Abstract
Background
The role of the geriatric nutritional risk index (GNRI) as a prognostic factor in intensive care unit (ICU) patients with acute kidney injury (AKI) remains uncertain.
Objectives
The aim of this study was to investigate the impact of the GNRI on mortality outcomes in critically ill patients with AKI.
Methods
For this retrospective study, we included 12,058 patients who were diagnosed with AKI based on ICD-9 codes from the eICU Collaborative Research Database. Based on the values of GNRI, nutrition-related risks were categorized into four groups: major risk (GNRI < 82), moderate risk (82 ≤ GNRI < 92), low risk (92 ≤ GNRI < 98), and no risk (GNRI ≥ 98). Multivariate analysis was used to evaluate the relationship between GNRI and outcomes.
Results
Patients with higher nutrition-related risk tended to be older, female, had lower blood pressure, lower body mass index, and more comorbidities. Multivariate analysis showed GNRI scores were associated with in-hospital mortality. (Major risk vs. No risk: OR, 95% CI: 1.90, 1.54–2.33, P < 0.001, P for trend < 0.001). Moreover, increased nutrition-related risk was negatively associated with the length of hospital stay (Coefficient: -0.033; P < 0.001) and the length of ICU stay (Coefficient: -0.108; P < 0.001). The association between GNRI scores and the risks of in-hospital mortality was consistent in all subgroups.
Conclusions
GNRI serves as a significant nutrition assessment tool that is pivotal to predicting the prognosis of critically ill patients with AKI.
Similar content being viewed by others
Introduction
Malnutrition is a prevalent condition observed in intensive care units (ICUs), affecting approximately 30–50% of critically ill patients [1]. The nutritional status of these patients is often linked to unfavorable clinical outcomes, including higher mortality rates, reduced quality of life, prolonged hospital length of stay (LOS), and increased healthcare costs [2, 3]. The metabolic profile of malnourished patients in the ICU also differs from that of critically ill patients without malnutrition [4]. Early identification and timely intervention of nutritional risks are important in improving the prognosis of ICU patients.
Acute kidney injury (AKI) is a frequently encountered complication in ICU patients, characterized by a sudden decline in kidney function. It is strongly associated with unfavorable outcomes, such as increased readmission rates, higher mortality, extended hospital stays, diminished health-related quality of life, and an elevated risk of developing chronic kidney disease (CKD) and end-stage renal disease [5, 6]. During the progression of kidney disease, the utilization of different nutrients varies, leading to metabolic and nutritional abnormalities [7]. A prospective cohort study on ICU patients with AKI revealed a prevalence of 67% for moderate malnutrition and 15% for severe malnutrition [8]. Timely provision of rational and effective nutritional support was also shown to be important in the management of AKI patients [9]. However, the nutritional status assessment was not a routine evaluation when AKI patients were admitted to ICU. The use of appropriate nutrition assessment tools is of clinical importance to refrain the adverse effects of malnutrition on critically ill AKI patients.
The Geriatric Nutritional Risk Index (GNRI), initially introduced by Bouillanne et al. in 2005, has established itself as a reliable tool for evaluating the nutritional status of patients and identifying those who are susceptible to malnutrition [10]. This metric, with a simple calculation, has shown its value in refining the prognosis of morbidity and mortality in patients with critical illness. In a retrospective study of patients with acute respiratory distress syndrome (ARDS) in ICU, it was found that GNRI upon admission was significantly associated with 30-day mortality [11]. In an observational study of 401 ICU patients, the prognostic significance of GNRI was established in elderly individuals with sepsis [12]. The role of the GNRI as a prognostic factor in ICU patients with AKI remains uncertain. Therefore, the objective of this study was to investigate whether GNRI contributed to identifying critically ill patients with AKI at high risk.
Methods
Population selection criteria
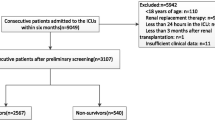
The paper selected patients with AKI based on their ICD-9 code (584*) and used several inclusion criteria to enroll participants. These criteria included the patients being over 18 years of age, the availability of albumin/BMI data, no cancer that could affect survival, and an ICU stay of more than 2 days. The study included a total of 12,058 patients who met these selection criteria (Fig. 1).
