compared to the levels in the hybrid offspring. This outcome supports previous findings, providing additional evidence that exogenous proteins can be expressed not only in genetically modified soybeans and hybrid wild soybean offspring but also in backcrossed offspring.While the expression levels are significantly lower than those in GM soybeans, they still confer glyphosate tolerance to the backcrossed offspring, thereby confirming the risk of wild soybeans evolving into superweeds owing to gene flow from the GM soybeans.
Fitness analysis of backcross progeny
Previous studies have demonstrated that, under consistent planting times, the flowering period of the F1 generation coincided with that of wild soybeans for 23 days, whereas the flowering period of the F2 generation overlapped with that of wild soybeans for 21 days. These findings confirmed the presence of favourable conditions for the spread of foreign genes in hybrid progenies to the wild soybean population [20]. Therefore, further investigation into the fitness of hybrid progenies and backcross progenies with wild soybeans is warranted.
There were no significant differences observed between BC1F2-negative and positive heterozygote and homozygous plants in vegetative and reproductive growth. This implies that the insertion of the epsps gene does not visibly influence the growth and development of wild soybeans. In the absence of herbicide selection pressure, the aboveground biomass and hundred-grain weight of the BC1F2 generation soybeans were notably higher than those of wild soybeans. Additionally, no significant differences were observed in pollen germination rate, emergence rate, filled seeds per plant and pods per plant when compared to wild soybeans. The findings indicated that the hybrid offspring of transgenic and wild soybeans incurred no fitness cost in terms of nutrition and reproduction, which proved advantageous for the spread of foreign genes within the wild soybean population. While current studies have predominantly concentrated on the fitness of hybrid offspring from transgenic and wild soybeans, there is a scarcity of research on the fitness of backcross progeny.
As per Kuroda et al. [16], the hybrid offspring resulting from the crossbreeding of cultivated and wild soybeans possess a fraction of artificially domesticated genes derived from cultivated soybeans, which may result in lower fitness than wild soybeans. Upon crossing transgenic soybeans with the epsps gene, developed by NAU, with wild soybeans from ten distinct regions, a majority of the F1 hybrid offspring displayed decreased germination rates and diminished biomass, number of pods per plant, plant height, and hundred-grain weight in comparison to wild soybeans. These factors might pose a disadvantage for the survival of the hybrid offspring in natural environments. [12]. Nonetheless, comparable outcomes have been documented in various other studies concerning the fitness of hybrid offspring resulting from the crossbreeding of transgenic soybeans with the epsps gene and wild soybeans.
In the study conducted by Guan et al. [17], a significantly low pod-setting rate was observed in the hybrid offspring resulting from the crossbreeding of herbicide-tolerant transgenic soybean AG5601 with the epsps gene and Beijing wild soybeans. Moreover, there was no substantial difference in fitness between hybrid offspring with and without the epsps gene, indicating that the herbicide resistance gene may not negatively impact the growth of introduced wild soybeans. Kan et al. [18] investigated the fitness of hybrid offspring resulting from the crossbreeding of four wild soybean materials with glyphosate-resistant transgenic soybeans carrying the epsps gene, conducting the study under net-house conditions. Without the influence of glyphosate selection pressure, no notable differences in fitness indicators were noted between the hybrid offspring and their wild soybean counterparts [18]. In a two-year field study conducted in Korea, Yook et al. [19] evaluated the potential weed risk linked to the hybrid offspring resulting from gene flow between glyphosate-resistant and wild soybeans. Their findings illustrate that, owing to pollen-mediated gene flow and the enhanced fitness of hybrid offspring, transgenic soybeans have the potential to spread into wild populations and endure in local agricultural ecosystems. The findings from the 3-year experiment showed that hybrids resulting from the crossbreeding of GM soybeans with wild soybeans exhibited decreased seed germination and increased seed productivity compared to GM soybeans. Notably, these characteristics, particularly in F2 and F3 hybrids, resembled those of wild soybeans [23]. These findings also suggest that without glyphosate application, the fitness of wild soybeans expressing the epsps gene remained relatively stable in comparison to wild soybeans and even showed a significant increase in certain fitness indicators. Hence, the invasive potential of hybrid offspring between transgenic and wild soybeans might surpass that of wild soybean parents, contributing to the dissemination of exogenous epsps genes in wild soybean populations.