Data extraction
The research was conducted using data from the eICU Collaborative Research Database, which included 20,859 admissions from 208 hospitals in the United States during the period of 2014 to 2015. Access to the database was granted to the author through the completion of the Protecting Human Research Participants exam (name: david chou, ID: 11660851). The study’s DOI is https://doi.org/10.13026/C2WM1R. The collected data encompassed various aspects, including demographics, vital signs, diagnoses and comorbidities, laboratory parameters, treatment details, and Acute Physiology and Chronic Health Evaluation IV (APACHE IV) (Supplementary 1).
Grouping and outcomes
GNRI was calculated using the formula: GNRI = 14.89 × albumin + 41.7 × (actual BMI/ideal BMI). The optimal BMI was defined as 22 kg/m2. In cases where a patient’s actual BMI equaled or exceeded the optimal value, the ratio of their actual BMI to the optimal BMI was set at 1. Four risk grades were defined: major risk (GNRI < 82), moderate risk (82 ≤ GNRI < 92), low risk (92 ≤ GNRI < 98), and no risk (GNRI ≥ 98).10 The primary endpoint was in-hospital mortality, while secondary endpoints included the duration of hospital stay (days) and ICU stay (days).
Statistical analysis
Continuous variables with a normal distribution were presented as mean ± standard deviation (SD) and analyzed using analysis of variance (ANOVA) to compare between groups. Skewed continuous variables were described as median with interquartile range (IQR) and compared using the Kruskal-Wallis test. Categorical variables were reported as numbers and percentages and compared between groups using the Chi-square test.
The association between GNRI and in-hospital mortality was assessed using binary logistic regression analysis, with odds ratios (OR) (Supplementary 1) and 95% confidence intervals (CI) reported. A trend analysis was performed (Supplementary 1). Covariates for the multivariable regression model were selected based on significant associations in univariate analysis (P < 0.05). Model 1 was unadjusted, Model 2 included adjustments for age, sex, and ethnicity, and Model 3 included additional adjustments for systolic blood pressure, stroke, chronic kidney disease, atrial fibrillation, respiratory failure, platelet, hemoglobin, alanine aminotransferase (ALT), creatine, dialysis, mechanical ventilation, and APACHE IV, estimated glomerular filtration rate (eGFR). The reference group for the analysis was the no risk group.
The non-linear association between GNRI and in-hospital mortality was assessed using the adjusted restricted cubic spline (RCS) model, which included the variables from Model 3. And four knots (5th, 35th, 65th, and 95th percentile of GNRI) were selected for the analysis.
To examine the relationship between GNRI and secondary outcomes, multiple linear regression was employed. The analysis included the variables from Model 3, with GNRI included as a continuous variable. Reinforcing the robustness of our study results, Poisson regression analysis was additionally conducted.
Subgroup analysis was conducted to assess the association between GNRI and in-hospital mortality across different subgroups, with the calculation of P-values for interaction. In the subgroup analysis, univariate binary logistic regression analysis was employed to determine the odds ratio (OR) values. Patients were categorized into two groups: the “No risk” group (GNRI ≥ 98) served as the reference group, while the “At-risk” group consisted of patients with GNRI < 98.
Statistical significance was set at P < 0.05 for all tests, which were conducted using R software (version 4.2.1, R-project®; R Foundation for Statistical Computing, Vienna, Austria).
Results
Subjects and baseline characteristics
Patients were categorized into four groups based on their GNRI levels. Table 1 provided an overview of the characteristics of each group. Patients in higher-risk groups were predominantly older and female, with lower blood pressure and body mass index. Additionally, they exhibited a higher prevalence of diagnoses and comorbidities, such as congestive heart failure, shock, atrial fibrillation, diabetes, respiratory failure, chronic kidney disease, and sepsis, while having lower rates of acute coronary syndrome and hypertension. Patients with higher nutrition-related risk also displayed elevated levels of white blood cells, ALT, aspartate aminotransferase (AST), blood nitrogen urea, and APACHE IV scores, while having lower levels of red blood cells, platelets, hemoglobin, glucose, and albumin. Furthermore, they received increased vasopressin, antibiotic, mechanical ventilation, and dialysis therapy.
Association between GNRI and outcomes
The overall in-hospital mortality was 18.5%. There was a significant increase in mortality as the nutrition-related risk groups advanced (Major risk vs. No risk: 25.2% vs. 9.1%, P < 0.001) (Table 2). In model 1, higher nutrition-related risk was associated with an increased risk of in-hospital mortality (Major risk vs. No risk: OR, 95% CI: 3.34, 2.80–3.99, P < 0.001). In model 2, the association remained significant (Major risk vs. No risk: OR, 95% CI: 3.18, 2.66–3.80, P < 0.001). In model 3, considering additional confounding variables, the relationship between GNRI and in-hospital mortality weakened, but GNRI remained an independent factor associated with an increased risk of in-hospital mortality (Major risk vs. No risk: OR, 95% CI: 2.03, 1.65–2.50, P < 0.001) (Table 3).
Figure 2 employed the RCS model to depict the association between GNRI and MACE. Model 3, after accounting for relevant confounding factors, substantiated a monotonically decreasing relationship between GNRI and in-hospital mortality (non-linear p = 0.281). A significant reduction in the risk of in-hospital mortality was observed as GNRI levels increased.
Additionally, an elevated nutrition-related risk was linked to prolonged durations of ICU stay (Major risk vs. No risk: 3.8, 2.1–7.5 vs. 2.7, 1.7–4.8, P < 0.001) and hospital stay (Major risk vs. No risk: 10.2, 5.8–18.3 vs. 6.7, 4.0-11.2, P < 0.001) (Table 2). Multiple linear regression analysis revealed a negative association between GNRI and both the length of hospital stay (Coefficient: -0.033; P < 0.001) and the length of ICU stay (Coefficient: -0.108; P < 0.001) (Table 4). The results from Poisson regression analysis corroborate the linear regression findings (Table S1).
Subgroup analysis
The association between nutrition-related risk and in-hospital mortality was found to be positive across all subgroups, with no significant interactions identified in the subgroup analysis, as illustrated in Fig. 3.
Subgroup analysis of the association between in-hospital mortality and nutrition-related risk. Patients were divided into two groups (No risk group, at risk group). No risk group was defined as GNRI ≥ 98 and served as reference group, at risk group was defined as GNRI < 98. Abbreviation: GNRI: geriatric nutritional risk index; OR: odds ratio; CI: confidence interval; APACHE IV: Acute Physiology and Chronic Health Evaluation IV
Discussion
This study highlighted the significance of GNRI, a composite measure involving serum albumin levels and the ratio of body weight to ideal body weight, as a prognostic indicator for AKI patients in the ICU. We found a significant association between low GNRI and increased risks of in-hospital mortality, prolonged ICU stay, and prolonged hospital stay. Furthermore, a monotonically decreasing association between GNRI and in-hospital mortality was observed. These findings emphasized the clinical relevance of GNRI in assessing the prognosis of critically ill patients with AKI, supporting its utility as a nutritional status indicator in this population.
Previous studies have established the prognostic significance of GNRI in various patient populations, including elderly individuals, [10] heart failure patients, [13] those with chronic obstructive pulmonary disease, [14] and cancer patients [15]. Recent investigations have also explored the role of GNRI in individuals with kidney disease. In a recent observational study focusing on patients with CKD, it was found that low GNRI, particularly in conjunction with elevated C-reactive protein levels, was associated with a higher prevalence of severe abdominal aortic calcification [16]. Additionally, a prospective cohort analysis of individuals with moderate to severe CKD discovered that reduced GNRI was substantially linked to a higher risk of development to dialysis, indicating poor prognosis and low quality of life in CKD patients [17]. An expanding body of research has solidified the significance of GNRI as an indicator of nutritional status in the development of AKI. For instance, a study revealed a notable association between GNRI and the incidence of AKI in elderly individuals who underwent cardiac surgery [18]. In a retrospective cross-sectional study, it was reported that a higher incidence of contrast-induced acute kidney injury (CI-AKI) was linked to lower GNRI values in patients undergoing CAG [19]. Additionally, a meta-analysis incorporating 13 observational studies confirmed that reduced GNRI levels were associated with an increased risk of AKI [20]. While prior research has established the connection between malnutrition and the occurrence of AKI, our study diverges by examining the relationship between GNRI and the clinical outcome of critically ill patients who have developed AKI. Furthermore, malnutrition was found to contribute to unfavorable prognoses in individuals with AKI. From another new perspective, more clinical evidence on the relationship between AKI and GNRI is provided. Moreover, across all subgroups, an elevated nutrition-related risk was strongly associated with increased in-hospital mortality among AKI patients in the ICU, thus confirming the predictive reliability of GNRI for assessing prognosis. Our study strongly supports the need for physicians to identify malnutrition with AKI in their daily practice.