Conclusion
In conclusion, our study has demonstrated that wild soybeans carrying exogenous genes can exhibit significant fitness advantages when subjected to glyphosate selection pressure. These findings shed light on the potential ecological consequences of gene flow from genetically modified soybeans to wild populations. Without the application of glyphosate, there were no notable differences in fitness observed between the backcrossed progeny BC1F2 and wild soybeans, suggesting the potential continuous spread of exogenous genes within the wild soybean population. Nonetheless, the expression status of exogenous genes remains unclear with the increase of backcross generations.To fully assess the long-term implications, it is imperative to conduct further validation studies that investigate the expression levels of these genes across successive backcross generations. This research provides valuable insights into the potential ecological risks associated with genetically modified soybeans and underscores the importance of continued monitoring and research in this field.
PCR detection results of partially surviving plants after glyphosate spraying. Panel A shows the epsps gene, and panel B represents the endogenous reference gene Actin. M is the DNA ladder marker; CK + is the positive control (DNA from GM plants); CK- is the negative control (DNA from wild soybeans); 1–20 represents the DNA of surviving soybean plants after treatment (1–5: GM soybeans, 6–10:F1, 11–15: BC1F1;16–20: BC1F2). Uncropped full-length gels are presented in Supplementary Fig. 1 and Supplementary Fig. 2
Data availability
The data used in this study are available from the corresponding author on reasonable request.
References
United States Department of Agriculture. World agricultural production [R]. Washington DC: United States Department of Agriculture; 2022.
World Agricultural Outlook Board. World Agricultural Supply and demand estimates [R]. Washington DC: United States Department of Agriculture; 2022.
Pellegrino E, Bedini S, Nuti M, Ercoli L. Impact of genetically engineered maize on agronomic, environmental and toxicological traits: a meta-analysis of 21 years of field data. Sci Rep. 2018;8:3113. https://doi.org/10.1038/s41598-018-21284-2.
Yu HL, Wu KM. Commercialization strategy of transgenic soybean in China. Biotechnol Bull. 2023;39:1–15.
Delannay X, Bauman TT, Beighley DH, Buettner MJ, Coble HD, DeFelice MS, et al. Yield evaluation of a glyphosate-tolerant soybean line after treatment with glyphosate. Crop Sci. 1995;35:1461–7.
Snow AA, Andersen B, Jørgensen RB. Costs of transgenic herbicide resistance introgressed from Brassica napus into weedy B. rapa. Mol Ecol. 1999;8:605–15.
Song ZP, Lu BR, Wang B, Chen JK. Fitness estimation through performance comparison of F1 hybrids with their parental species Oryza rufipogon and O. sativa. Ann Bot. 2004;93:311–6.
Lu BR. Analysis of fitness effect and its application in assessing environmental risk caused by transgene flow. Biotechnol Bull. 2015;31:7–16.
Pandolfo CE, Presotto A, Carbonell FT, Ureta S, Poverene M, Cantamutto M. Transgene escape and persistence in an agroecosystem: The case of glyphosate-resistant Brassica rapa L. in Central Argentina. Environ Sci Pollut Res Int. 2018;25:6251-64. https://doi.org/10.1007/s11356-017-0726-3.10.
Li YH, Zhou GY, Ma JX, Jiang W, Jin LG, Zhang Z,. De novo assembly of soybean wild relatives for pan-genome analysis of diversity and agronomic traits. Nat Biotechnol. 2014;32:1045-52.
Li RF, Wang D, Li Z, Ding H, Zhang X. Research progress on the genetic diversity of wild soybean in China. Agric Sci Technol. 2017;18:2326–30.
Liu JY, Sheng ZW, Hu YQ, Liu Q, Qiang S, Song XL, et al. Fitness of F1 hybrids between 10 maternal wild soybean populations and transgenic soybean. Transgen Res. 2021;30:105–19.
Xu B. A decade of wild soybean (G. Soja) research in China. J Jilin Agric Sci. 1989;14:5–13.
Liu YC, Du HL, Li PC, Shen Y, Peng H, Liu S et al. Pan-genome of wild and cultivated soybeans. Cell. 2020;182:162 – 76.e13.