Although GNRI itself cannot be directly modified upon admission, several measures can improve patient prognosis. Firstly, early and comprehensive nutritional assessments should be conducted, followed by the development of individualized nutrition plans based on each patient’s specific needs. For patients unable to meet their nutritional requirements through normal diet alone, high-calorie and high-protein oral supplements or enteral nutrition should be considered to enhance their nutritional status. Additionally, dietitians and physical therapists should be integral members of the multidisciplinary care team, providing expert guidance and tailored exercise programs to maintain muscle mass and function. Strict glycemic control and inflammation management are also crucial to mitigate the negative impact of malnutrition on patient outcomes. Continuous training and education for ICU staff on nutritional management, as well as educating patients and their families on the importance of nutrition, can significantly enhance care quality. Finally, implementing standardized nutritional protocols ensures that all ICU patients, especially those at high nutritional risk, receive consistent and effective nutritional care. These comprehensive measures are expected to significantly reduce the risk of adverse outcomes in high-GNRI patients and improve their overall prognosis.
Several potential pathological mechanisms may explain the results of our findings. One possible mechanism underlying the influence of nutritional risk on the prognosis of AKI patients could be attributed to the potential upregulation of inflammation. Malnutrition was shown to up-regulate inflammation, disrupt immune cell function, and promote immunosuppression [21, 22]. In alignment with the findings of this study, patients with elevated GNRI exhibited notably higher baseline levels of white blood cells. High levels of proinflammatory status can significantly worsen nutritional status. Since AKI patients in ICU are often in a heightened proinflammatory state, the inflammation is further aggravated in malnourished patients, leading to a higher risk of death. Moreover, nutritional risks may influence the prognosis of patients with AKI by inducing metabolic disorders. Malnutrition is associated with different metabolic characteristics in the early stages of critical illness [4]. Alterations in metabolites related to redox states and increased degradation products of ATP may contribute to heightened cytotoxicity [4]. Due to acute loss of renal balance, patients with AKI are particularly vulnerable to metabolic disorders, including disturbances in blood sugar, lipids, electrolytes, and acid-base balance. Malnutrition in AKI patients can result in aggravated metabolic disorders and increased risks of morbidity and mortality. This is also consistent with our finding that AKI patients with lower GNRI had significantly higher levels of glucose and potassium.
This study presented the initial findings regarding the correlation between GNRI and mortality among AKI patients in the ICU. We found that GNRI independently predicted the risk of in-hospital mortality, prolonged length of ICU stays, and hospital stay among AKI patients in the ICU. Moreover, in this study, we assessed the interaction between GNRI groups and stratified factors, including age (< 65/≥65 years), sex(male/female), chronic kidney disease(with/without), mechanical ventilation(with/without), dialysis(with/without) and APACHE IV (< 70/≥70 years). The results of subgroup analysis indicated that our findings were consistent in all subgroups.
Despite the importance being mentioned, some limitations need to be recognized. First, the nature of the retrospective study prohibits causal interpretations of the association and cannot exclude the effect of inherent bias. Moreover, the data used to conduct the study were collected from 2014 to 2015, which was approximately eight years prior to the present day. This temporal gap may limit the contemporary relevance and applicability of the findings. In addition, data on time-dependent changes in BMI and albumin were not obtained, so we were unable to examine the relationship between changes in albumin and BMI and the outcomes. Third, due to missing data, inflammatory markers (such as PCT, CRP, ferritin, IL-6, and D-dimer) were not included. As inflammation is a possible mechanism that explains malnutrition increases the risk of mortality in AKI patients, this can be a potential confounding factor for the association in this study. The absence of specific timing data on patient events precluded the use of Cox regression in our study. Finally, possible subsequent nutritional treatments which may affect nutritional scores were not considered.
The evaluation of malnutrition is of utmost importance in predicting the prognosis of critically ill patients with AKI. A low GNRI level can serve as a valuable indicator of unfavorable outcomes in AKI patients within the ICU environment. The nutritional risks of critically ill AKI patients should not be overlooked, and the utilization of GNRI can effectively identify AKI patients with malnutrition who are at a heightened risk of mortality in the ICU. Given the fact that malnutrition assessment is not routinely evaluated in the ICU, improving nutritional support for critically ill patients with AKI, particularly those presenting with a low GNRI upon admission to the ICU, is of utmost importance.
Data availability
The dataset supporting the conclusions of this article is available from the eICU Collaborative Research Database (DOI: https://doi.org/10.13026/C2WM1R). The authors have been granted access to the database through the completion of the Protecting Human Research Participants exam (name: david chou, ID: 11660851). Please contact the corresponding author for more information about the dataset.
References
Powers J, Samaan K. Malnutrition in the ICU patient population. Crit Care Nurs Clin North Am Jun. 2014;26(2):227–42. https://doi.org/10.1016/j.ccell.2014.01.003.
Lim SL, Ong KC, Chan YH, Loke WC, Ferguson M, Daniels L. Malnutrition and its impact on cost of hospitalization, length of stay, readmission and 3-year mortality. Clin Nutr Jun. 2012;31(3):345–50. https://doi.org/10.1016/j.clnu.2011.11.001.
Correia MI, Waitzberg DL. The impact of malnutrition on morbidity, mortality, length of hospital stay and costs evaluated through a multivariate model analysis. Clin Nutr Jun. 2003;22(3):235–9. https://doi.org/10.1016/s0261-5614(02)00215-7.
Mogensen KM, Lasky-Su J, Rogers AJ, et al. Metabolites Associated with Malnutrition in the Intensive Care Unit are also Associated with 28-Day mortality. JPEN J Parenter Enter Nutr Feb. 2017;41(2):188–97. https://doi.org/10.1177/0148607116656164.
Mercado MG, Smith DK, Guard EL. Acute kidney Injury: diagnosis and management. Am Fam Physician Dec. 2019;1(11):687–94.
Shaikhouni S, Yessayan L. Management of Acute kidney Injury/Renal replacement therapy in the Intensive Care Unit. Surg Clin North Am Feb. 2022;102(1):181–98. https://doi.org/10.1016/j.suc.2021.09.013.
Fiaccadori E, Cremaschi E. Nutritional assessment and support in acute kidney injury. Curr Opin Crit Care Dec. 2009;15(6):474–80. https://doi.org/10.1097/MCC.0b013e328332f6b2.
Guimaraes SM, Lima EQ, Cipullo JP, Lobo SM, Burdmann EA. Low insulin-like growth factor-1 and hypocholesterolemia as mortality predictors in acute kidney injury in the intensive care unit. Crit Care Med Dec. 2008;36(12):3165–70. https://doi.org/10.1097/CCM.0b013e318186ab70.
Antoniotti R, Sabatino A, Regolisti G et al. [Nutritional support in acute kidney injury]. G Ital Nefrol Mar-Apr 2014;31(2)Supporto nutrizionale nel danno renale acuto.
Bouillanne O, Morineau G, Dupont C, et al. Geriatric nutritional risk index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr Oct. 2005;82(4):777–83. https://doi.org/10.1093/ajcn/82.4.777.
Yoo JW, Ju S, Lee SJ, Cho YJ, Lee JD, Kim HC. Geriatric nutritional risk index is associated with 30-day mortality in patients with acute respiratory distress syndrome. Med (Baltimore) Jun. 2020;19(25):e20671. https://doi.org/10.1097/MD.0000000000020671.