Liu B, Xue K, Liu LP. Progress on the gene flow from GM soybeans to wild soybeans. J Ecol Rural Environ. 2020;7:833–41. https://doi.org/10.19741/j.issn.1673-4831.2019.0407.
Kuroda Y, Kaga A, Tomooka N, Yano H, Takada Y, Kato S, et al. QTL affecting fitness of hybrids between wild and cultivated soybeans in experimental fields. Ecol Evol. 2013;3:2150–68.
Guan ZJ, Zhang PF, Wei W, Mi XC, Kang DM, Liu B. Performance of hybrid progeny formed between GM herbicide-tolerant soybean and its wild ancestor. AoB Plants. 2015;7:plv121.
Kan GZ, Tong ZF, Hu ZB, Ma DY, Zhang GZ, Yu DY. Fitness of hybrids between wild soybeans (Glycine soja) and the glyphosateresistant transgenic soybean (Glycine max). Soybean Sci. 2015;34:177–84.
Yook MJ, Park HR, Zhang CJ, Lim S, Jeong S, Chung YS, et al. Environmental risk assessment of glufosinate-resistant soybean by pollen-mediated gene flow under field conditions in the region of the genetic origin. Sci Total Environ. 2021;762:143073.
Liu L, Zhang L, Fu J, Shen W, Fang Z, Dai Y, et al. Fitness and ecological risk of hybrid progenies of wild and herbicide-tolerant soybeans with epsps gene. Front Plant Sci. 2022;13:922215.
Ministry of Agriculture and Rural Affairs of the PRC. Administrative Measures for the safety evaluation of agricultural genetically modified organisms. http://www.moa.gov.cn/ztzl/zjyqwgz/zcfg/202206/t20220607_6401864.htm (2022). Accessed 23 Jul 2024.
Rott M, Lawrence T, Green M. Detection and quantification of roundup ready soy in food samples using conventional and real-time polymerase chain reaction. ACS Symp Ser. 2007;952:13–38.
Zhang L, Liu L, Fang Z, Shen W, Dai Y, Jia R, et al. Fitness changes in wild soybean caused by gene flow from genetically modified soybean. BMC Plant Biol 2. 2023;23424. https://doi.org/10.1186/s12870-023-04398-2.
Stewart CN, Halfhill MD, Warwick SI. Transgene introgression from GM crops to their wild relatives. Nat Rev Genet. 2003;4:806–17.
Lu BR, Yang C. Gene flow from GM rice to its wild relatives: assessing potential ecological consequences. Biotechnol Adv. 2009;27:1083–91.
Lu BR, Yang X, Ellstrand NC. Fitness correlates of crop transgene flow into weedy populations: a case study of weedy rice in China and other examples. Evol Appl. 2016;9:857–70.
Beres ZT, Yang X, Jin L, Zhao W, Mackey DM, Snow AA. Overexpression of a native gene encoding 5-enolpyruvylshikimate-3-phosphate synthase (epsps) may enhance fecundity in Arabidopsis thaliana in the absence of glyphosate. Int J Plant Sci. 2018;179:390–401. https://doi.org/10.1086/696701.
Huang Y, Wang YY, Qiang S, Song X, Dai W. Fitness of F1 hybrids between stacked transgenic rice T1c-19 with cry1C*/bar genes and weedy rice. J Integr Agric. 2019;18:2793–805.
Acknowledgements
Not applicable.
Funding
This research was supported by the National Natural Science Foundation of China (32171656), STI 2030-Major Projects (2022ZD0402105; 2023ZD04062), the Basic Scientific Research Program of National Nonprofit Research Institutes (ZX2023QT010), and the Natural Science Foundation of Jiangsu Province (G rants No BK 20200157).
Author information
Authors and Affiliations
Contributions
L.L. conducted the experiments, analyzed the data, and wrote the manuscript. LL and BL designed the research. L.Z., Z.F., W.S., X.Y., Z.R. and Q.Y. participated in the experiments and assisted in analyzing the data. J.L. and B.L. reviewed and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All experiments and methods were performed in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, L., Zhang, L., Fang, Z. et al. Glyphosate resistance and no fitness cost in backcross offspring of wild soybean and transgenic soybean with epsps gene. BMC Plant Biol 24, 849 (2024). https://doi.org/10.1186/s12870-024-05559-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-024-05559-7