Lee JS, Choi HS, Ko YG, Yun DH. Performance of the Geriatric Nutritional Risk Index in predicting 28-day hospital mortality in older adult patients with sepsis. Clin Nutr Oct. 2013;32(5):843–8. https://doi.org/10.1016/j.clnu.2013.01.007.
Kinugasa Y, Kato M, Sugihara S, et al. Geriatric nutritional risk index predicts functional dependency and mortality in patients with heart failure with preserved ejection fraction. Circ J. 2013;77(3):705–11. https://doi.org/10.1253/circj.cj-12-1091.
Matsumura T, Mitani Y, Oki Y, et al. Comparison of Geriatric Nutritional Risk Index scores on physical performance among elderly patients with chronic obstructive pulmonary disease. Heart Lung Nov-Dec. 2015;44(6):534–8. https://doi.org/10.1016/j.hrtlng.2015.08.004.
Lidoriki I, Schizas D, Frountzas M, et al. GNRI as a prognostic factor for outcomes in Cancer patients: a systematic review of the literature. Nutr Cancer. 2021;73(3):391–403. https://doi.org/10.1080/01635581.2020.1756350.
Harada K, Suzuki S, Ishii H, et al. Nutrition Status predicts severity of vascular calcification in non-dialyzed chronic kidney disease. Circ J Feb. 2017;24(3):316–21. https://doi.org/10.1253/circj.CJ-16-0911.
Kuo I-C, Huang J-C, Wu P-Y, Chen S-C, Chang J-M, Chen H-C. A low Geriatric Nutrition Risk Index is Associated with Progression to Dialysis in patients with chronic kidney disease. Nutrients. 2017;9(11):1228.
Usta S, Engin M. Investigation of the effects of preoperative nutritional status scores on renal injury after cardiac surgery in elderly patients. Eur Rev Med Pharmacol Sci Dec. 2022;26(24):9345–52. https://doi.org/10.26355/eurrev_202212_30685.
Li D, Chen Z, He W, et al. The association between nutritional risk and contrast-induced acute kidney injury in patients undergoing coronary angiography: a cross-sectional study. Nutr J Sep. 2022;16(1):56. https://doi.org/10.1186/s12937-022-00810-z.
Kazemian S, Tavolinejad H, Rashedi S, Yarahmadi P, Farrokhpour H, Kolte D. Meta-analysis on the Association between Nutritional Status and outcomes after Transcatheter aortic valve implantation. Am J Cardiol. Jan 2023;1:186:109–16. https://doi.org/10.1016/j.amjcard.2022.10.016.
Kalantar-Zadeh K, Stenvinkel P, Bross R, et al. Kidney insufficiency and nutrient-based modulation of inflammation. Curr Opin Clin Nutr Metab Care Jul. 2005;8(4):388–96. https://doi.org/10.1097/01.mco.0000172578.56396.9e.
Alwarawrah Y, Kiernan K, MacIver NJ. Changes in Nutritional Status Impact Immune Cell metabolism and function. Front Immunol. 2018;9:1055. https://doi.org/10.3389/fimmu.2018.01055.
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
Dong Zhao: Conceptualization, Writing- Original draft preparation. Dawei Zhou: Investigation, Methodology. Tong Li: Software, Visualization. Chao Wang: Data Curation, Supervision. Shuyang Fei: Writing - Review & Editing. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Informed consent for data use has been described in previous studies with the subject’s own authorization. Additionally, the current research protocol has been approved by the Institutional Review Committee of Beijing Tongren hospital, and the entire research process follows the Declaration of Helsinki. Informed consent was obtained from all the subjects and/or their legal guardians.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhao, D., Zhou, D., Li, T. et al. The relationship between Geriatric Nutritional Risk Index (GNRI) and in-hospital mortality in critically ill patients with Acute Kidney Injury (AKI). BMC Anesthesiol 24, 313 (2024). https://doi.org/10.1186/s12871-024-02689-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12871-024-02689-1